Abstract
Background
Liquid based cytology (LBC) has been widely used for cervical cancer screening. Despite numerous studies and systematic reviews, few large studies have focused on biopsy-confirmed cervical lesions and controversy remains about its diagnostic accuracy. The aim of our study was to assess LBC for detecting biopsy-confirmed cervical intraepithelial neoplasia (CIN) and cancer.
Methods
We performed a pooled analysis of LBC using data from 13 population-based, cross-sectional, cervical-cancer screening studies performed in China from 1999 to 2008. Participants (n = 26782) received LBC and HPV testing. Screen-positive women were referred for colposcopy and biopsy. We analyzed the accuracy of LBC for detecting biopsy-confirmed CIN2 or worse lesion (CIN2+) as well as CIN3 or worse lesion (CIN3+).
Results
Of 25830 women included in the analysis, CIN2+ was found in 107/2612(4.1%) with ASC, 142/923 (15.4%) with LSIL, 512/784 (65.3%) with HSIL, 29/30 (96.7%) with SCC, 4/27(14.8%) with AGC, and 0.4% (85/21454) with normal cytology results. No invasive cancers had ASC, AGC or cytological normal slides. The overall sensitivity, specificity, PPV, NPV and accuracy of LBC for detecting CIN2+ were 81.0%, 95.4%, 38.3%, 99.3 % and 94.9% respectively. Although HC2 was more sensitive than LBC, the specificity, PPV and overall accuracy of LBC were higher than those of HC2, at 85.2%, 18.6% and 85.5%, respectively.
Conclusions
The results indicate that performance of LBC can effectively predict a risk of existing CIN2+ and may be a good screening tool for cervical cancer prevention in a developing country.
Keywords: Pool analysis, liquid-based cytology, Population-based, cervical cancer, screening
Introduction
Because of China’s large population and the imbalance of economic development, the disease burden of cervical cancer varies greatly in China. Women in urban areas may have the opportunity to access cervical screening. However, in the rural areas, due to lack of medical resources, the incidence of cervical cancer is relatively high and has reached 81 per hundred thousand in some areas [1]. To explore a strategy for cervical cancer screening suited to conditions in China, the Cancer Institute and Hospital of the Chinese Academy of Medical Science (CICAMS) in Beijing, China, in collaboration with institutions at home and aboard, conducted a series of population based, cross sectional cervical cancer screening studies from 1999–2008 [2–15].
LBC was first introduced into China in 1999, and was evaluated in all the studies. LBC is now widely used for cervical cancer screening in China. LBC is preferred to conventional cytology for its lower rate of unsatisfactory slides, shorter time needed for interpretation, and the ability to use the same sample for HPV and other molecular testing. Although some studies have reported that LBC increased the detection of cytological abnormalities and showed improved sensitivities, few large studies have focused on biopsy-confirmed cervical lesions[16, 17], and none were based on an indigent population at high risk for the development of cervical cancer. To better delineate cervical cancer screening diagnoses and outcomes of abnormal LBC, we performed a pooled analysis of LBC screening at CICAMS using the data from 13 population-based, cross-sectional, cervical-cancer screening studies. We assessed the overall accuracy of LBC for detection of biopsy-confirmed cervical intraepithelial neoplasia (CIN) and cancer in high incidence areas in China.
Materials and Methods
Participants
CICAMS in collaboration with the Cleveland Clinic (Cleveland, Ohio, USA), Program for Appropriate Technology in Health (PATH) (Seattle, Washington, USA), and the International Agency for Research on Cancer (IARC) (Lyon, France) screened women from 1999 to 2008. Our pooled analysis used individual patient data from 13 cross-sectional, population-based studies that had LBC done at CICAMS. The 13 studies were: 4 projects of Shanxi Province Cervical Cancer Screening Study (SPOCCS 1, SPOCCS 2, SPOCCS 3-Henan, and SPOCCS 3-Xiangyuan) performed with the Cleveland Clinic from 1999 to 2006[2, 3, 5–10, 15], 5 projects of the Screening Technologies to Advance Rapid Testing (START 2003, 2004, 2005, 2006 and 2007) done with PATH[11, 15], 1 project performed in 2004 with IARC in Yangcheng, Shanxi province[4, 15], the FastHPV trial in 2007[14, 15], the HPV and CIN Prevalence Survey performed in Jiangsu in 2008[12, 15], and the HC2 clinical trial in 2008[13, 15]. Eligible women were 15–59 years old, sexually active, not pregnant, had an intact uterus, and had no history of CIN, cervical cancer or pelvic radiation. None had been screened for cervical cancer in the past 5 years, and all provided written informed consent. Recruitment for all studies was based on community lists to minimize selection bias. The Human Subjects Review Boards of CICAMS, Cleveland Clinic, PATH, and IARC approved these studies.
Procedures
Women included in the pooled analysis all concurrently received LBC (SurePath, BD Diagnostics, Franklin Lakes, NJ, USA or ThinPrep, Hologic, Bedford, MA, USA), HPV DNA testing with Hybrid Capture 2 (HC2) assay (Qiagen, Gaithersburg, MD, USA), and direct visual inspection of the cervix after the application of 5% acetic acid solution (VIA). The study methods for each individual study have been outlined in detail elsewhere [2–15], and are described briefly below.
All participants underwent collection of cervical specimens and VIA in the local health centers. All spesimens were collected by physicians. Specimens for HPV tests were obtained from the endocervix with a conical-shaped brush. The conical brushes were placed in Universal Collection Media (UCM, Digene Corp.). Specimens for liquid-based cytology were obtained with a broom-type sampling device (Cervex-Brush; Rovers Medical Devices B.V., Oss, The Netherlands), and then transferred into a vial containing a fixative liquid. For processing the liquid-based cervical samples, 9 studies had chosen to use the SurePath slide preparation technique in which cervical samples were placed into SurePath Preservative Fluid; 3 studies (SPOCCS 1, START 2004, and the HPV and CIN prevalence study) had chosen to use ThinPrep slide preparation technique in which cervical samples were placed in PreservCyt solution; and in 1 study (START 2003) samples from the first half of screening participants were placed in SurePath Preservative Fluid and samples from the second half of screening participants were placed in PreservCyt solution[18]. All Specimens were stored at room temperature and sent weekly to CICAMS (Beijing, China) to be processed in a centralized laboratory. Following the collection of cervical specimens, all participants underwent VIA. The participants also used VILI (visual inspection with lugol’s iodine) if VIA was negative In six of the studies (START 2004, START 2005, START 2006, IARC-Yangcheng, FastHPV, HC2 trial). The definition of positive VIA and VILI results had been described elsewhere in detail [11]. All visual inspection was done by trained Chinese gynecologists.
In the centralized laboratory of CICAMS, HPV tests and LBC were performed. For LBC slide systems ThinPrep2000 and SurePath, were used respectively. Cytology results were reported according to the Bethesda System (TBS). The cytological classifications were: normal; atypical squamous cells (ASC); low-grade squamous intraepithelial lesion (LSIL); high-grade squamous intraepithelial lesion (HSIL); squamous cell carcinoma (SCC); atypical glandular cells (AGC); adenocarcinoma in situ (AIS); or adenocarcinoma. To mach the data, ASC represents atypical squamous cells- undetermined significance (ASC-US) for the projects conducted before 2003, that is, SPOCCS 1 and SPOCCS 2, according to TBS 1991; for the projects conducted from 2003, ASC represents the combined categories of ASC-US and ASC-H (atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion), in accordance with TBS 2001[19].
The cytological diagnoses were made by cytopathologists of CICAMS. In addition, in the 5 START studies, all abnormal cytology slides and a proportion of negative slides that were randomly selected were reviewed by an external international expert; any disagreements were reviewed together with the external consultant and the cytopathologists of CICAMS, and a consensus was reached for the final diagnoses. For HPV tests, carcinogenic HPV DNA testing was done with the high-risk probe set of HC2, which detects a pool of 13 carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). HPV DNA positive was defined according to the manufacturer’s recommendation positive cut point of 1.0 relative light units per cutoff (1.0 RLU/CO; approximately equal to 1.0 pg DNA per mL)[2 ] In addition to using the specimen collected directly by physicians, 7 studies (14329 samples) used the liquid remaining after LBC slide preparation for HPV DNA testing (SPOCCS 1, all 5 START studies, and IARC-Yangcheng).
Regardless of screening status, all women in SPOCCS 1 underwent colposcopy and biopsy after VIA. In SPOCCS 2, SPOCCS 3, START 2003 and START2004, women who were positive on any screening test were referred for colposcopyand biopsy, and in the other studies, women underwent colposcopyand biopsy if they had ASCUS and were HPV positive, or if their cytology was ASC-H+, that is, ASC-H, LSIL, HSIL, SCC, AGC, AIS, or adenocarcinoma. Directed biopsy was taken from all visible cervical lesions. When the four-quadrant punch biopsy method was indicated, biopsies were taken at positions of 2, 4, 8, and 10 o’clock depending on the quadrant and an endocervical curettage was performed. Histological diagnoses were categorized as negative, CIN1, CIN2, CIN3, SCC, AIS, or adenocarcinoma. All of the biopsies were performed within 2 months of cytology sampling in the local health centers. Biopsydiagnoses were made by pathologists of CICAMS, were reviewed by international experts in 11 studies.
Verification of disease endpoint
Our study combined individual raw data from 13 studies and estimated pooled sensitivity and specificity for the detection of histological CIN2+ (that is, lesions CIN2 or worse) and CIN3+ (CIN3 or worse). In all the studies, the clinical results and other test results were masked from the laboratory personnel that did the HPV analyses and the cytopathologists and histopathologists who did the reviews and made diagnoses did not know the results of other tests. Results of the specific screening tests were masked from colposcopists, but they were aware that one of the tests was positive.
To minimize verification bias, we unified the criteria to verify disease status for final diagnoses and applied it to all participants. The gold standard diagnosis was histologically confirmed biopsy result. However, women with no biopsy results, but negative or ASC-US on cytology and negative for HPV DNA, were deemed to be true negatives (very low risk for high-grade lesions) on the basis of findings from SPOCCS1 that there was only one CIN 2 case and no CIN 3+ cases of those women (one [0·07%] of 1511). Women without a biopsy were also categorized as disease negative if they were positive for HPV or had an inadequate HPV test, but had negative cytology and a negative colposcopic examination. Women who had no biopsy were judged as having incomplete data if they had ASC-US and were positive or inadequate HPV; had ASC-H+; were HPV positive with negative cytology, but had missing or positive colposcopy; had unsatisfactorycytology results; or had inadequate HPV testing results (that is, the specimen was not sufficient for HC2 testing) in circumstances other when both cytology and colposcopy was negative.
Statistical analysis
We assessed the accuracy of LBC testing for the detection of CIN2+ and CIN3+. Women with incomplete data were excluded from the analyses. Sensitivities were pooled with fixed-effect model, and specificities were pooled with random-effect model since inter study heterogeneity was statistically significant. We used forest plots to display the variations of sensitivity and specificity in the individual studies and pooled measures. Q test and I2 test were used to assess the heterogeneity between studies. Sensitivities, specificities, positive predictive values, and negative predictive values were calculated. Pooled-analyses were done with Meta-Disc 1·4 (Meta-analysis of Diagnostic and Screening Tests, version 1·4). The corrected accuracy was computed with the estimated number of cases where the lesion was CIN2+ and CIN3+. Trends in pathology with cytological results were calculated using the Cochran Armitage trend test. Continuous variables were examined by calculating the means, medians, and SDs. A P-value of less than or equal to 0.05 was considered statistically significant. R 2.11.1 software (www.r-project.org) was used for all other analyses.
Result
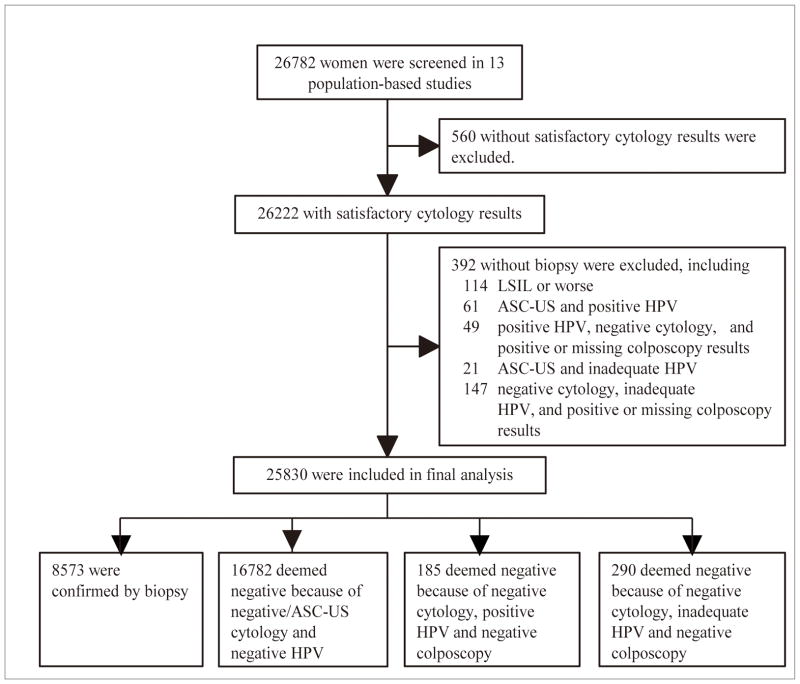
26782 women from 13 population-based studies were screened. Of these, 560 (2.1%) women were excluded because they did not have satisfactory cytology results (Figure 1). Altogether, 26222 women had satisfactory cytological results. The mean age of the women who were included in the cytologic analysis was 40.07±6.32 years old (range from18 to 59); 1063 women were <30 years old and 1497 women were >50 years old. The majority of women were from rural areas (26116/26222, 99.6%).
Figure 1.
Flow chart of study participants
The LBC diagnoses for each project are shown in Table 1. The rate of abnormal cytology, including ASC, LSIL, HSIL, AGC and SCC, was 17.4% among 26222 women, and ranged from 8.9% in the START 2005 trial to 25.7% in the SPOCCS 1 trial. The ASC rate was 10.3%, ranging from 6.3% in START 2005 to 14.9% in SPOCCS 1; the rate for LSIL, HSIL, and SCC combined was 7.1%, ranging from 2.6% in START 2005 to 10.9% in SPOCCS 1. For AGC, the overall rate was 0.1%. The ratio for ASC compared to LSIL, HSIL and SCC combined was 1.5 (range 1.1 – 3.2).
Table 1.
Reporting rates of LBC for various Bethesda categories by project
| Project (year; location) | LBC, n (%)
|
||||||
|---|---|---|---|---|---|---|---|
| Normal | ※ ASC | LSIL | HSIL | AGC | SCC | Total | |
| SPOCCS 1 (1999; Xiangyuan County, Shanxi Province) | 1480 (74.26) | 296 (14.85) | 91 (4.57) | 96 (4.82) | 18 (0.90) | 12 (0.60) | 1993 (100.00) |
| SPOCCS 2 (2001;Xiangyuan andYangcheng County, Shanxi Province) | 6643 (78.18) | 990 (11.65) | 535 (6.30) | 307 (3.61) | 15 (0.18) | 7 (0.08) | 8497 (100.00) |
| SPOCCS 3-Henan (2006; Ximi, Henan Province) | 787 (89.53) | 67 (7.62) | 13 (1.48) | 10 (1.14) | 0 (0.00) | 2 (0.23) | 879 (100.00) |
| SPOCCS 3-Xiangyuan (2006; Xiangyuan County, Shanxi Province) | 734 (83.03) | 114 (12.90) | 16 (1.81) | 19 (2.15) | 0 (0.00) | 1 (0.11) | 884 (100.00) |
| START 2003 (2003; Xiangyuan County, Shanxi Province) | 1666 (83.80) | 202 (10.16) | 52 (2.62) | 62 (3.12) | 0 (0.00) | 6 (0.30) | 1988 (100.00) |
| START 2004 (2004; Xiushui County, Jiangxi Province) | 2017 (85.68) | 184 (7.82) | 49 (2.08) | 103 (4.38) | 0 (0.00) | 1 (0.04) | 2354 (100.00) |
| START 2005 (2005; Wudu County, Gansu Province) | 1863 (91.06) | 129 (6.30) | 30 (1.47) | 22 (1.08) | 1 (0.05) | 1 (0.05) | 2046 (100.00) |
| START 2006 (2006; Qinxian County, Shanxi Province) | 2000 (85.14) | 240 (10.22) | 51 (2.17) | 58 (2.47) | 0 (0.00) | 0 (0.00) | 2349 (100.00) |
| START 2007 (2007; Xiangyuan and Wuxiang County, Shanxi) | 2018 (86.24) | 214 (9.15) | 46 (1.97) | 62 (2.65) | 0 (0.00) | 0 (0.00) | 2340 (100.00) |
| IARC-Yangcheng (2004; Yangcheng County, Shanxi Province) | 603 (81.93) | 81 (11.01) | 20 (2.72) | 30 (4.08) | 2 (0.27) | 0 (0.00) | 736 (100.00) |
| FastHPV trial (2007; Qinxian County, Shanxi Province) | 674 (82.40) | 96 (11.74) | 16 (1.96) | 30 (3.67) | 1 (0.12) | 1 (0.12) | 818 (100.000 |
| Prevalence survey (2008; Binhai and Jintan County, Xuzhou City, Jiangsu Province) | 257 (85.38) | 28 (9.30) | 10 (3.32) | 5 (1.66) | 1 (0.33) | 0 (0.00) | 301 (100.00) |
| HC2 trial (2008; Xiangyuan County, Shanxi Province) | 909 (87.66) | 69 (6.65) | 36 (3.47) | 22 (2.12) | 1 (0.10) | 0 (0.00) | 1037 (100.00) |
| Total | 21651 (82.57) | 2710 (10.33) | 965 (3.68) | 826 (3.15) | 39 (0.15) | 31 (0.12) | 26222 (100.00) |
To mach the data, ASC represents ASC-US for the projects conducted before 2003, according to TBS 1991; for the projects conducted from 2003, ASC represents the combined categories of ASC-US and ASC-H, in accordance with TBS 2001.
Among 26222 participants with satisfactory cytology results, 392 without biopsy results were excluded (Figure 1). 25830 women were included in the final analysis. The presence of CIN and SCC according to cytology diagnosis in studies that used TBS 1991and TBS 2001 terminology is shown in Tables 2 and 3, respectively. The overall CIN2+ rate, the rate of LSIL, HSIL and SCC combined, and LSIL/HSIL+ (including HSIL and SCC) ratio prior to 2003 were 4.4%, 10.0% and 1.5 respectively, and were higher than those of the studies conducted from 2003 to 2008 (2.7%, 4.5% and 0.8, respectively, p<0.0001). Using data from all 13 studies, 3.4% of women had CIN2+. CIN 2 +was found in 107/2612(4.1%) with ASC, 142/923 (15.4%) with LSIL, 512/784 (65.3%) with HSIL, 29/30 (96.7%) with SCC and 4/27(14.8%) with AGC. No histologic adenocarcinoma was identified. Of 21454 women with normal cytology results, 0.4% (85/21454) had a CIN2 or CIN3 diagnosis. No invasive cancers had ASC, AGC or cytologically normal slides.
Table 2.
Correspondence between LBC diagnoses using TBS 1991 and outcomes of biopsy for the 2 projects before 2003.
| LBC | Biopsy, n (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Normal | CIN1 | CIN2 | CIN3 | SCC | Total | |
| Normal | 7914 (97.72) | 136 (1.68) | 37 (0.46) | 12 (0.15) | 0 (0.00) | 8099 (100.00) |
| ASC-US | 1158 (90.40) | 83 (6.48) | 26 (2.03) | 14 (1.09) | 0 (0.00) | 1281 (100.00) |
| LSIL | 351 (56.52) | 171 (27.54) | 57 (9.18) | 41 (6.60) | 1 (0.16) | 621 (100.00) |
| HSIL | 75 (18.61) | 76 (18.86) | 91 (22.58) | 138 (34.24) | 23 (5.71) | 403 (100.00) |
| AGC | 18 (81.82) | 1 (4.55) | 2 (9.09) | 1 (4.55) | 0 (0.00) | 22 (100.00) |
| SCC | 0 (0.00) | 1 (5.26) | 3 (15.79) | 6 (31.58) | 9 (47.37) | 19 (100.00) |
|
| ||||||
| Total | 9516 (91.11) | 468 (4.48) | 216 (2.07) | 212 (2.03) | 33 (0.32) | 10445 (100.00) |
Table 3.
Correspondence between LBC diagnoses using TBS 2001 and outcomes of biopsy for the 11 projects conducted from 2003–2008.
| LBC | Biopsy, n (%)
|
|||||
|---|---|---|---|---|---|---|
| Normal | CIN1 | CIN2 | CIN3 | SCC | Total | |
| Normal | 13169 (98.61) | 150 (1.12) | 25 (0.19) | 11 (0.08) | 0 (0.00) | 13355 (100.00) |
| ASC-US | 1084 (87.21) | 119 (9.57) | 24 (1.93) | 16 (1.29) | 0 (0.00) | 1243 (100.00) |
| ASC-H | 51 (57.95) | 10 (11.36) | 17 (19.32) | 10 (11.36) | 0 (0.00) | 88 (100.00) |
| LSIL | 138 (45.70) | 121 (40.07) | 33 (10.93) | 10 (3.31) | 0 (0.00) | 302 (100.00) |
| HSIL | 68 (17.85) | 53 (13.91) | 99 (25.98) | 153 (40.16) | 8 (2.10) | 381 (100.00) |
| AGC | 4 (80.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 5 (100.00) |
| SCC | 0 (0.00) | 0 (0.00) | 1 (9.09) | 3 (27.27) | 7 (63.64) | 11 (100.00) |
|
| ||||||
| Total | 14514 (94.34) | 453 (2.94) | 199 (1.29) | 204 (1.33) | 15 (0.10) | 15385 (100.00) |
Excluding cases with AGC, the CIN2+ rate generally increased with cytology grade (chi-square=7313.4, P<0.0001). In the 11 studies that used TBS 2001 classification, CIN2+ was present in 3.2% of cases withASC-US and in 30.7% of women with ASC-H.
The sensitivities and specificities of LBC testing for detecting CIN2+ are shown in Figure 2 and for detecting CIN3+ in Figure 3. For the 2 studies using TBS 1991 terminology, the threshold for a positive LBC test was LSIL (that is, either LSIL, HSIL, SCC, AGC, AIS, or adenocarcinoma) and for the other 11 studies using TBS 2001, it was ASC-H (that is, ASC-H, LSIL, HSIL, SCC, AGC, AIS, or adenocarcinoma). The pooled sensitivities for CIN2+ and CIN3+ were 81% and 89% respectively. The sensitivity varied little by project. The pooled specificity of LBC for CIN2+ was 95%, with 11 of 13 projects having specificities of 96% or above. The pooled specificity of LBC for CIN3+ was 94%, with 10 of 13 studies having specificities of 95% or above. Specificities varied significantly between studies done before 2003 and those done from 2003 for both CIN 2+ and CIN3+ (both p<0·0001). The pooled specificities of LBC for both CIN2+ and CIN3+ had narrow confidence intervals (CI) which varied less than 2% (95%CI: 95% - 96% for CIN2, and 94% to 95% for CIN3).
Figure 2.
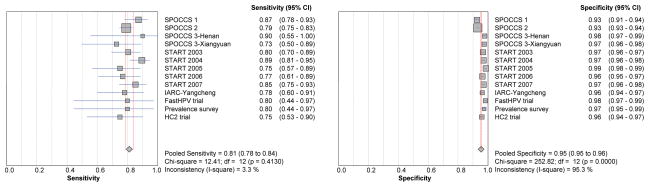
Forest plots of pooled and individual study sensitivities and specificities of LBC for CIN2+
Figure 3.
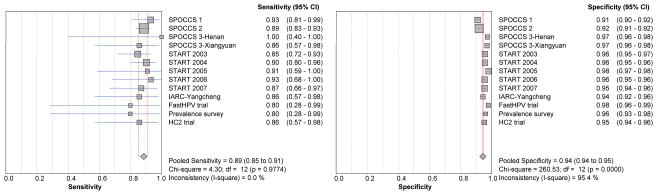
Forest plots of pooled and individual study sensitivities and specificities of LBC for CIN3+
To compare the accuracy of LBC and HC2 testing, the results in the 25404/25830 women who had adequate HPV testing and satisfactory LBC were examined. Although the sensitivities of HC2 for detecting CIN2+ and CIN3+ were significantly higher than the sensitivities of LBC (all p values <0.0001), the specificities, PPV and accuracy of LBC for detecting CIN2+ and CIN3+ were greater than those of HC2 (p<0.0001) (Table 4).
Table 4.
Accuracy of LBC compared to HC2 testing for detecting CIN2+ and CIN3+
| Detecting | Sensitivity % 95%CI | Specificity % 95%CI | PPV % 95%CI | NPV % 95%CI | Yodden % 95%CI | Accuracy % 95%CI |
|---|---|---|---|---|---|---|
| LBC (N=25404) | ||||||
| CIN2+ | 81.0 | 95.4 | 38.3 | 99.3 | 76.4 | 94.9 |
| 78.2–83.5 | 95.1–95.7 | 36.0–40.5 | 99.2–99.4 | 73.3–79.2 | 94.6–95.2 | |
| CIN3+ | 88.5 | 94.3 | 22.0 | 99.8 | 82.8 | 94.2 |
| 85.2–91.3 | 94.0–94.6 | 20.1–24.0 | 99.7–99.8 | 79.2–85.9 | 93.9–94.5 | |
| HC2 (N=25404) | ||||||
| CIN2+ | 96.3 | 85.2 | 18.6 | 99.9 | 81.5 | 85.5 |
| 94.8–97.4 | 84.7–85.6 | 17.4–19.7 | 99.8–99.9 | 79.5–83.1 | 85.1–86.0 | |
| CIN3+ | 97.4 | 83.9 | 9.9 | 99.9 | 81.2 | 84.1 |
| 95.4–98.6 | 83.4–84.3 | 9.0–10.8 | 99.9–100.0 | 78.8–82.9 | 83.6–84.5 |
In 10656 cases, HPV testing was performed on the liquid that remained after a sample of the LBC was removed for cytology, and in these cases, the unsatisfactoryrate was 0.1% (9/10656). The accuracy of HPV testing performed on the remaining liquid sample was similar to that performed on the physician obtained cervical swabs (sensitivity: 94.9%, specificity: 88.3%, Yodden: 83.9%, Accuracy: 88.5%, PPV: 15.9%, NPV: 99.9% for detecting CIN2+).
Discussion
This study with pooled analysis from multiple studies performed in China and including large numbers of women with abnormal cytology results is, to our knowledge, the first report of a large-scale population-based study to examine the diagnostic accuracy of LBC as a primary screening tool for histologically confirmed CIN2+ and CIN3+ detection in China.
Over 99% of the women in this study lived in rural areas. None of the study participants, regardless of residence, had undergone cervical screening in the 5 years prior to study enrollment. The prevalence of CIN2+ ranged from 1.2% to 4.4%. These rates are least 8 times higher than those reported in developed countries[20].
The unsatisfactory rate for LBC specimens in our study was 2.1%, which is consistent with other studies, and lower than that observed in the studies using conventional Pap smears [16, 21].
Our overall rates for ASC (10.3%) and for LSIL, HSIL and SCC combined (7.1%) were higher than those reported by other studies[21]. Nevertheless, the ratio of ASC to LSIL, HSIL and SCC combined, which was 1.52 in this study, is consistent with most high-quality studies[21], and indicates that the higher rates of abnormal cytology reports in our center were unlikely due to over-interpretations.
As not unexpected with screening tests, the correlation between LBC and biopsy diagnosis was not perfect. However, the proportion of women with negative outcomes fell as the severity of the LBC diagnosis increased, from 87.8% among women with ASC, to 53.1% after LSIL, 18.2% after HSIL, and 0.0% after a SCC smear. Conversely, the probability of a biopsy showing CIN 2+ rose with increasing grade of cytological abnormality, from 4.1% among women with ASCUS, to 15.6%, 65.3% and 96.7% among those with LSIL, HSIL, and SCC, respectively. Similarly, the risk of invasive cancer rose from 0.11% among women with LSIL, to 3.9% among women with HSIL, and 53.3% among those with SCC smears. The vast majority (over 98%) of cytologically normal cases also had normal histology. CIN3 and CIN2 were present in few cases (0.1% and 0.3%, respectively) of normal cytology, and no invasive cancers were found in women with smears called ASC or normal.
As in other studies, ASC constituted the majority (59.3%) of abnormal cytology interpretations, and among these, few had positive biopsy findings. Using TBS 2001 classification in our studies conducted from 2003 helped to refine the risk of CIN2+ among women with ASC: while ASC-H constituted only 6.6% of cases called ASC, CIN2+ was present in 30.7% of women with ASC-H and in only 3.2% in those with ASCUS. Although not part of our studies, the literature has shown that women with ASCUS can be further triaged using HR-HPV DNA [22, 23]; LBC has the advantage over conventional Pap in that HPV testing can be performed on residual LBC material. In our studies, most LBC had sufficient residual material for testing: only 0.1% of cases could not be tested because of insufficient material.
In our studies, AGC was uncommon and was reported in only 0.1% of cases. None of these women were found to have a glandular lesion on further investigation; 18.5% (5/27) had CIN and 81.5% had negative evaluations. The low detection rates for cervical granular lesions in this study were also indicated by literature [21, 24]. Although we cannot exclude the possibility that AGC was underdiagnosed at our institution, the finding that squamous lesions predominate among women with AGC smears has been reported generally [24, 25]. Since the reliable diagnoses for women with AGC may require cervical conization, endometrial biopsy, and other potentially invasive and costly procedures [23], the AGC smears in these studies deserves further validation.
By pooling our results from several studies, we have presented an overall picture of the sensitivity, specificity, PPV and NPV of LBC. Over the last 10 years, numerous studies have reported the accuracy of LBC[16, 17, 26–32], but only a limited number have used biopsy-confirmed cervical lesions as the reference standard[16, 17, 26, 31] and few studies have performed population based studies with such a large number of women with cervical abnormalities[16, 30, 31]. Our overall sensitivity, specificity, PPV and NPV for detecting CIN2+ were 81.0%, 95.4%, 38.3% and 99.3 % respectively; and for detecting CIN3+ were 88.5%, 94.3%, 22.0% and 99.8% respectively. The studies appeared homogeneous in their estimates of sensitivities. Statistically significant heterogeneity was detected among the estimates for specificity for detection of both CIN2+ and CIN3+. This is attributed to the difference in specificities in projects carried out before 2003 as compared to those from 2003 onwards. Nevertheless, specificities in all cases were over 90%, and we do not think that the differences before and from 2003 were significant from a practical or “clinical” perspective.
The overall accuracy of LBC in this study appears to be higher than those in most reports on conventional Pap smear and LBC [16, 17, 26, 30, 31]. But there were several important differences among our study and other studies. Firstly, our study population consisted of women who had rarely or never been screened and who had a high prevalence of high grade CIN2+. Most other studies involved women who were well screened, having been enrolled from areas in which cytologic screening had been in place for many years, and therefore comparably fewer CIN2+ lesions were present[16, 17, 31]. Secondly, our interval from screening to colposcopy examination and biopsy was generally less than 2 months. Other studies have included lesions detected within one year or more from screening to colposcopy [16, 31]. It is possible that immediate referral led to detection of a higher proportion of lesions that otherwise would have regressed, and thus increased the accuracyof the LBC screen. Finally, published reports have been a summary of test results from multiple laboratories, [16, 30, 31], whereas the data in our study were all from one laboratory. The potential of variation of test accuracy from different laboratories was avoided in our study.
When we compared LBC to HC2 testing, we found that LBC testing had higher accuracies for detecting CIN2+ and CIN3+ than HC2 (94.9% and 94.2% versus 85.5% and 84.1%). This was primarily due to the higher specificity of LBC. However, HC2 was more sensitive than LBC for detecting CIN2+ and CIN3+.
This study shows that the performance of LBC is effective for the detection of CIN2+ and cervical cancer in a high incidence area. All the studies summarized in this paper were performed in a high quality laboratory in Beijing, and may not be generalizable to all the cytology laboratories in China. However, the results from this study can serve as a relevant baseline for the risk of histological abnormalities after an abnormal LBC diagnosis and indicate that if centralization of cytology services to a high quality laboratory is possible, then LBC may be a good screening tool for cervical cancer prevention in a developing country.
Acknowledgments
We thank the local doctors and the women who participated in our study from Beijing, Gansu, Jiangsu, Jiangxi, Henan and Shanxi.
Grant supports
This work was supported by the Fogarty International Clinical Research Scholars Program (Fogarty International Center, National Institutes of Health) (R24 TW007988); and the Academic Capacity Development Program of the Beijing Municipal Commission of Education grant (XK100230447).
Footnotes
Conflict of interest:
All the authors declare that they have no conflict of interest.
References
- 1.Shi JF, Qiao YL, Smith JS, et al. Epidemiology and prevention of human papillomavirus and cervical cancer in China and Mongolia. Vaccine. 2008;26:M53–59. doi: 10.1016/j.vaccine.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–444. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 3.Belinson JL, Qiao YL, Pretorius RG, et al. Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer. 2003;13:819–826. doi: 10.1111/j.1525-1438.2003.13611.x. [DOI] [PubMed] [Google Scholar]
- 4.Dai M, Bao YP, Li N, et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: a population-based study. Br J Cancer. 2006;95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belinson JL, Hu S, Niyazi M, et al. Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: Implications for vaginal self-collection. Int J Cancer. 2010;127:1151–1157. doi: 10.1002/ijc.25144. [DOI] [PubMed] [Google Scholar]
- 6.Pan Q, Belinson JL, Li L, et al. A thin-layer, liquid-based pap test for mass screening in an area of China with a high incidence of cervical carcinoma. A cross-sectional, comparative study. Acta Cytol. 2003;47:45–50. doi: 10.1159/000326474. [DOI] [PubMed] [Google Scholar]
- 7.Belinson JL, Pretorius RG, Zhang WH, Wu LY, Qiao YL, Elson P. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. 2001;98:441–444. doi: 10.1016/s0029-7844(01)01454-5. [DOI] [PubMed] [Google Scholar]
- 8.Pretorius RG, Kim RJ, Belinson JL, Elson P, Qiao YL. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. J Low Genit Tract Dis. 2006;10:5–9. doi: 10.1097/01.lgt.0000192694.85549.3d. [DOI] [PubMed] [Google Scholar]
- 9.Belinson JL, Pan QJ, Biscotti C, et al. Primary screening with liquid-based cytology in an unscreened population in rural China, with an emphasis on reprocessing unsatisfactory samples. Acta Cytol. 2002;46:470–474. doi: 10.1159/000326863. [DOI] [PubMed] [Google Scholar]
- 10.Pretorius RG, Zhang WH, Belinson JL, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191:430–434. doi: 10.1016/j.ajog.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Cagle AJ, Hu SY, Sellors JW, et al. Use of an expanded gold standard to estimate the accuracy of colposcopy and visual inspection with acetic acid. Int J Cancer. 2010;126:156–161. doi: 10.1002/ijc.24719. [DOI] [PubMed] [Google Scholar]
- 12.Hu SY, Hong Y, Zhao FH, et al. Prevalence of HPV infection and cervical intraepithelial neoplasia and attitudes towards HPV vaccination among women aged 18–25 in Jiangsu province. Chin J Cancer Res. 2011;23:25–32. doi: 10.1007/s11670-011-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CY, Bian ML, Gen L, et al. Clinical trial on evaluation of Hybrid Capture for Human Papillomavirus in screening for cervical cancer and cervical intraepithelial neoplasia. China Cancer. 2009;18:1008–1011. [Google Scholar]
- 14.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol. 2010;11:1160–1171. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco G, Cuzick J, Pierotti P, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007;335:28. doi: 10.1136/bmj.39196.740995.BE. http://www.bmj.com/content/335/7609/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strander B, Andersson-Ellström A, Milsom I, Rådberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program : a prospective randomized study. Cancer. 2007;111:285–291. doi: 10.1002/cncr.22953. [DOI] [PubMed] [Google Scholar]
- 18.Zhao FH, Hu SY, Bian JJ, et al. Comparison of ThinPrep and SurePath liquid-based cytology and subsequent human papillomavirus DNA testing in China. Cancer Cytopathol. 2011;119:387–394. doi: 10.1002/cncy.20177. [DOI] [PubMed] [Google Scholar]
- 19.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 20.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol. 2004;191:105–113. doi: 10.1016/j.ajog.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224–1229. doi: 10.5858/2004-128-1224-BIARRP. [DOI] [PubMed] [Google Scholar]
- 22.Bruner KS, Davey DD. ASC-US and HPV Testing in Women Aged 40 Years and Over. Diagn Cytopathol. 2004;31:358–361. doi: 10.1002/dc.20144. [DOI] [PubMed] [Google Scholar]
- 23.Wright TJ, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 24.DeSimone CP, Day ME, Tovar MM, Dietrich CR, Eastham ML, Modesitt SC. Rate of pathology from atypical glandular cell Pap tests classified by the Bethesda 2001 nomenclature. Obstet Gynecol. 2006;107:1285–1291. doi: 10.1097/01.AOG.0000218705.87329.4a. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Montes TP, Farinola MA, Zahurak ML, Bristow RE, Rosenthal DL. Clinical utility of atypical glandular cells (AGC) classification: cytohistologic comparison and relationship to HPV results. Gynecol Oncol. 2007;104:366–371. doi: 10.1016/j.ygyno.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap Test with the conventional Pap Test, including follow-up of HSIL cases. Acta Cytol. 2001;45:709–714. doi: 10.1159/000328292. [DOI] [PubMed] [Google Scholar]
- 27.Klinkhamer PJ, Meerding WJ, Rosier PF, Hanselaar AG. Liquid-based cervical cytology. Cancer. 2003;99:263–271. doi: 10.1002/cncr.11673. [DOI] [PubMed] [Google Scholar]
- 28.Kirschner B, Simonsen K, Junge J. Comparison of conventional Papanicolaou smear and SurePath liquid-based cytology in the Copenhagen population screening programme for cervical cancer. Cytopathology. 2006;17:187–194. doi: 10.1111/j.1365-2303.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 29.Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–32. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 30.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–177. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 31.Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009;302:1757–1764. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 32.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]