Abstract
The rate at which alcohol (ethanol) is consumed has direct impact on its behavioral and subjective effects. For this reason, alterations in the pattern of ethanol consumption as a function of drinking history might be critical to the development and maintenance of alcoholism. Furthermore, because pharmacological interventions aimed at disrupting the motivation to consume ethanol are dependent on the brain/plasma concentrations present when an individual is most likely to engage in consumption of this substance, characterizing temporal drinking patterns might be useful to determine the timing of such treatments. The primary goal of the present study was to evaluate alterations in the timecourse of daily binge (Drinking-in-the-dark; DID) ethanol consumption. We gave 14 daily 2 hour DID ethanol or water access sessions to male C57BL/6J (B6) mice using a state of the art volumetric drinking monitoring device. We then, primarily as a proof-of-principle, used the GABAB allosteric modulator GS39783 (GS) to determine how this compound influenced the timecourse of binge-like ethanol intake. The rate of ethanol consumption increased dramatically over sessions with the majority occurring in the first few minutes of the final session. Additionally, ethanol consumption occurring immediately following access was almost completely abolished in mice pretreated with GS; an effect which was ethanol-specific only at this early time interval. These data characterize progressive alterations in the rate of ethanol intake using the DID model and suggest that careful consideration of prior ethanol history and timing of drug administration are warranted when interpreting results of preclinical drug administration studies.
The within-session rate and amount of alcohol (ethanol) consumption, as well as the frequency of such drinking sessions, all have direct impact on an individual’s propensity to develop an alcohol use or abuse disorder (Li et al., 2007). Although each of these variables has independent predictive value, together they have been suggested to offer a more comprehensive clinical outcome measure (Allen, 2003). Despite this, very few preclinical animal studies have focused on the collective relationship of these measures. This is often because experiments are designed in ways that make this difficult or impractical to evaluate, or simply because the methodological constraints of a particular model do not allow it.
However, one relatively recent mouse model of binge-like alcohol intake, known as ‘drinking-in-the-dark (DID; (Rhodes et al., 2007), is being used with increasing frequency to test hypothesis related to the rate, amount, and frequency of alcohol consumption. Under this model, alcohol preferring C57BL/6J inbred mice consume large quantities of 20% unsweetened ethanol solution when provided with 2 hours of access 3 hours into the dark cycle(Rhodes et al., 2005). Importantly, the levels of intake that occur in this limited-access model lead to elevated blood ethanol concentrations (BECs) that meet the definition of ‘binge as defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA; ≥80 mg/dl), as well as overt signs of behavioral intoxication (Linsenbardt et al., 2011, Moore et al., 2007, Rhodes et al., 2007). Furthermore, binge-like ethanol intake occurs daily over many repeated DID sessions, and leads to the development of both metabolic and behavioral tolerance(Linsenbardt and Boehm, 2011, Linsenbardt et al., 2011). These factors, together with the ease with which the procedures can be performed, we believe make DID an ideal model for studying the temporal aspects of repeated heavy drinking.
To this end, differences in the rate of ethanol vs. water intake within a single DID session have been observed, with fewer bouts occurring in those animals given access to ethanol versus those given access to water(Rhodes et al., 2007). Furthermore, we have observed alterations in the rate of ethanol intake over multiple DID sessions, with more ethanol intake occurring during the first half of the 2 hour sessions with each subsequent daily DID session (Linsenbardt and Boehm, 2009, Linsenbardt et al., 2011). The measures of the rate of ethanol intake over DID sessions in these last studies were rather crude however, and do not speak to rate differences that might occur on a microstructural scale over repeated binge-like intake sessions. This last point is one for which we have developed a keen appreciation, as one of our (and many other labs) primary goals is to use this model to test the efficacy of pharmacological agents. Because pharmacological interventions aimed at disrupting the motivation to consume ethanol are dependent on the brain/plasma concentrations of drug present when an individual is most likely to engage in consumption of this substance, characterizing temporal drinking patterns might be useful to determine the dose and timing of such treatments. Furthermore, alterations in the rate of ethanol intake as a function of alcohol experience might more generally inform us of how the pattern of consumption is involved in the maintenance of repeated binge-like consumption behavior in genetically predisposed populations. We are, after all, ultimately interested in providing treatment for those who have an extensive history of alcohol use and abuse, and by the same token, equally as interested in keeping such behavior from developing, particularly in genetically vulnerable populations.
The primary goal of the present study was to evaluate alterations in the timecourse of daily DID ethanol consumption. As a secondary goal, and primarily as a proof-of-principle, we used the GABAB positive allosteric modulator GS39783 to determine the influence of this compound on the timecourse of binge-like ethanol intake. GS39783 was chosen based on our recent findings that this compound alters ethanol-induced locomotor stimulation and sensitization (Kruse et al., 2012), and also other published work indicating that this compound reduces voluntary ethanol consumption in rats (Maccioni et al., 2008, Maccioni et al., 2007, Maccioni et al., 2012). Thus, use of GS39783 allowed us to directly test the influence of pharmacological manipulation on discrete temporal aspects of DID ethanol intake, while also providing additional information on the role of the GABAB receptor system on the positive motivational effects of ethanol. Because we have previously observed home-cage locomotor activity alterations during DID ethanol intake sessions, and because drug-induced alterations in motor behavior might directly affect fluid intake, home cage activity was also simultaneously monitored during each DID session.
Methods
Animals
Male C57BL/6J mice (8-week old) were purchased from Jackson Laboratory (Bar Harbor, ME) and shipped to the Purdue School of Science animal facility at Indiana University – Purdue University Indianapolis (IUPUI). Upon arrival animals were acclimated to single housing in standard shoebox mouse cages and a 12 hour reverse light/dark cycle with lights off at 7:00 AM for at least a week prior to testing. All animals had ad lib access to food and water except during ethanol access sessions when only ethanol was available, and (in some cases) during home cage locomotor behavioral testing. All procedures were approved by the Purdue School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academic Press, 2003).
Ethanol and Drug Solutions
Ethanol drinking and injection solutions (20% v/v) were made with 190 proof ethanol purchased from Pharmco, Inc (Brookfield, CT) and regular tap water or 0.9% physiological saline, respectively. Drinking solutions were made fresh every other day and stored in sealed fluid reservoirs connected to the Volumetric Drinking System (VDM) fluid dispensation system (Columbus Instruments Inc., Columbus, OH). Injection solution was made immediately prior to use.
GS39783 was purchased from Sigma Aldrich (St. Louis, MO) and suspended in 30 μl Tween 80 and physiological saline for administration of the 30 mg/kg dose. Tween 80 constituted 0.6% of the total solution volume. The 0 mg/kg (vehicle) dose, used as a control for GS39783, simply contained 30 μl of Tween 80 in physiological saline. We chose to administer the 30 mg/kg dose based on previous data from our lab where alterations in ethanol-induced behavioral effects were observed at his dose (Kruse et al., 2012).
Drinking in the Dark (DID)
DID procedures performed in our lab have been recently described (Linsenbardt and Boehm, 2009, Linsenbardt and Boehm, 2011, Linsenbardt et al., 2011). Three hours into the dark cycle each day, animals received access to an unsweetened 20% ethanol solution or tap water for 2 hours. This 2 hour access period was used for each experiment on every DID session – animals were never given more or less than 2 hours of DID fluid access. However, in lieu of the standard ball-bearing sipper tubes typically used for DID procedures in our lab, water bottles were replaced with specialized volumetric sipper tubes (Columbus Instruments, Columbus, OH) containing ethanol or tap water. Each specialized volumetric drinking tube housed a secondary electrically isolated fluid delivery tube that continually provided 20μl fluid beads to a standard sized drinking orifice on the outer sipper spout. When each fluid bead was consumed the circuit between the larger tube and the smaller tube house within that larger tube was open. The opening of this circuit signaled the consumption of the fluid bead and immediately triggered the pump to deliver another fluid bead to the orifice thus closing the circuit. The total volume of fluid consumed was recorded in one minute epochs for each 2 hour DID session.
Home Cage Locomotor Apparatus
Home cage locomotion was monitored using a CI Multi-Device Interface (Columbus Instruments Inc., Columbus, OH) in conjunction with a Dell computer. The dimensions and other technical specifications of this apparatus have been previously described (Linsenbardt et al., 2011). Briefly, ambulatory activity was detected by the interruption of photocell beams positioned along the walls of each animals standard shoebox mouse cage (40 independent photocell beam enclosures in total). Each of the 40 photocell beam enclosures was housed in a designated mouse vivarium for these studies where the animals remained for the duration of all experiments. Data were collected in 1-min time intervals for a total of 2 hours during each DID session and translated into ambulatory counts using the provided software (version 1.4.0).
Blood Sampling
For the determination of blood ethanol concentrations (BECs), 50μl peri-orbital sinus bloods were drawn following behavioral testing in experiment 1 on days 15 and 16. Blood samples were not collected for experiment 2 as it was our intent to use these animals for a follow up study in which the association between ethanol intake and the blood sampling procedures might have been detrimental. Samples were centrifuged and plasma was withdrawn and stored at −20°C. BECs were then determined using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Procedures
Experiment 1: Alterations in the Timecourse of Intake and Home Cage Locomotion
The goal of experiment 1 was to determine if alterations in the rate of fluid consumption and concomitant home cage locomotor activity changed dynamically as a function of ethanol history. Out of the 40 animals in this experiment, 20 were given access to ethanol and the remaining 20 were given access to water for 14 consecutive days using the DID procedures outlined above. On day 15, the type of fluid presented was switched in half of the mice; water consuming mice received ethanol (WE) and vice versa (EW). The other half continued to receive their previously assigned ethanol (EE) or water (WW) solution. This allowed for a within and between subjects analysis of intake and home cage locomotor activity as a function of previous ethanol history.
Three hours following the onset of the ‘lights off phase of the light cycle on the 16th day (24 hours following the previous days DID session), each animal was given a 2.0 g/kg ethanol injection (i.p.) and was immediately placed back into the home cage for 2 hours while locomotor activity was recorded. This was done primarily to evaluate possible alterations in ethanol metabolism, but also to assess how alcohol history might influence ethanol-induced home cage locomotion. Blood was sampled immediately after bottles were removed on day 15 and 2 hours following ethanol injections on day 16 for determination of BECs.
Experiments 1 Statistics: General Linear Model Analysis
Mean total (2 hour) fluid intake and home cage locomotor activity of the ethanol and water consuming groups on days 1–14 for experiment 1 were analyzed using a mixed 2-way analysis of variance (ANOVA) with day as the within subject’s factor and fluid assignment group (ethanol or water) as the between groups factor. To determine if there were differences in mean intake on days 1–14 between day 15 assignment groups, fluid history subdivisions were analyzed separately such that the WW and WE groups were compared and the EE and EW groups were compared using ANOVA with intake on days 1–14 as the within subjects factor and the day 15 group assignment as the between subjects factor. Mean total fluid intake and home cage locomotion on day 15 were analyzed separately using a two-way ANOVA with repeated fluid assignment (days 1–14) and day 15 fluid assignment as factors. A T-test was also used to compare the first 15 minutes of intake data in the WE and EE groups on day 15. Day 16 was analyzed identically to day 15, except as DID procedures were not performed, only 2 hour home cage locomotion was analyzed.
Experiment 2: Effects of the Positive GABAB Allosteric modulator GS39783 on the Timecourse of Intake and Home Cage Locomotion
The goal of experiment 2 was to determine the influence of GS39783 on the timecourse of binge-like ethanol intake and home cage locomotor activity. In total, 40 mice were given 4 consecutive days access to ethanol (E; N=20) or water (W; N=20) using the DID procedures detailed above. Our decision to use a water drinking control group for detecting possible non-specific drug effects over an alternative two-bottle (1 ethanol and 1 water) DID procedure was to be consistent with experiment 1 and also because previous studies have shown that single bottle DID procedures produce 40% higher BECs than when 2 bottles are used using otherwise identical DID procedures (Rhodes et al., 2007). On the 5th day, half of the mice from each fluid consuming group (N=10) were given GS39783 (30 mg/kg i.p.) and half were given vehicle 15 minutes prior to DID fluid access. We used the 15 minute pretreatment interval to be consistent with other data from our lab in which the 30 mg/kg dose was used to alter ethanol-induced behavioral effects (Kruse et al., 2012). We chose to administer drug on day 5 to be consistent with previous data from our lab evaluating the effects of GABAergic drugs on ethanol intake using DID procedures (Moore et al., 2007).
Experiments 2 Statistics: General Linear Model Analysis
Mean water intake and home cage locomotion of the W and E groups on days 1–4 of experiment 2 were analyzed using a mixed two-way ANOVA with days as the within subject’s factor and fluid assignment as the between groups factor. To determine between groups differences in fluid intake and home cage locomotion on day 5 a multi-factorial ANOVA was conducted with fluid assignment (Water vs. Ethanol), and drug assignment (30mg/kg GS39783 vs. 0mg/kg GS39783) as factors. Subsequent analyses were conducted on each drug injection time interval separately.
Experiments 1–2 Correlations and Post-hoc Testing
For experiment 1, Pearson’s correlation was used to measure the linear relationship between intake and BEC (days 15). Tukey post-hoc tests were performed when appropriate for both experiments. Data were considered significant at p<0.05.
Experiments 1–2 Statistics: Non-linear/Polynomial Analysis
After initial analysis of the discrete time interval data, it was clear that within-session daily intake and locomotor activity data were not linearly organized and the dynamic temporal order of the effects appeared critical to interpretation of the data. In order to better characterize these alterations, data from each one minute bin of each daily 120 min (2 hour) DID session were fit to 1st – 4th order polynomials according to fluid and/or treatment group as outlined above. The fit of each day’s data to one of the four polynomial equations were compared using an F-statistic to determine the equation that best described intake or locomotor activity data on a particular day. The higher order polynomial was used if the difference in sum of squares between the more simple and that higher order polynomial divided by the sum of squares of the higher order polynomial was greater than the relative increase in the degrees of freedom. In other words, if the higher order polynomial was better suited at describing the nature of the data, it was used preferentially over a lower order polynomial up to the highest (4th) order. Significant polynomial equation fitting indicated that intake/locomotion were systematically related to time within a given DID session. That the fit of each of the variables (intake and locomotion) varied as a function of day and fluid group indicated that the type of fluid assigned and the number of days of DID experience were each factors influencing within-DID session behavior. Daily differences (or lack thereof) between fluid groups as a function of DID experience in the strength and nature of the non-linear relationship imply that biologically relevant behavioral alterations occurred as a consequence of binge-like ethanol intake.
As might be expected from microstructural behavioral datasets, there were daily intake and locomotor activity measurements that did not perfectly fit one of the polynomial equations. In order to better characterize the most prominent temporal alterations, a finite impulse response filter (known most commonly as a central moving average) was calculated on 15 minute bin increments. Fifteen minute increments were chosen primarily because the divergence in ethanol and water intake on the final repeated DID session in experiment 1 (Day14) was greatest in approximately the first 15 minutes; the 95% confidence intervals of each of the most accurate polynomials for each group intersected at that this point in time on this day. For the sake of example, the first data point represented graphically in the figure insets depicts the average of the 1st 15 minutes, and the second data point represents the mean of the following 15 minutes excluding the 1st minute (minutes 2–16, and so on). We were most interested in using this analysis to characterize how the changes in intake and locomotion might have gradually occurred over each daily DID session. These types of data filtering are commonly used to smooth out short-term fluctuations and highlight the most prominent trends. The results of this data smoothing were then subject to polynomial curve fitting as detailed above. Notably, these analyses allowed us to better define peaks and troughs in behavior (see Figures 2 and 4 insets). The results of experiment 2 were analyzed using identical procedure as those detailed above except that drug was added as a factor.
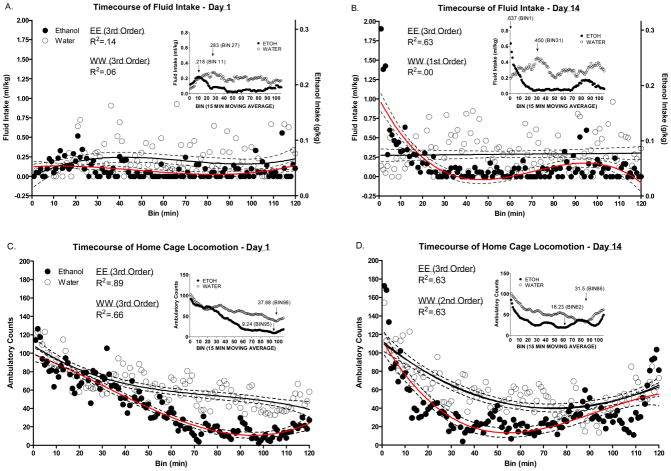
Figure 2.
Timecourse of fluid intake and home cage locomotor activity on days 1 vs. 14. The solid lines reflect the best fit polynomial line whereas the dotted lines reflect the 95% confidence intervals of that line. The R2 values represent the amount of variance in intake/locomotion that is explained by time (in minutes); the larger the R2 value the closer the polynomial curve fits the data. Insets reflect 15 minute central moving averages. The first data point/bin of this inset represents the mean of minutes 1–15, the second data point/bin represents the mean of minute 2–16, and so on. Mean daily fluid intake (A–B) and home cage locomotion (C–D) on days 1 and 14.
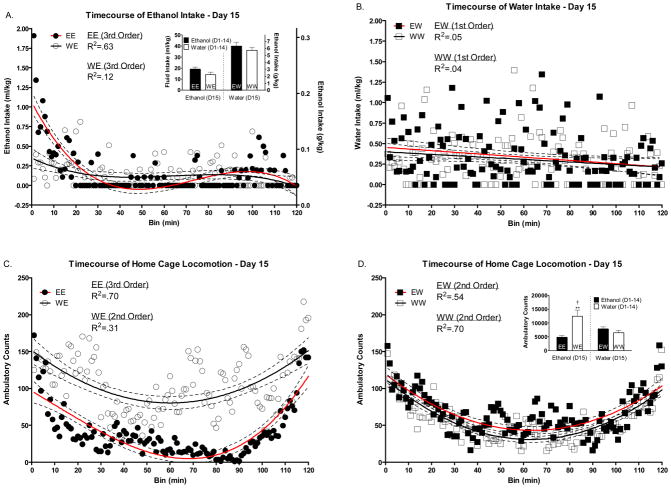
Figure 4.
Effect of 14 days of DID on Day 15 intake and home cage locomotor activity. The solid lines reflect the best fit polynomial line whereas the dotted lines reflect the 95% confidence intervals of that line. The R2 values represent the amount of variance in intake/locomotion that is explained by time (in minutes); the larger R2 the closer the polynomial curve fits the data. (A) Ethanol intake on day 15 in animals with 14 days of ethanol (EE) or water (WE) experience. (B) Water intake on day 15 in animals with 14 days of ethanol (EW) or water (WW) experience. Two hour cumulative intake on day 15 in all groups (Panel A inset). (C) Home cage locomotor activity on day 15 in animals with 14 days of ethanol (EE) or water (WE) experience. Home cage locomotor activity on day 15 in animals with 14 days of water (WW) or ethanol (EW) experience. Two hour cumulative home cage locomotor activity on day 15 in all groups (Panel D inset). *’s indicate differences from EE and WW groups (**p<.01). † indicates a trend towards a difference from EW group (†p=.06).
Results
Experiment 1: Days 1–14
General Linear Analysis
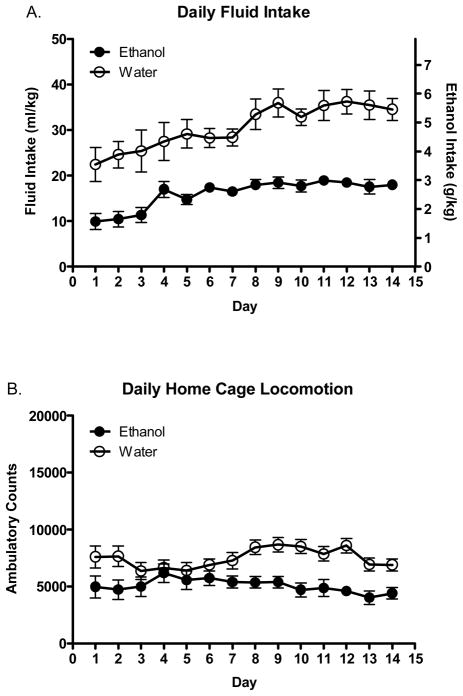
Total daily ethanol and water consumption on days 1–14 can be seen in Figure 1A. One animal from the ethanol assigned group died prior to the completion of this experiment so data from this individual was removed from all analyses. Additionally, on day 14 the drinking tube from one ethanol consuming animal leaked continuously for the majority of the DID access session for unknown reasons. Because we were unsure how much total ethanol might have been consumed on this day, we used the previous day’s intake and locomotor values for the daily repeated measures analysis for this individual. Analysis revealed significant main effects of fluid assignment [F(1, 37)=48.33 p<.0001] and day [F(13, 481)=7.15 p<.0001] with water assigned animals consuming more in volume by weight than ethanol, and overall fluid intake increasing over daily DID sessions. There were no significant differences in water or ethanol consumption over the first 14 days as a function of day 15 fluid assignment; there were no significant day 15 fluid group assignment effects or interactions (p’s>.05; data not shown). In other words, ethanol (EE and EW) and water (WW and WE) groups had similar drinking histories prior to day 15 fluid access.
Figure 1.
Total fluid intake and home cage locomotor activity on days 1–14. Mean daily fluid intake (A.) and home cage locomotor activity (B.) in ethanol and water consuming animals over 14 consecutive DID sessions.
Home cage locomotor activity on days 1–14 can be seen in Figure 1B. Analysis revealed significant main effects of fluid assignment [F(1, 37)=15.82 p<.001] with ethanol assigned animals having significantly lower locomotor activity than water consuming animals, and a significant day*fluid group interaction [F(13, 481)=1.98 p<.05]. Post hoc tests did not detect any between group differences on any particular day (p’s>.05). Similar to intake measures, there were no significant differences in locomotor activity over the first 14 days as a function of day 15 fluid assignment (p’s>.05; data not shown); ethanol (EE and EW) and water (WW and WE) groups displayed similar locomotor activity prior to day 15 fluid access. For this reason, all graphical representations of intake and home cage locomotor data in Figure 1 are collapsed on day 15 fluid group assignment.
Non-linear/Polynomial Analysis
Mean ethanol and water intake in each of the 120 minute bins on days 1 and 14 can be seen in Figure 2A and B respectively. On day 1, although both ethanol and water intake were best fit to 3rd order polynomials, there were no easily identifiable timecourse differences (i.e. the 95% confidence intervals of each of the fluids represented by the dotted lines were largely overlapping). By day 14, large differences in the timecourse of ethanol intake had developed whereas the timecourse of water intake was generally unchanged.
Mean home cage locomotor activity in each of the 120 minute bins on days 1 and 14 can be seen in Figure 2C and D respectively. On day 1, home cage locomotor activity differences emerged between ethanol and water groups after approximately 30 minutes and remained until the final minutes of the session. By day 14, differences between fluid groups in locomotor activity emerged approximately 20 minutes earlier than on day 1 but then dissipated in the final 30 minutes of the session.
Central moving average representations for ethanol and water intake on days 1 and 14 can be seen in Figure 2A and B insets. The volume of fluid consumed in the ethanol consuming animals at the identified peak intake bins was lower than the water consuming animals on day 1 (.065 ml/kg less fluid) but higher than the water group on day 14 (.187 ml/kg more fluid). Furthermore, although fluid intake increased within both the ethanol (.419 ml/kg increase) and water (.167 ml/kg increase) consuming groups from day 1 to day 14, when these peaks occurred and the direction of alterations in when the peaks occurred differed. Peak intake occurred later in the water consuming animals on days 1 (16 bins later) and 14 (30 bins later) compared to the ethanol consuming animals; the difference in time between peak intake increased approximately twofold from day 1 to day 14. The increase in the magnitude of this difference was driven primarily by the leftward shift in peak intake in the ethanol consuming group; peak ethanol intake occurred 10 bins earlier on day 14 compared to day 1 whereas peak water intake occurred 4 bins later. These analyses were simply a snap-shot of a change that hypothetically occurred progressively over each subsequent DID access day. To test the progressive nature of our observations, we ran several additional analyses based on these findings.
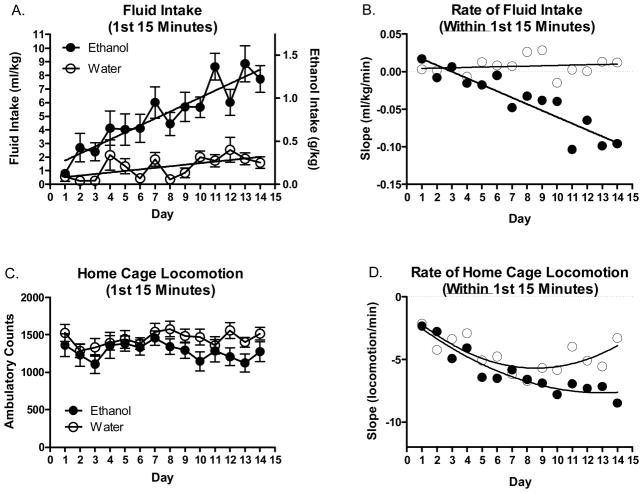
Analysis of the first 15 minutes of ethanol and water intake over all 14 days can be seen in Figure 3A+B. The results of this analysis indicated significant main effects of fluid group [F(1, 37)=39.74 p<.0001], day [F(13, 481)=9.03 p<.0001] and a fluid group*day interaction [F(13, 481)=4.00 p<.0001] which post hoc tests confirmed was due to significant increases in ethanol intake compared to water intake. Significantly higher (within group) ethanol intake during the first 15 minutes was first detected on day 4 (p<.05) and continued through day 14 (p<.0001). Water intake within this time frame never increased significantly (p’s>.05). Changes in fluid intake over days was also linear, with ethanol intake increasing consistently over each subsequent DID day [R2=.19; p<.0001], and water intake increasing only marginally [R2=.04; p<.01]. As would be expected based on the results of the ANOVA, the slopes of each fluid group were found to be significantly different [F=29.28; DFn/d=1/542; p<.0001]. By way of comparison, 41% of total ethanol intake on day 14 was consumed during the first 15 minutes while less than 2% of total water intake was consumed during the first 15 minutes on this day. To help explain alterations in the rate of ethanol intake even further, the slope of the best fit line of the first 15 minutes plotted as a function of day revealed a significant negative relationship in ethanol consuming animals [R2=.82; p<.001] but not in water consuming animals [R2=.03; p=.58]. As negative values indicate a decrease in intake from the 1st to the 15th minute, the lower (more negative) the value, the more quickly fluid consumption decreased over this 15 minute interval. Daily changes in the magnitude of the slope was influenced both by increases in intake earlier within the 15 minute epoch and also decreases in intake at the end of the 15 minute epoch; both characteristics which changed in the ethanol group but not the water group. Thus, not only did ethanol consuming animals consume progressively more fluid over each subsequent day during the first 15 minutes, they also consumed this greater overall volume progressively more quickly within those first 15 minutes (Figure 3B).
Figure 3.
Alterations in intake and home cage locomotor activity during the 1st 15 minutes of 14 days 1–14. (A) Mean daily fluid intake during the first 15 minutes of DID on days 1–14. (B) Alterations in the rate (slope) of ethanol intake during the first 15 minutes on days 1–14. Negative values indicate increases in the rate of intake whereas positive values indicate decreases. (C) Mean daily home cage locomotor activity during the first 15 minutes of DID on days 1–14. (D) Alterations in the rate (slope) of home cage locomotor activity during the first 15 minutes on days 1–14. As with intake data, negative values indicate increases in rate whereas positive values indicate decreases.
Identical comparisons were made for home cage locomotor activity (Figure 3C+D). Two-way ANOVA of the first 15 minutes of home cage locomotion did not detect any significant differences between or within groups (p’s>.05). However, non-linear regression of the locomotor alterations within the first 15 minutes indicated that the lines of the best fit polynomial (2nd order) were best explained by different curves (i.e. the groups differed in the rate of change over days) [F=11.31; DFn/d=3/22; p<.0001]. To determine if the rate of fluid intake during the first 15 minutes was predictive of the rate of locomotor activity during this same time period, the slope of the 1st 15 minutes of intake was regressed on the slope of the home cage locomotion during this time period (see supplementary figure 1). Results of this analysis (Panel A) indicated that whereas the rate of ethanol intake was positively associated with the rate of home cage locomotion in this group [R2=.52; p<.01], the rate of water intake was not significantly related to the rate of home cage locomotion [R2=.14; p=.19]. Thus, the more quickly ethanol was consumed during the first 15 minutes the more quickly locomotor activity decreased. In light of these results, it was also of interest to evaluate if the rate and/or amount of ethanol consumed during the first 15 minutes might predict the observed locomotor inactivity subsequent to that intake. Although the rate of ethanol consumption during the first 15 minutes over each of the 14 days was significantly associated with total locomotor activity following these first 15 minutes (minutes 16–120) in ethanol consuming animals [R2=.32; p<.05], this was not the case for water consuming animals [R2=.00; p=.85; supplementary figure 1 Panel B]. The amount (ml/kg) of fluid consumed was not significantly associated with subsequent locomotor activity in either ethanol [R2=.19; p=.12] or water consuming groups [R2=.01; p=.76] (data not shown).
Central moving average representations for home cage locomotion on days 1 and 14 can be seen in Figure 2C and D insets. Based on our observations that ethanol generally decreased home cage locomotor activity (see Figure 1B), we were primarily interested in troughs in this behavior. The ambulatory counts in the ethanol consuming animals at the identified troughs was lower than the water consuming animals on days 1 (28.44 fewer counts) and 14 (13.27 fewer counts). Furthermore, locomotor activity troughs were not as deep within the ethanol consuming animals on day 14 compared to day 1 (8.99 count increase) but were deeper in water consuming animals on day 14 compared to day 1 (6.18 count decrease). Troughs in locomotor activity occurred later in the water consuming animals on days 1 (3 bins later) and 14 (24 bins later) compared to the ethanol consuming animals; the difference in time between troughs in locomotion was 8 times greater on day 14 compared to day 1. The increase in the magnitude of this difference was driven primarily by the leftward shift in trough locomotion in the ethanol consuming group; trough locomotor activity in ethanol animals occurred 33 bins earlier on day 14 compared to day 1, whereas trough locomotor activity in water animals occurred 12 bins earlier.
Experiment 1: Day 15
General Linear Analysis
Total fluid consumption and home cage locomotor activity on day 15 can be seen in Figure 4A and 4D insets. Analysis of day 15 intake revealed a significant main effect of day 15 fluid assignment [F(1, 35)=67.69 p<.0001] with the water group drinking more fluid volume by weight than the ethanol group. There was no difference in mean ethanol intake between the EE group on day 1 and the WE group on day 15 (t = 1.55; df = 27; p>.10; data not shown); the days that correspond to the first ethanol exposure for each group respectively. That differences were not detected suggests that novelty of the entire DID procedure in the EE group on day 1 did not influence overall ethanol intake in this group on this day. However, mean water intake was lower in the WW group on day 1 compared to the EW group on day 15 (t = 2.89; df = 27; p<.01; data not shown); the days that correspond to the first water exposure for each group respectively. Thus, novelty of the DID procedure in the WW group on day 1 would appear to have influenced overall water intake in this group on this day. There were no differences in total ethanol intake between the EE and WE groups or total water intake between the WW and EW on day 15 (p>.05).
Analysis of total mean home cage locomotion on day 15 revealed a significant main effect of days 1–14 fluid assignment [F(1, 35)=7.55 p<.01] as well as a significant days 1–14 fluid assignment*day 15 fluid assignment interaction [F(1, 35)=14.47 p<.001]. The results of the post hoc test indicated that the WE group displayed robust locomotor stimulation compared to the EE and WW groups (p’s<.01). The difference between the WE and EW groups only showed a trend towards significance (p=.055). There were also differences in home cage locomotor activity between the EE group on day 1 and the WE group on day 15 (t = 3.64; df = 26; p<.01; data not shown), with the WE group displaying significantly higher activity (stimulation) compared to the EE group. There were no differences in home cage locomotor activity between the WW group on day 1 and the EW group on day 15 (t = 0.21; df = 27; p>.80; data not sown).
There were no differences in mean BEC between the EE (119.9 ± 14.8) and WE (106.5 ± 15.2) groups. However, consistent with much data from our lab, BECs were positively associated with ethanol intake in both the EE (R2=.46; N=10; p<.05) and WE (R2=.83; N=9; p<.001) groups as well as when collapsed on repeated fluid assignment group (R2=.61; N=19; p<.0001).
Non-linear/Polynomial Analysis
Mean ethanol and water intake in each of the 120 minute bins on day 15 can be seen in Figure 4A and B respectively. It is important to note that each panel in these figures illustrates groups given access to the same fluid type on day 15; groups differed only in the previous 14 day fluid assignment. While there were no differences in overall ethanol intake between the WE and EE groups (Figure 4A inset), there were large differences in the rate at which ethanol was consumed. As expected based on data from day 14, the ethanol experienced EE group drank significantly more ethanol than the ethanol naïve WE group during the first 15 minutes of the ethanol access session (t=3.79; df=17; p<.01). That the timecourse of home cage locomotion in the WE and EE groups were best fit to different order polynomials indicates that these groups differed in temporal structure on this day. There were no differences between the EW and WW groups in the rate of water intake or the timecourse of home cage locomotion. Because differences in the peaks and troughs of intake were identical to what would be expected based on previous results/figures, and because the generally higher locomotor activity in WE group was consistent with the main effects analysis with no real defining timecourse differences, we opted not to illustrate this data graphically.
Experiment 1: Day 16
Analysis of total mean home cage locomotion following ethanol injection on day 16 revealed significant main effects of the previous days (day 15) fluid assignment [F(1, 35)=11.67 p<.01] with animals that were given access to ethanol the previous day having significantly lower home cage locomotor activity than those that had access to water (data not shown). Surprisingly, there were no differences as a function of repeated fluid assignment group; 14 days of ethanol or water intake did not affect or interact with home cage locomotion on day 16 following ethanol injections. Together these results are suggestive of rapid sensitization to ethanol’s sedative effects.
Polynomial analysis did not detect any obvious differences in the rate of ethanol-induced home cage locomotor activity and are therefore not presented graphically. In general, all animals developed rapid locomotor sedation that lasted the majority of the 2 hour recording session.
There were no differences in BEC as a function of group 2 hours following ethanol injections [EE=78.54±7.09; EW=76.29±6.25; WE=86.26±5.54; WW=75.57±6.75]. We take this as evidence for no differences in metabolic tolerance as a function of repeated (EE) or single (WE) ethanol exposure(s).
Experiment 2: Days 1–5
General Linear Analysis
Data from one of the water consuming animals was not recorded accurately on days 2 and 3 due to an equipment malfunction and we therefore used the average water intake from days 1 and 4 for statistical analysis. Consistent with experiment 1, water animals drank more overall fluid volume by weight than ethanol consuming animals (data not shown [F(1, 38)=128.2 p<.0001]). Also consistent with experiment 1, significantly higher (within group) ethanol intake during the first 15 minutes was first detected on day 4 (p<.05). Water intake within this time frame never increased significantly (p’s>.05). There were no consistent differences in home cage locomotor activity between fluid groups on days 1–4. This is consistent with experiment 1 in that locomotor activity was generally similar on the 4th day of DID access. However, there was a significant main effect of day [F(3, 152)=7.13 p<.001] with locomotor activity generally decreasing over days. Importantly, there were no differences in mean fluid intake or home cage locomotor activity as a function of day 5 treatment assignment (p’s>.05).
The effects of GS39783 on fluid consumption can be seen in Figure 5. Analysis indicated main effects of drug treatment [F(1, 36)=13.07 p<.001] and fluid [F(1, 36)=11.26 p<.01], with animals treated with GS39783 generally consuming less fluid and, as expected, water animals consuming more fluid than ethanol animals (Figure 5A). There were no significant differences or interactions between groups in 2 hour home cage locomotor activity (Figure 5C).
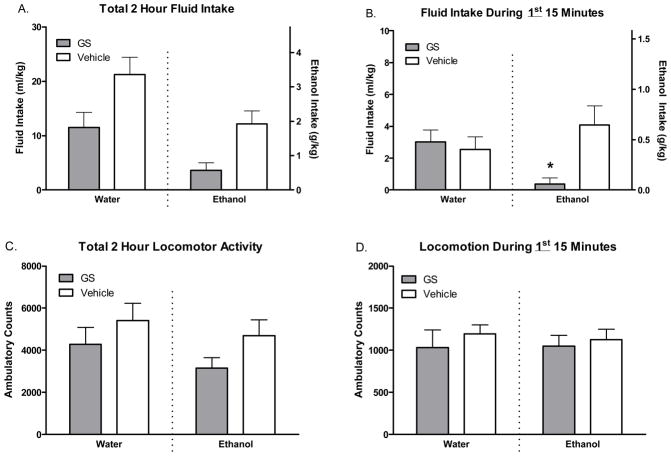
Figure 5.
Fluid intake and home cage locomotion following GS39783. Two hour cumulative fluid intake (A) and concomitant home cage locomotor activity (C) following GS39783 or vehicle pretreatment. Fluid intake (B) and concomitant home cage locomotor activity (D) during the first 15 minutes of DID access in animals pretreated with GS39783 or vehicle.
The first 15 minutes of day 5 were analyzed in order to evaluate drug effects that might have occurred at a time when peak ethanol intake was expected to occur (Figure 5C+D). Analysis of fluid intake revealed a significant treatment*fluid interaction [F(1, 36)=6.31 p<.05], which post hoc tests revealed was due to significantly lower intake in the ethanol group treated with GS39783 vs. that treated with vehicle (p<.05). Remarkably, only 1 of the 10 animals consumed any ethanol during the first 15 minutes following GS39783 treatment whereas 8 of the 10 animals given vehicle injections consumed ethanol. There were no effects of fluid or treatment on locomotor activity during the first 15 minutes. Thus, there were ethanol-intake-specific effects in the first 15 minutes of the DID session.
The final 15 minutes of the session were analyzed to determine if drug effects 1) diminished or increased over time or 2) interacted with ethanol’s sedative effects (data not shown). Analysis of fluid intake revealed significant main effects of fluid [F(1, 36)=7.79 p<.01] and treatment [F(1, 36)=6.31 p<.05] with higher fluid intake in water group and generally lower fluid intake in GS39783 treated animals. Analysis of locomotor activity revealed significant main effects of treatment only [F(1, 36)=10.98 p<.01], with generally lower activity in animals treated with GS39783. Thus, in the final 15 minutes, GS39783 had non-specific effects on both fluid intake and locomotor activity. We opted to not include the results of the polynomial analysis in either the text or in graphical form here as we did not feel they provided any obvious interpretational differences than those just reported above. The interested reader is referred to the supplementary materials for these comparisons (supplementary figure 2). We have also included a similar supplementary figure (supplementary figure 3) of the 5th day of testing of experiment 1 as this is a typical endpoint for DID studies.
Discussion
The results of these experiments suggest that there is a dynamic relationship between ethanol intake and concomitant home cage locomotor activity that 1) changes as a function of within- and between-DID sessions, and 2) can be differentially altered using the GABAB positive allosteric modulator GS39783.
Cumulative Daily Intake and Home Cage Locomotion
When the 2 hour cumulative totals were evaluated, generally larger volumes of fluid and greater home cage locomotor activity were observed in water assigned animals compared to those assigned to ethanol. These results are consistent with previous findings in our lab with one notable exception. We have previously observed significant increases in home cage locomotor activity during the first day of DID ethanol exposure (locomotor stimulation), with progressive decreases in locomotor activity observed following each subsequent DID session to levels below those of water consuming controls (Linsenbardt and Boehm, 2011, Linsenbardt et al., 2011). However, we did not observe significant acute locomotor stimulation on day 1 in these studies. Rather, animals given access to ethanol in experiment 1 displayed a trend towards a significant decrease in home cage locomotion on the first day (df=37, t=1.44, p=.06), and this observation appeared to dissipate and then re-emerge following additional exposures were it was generally sustained. Slight home cage locomotor stimulation was observed on day 1 in experiment 2, but this effect did not reach statistical significance (p>.05; data not shown). The apparent lack of significant acute stimulation on the first day in these studies we believe may due in part to variable ethanol intake attributable to habituation to the specialized sipper tubes, as standard ball-bearing sipper tubes were used in all previous studies where stimulation was observed. This view is supported by several observations. First, total home cage locomotor activity on day 1 was significantly positively associated with total ethanol intake on this day (R2=.64, p<.0001). However, because there was a substantial number of individuals whose ethanol consumption was very low on the first day, mean locomotor activity of the entire group do not appropriately illustrate this relationship. Significant locomotor stimulation observed on day 15 in ethanol naïve (WE) animals provide additional support that ethanol-induced locomotor stimulation occurs when ethanol is novel, as sufficient levels of intake occurred on this day in the majority of animals presumably because they were habituated to the sipper tubes. However, as mean ethanol intake on day 15 in the WE group were comparable to mean ethanol intake in naïve animals on day 1, the novelty of the DID procedures or other unknown factors also likely contributed to the lack of locomotor stimulation on day 1. Nevertheless, that binge-like ethanol intake in B6 mice is associated with within-session home cage locomotor activity alterations 1) highlights the relevance of monitoring this behavior, and 2) suggests it may be important for the maintenance of binge-like ethanol intake over daily repeated sessions.
Alterations in the Rate of Intake and Home Cage Locomotion
While evaluating cumulative 2 hour totals was important, we gained a much more comprehensive understanding of these behaviors from the discrete time interval analysis. That the rate of ethanol intake increased over DID sessions was not altogether surprising given that we had previous evidence of such an effect using very similar experimental procedures (Linsenbardt and Boehm, 2009, Linsenbardt et al., 2011). However, the degree to which ethanol intake changed from the first to the 14th ethanol exposure was remarkable. Not only was more ethanol consumed during the first 15 minutes in each subsequent DID session, but the rate of that intake during those 15 minutes increased dramatically. We take this as evidence of increased motivation to consume ethanol rather than simply habituation to the DID procedures, as there were no meaningful increases in the rate of water intake in water consuming animals across DID sessions. It is not immediately clear what factors may have influenced this change in motivation. However, possibilities include alterations in either preabsorptive effects such as overcoming aversive taste qualities and habituation to the novel ethanol tastant, and/or postabsorptive effects directly related to the ethanol’s physiological effects. It is also possible that the observed shift in ethanol consumption is in some way related to a possible transition to a dependence-like state. Future studies will be necessary to flush out these possibilities.
The timecourse of home cage locomotor activity alterations were also quite interesting. While on both the 1st and 14th days of testing ethanol consuming animals displayed hypolocomotor activity compared to water drinking controls, this occurred more quickly on the 14th session compared to the 1st. This effect may be attributable to differences in the rate of ethanol intake between days, as consumption of more ethanol in a shorter period of time elicits faster increasing and higher peaking BECs (Eckardt et al., 1998). A significant positive relationship between the rate of ethanol intake and the rate of home cage locomotor activity support this view (see suppl. Fig 2). However, there is at least one alternative explanation. As approach towards the sipper tube is necessary for initiating intake, and these approach behaviors would presumably be captured as home cage ambulatory activity, it is possible that faster decreases in locomotion are a direct reflection of decreases in ambulation necessary for fluid intake. In this case, rather than interpreting decreases as ethanol-induced locomotor sedation, they might be interpreted as decreased frequency in bout initiation (i.e. fewer bouts). This assumes that the primary purpose of locomotor activity during the session was for the purposes of fluid intake, and this would appear to be the case for ethanol consuming animals as ethanol intake occurred almost exclusively at times when locomotion was also most evident. However, because water intake at each bin was not directly proportional to home cage locomotion that occurred at that epoch, home cage locomotion in the water consuming animals was not primarily water intake-oriented.
Pharmacological Manipulation with GS39783
The results of the pharmacological manipulation with GS39783 further highlight the importance of monitoring behavioral variables with high temporal resolution. Had we only recorded 2 hour totals, we would have reached the conclusion that GS39783 non-specifically reduced fluid intake, perhaps as a direct result of the drug’s motor sedative effects. However, given that GS39783 abolished ethanol intake almost entirely during the first 15 minutes, and did not affect water intake or locomotor activity within this timeframe, suggests that the observed drug effects were ethanol-intake specific. The implications of this finding are intriguing, as decreasing or eliminating the ethanol ‘front-loading sufficiently decreased total ethanol intake within an entire given drinking session. This result provides compelling clinical implications. The focus of our interventions might be shifted toward decreasing the amount of ethanol intake at an early discrete time interval–at the onset of a binge-drinking session rather than simply reducing total consumption of ethanol. Of course, the goal is to reduce overall ethanol intake within a given session, but the timing of pharmacotherapeutics with short half-lives or slower rates of actions could be adjusted such that their peak plasma concentrations occur at the onset of typical drinking sessions when intake is most rapid.
There are also interesting implications of these findings with respect to the GABAB receptor system and binge-like ethanol intake. The GS39783 compound (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitropyrimidine-4,6-diamine) binds to sites distinct from those of endogenously produced GABA and increase GABAs potency and maximal efficacy (Urwyler et al., 2003). This compound is functionally distinct from GABAB agonists such as Baclofen, in that its facilitory effects on GABAB receptor activity are dependent on the presence of endogenously regulated GABA. Therefore, indirectly facilitating the action of GABA at GABAB receptors may be a promising mechanism by which to decrease binge-like ethanol intake. Indeed, positive allosteric modulation of GABAB receptors with GS39783 (Maccioni et al., 2008, Maccioni et al., 2007, Maccioni et al., 2012) and other compounds (Maccioni et al., 2009, Maccioni et al., 2010) have been shown to effectively decrease voluntary ethanol intake under several experimental conditions. However, these data were generated for the purposes of this manuscript wholly as a proof-of-principle; the rate of intake and other variables such as concomitant home cage locomotion have the potential to (and did) interact with drug pharmacology. Clearly, additional doses of GS39783 are necessary to further test this drugs potential utility as a pharmacotherapeutic. Evaluating the effect of this compound over several or many such sessions would also be highly informative given the increases in the rate of intake observed in experiment 1.
Conclusions
These results further characterize the DID model and strongly suggest that B6 mice given daily access to ethanol under these procedures alter their behavior to maximize ethanol’s pharmacological effects. These data extend recent results detailing the microstructure of 4 days of DID in animals selectively bred for high BECs(Barkley-Levenson and Crabbe, 2012), and emphasize the importance of evaluating progressive changes in ethanol-related behavior with fine temporal resolution. These data also provide evidence supporting the notion that reducing ethanol intake at the onset of a binge drinking session is particularly effective at reducing overall intake within that session. Together, these data suggest that re-evaluating previously tested therapeutics with a focus on the within- and between-session rate of ethanol intake might alter previous interpretations of both published and unpublished data. This possibility extends beyond ethanol studies, as many similar drug self-administration models are currently in use today.
Supplementary Material
Acknowledgments
This work was supported by NIAAA grant #’s AA015434 (SLB), AA016789 (SLB), and AA07462 (DNL).
References
- ALLEN JP. Measuring outcome in interventions for alcohol dependence and problem drinking: executive summary of a conference sponsored by the national institute on alcohol abuse and alcoholism. Alcohol Clin Exp Res. 2003;27:1657–60. doi: 10.1097/01.ALC.0000091223.72517.13. [DOI] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM, CRABBE JC. Ethanol drinking microstructure of a high drinking in the dark selected mouse line. Alcohol Clin Exp Res. 2012;36:1330–9. doi: 10.1111/j.1530-0277.2012.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKARDT MJ, FILE SE, GESSA GL, GRANT KA, GUERRI C, HOFFMAN PL, KALANT H, KOOB GF, LI TK, TABAKOFF B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- KRUSE LC, LINSENBARDT DN, BOEHM SL., 2ND Positive allosteric modulation of the GABA(B) receptor by GS39783 attenuates the locomotor stimulant actions of ethanol and potentiates the induction of locomotor sensitization. Alcohol. 2012;46:455–62. doi: 10.1016/j.alcohol.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI TK, HEWITT BG, GRANT BF. The Alcohol Dependence Syndrome, 30 years later: a commentary. the 2006 H. David Archibald lecture. Addiction. 2007;102:1522–30. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- LINSENBARDT DN, BOEHM SL., 2ND Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSENBARDT DN, BOEHM SL., 2ND Role of Novelty and Ethanol History in Locomotor Stimulation Induced by Binge-Like Ethanol Intake. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSENBARDT DN, MOORE EM, GRIFFIN KD, GIGANTE ED, BOEHM SL., 2ND Tolerance to Ethanol’s Ataxic Effects and Alterations in Ethanol-Induced Locomotion Following Repeated Binge-Like Ethanol Intake Using the DID Model. Alcohol Clin Exp Res. 2011;35:1246–55. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACCIONI P, CARAI MA, KAUPMANN K, GUERY S, FROESTL W, LEITE-MORRIS KA, GESSA GL, COLOMBO G. Reduction of alcohol’s reinforcing and motivational properties by the positive allosteric modulator of the GABA(B) receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res. 2009;33:1749–56. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- MACCIONI P, FANTINI N, FROESTL W, CARAI MA, GESSA GL, COLOMBO G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783--comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res. 2008;32:1558–64. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- MACCIONI P, PES D, ORRU A, FROESTL W, GESSA GL, CARAI MA, COLOMBO G. Reducing effect of the positive allosteric modulator of the GABA(B) receptor, GS39,783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacology (Berl) 2007;193:171–8. doi: 10.1007/s00213-007-0776-1. [DOI] [PubMed] [Google Scholar]
- MACCIONI P, THOMAS AW, CARAI MA, GESSA GL, MALHERBE P, COLOMBO G. The positive allosteric modulator of the GABA(B) receptor, rac-BHFF, suppresses alcohol self-administration. Drug Alcohol Depend. 2010;109:96–103. doi: 10.1016/j.drugalcdep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- MACCIONI P, ZARU A, LOI B, LOBINA C, CARAI MA, GESSA GL, CAPRA A, MUGNAINI C, PASQUINI S, CORELLI F, HYYTIA P, LUMENG L, COLOMBO G. Comparison of the Effect of the GABA(B) Receptor Agonist, Baclofen, and the Positive Allosteric Modulator of the GABA(B) Receptor, GS39783, on Alcohol Self-Administrationin 3 Different Lines of Alcohol-Preferring Rats. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE EM, SERIO KM, GOLDFARB KJ, STEPANOVSKA S, LINSENBARDT DN, BOEHM SL., 2ND GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–13. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES JS, BEST K, BELKNAP JK, FINN DA, CRABBE JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- RHODES JS, FORD MM, YU CH, BROWN LL, FINN DA, GARLAND T, JR, CRABBE JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- URWYLER S, POZZA MF, LINGENHOEHL K, MOSBACHER J, LAMPERT C, FROESTL W, KOLLER M, KAUPMANN K. N,N′-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function. J Pharmacol Exp Ther. 2003;307:322–30. doi: 10.1124/jpet.103.053074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.