Abstract
Activation of invariant natural killer T (iNKT) cells and signaling through receptor for advanced glycation end products (RAGE) are known to independently mediate lung ischemia-reperfusion injury. This study tests the hypothesis that activation of RAGE specifically on iNKT cells via alveolar macrophage-produced high mobility group box 1 (HMGB1) is critical for the initiation of lung ischemia-reperfusion injury. A murine in vivo hilar clamp model was utilized, which demonstrated that RAGE−/− mice were significantly protected from ischemia-reperfusion injury. Treatment of WT mice with soluble RAGE (a decoy receptor), or anti-HMGB1 antibody, attenuated lung ischemia-reperfusion injury and inflammation, whereas treatment with recombinant HMGB1 enhanced ischemia-reperfusion injury in WT mice but not RAGE−/− mice. Importantly, lung dysfunction, cytokine production and neutrophil infiltration were significantly attenuated after ischemia-reperfusion in Jα18−/− mice reconstituted with RAGE−/− iNKT cells (versus WT iNKT cells). In vitro studies demonstrated that, after hypoxia-reoxygenation, alveolar macrophage-derived HMGB1 augmented IL-17 production from iNKT cells in a RAGE-dependent manner. These results suggest that HMGB1-mediated RAGE activation on iNKT cells is critical for initiation of lung ischemia-reperfusion injury and that a crosstalk between macrophages and iNKT cells via the HMGB1/RAGE axis mediates IL-17 production by iNKT cells causing neutrophil infiltration and lung ischemia-reperfusion injury.
Keywords: HMGB1, alveolar macrophages, iNKT cells, RAGE, IL-17, lung transplantation
Introduction
Lung ischemia-reperfusion (IR) injury is the primary cause of mortality and morbidity in patients undergoing lung transplantation (1, 2). Lung transplantation has the worst outcome of all solid organ transplants, and IR injury contributes to primary graft failure and enhances the risk for development of bronchiolitis obliterans (3–5). We have previously demonstrated that the activation of invariant natural killer T (iNKT) cells and iNKT cell-produced IL-17 are key early events that initiate neutrophil infiltration and lung IR injury (6). In addition, we have shown that mice deficient in the receptor for advanced glycation end products (RAGE) are significantly protected from lung IR injury (7). Although it is known that RAGE can mediate T cell responses, the mechanism for iNKT cell activation and IL-17 production after IR remains unknown. Thus, the present study investigates the role of RAGE activation on iNKT cells in IL-17 production and neutrophil infiltration during lung IR injury.
RAGE belongs to an immunoglobulin superfamily of cell-surface molecules, and in addition to its full-length, membrane-bound form, a soluble form of RAGE (sRAGE) can be generated extracellularly by proteolytic cleavage or alternate splicing of membrane RAGE (8–11). sRAGE can act as a ligand decoy to block RAGE signaling (12, 13) and attenuate lung injury after IR or lipopolysaccharide administration (14, 15). In lung transplant patients, higher plasma levels of sRAGE correlate with longer duration of mechanical ventilation (16, 17).
RAGE binds to various ligands including high mobility group box 1 (HMGB1), S100 proteins, amyloid β-peptide and advanced glycation end products (AGE), which are highly expressed under inflammatory conditions (18, 19). However, HMGB1 has a 7-fold higher affinity for RAGE than other ligands, and the HMGB1/RAGE axis has been implicated in the pathogenesis of various inflammatory processes (20–26). HMGB1 is a nuclear alarmin rapidly released by activated macrophages, dendritic cells or endothelial cells (27, 28). HMGB1 can act as a chemokine leading to recruitment of monocytes, T cells and dendritic cells, NF-κβ activation and proinflammatory cytokine production via its binding with RAGE as well as potential interaction with toll-like receptors (29, 30). Recent studies suggest that RAGE expression on T cells can play an important role in adaptive immune responses (31–33). Moreover, transgenic mice with targeted overexpression of dominant-negative RAGE in CD4+ T cells are resistant to inflammation and experimental autoimmune encephalomyelitis (34). However, the precise role of the HMGB1/RAGE axis in the activation of T cells remains undefined in lung IR injury and was the focus of the current study.
Using a murine in vivo hilar clamp model of lung IR as well as an in vitro hypoxia-reoxygenation model, the current study demonstrates that the HMGB1/RAGE axis mediates iNKT cell activation and IL-17 production after lung IR. This study presents a novel signaling paradigm by demonstrating that a crosstalk between alveolar macrophage-produced HMGB1 and RAGE-mediated iNKT cell activation is a critical event for IL-17 production, neutrophil activation and subsequent lung IR injury.
Materials and Methods
Detailed methods are described in the online supplement.
Animals
This study utilized 8–12 week old male C57BL/6 WT (Jackson Laboratory, Bar Harbor, ME), Jα18−/− (35) and RAGE−/−(36) mice. Jα18−/− and RAGE−/−mice were congenic with C57BL/6. Mice underwent either sham surgery or lung IR. This study conformed to the National Institutes of Health guidelines and was conducted under animal protocols approved by the University of Virginia’s Institutional Animal Care and Use Committee.
Lung IR model and reagents
An in vivo hilar clamp model of lung IR was used wherein mice undergoing IR were subjected to 1 hour left lung ischemia (via left hilar occlusion) followed by 2 hours of reperfusion as previously described (37). Sham animals received the same surgery but without hilar occlusion. Intratracheal treatment of mice with human recombinant HMGB1 (rHMGB1; 10 μg/mouse; R&D Systems, Minneapolis, MN), human sRAGE (20 μg/mouse; MyBioSource, Inc., San Diego, CA) or anti-RAGE antibody (100μg/mouse; Sigma Aldrich, St. Louis, MO) was performed 5 min before ischemia.
Pulmonary function
Pulmonary function was evaluated using an isolated, buffer-perfused lung system (Hugo Sachs Elektronik, March-Huggstetten, Germany) as previously described (37), and hemodynamic and pulmonary function parameters were recorded by the PULMODYN data acquisition system (Hugo Sachs Elektronik). Lungs were maintained on the system for a 5-minute equilibration period before data was recorded for an additional 5 minutes.
Cytokine analysis
Cytokine concentrations in BAL fluid were quantified using the Bioplex Bead Array technique and a multiplex cytokine panel assay (Bio-Rad Laboratories, Hercules, CA) as previously described (6).
HMGB1 measurement
HMGB1 was measured using an ELISA kit per the manufacturer’s instructions (IBL International, Hamburg, Germany).
Lung wet/dry weight
Fresh lungs from separate groups of animals were weighed and desiccated until a stable dry weight was achieved. Lung wet/dryweight was then calculated as an indicator of edema.
Immunohistochemistry
Immunostaining to identify neutrophils was performed as described previously (37).
Purification and adoptive transfer of iNKT cells
Reconstitution of Jα18−/−mice was performed by adoptive transfer of primary iNKT cells as previously described (6). After lysis of red blood cells, splenocytes were suspended in FACS staining buffer containing anti-mouse CD16/CD32 mAb (eBioscience) to block non-specific FcR binding. Cells were then incubated with CD1d tetramer-Alexa647 (NIH Tetramer Facility, Emory University) and enriched by positive magnetic bead selection using anti-Alexa647 microbeads (Miltenyi Biotec, Auburn, CA). The enriched cells were stained with FITC-conjugated anti-CD19 and PE-conjugated anti-TCRβ (eBioscience) and sorted using a FACSVantage SE Turbo Sorter (Becton Dickinson) resulting in cell populations of >98% pure CD1d tetramer+CD4+ iNKT cells. Purified iNKT cells (2.5×105) were injected into Jα18−/−mice via tail vein injection 4 days prior to surgery.
In vitro hypoxia-reoxygenation (HR)
Primary iNKT cells were isolated (as described above) and cultured overnight in RPMI media containing 10% fetal bovine serum and1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Alveolar macrophages (MH-S murine alveolar macrophage cell line; ATCC, Manassas, VA) were grown in complete medium consisting of DMEM with 4.5 g/l glucose containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C with 5% CO2 and 95% O2. For exposure to HR, 24-well culture plates were placed in a humidified, sealed hypoxic chamber (Billups- Rothenberg, Del Mar, CA) that was purged with 95% N2 and 5% CO2 for 25 min to establish hypoxia as described previously (38). The chamber was then placed in an incubator for 3 hours after which it was opened and the culture media was immediately analyzed for O2 concentration using a blood-gas analyzer (Chiron Diagnostics). The partial percentage of O2 in culture media after hypoxia was consistently found to be 5% versus 21% in normoxic cultures. Reoxygenation was achieved by transferring the plates to a normoxic, humidified incubator (37°C, 5% CO2 and 95% O2) for 1 hour. Conditioned media transfer (CMT) experiments were performed by exposing MH-S cells to HR, with or without 1 μg/ml sRAGE or 10 μg/ml anti-HMGB1 antibody treatment, followed by transfer of this conditioned media to washed iNKT cells, which were then exposed to HR. IL-17A in culture media was measured by ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6.0 software, and data are presented as the mean ± standard error of the mean. One-way ANOVA with post-hoc Bonferroni’s multiple comparisons, Mann-Whitney U-test, or Student’s t-test were used as appropriate to compare experimental groups. Statistical significance was set at P<0.05.
Results
Pulmonary dysfunction and lung edema after IR is mediated by the HMGB1/RAGE axis
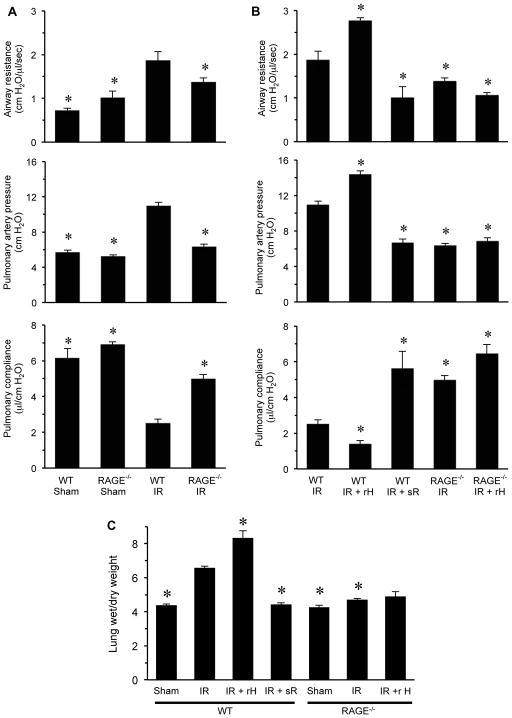
To investigate the importance of the HMGB1/RAGE axis in lung IR injury, pulmonary function was measured in WT and RAGE−/− mice after sham surgery or IR (Figure 1A). Significant pulmonary dysfunction occurred after IR in WT mice as indicated by increased airway resistance and pulmonary artery pressure as well as decreased pulmonary compliance. Pulmonary dysfunction after IR was significantly attenuated in RAGE−/− mice compared to WT mice after IR. Pulmonary function was similar between WT and RAGE−/− sham mice. These results confirm that RAGE is an important mediator of lung dysfunction after IR.
Figure 1. Pulmonary dysfunction and edema after IR is mediated by the HMGB1/RAGE axis.
Lung function was measured in WT and RAGE−/− mice after IR. (A) A significant increase in airway resistance and pulmonary artery pressure as well as a decrease in pulmonary compliance occurred in WT mice after IR versus sham. Pulmonary dysfunction after IR was significantly attenuated in RAGE−/− mice versus WT mice. (B) Pulmonary function was measured in WT and RAGE−/− mice after IR with or without treatment with recombinant HMGB1 (rH) or sRAGE (sR). A significant enhancement in airway resistance and pulmonary artery pressure and a decrease in pulmonary compliance occurred after IR in WT mice treated with rHMGB1 compared to WT mice undergoing IR alone. However, rHMGB1 had no significant effects in RAGE−/− mice. Treatment of WT mice with sRAGE significantly attenuated lung dysfunction in WT mice after IR compared to WT mice undergoing IR alone. (C) Pulmonary edema was assessed by measuring lung wet/dry weight in WT and RAGE−/− mice after sham surgery or lung IR. A significant increase in wet/dry weight occurred after IR in WT mice versus sham, which was significantly attenuated in RAGE−/− mice. Lung edema was significantly enhanced after IR in WT mice treated with recombinant HMGB1 (rH) versus WT mice undergoing IR alone. Treatment of RAGE−/− mice with recombinant HMGB1 after IR failed to enhance lung edema versus untreated RAGE−/− mice after IR. Treatment with sRAGE (sR) significantly attenuated lung edema in WT mice undergoing lung IR versus WT mice undergoing lung IR alone. *P<0.05 versus WT IR; n=5–8/group.
Lung dysfunction after IR was significantly worsened in WT mice treated with rHMGB1 (10 μg/mouse intratracheally 5 min before ischemia) as exemplified by increased airway resistance and pulmonary artery pressure as well as decreased pulmonary compliance (Figure 1B). However, rHMGB1 had no significant effect on lung function after IR in RAGE−/− mice. Treatment of WT mice with sRAGE (20 μg/mouse intratracheally 5 min before ischemia) significantly attenuated lung dysfunction after IR compared to WT mice undergoing IR alone (Figure 1B). Treatment of WT mice with sRAGE improved lung dysfunction to levels similar to that of RAGE−/− mice. rHMGB1 did not significantly alter lung function in WT or RAGE−/− sham mice (Figure S1).
In addition, pulmonary edema (wet/dry weight) was measured in separate groups of mice (Figure 1C). There was no difference in lung wet/dry weight between WT and RAGE−/− sham mice. A marked increase in edema occurred in WT mice after IR compared to sham, which was significantly reduced in RAGE−/− mice after IR. Treatment with rHMGB1 significantly enhanced edema after IR in WT mice, but not in RAGE−/− mice. Furthermore, treatment of WT mice with sRAGE significantly attenuated edema after IR compared to WT mice undergoing IR alone (Figure 1C). These results suggest that RAGE signaling plays a crucial role in mediating lung IR injury and that RAGE is required for rHMGB1 to enhance lung dysfunction after IR.
Inflammation after IR is mediated by the HMGB1/RAGE axis
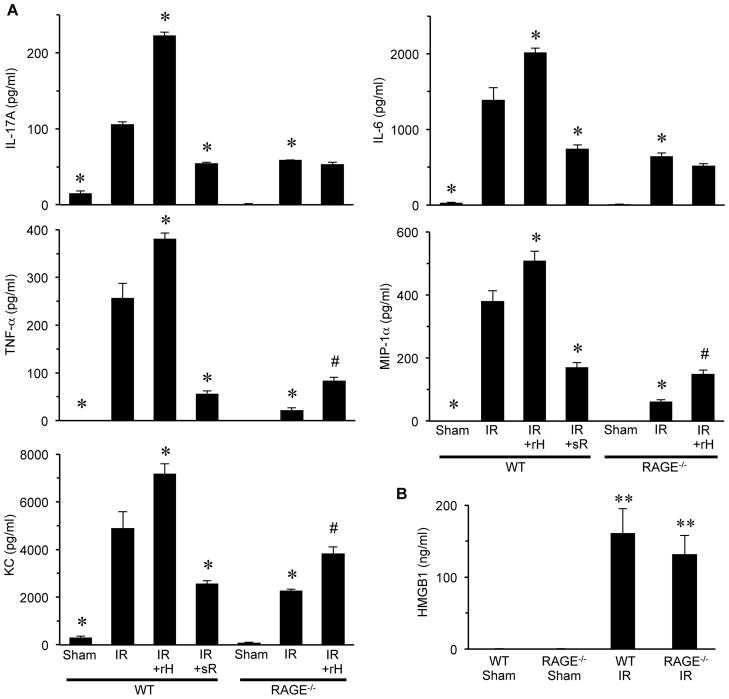
The expression of proinflammatory cytokines and chemokines was measured in BAL fluid to assess pulmonary inflammation. A significant induction of IL-17, TNF-α, KC (CXCL1), IL-6 and MIP-1α (CCL3) occurred after IR in WT mice, which were all significantly attenuated in RAGE−/− mice (Figure 2A). Treatment with rHMGB1 significantly enhanced cytokine production after IR in WT mice; however, this was significantly attenuated or blocked in RAGE−/− mice. Although a small but significant increase in TNF-α, MIP-1α and KC occurred in rHMGB1-treated RAGE−/− mice after IR, the enhancement of IL-17 and IL-6 were completely blocked in these mice. Furthermore, sRAGE treatment significantly attenuated proinflammatory cytokine production in WT mice after IR (Figure 2A).
Figure 2. The HMGB1/RAGE axis mediates proinflammatory cytokine production after lung IR.
(A) Cytokine levels (IL-17A, TNF-α, KC, MIP-1α and IL-6) were measured in BAL fluid of WT and RAGE−/− mice after sham surgery or lung IR. A significant, multifold increase of each cytokine occurred after IR in WT mice compared to sham, which was significantly attenuated in RAGE−/− mice after IR. A significant enhancement in cytokine production occurred after IR in WT mice treated with rHMGB1 versus WT mice undergoing IR alone. However, recombinant HMGB1 (rH) treatment of RAGE−/− mice undergoing IR did not enhance IL-17A or IL-6 expression and induced only a small, but significant, increase in TNF-α, MIP-1α and KC expression compared to RAGE−/− mice undergoing IR alone. Treatment with sRAGE (sR) significantly attenuated cytokine production in WT mice undergoing IR versus WT mice undergoing IR alone. (B) Expression of HMGB1 in BAL fluid was not detectable in WT or RAGE−/− sham mice but was significantly increased in both after IR. *P<0.05 versus WT IR, #P<0.05 versus RAGE−/−IR, **P<0.001 versus Sham; n=5–8/group.
HMGB1 levels in BAL fluid were not detected in sham animals, but a large increase in HMGB1 occurred in both WT and RAGE−/− mice after IR (Figure 2B). Although rHMGB1 did not significantly induce IL-17 production in sham RAGE−/− mice, rHMGB1 did result in a small but significant increase in IL-17 production in sham WT mice (Figure S1). These results suggest that HMGB1 exacerbates lung inflammation after IR and that blockage of ligand binding to RAGE via sRAGE attenuates proinflammatory cytokine production.
Neutrophil infiltration and activation after IR are mediated by the HMGB1/RAGE axis
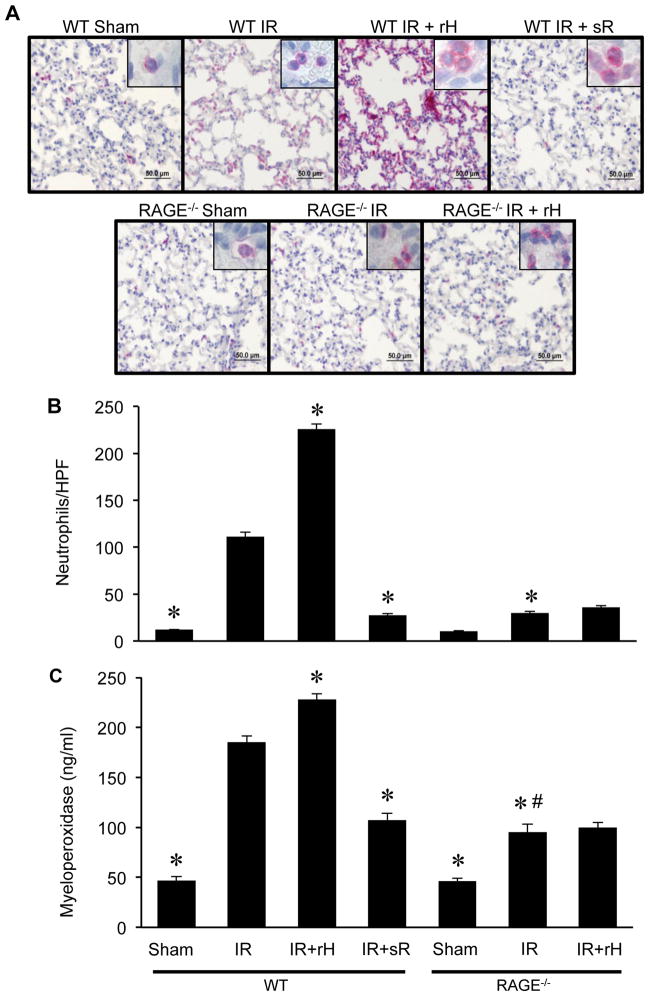
To determine the role of the HMGB1/RAGE axis in neutrophil infiltration, neutrophils were quantified in lung sections by immunohistochemistry (Figure 3A and B). Neutrophil numbers were similar between WT and RAGE−/− sham mice. Significant infiltration of neutrophils occurred in WT mice after IR, which was significantly attenuated in RAGE−/− mice. Treatment with rHMGB1 significantly enhanced neutrophil infiltration after IR in WT mice but not RAGE−/− mice. Treatment with sRAGE significantly attenuated neutrophil infiltration in WT mice after IR.
Figure 3. The HMGB1/RAGE axis mediates neutrophil infiltration and activation after IR.
(A) Immunohistochemical staining of lung sections was performed to assess neutrophil infiltration after lung IR in WT and RAGE−/− mice. Representative images of immunostaining are depicted. Neutrophils are stained red, sections are counterstained with hematoxylin, and images are at 40X magnification (bar=50 μm). Insets show higher magnification images (100X) to reveal neutrophil staining and morphology. (B) The number of neutrophils per high power field (neutrophils/HPF) significantly increased after IR in WT mice compared to sham, which was attenuated in RAGE−/− mice after IR. A significant enhancement in neutrophil numbers occurred in lungs of WT mice undergoing IR with treatment with recombinant HMGB1 (rH) versus WT mice undergoing IR alone. However, treatment of RAGE−/− mice undergoing lung IR with recombinant HMGB1 failed to enhance neutrophil numbers compared to untreated RAGE−/− mice after IR. Neutrophil infiltration was significantly attenuated in sRAGE (sR)-treated WT mice undergoing IR versus WT mice undergoing IR alone. (C) Neutrophil activation and infiltration into alveolar air spaces was also assessed by measuring myeloperoxidase levels in BAL fluid of WT and RAGE−/− mice after sham surgery or lung IR. Myeloperoxidase, a peroxidase enzyme abundantly present in neutrophil granules, was used as an indication of neutrophil activation and infiltration into alveolar airspaces. A significant increase in myeloperoxidase levels occurred after IR in WT mice versus sham, which was significantly attenuated in RAGE−/− mice. Myeloperoxidase levels were significantly enhanced after IR in WT mice treated with recombinant HMGB1 (rH) versus WT mice undergoing IR alone. Treatment of RAGE−/− mice with recombinant HMGB1 after IR failed to enhance myeloperoxidase levels versus untreated RAGE−/− mice after IR. Treatment with sRAGE (sR) significantly attenuated myeloperoxidase levels in WT mice undergoing lung IR versus WT mice undergoing lung IR alone. *P<0.05 versus WT IR, #P=0.001 versus RAGE−/−Sham; n=5–8/group.
Myeloperoxidase, a peroxidase enzyme abundantly present in neutrophil granules and released upon activation, was measured in BAL fluid to assess neutrophil activation and infiltration into alveolar airspaces (Figure 3C). There was no difference in myeloperoxidase levels between WT and RAGE−/− sham mice. A marked increase in myeloperoxidase occurred in WT mice after IR compared to sham, which was significantly reduced in RAGE−/− mice after IR. Treatment with rHMGB1 significantly enhanced myeloperoxidase after IR in WT mice, but not in RAGE−/− mice. Furthermore, treatment of WT mice with sRAGE significantly attenuated myeloperoxidase after IR compared to WT mice undergoing IR alone. These results suggest that the HMGB1/RAGE axis mediates neutrophil infiltration and activation after IR.
RAGE signaling in iNKT cells mediates lung dysfunction and inflammation after IR
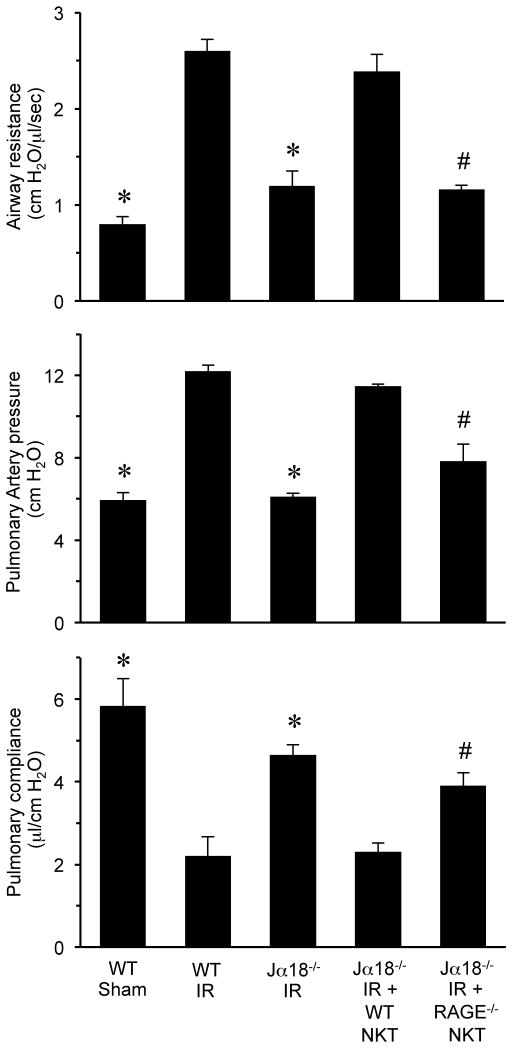
To investigate the role of RAGE signaling specifically in iNKT cells, pulmonary function was assessed after IR in Jα18−/− mice (iNKT cell-deficient) that were reconstituted with iNKT cells from WT or RAGE−/− mice. Lung dysfunction after IR was significantly attenuated in Jα18−/−mice, and reconstitution with WT iNKT cells restored dysfunction to WT levels (Figure 4). However, reconstitution of Jα18−/− mice with RAGE−/− iNKT cells failed to restore lung dysfunction, which remained significantly attenuated after IR compared to WT mice as demonstrated by decreased airway resistance and pulmonary artery pressure as well as increased pulmonary compliance (Figure 4). There was no difference in lung function in sham Jα18−/− mice with or without rHMGB1 treatment (Figure S1). These results establish a key role for RAGE activation specifically on iNKT cells in pulmonary dysfunction after IR.
Figure 4. Lung dysfunction after IR is mediated by RAGE activation on iNKT cells.
Pulmonary function was measured after IR in Jα18−/− mice that were reconstituted with iNKT cells purified from either WT or RAGE−/− mice as indicated. Lung dysfunction was significantly attenuated in Jα18−/− mice reconstituted with RAGE−/− iNKT cells (Jα18−/− IR + RAGE−/− NKT) compared to Jα18−/− mice reconstituted with WT iNKT cells (Jα18−/− IR + WT NKT). *P<0.05 versus WT IR, #P<0.05 versus Jα18−/− IR + WT NKT; n=5/group.
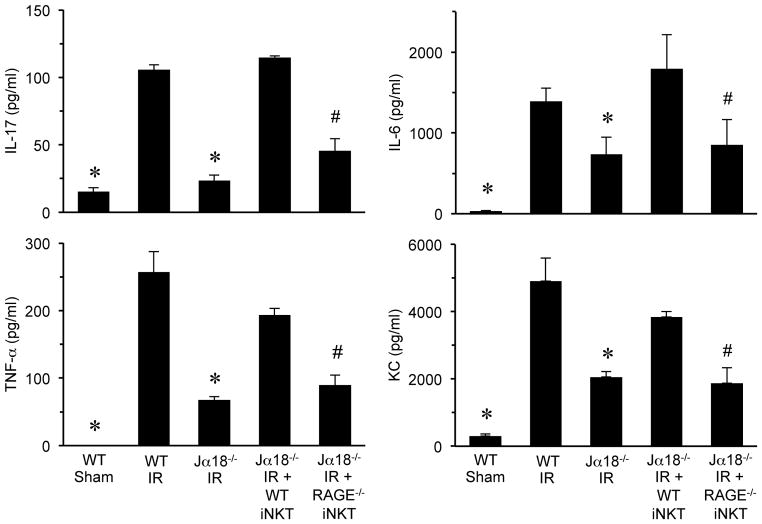
Moreover, the expression of proinflammatory cytokines (IL-17A, TNF-α, KC and IL-6) after IR in Jα18−/− mice reconstituted with RAGE−/− iNKT cells was significantly attenuated compared to Jα18−/− mice reconstituted with WT iNKT cells (Figure 5). There was no difference in IL-17 production in sham Jα18−/− mice with or without rHMGB1 treatment (Figure S1). These results implicate RAGE activation specifically on iNKT cells as a critical mediator of inflammation, especially iNKT cell-derived IL-17 production, after lung IR.
Figure 5. Proinflammatory cytokine production after lung IR is mediated by RAGE activation on iNKT cells.
Proinflammatory cytokine levels (IL-17A, TNF-α, KC and IL-6) were significantly increased after IR in WT mice versus sham and were significantly attenuated in Jα18−/− mice after IR. Cytokine production was restored after IR in Jα18−/− mice reconstituted with WT iNKT cells (Jα18 IR + WT NKT) but was significantly attenuated after IR in Jα18−/− mice reconstituted with RAGE−/− iNKT cells (Jα18−/− IR + RAGE−/− NKT). *P<0.05 versus WT IR, #P<0.05 versus Jα18−/− IR + WT NKT; n=5/group.
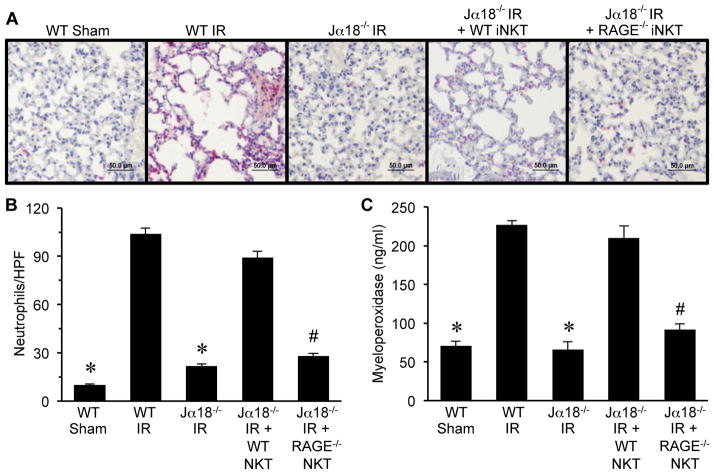
RAGE-dependent activation of iNKT cells mediates neutrophil infiltration after IR
To determine if RAGE activation on iNKT cells contributes to neutrophil infiltration and activation after IR, quantification of neutrophils (by immunohistochemistry) and myeloperoxidase were performed. Both neutrophil numbers (Figure 6A and B) and myeloperoxidase levels (Figure 6C) were significantly attenuated after IR in Jα18−/− mice reconstituted with RAGE−/− iNKT cells versus reconstitution with WT iNKT cells. Neutrophil numbers and myeloperoxidase levels in Jα18−/− mice were similar between IR and sham animals (data not shown). These results demonstrate that RAGE activation on iNKT cells is a key mediator of neutrophil infiltration after lung IR.
Figure 6. RAGE activation on iNKT cells mediates neutrophil infiltration and activation after IR.
Neutrophil infiltration and activation were assessed in lungs by immunohistochemistry and quantification of myeloperoxidase in BAL fluid. (A) Representative immunohistochemistry of lung sections. Neutrophils are stained red, sections are counterstained with hematoxylin, and images are at 40X magnification (bar=50 μm). (B) The number of neutrophils per high power field (neutrophils/HPF) was significantly increased after IR in WT mice (WT IR) versus sham (WT Sham) and was attenuated in Jα18−/− mice after IR (Jα18−/− IR). Neutrophil infiltration was restored after IR in Jα18−/− mice reconstituted with WT iNKT cells (Jα18−/− IR + WT NKT) but was significantly attenuated after IR in Jα18−/− mice reconstituted with RAGE−/− iNKT cells (Jα18−/− IR + RAGE−/− NKT). (C) Myeloperoxidase levels in BAL fluid were significantly increased in WT mice after IR and were attenuated in Jα18−/− mice after IR. Myeloperoxidase levels after IR were restored in Jα18−/− mice reconstituted with WT iNKT cells but were significantly attenuated after IR in Jα18−/− mice reconstituted with RAGE−/− iNKT cells. *P<0.05 versus WT IR, #P<0.05 versus Jα18−/− IR + WT NKT; n=5/group.
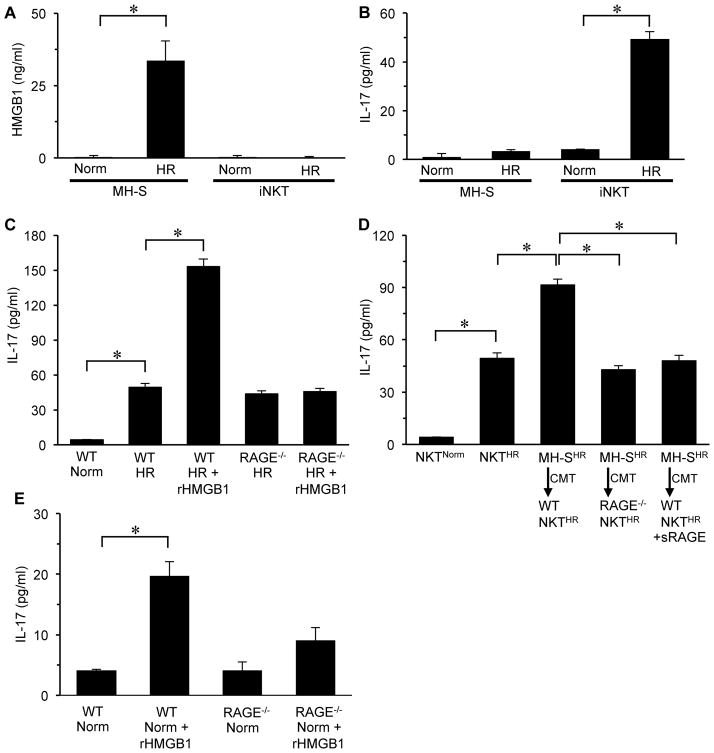
Alveolar macrophage-produced HMGB1 modulates RAGE-mediated activation of iNKT cells
HMGB1 is known to be rapidly released by activated macrophages among other cells (27, 30). To investigate potential crosstalk between alveolar macrophages and iNKT cells via HMGB1 after IR, we used an in vitro model of acute hypoxia-reoxygenation (HR) as a surrogate for lung IR. HR-exposed MH-S cells (a murine alveolar macrophage cell line) resulted in a significant increase in HMGB1 release compared to normoxia (Figure 7A). However, exposure of WT iNKT cells to HR did not induce detectable HMGB1 (Figure 7A). IL-17 production was significantly induced by HR-exposed iNKT cells but not by HR-exposed MH-S cells (Figure 7B). IL-17 production was significantly enhanced by HR-exposed WT iNKT cells after treatment with rHMGB1 compared to HR-exposed WT iNKT cells alone (Figure 7C). IL-17 production by HR-exposed RAGE−/− iNKT cells was similar to HR-exposed WT iNKT cells, and treatment with rHMGB1 failed to augment IL-17 production in HR-exposed RAGE−/− iNKT cells (Figure 7C). These results demonstrate that RAGE signaling in iNKT cells is responsible for the rHMGB1-mediated enhancement of IL-17 after HR.
Figure 7. Alveolar macrophage-produced HMGB1 mediates IL-17 production by iNKT cells after hypoxia-reoxygenation (HR) via RAGE activation.
An in vitro model of HR was used to evaluate the effect of macrophage (MH-S cell)-derived HMGB1 on IL-17 production by primary iNKT cells. (A) HR-exposed MH-S cells produced significantly greater amounts of HMGB1 versus normoxic (Norm) controls. There was no detectable production of HMGB1 in either normoxic- or HR-exposed iNKT cells. (B) HR-exposed iNKT cells had significantly increased IL-17 production versus normoxic controls. There was no significant production of IL- 17 by either normoxic- or HR-exposed MH-S cells. (C) Treatment of HR-exposed WT iNKT cells with recombinant HMGB1 (rHMGB1) significantly enhanced IL-17 production, which was blocked in RAGE−/− iNKT cells treated with rHMGB1. (D) IL-17 production was significantly enhanced upon conditioned media transfer (CMT) from HR-exposed MH-S cells (MH-SHR) to HR-exposed WT iNKT cells (WT NKTHR), but not to RAGE−/− iNKT cells (RAGE−/− NKTHR). Pretreatment of HR-exposed MH-S cells with sRAGE blocked the enhancement of IL-17 production after CMT to HR-exposed WT iNKT cells. *P<0.05, n = 5–8/group. (E) Treatment of normoxic WT iNKT cells with recombinant HMGB1 (rHMGB1) significantly increased IL-17 production versus normoxia alone. There was no significant difference in IL-17 production between normoxic RAGE−/− iNKT cells with or without rHMGB1 treatment. *P<0.05; n=4–6/group.
Conditioned media transfer (CMT) experiments were performed to evaluate potential crosstalk between macrophages and iNKT cells via RAGE signaling (Figure 7D). CMT from HR-exposed MH-S cells to HR-exposed WT iNKT cells resulted in a significant enhancement of IL-17 production, which was completely blocked by CMT to RAGE−/− iNKT cells (Figure 7D). Treatment of HR-exposed MH-S cells with sRAGE and subsequent CMT to HR-exposed WT iNKT cells also blocked the enhancement of IL-17 production (Figure 7D). Treatment of normoxic WT iNKT cells with rHMGB1 induced a small but significant increase in IL-17 production compared to normoxic WT iNKT cells alone; however, rHMGB1 did not significantly induce IL-17 production by normoxic RAGE−/− iNKT cells (Figure 7E). These results demonstrate that an alveolar macrophage-derived RAGE ligand activates iNKT cells after HR to produce IL-17, and treatment with sRAGE attenuates this crosstalk. When taken together with results from Figure 7C, the likely macrophage-derived RAGE ligand is HMGB1.
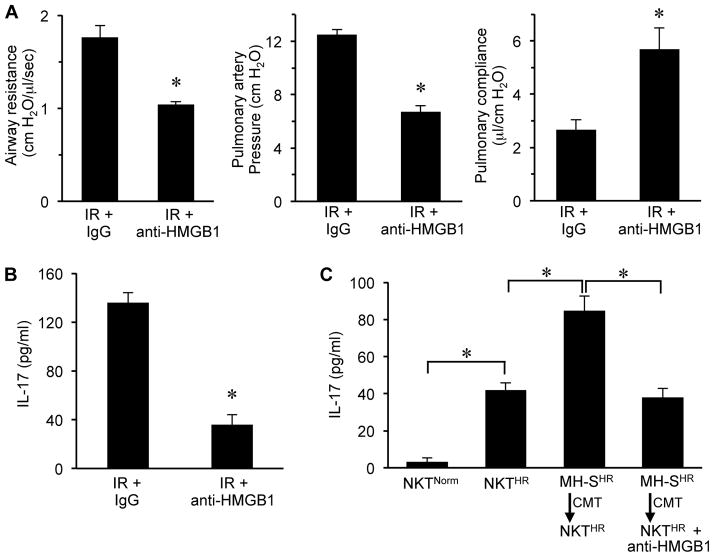
HMGB1 directs RAGE-mediated lung IR injury and activation of iNKT cells
The results of the above experiments suggest that HMGB1 is a key ligand for RAGE-mediated IR injury and activation of iNKT cells, however these experiments do not specifically establish HMGB1 as the predominant ligand since sRAGE can bind to many RAGE ligands. To address this, two key experiments were performed to test if anti-HMGB1 neutralizing antibody has the same protective effects as sRAGE. WT mice underwent lung IR and treatment with either control IgG antibody or anti-HMGB1 neutralizing antibody, and lung function was evaluated (Figure 8A). Anti-HMGB1 antibody significantly attenuated lung dysfunction after IR by decreasing airway resistance and pulmonary artery pressure as well as improving pulmonary compliance. In addition, IL-17 expression in lungs after IR was abolished by anti-HMGB1 antibody treatment (Figure 8B). This attenuation of lung dysfunction and IL-17 production was very similar to the results obtained with sRAGE in Figures 1 and 2. Furthermore, treatment of HR-exposed MH-S cells with anti-HMGB1 antibody and subsequent CMT to HR-exposed WT iNKT cells blocked the enhancement of IL-17 production (Figure 8C); results that are very similar to those obtained with sRAGE in Figure 7D. Taken together, these results confirm that the primary RAGE ligand that mediates lung dysfunction after IR is HMGB1 and that alveolar macrophage-produced HMGB1 plays a critical role in iNKT cell-dependent IL-17 production.
Figure 8. Anti-HMGB1 antibody attenuates lung dysfunction and IL-17 production after IR.
Lung function (A) and IL-17 expression in BAL fluid (B) were assessed after IR in WT mice treated with control IgG antibody or anti-HMGB1 neutralizing antibody intratracheally 5 min prior to ischemia. Lung dysfunction and IL-17 expression were significantly attenuated by anti-HMGB1 antibody treatment. (C) Pretreatment of HR-exposed MH-S cells with anti-HMGB1 significantly attenuated IL-17 production after conditioned media transfer (CMT) to HR-exposed WT iNKT cells. *P<0.01; n=5–7/group.
Discussion
The results of the current study define a pivotal role for the HMGB1/RAGE axis in the initiation of lung IR injury via iNKT cell activation as demonstrated by the fact that Jα18−/− mice reconstituted with RAGE−/− iNKT cells failed to restore lung dysfunction, injury and IL-17 production after IR. Furthermore, treatment with sRAGE or anti-HMGB1 antibody significantly attenuated lung dysfunction, injury and IL-17 production after IR, thereby blocking the proinflammatory effects of HMGB1. Finally, using an in vitro model, we showed that alveolar macrophages communicate with iNKT cells after HR via the HMGB1/RAGE axis, thereby underlining the importance of macrophage-produced HMGB1 on RAGE-mediated activation of iNKT cells that ultimately leads to inflammation, neutrophil infiltration and lung injury after IR.
High levels of RAGE expression in the lung, especially type I epithelial cells, are well documented (15), and RAGE has been implicated as a marker of type I epithelial cell injury in acute lung injury models (33). However, the role of RAGE and its ligands in the context of innate immune cells such as T lymphocytes is unknown in the setting of IR. Recent studies have shown that RAGE affects T cell activation and differentiation in mouse models of inflammation (31, 32). RAGE is able to modulate T cell phenotype by regulating the secretion of cytokines such as IFN-γ and by signaling through intermediaries such as NF-κB, MAP kinase, Jak/STAT and PI3K/Akt (31, 39, 40). In the present study, using both in vivo and in vitro models, we have demonstrated that RAGE signaling in iNKT cells plays a crucial role in lung inflammation and cytokine production after IR. The adoptive transfer experiments demonstrated the importance of RAGE activation specifically on iNKT cells in the pathogenesis of lung IR injury. This data represents a novel paradigm wherein the role of RAGE has been implicated in the context of the immunomodulation and proinflammatory signaling of iNKT cells after lung IR.
Our results demonstrate a critical role for the HMGB1/RAGE axis in lung IR injury as treatment with rHMGB1 exacerbated lung dysfunction and injury in WT, but not RAGE−/−, mice. We also observed an increased expression of HMGB1 (Figure 2B), but not S100A/B (data not shown), another RAGE ligand, in lungs of WT mice after IR. In addition to RAGE, HMGB1 is capable of binding to toll-like receptor (TLR)-2, TLR-4 and TLR-9 (22, 41). Thus it is plausible that TLRs may also play a role in HMGB1-mediated lung IR injury. However, rHMGB1 failed to augment lung dysfunction and cytokine production in RAGE−/− mice, suggesting that RAGE, and not TLRs, is the predominant target of HMGB1 during lung IR. Furthermore, our data suggest that alveolar macrophage-derived HMGB1 activates iNKT cells after HR to enhance IL-17 production. The fact that macrophages can produce HMGB1 under inflammatory conditions has been demonstrated in recent studies where the translocation of HMGB1 from the nucleus to cytoplasm requires inflammasome and caspase activity (42–45). On the other hand, extracellular HMGB1 can induce cytokine release from macrophages via its interactions with TLR-4 (46). Thus neutralizing HMGB1 can be of paramount importance for the mitigation of inflammation and disease pathogenesis, as has been shown in models of colitis, liver IR, sepsis and arthritis (25, 41, 43).
Because sRAGE readily binds to HMGB1 in circulation, thus serving as a decoy receptor, we postulate that sRAGE treatment prevents HMGB1 (one source being alveolar macrophages) from activating iNKT cells via RAGE, resulting in protection from IR injury. A previous study by Sternberg et al. has similarly shown that sRAGE attenuates lung IR injury (14). However, our current study importantly establishes a mechanism by which sRAGE affords protection from IR injury. Our results suggest that sRAGE blocks the effects of HMGB1-mediated IL-17 production by iNKT cells after HR. It has also been shown that sRAGE can be used as a biomarker as well as a therapeutic agent to neutralize inflammation (8, 9, 16, 47, 48). Other studies have shown similar protective effects with the use of neutralizing antibody to HMGB1 (23, 24, 45, 49–51), and our own results with anti-HMGB1 antibody confirms that the major ligand for RAGE-mediated lung IR injury and iNKT cell activation is HMGB1. However, regarding therapeutic efficacy, sRAGE, an endogenously secreted molecule that can attenuate proinflammatory signaling, is likely to be more clinically relevant as a therapeutic strategy to prevent IR injury in lung transplant patients.
We acknowledge that a limitation of this study is that the mouse hilar clamp model of lung IR injury does not truly represent a clinical lung transplant scenario. However, this model does allow for a detailed analysis of the molecular and cellular mechanisms and innate immune responses that occur in response to IR, as a model of primary graft dysfunction. The molecular and cellular events observed in the murine lung IR model appear to be clinically relevant as recent studies have provided evidence that iNKT cells and IL-17 play a key role in mediating primary graft dysfunction in clinical lung transplantation (52–55). For example, Hodge et al. showed that IL-17 production by NKT cells is increased in transplant patients, suggesting that these cells may play an important role in lung transplant rejection (52). Thus, increased expression of iNKT cell-derived IL-17 (via the HMGB1/RAGE axis as shown in the present study) may partially explain the link between early post-transplant acute lung injury (primary graft dysfunction) and shortened allograft survival in lung transplant patients since IL-17 expression is associated with destructive adaptive immune responses.
In summary, the present study demonstrates that in the setting of lung IR injury: a) HMGB1 is a potent inflammatory molecule that leads to RAGE-mediated activation of iNKT cells, b) the HMGB1/RAGE axis induces IL-17 and TNF-α production leading to infiltration of neutrophils, and c) the crosstalk between alveolar macrophages and iNKT cells via the HMGB1/RAGE axis is a novel signaling paradigm in the context of lung IR injury. The implication of RAGE-mediated signaling events in T cells furthers our knowledge of IR injury and may help in the design of novel therapeutic strategies that target T cells for preventing IR injury in patients undergoing lung transplantation.
Supplementary Material
Lung function was measured in WT, RAGE−/− and Jα18−/− mice after sham surgery with or without recombinant HMGB1 (rH) treatment.
Acknowledgments
This work was supported by NIH grant R01HL077301 (V.E.L.) and a Research and Development grant from the University of Virginia (A.K.S.).
Abbreviations
- HMGB1
high mobility group box 1
- RAGE
receptor for advanced glycation end products
- iNKT cells
invariant natural killer T cells
- IR
ischemia-reperfusion
- HR
hypoxia-reoxygenation
- TLR
Toll like receptor
- CMT
conditioned media transfer
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–7. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 2.Ailawadi G, Lau CL, Smith PW, Swenson BR, Hennessy SA, Kuhn CJ, et al. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg. 2009;137:688–94. doi: 10.1016/j.jtcvs.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant. 2009;28:1031–49. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–5. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–11. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–49. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaPar DJ, Hajzus VA, Zhao Y, Lau CL, French BA, Kron IL, et al. Acute hyperglycemic exacerbation of lung ischemia-reperfusion injury is mediated by receptor for advanced glycation end-products signaling. Am J Respir Cell Mol Biol. 2012;46:299–305. doi: 10.1165/rcmb.2011-0247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011;44:601–4. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Englert JM, Ramsgaard L, Valnickova Z, Enghild JJ, Oury TD. Large scale isolation and purification of soluble RAGE from lung tissue. Protein Expr Purif. 2008;61:99–101. doi: 10.1016/j.pep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, et al. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279:50019–24. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan XY, Clancy BM, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79:1379–86. doi: 10.1016/j.bcp.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, et al. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg. 2008;136:1576–85. doi: 10.1016/j.jtcvs.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1–L5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180:1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant. 2007;26:675–80. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 19.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 24.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 27.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–8. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–9. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 29.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–1. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 31.Akirav EM, Preston-Hurlburt P, Garyu J, Henegariu O, Clynes R, Schmidt AM, et al. RAGE expression in human T cells: a link between environmental factors and adaptive immune responses. PLoS One. 2012;7:e34698. doi: 10.1371/journal.pone.0034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–8. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–15. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, et al. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med. 2003;9:287–93. doi: 10.1038/nm831. [DOI] [PubMed] [Google Scholar]
- 35.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 36.Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, et al. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L105–13. doi: 10.1152/ajplung.00470.2006. [DOI] [PubMed] [Google Scholar]
- 39.Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010;24:1043–55. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, et al. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–93. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of γδ T cells with macrophages. Hepatology. 2012;57:373–84. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 42.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–15. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, et al. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2012 doi: 10.1016/j.jss.2012.07.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–35. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koyama H, Yamamoto H, Nishizawa Y. Endogenous Secretory RAGE as a Novel Biomarker for Metabolic Syndrome and Cardiovascular Diseases. Biomark Insights. 2007;2:331–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 51.Liu A, Fang H, Dirsch O, Jin H, Dahmen U. Oxidation of HMGB1 causes attenuation of its pro-inflammatory activity and occurs during liver ischemia and reperfusion. PLoS One. 2012;7:e35379. doi: 10.1371/journal.pone.0035379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodge G, Hodge S, Li-Liew C, Reynolds PN, Holmes M. Increased natural killer T-like cells are a major source of pro-inflammatory cytokines and granzymes in lung transplant recipients. Respirology. 2012;17:155–63. doi: 10.1111/j.1440-1843.2011.02075.x. [DOI] [PubMed] [Google Scholar]
- 53.Snell GI, Levvey BJ, Zheng L, Bailey M, Orsida B, Williams TJ, et al. Interleukin-17 and airway inflammation: a longitudinal airway biopsy study after lung transplantation. J Heart Lung Transplant. 2007;26:669–74. doi: 10.1016/j.healun.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Verleden SE, Vos R, Vandermeulen E, Ruttens D, Vaneylen A, Dupont LJ, et al. Involvement of interleukin-17 during lymphocytic bronchiolitis in lung transplant patients. J Heart Lung Transplant. 2013;32:447–53. doi: 10.1016/j.healun.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lung function was measured in WT, RAGE−/− and Jα18−/− mice after sham surgery with or without recombinant HMGB1 (rH) treatment.