Abstract
Our previous studies using rat models of incisional pain have demonstrated that tissue lactate levels increase and pH decreases for several days after incision, suggesting the presence of an ischemic-like condition. The purpose of this study was to evaluate the time course and the extent of tissue hypoxia that develops in incised muscle and skin. We directly measured oxygen tension at several time points after incisions of the gastrocnemius muscle, the paraspinal skin, and the plantar hindpaw in anesthetized rats using an oxygen-sensitive microelectrode. In vivo hypoxia of the incised tissues was also evaluated immunohistochemically using a hypoxia marker pimonidazole hydrochloride. To minimized inter-subject variability, unincised contralateral tissues were used as a control. Tissue oxygen tension was decreased in both skeletal muscle and skin compared to control, for several days after incision: when measured directly, oxygen tension decreased immediately and remained low for several days after incisions. Pimonidazole immunostaining revealed hypoxic areas in incised muscle and skin for several days. By postoperative day 10, tissue oxygen tension recovered to that of control tissue. These results support the evidence that a hypoxic condition is present in deep tissue after incisions and that an ischemic-like mechanism may contribute to postoperative pain.
Keywords: Incision, Hypoxia, Deep tissue, Ischemia, Incisional pain, Hyperalgesia
INTRODUCTION
In order to improve patient outcome, reduce perioperative morbidity and decrease healthcare costs following surgery, it is critical that we improve acute postoperative pain management (1). To better understand mechanisms of postoperative pain, we have developed and characterized rat models of incisional pain (2, 3), which show similarities to human postoperative pain and the responses of humans to a small incision (4). Previous studies indicate that the mechanisms for pain-related behaviors following an incision are different from preclinical models of inflammatory or neuropathic pain (5, 6). Therefore, it is important to understand the underlying pathophysiologic mechanisms in these postsurgical pain models, in order to explore the effective targets for the improved treatment of pain in patients undergoing surgery.
Our previous studies using incisions in several tissues have demonstrated that tissue lactate levels increase and tissue pH decreases on the day of incision and for several days afterwards (7, 8). These findings suggest that an ischemic-like condition is present in both skin and muscle after surgery. Many animal and human studies demonstrated a decrease in the cutaneous oxygen tension after incision (9, 10). Oxygen tension of incised deep tissue like skeletal muscle was rarely studied even though muscle is commonly injured after surgery. Decreases in blood flow to skin, caused by thermoregulatory vasoconstriction during hypothermia, occurs routinely without causing pain, whereas ischemic pain occurs when there is reduced blood flow to muscle (11, 12), suggesting that ischemic pain is a characteristic of muscle rather than skin (13). Indeed, protons and lactate have been proposed as mediators that together cause nociception in ischemic muscular pain (13–15). Therefore, it would be of considerable interest to evaluate the time course and extent of tissue hypoxia that develops in incised tissue especially skeletal muscle.
In the present study, we hypothesized that oxygen tension will be less in incised tissue, especially in the incised skeletal muscle, compared to the unincised contralateral control tissue. We directly measured tissue oxygen tension at several time points after incisions of the gastrocnemius muscle, paraspinal skin and the plantar hindpaw in anesthetized rats using an oxygen-sensitive microelectrode. We also evaluated in vivo hypoxia of the incised skeletal muscle and skin immunohistochemically using pimonidazole hydrochloride, a marker for hypoxia.
MATERIALS AND METHODS
General
All experimental procedures were approved by The University of Iowa Animal Care and Use Committee, Iowa City, Iowa. The animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals by National Institutes of Health.
Seventy-two adult male Sprague–Dawley rats (250–300 g; Harlan, Indianapolis, IN) were used; 46 rats for the direct measurement of tissue oxygen tension and 26 rats for immunohistochemistry. Rats were housed in groups of two to three in clear plastic cages, with a 12-h light-dark cycle. Food and water were available ad libitum.
Incisions
A gastrocnemius incision (Fig. 1A) was made under 1.5–2 % isoflurane anesthesia delivered via a nose cone similar to that described previously (3). After sterile preparation of the right posterior hindlimb, 3–4 cm of skin was incised. The cutaneous tissue was separated from the underlying muscle and the fascia between the 2 bodies of the gastrocnemius muscle was split and separated using blunt dissection. The insertion of the muscles remained intact. The skin was closed with 4 5-0 nylon sutures and the wound was covered with antibiotic ointment. Sutures were removed on postoperative day 3 (POD3)1.
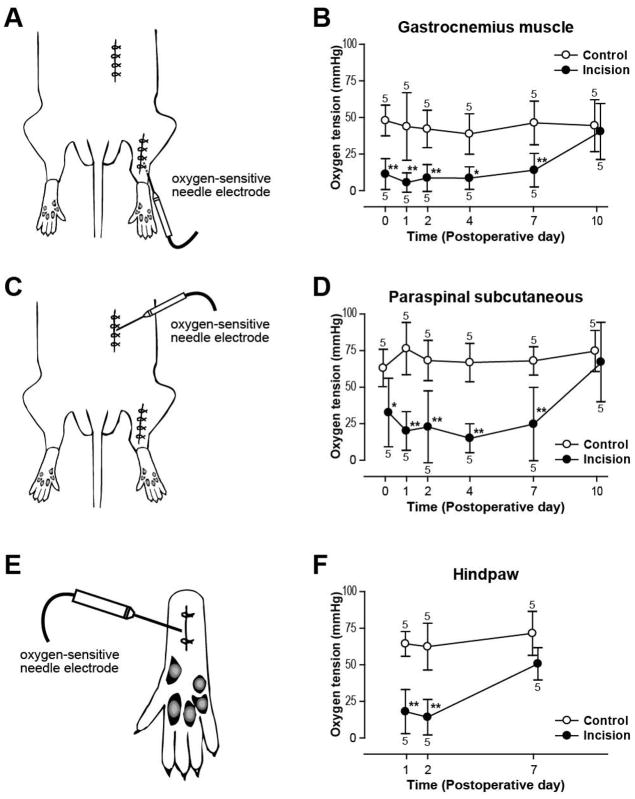
Figure 1.
Changes in tissue oxygen tension produced by gastrocnemius muscle (A, B), paraspinal skin (C, D) and plantar (E, F) incision. (A): Schematic of the hindquarter of the rat depicting the electrode insertion into an incised gastrocnemius incision. (B): Time course of changes in muscle oxygen tension after gastrocnemius incisions. (C): The location of oxygen electrode inserted into a paraspinal incision. (D): Time course of changes in subcutaneous oxygen tension after paraspinal incisions. (E): Schematic of the hindpaw of the rat depicting the electrode insertion in an incised paw. (F): Time course of changes in tissue oxygen tension after plantar incision. Results in (B), (D), and (F) are expressed as mean ± SD. Control value is the oxygen tension in the contralateral unincised site. Numeric values associated with each data point represent sample size at each time point (n = 5 per time point). *p < 0.01; **p < 0.001 vs. control by Bonferroni post hoc tests.
A paraspinal incision (Fig. 1B) was made after shaving and sterile preparation of the posterior hindquarter. A 3-cm incision was made through the hairy skin down to the fascia of the paraspinous muscles. The skin was closed with 4 sutures of 5-0 nylon and treated as described above; sutures were removed on POD3.
The plantar incision (Fig. 1C) was previously described (2). Briefly, the plantar aspect of the right hindpaw was prepared in a sterile manner, and a 1-cm longitudinal incision though skin, fascia and muscle was made. The skin was closed with 2 5-0 nylon sutures and treated as described above; sutures were removed on POD2.
Direct measurement of tissue oxygen tension
Oxygen-sensitive microelectrode
A polarographic oxygen-sensitive microelectrode mounted in the lumen of a 16-gauge needle (DO-166-NP: Lazar Research Laboratories, Los Angeles, CA) was used to measure tissue oxygen tension. The millivolt output of the oxygen probe was digitized using a 1401 Plus Laboratory Interface and Spike2 software (Cambridge electronic Design, Cambridge, United Kingdom). The electrode was calibrated using a two point method according to the manufacturer’s instructions. The low point calibration was made with 0.1% NaCl solution in double-distilled water bubbled with 100% nitrogen for 30 min. Blood gas analyses of this solution yielded oxygen tension of approximately 5 mmHg (1 mmHg = 133.322 Pa). For the high point (span point) calibration, a 0.1% NaCl solution equilibrated with room air for at least 1 hour was used. Blood gas analyses of this solution yielded oxygen tension of 148–155 mmHg. While the probe tip was completely immersed into this solution, the span point was adjusted so that the output read a value in the manufacturer-supplied calibration table at a given atmospheric pressure and temperature of the laboratory room (e.g., at 25 °C and 760 mmHg, the span point was adjusted to a value of 152 mmHg). This table provided pre-calculated dissolved oxygen tension values in 0.1% NaCl solution that was equilibrated to a wide range of different barometric pressure and temperature.
The linearity of the electrode response within the calibration range was checked using a third solution with different oxygen tension: 0.1% NaCl solution bubbled with 95% nitrogen/5% oxygen for 30 min. Blood gas analysis of this solution yielded oxygen tension of 32 mmHg. Oxygen tension of each solution was measured using both the oxygen-sensitive electrode and the blood gas analyzer (ABL5, Radiometer; Copenhagen, Denmark; measurements corrected to the actual temperature of the solutions, usually 25 °C) simultaneously, and a three-point standard curve was constructed by plotting the electrode’s readings versus the gas analyzer’s readings. The standard curve showed linearity within the calibration range (r2 = 0.989).
Measurement protocol
Rats were separated into 2 groups. In order to minimize the number of rats, both a unilateral gastrocnemius and paraspinal incision were made in one group. Both incisions were studied. In a separate group, only a unilateral plantar incision was made. Tissue oxygen tension was measured 1 h, 1, 2, 4, 7, and 10 days following gastrocnemius and paraspinal incision and 1, 2, and 7 days following hindpaw incision. Measurements at each site were taken only once in each rat. To prevent confounding due to bleeding caused by the needle electrode, multiple passes through the tissue or additional manipulation after insertion was avoided. The oxygen-sensitive electrode was recalibrated after each tissue measurement.
On the day of the measurement, anesthesia was induced with 3% isoflurane in air in a sealed box. Rats were intubated endotracheally with a 16-gauge plastic catheter. Anesthesia was maintained with 1–2% isoflurane in air. Rectal temperature was measured and maintained at 36 ± 1 °C with a heating pad and a radiant heat lamp. The common carotid artery was cannulated with polyethylene (PE) 50 tubing for the measurement of the mean arterial blood pressure and arterial blood gases. Mean arterial blood pressure was maintained above 90 mmHg. Arterial and end-tidal pCO2 were monitored during spontaneous ventilation. In 10 rats, mechanical ventilation was needed to support respiration so that end-tidal pCO2 was maintained in the range of 35–45 mmHg for all rats. To prevent hypovolemia, rats were given free access to sufficient water before the experiments, and a total of no more than 0.9 mL of blood was removed from each rat to measure arterial blood gases. Duration of anesthesia was 80–120 min for the gastrocnemius/paraspinal incision group, and 50–90 min for the hindpaw incision group.
For the gastrocnemius muscle measurements, a 16-gauge needle was used to pierce the skin 1-cm lateral from the distal end of the skin incision, to facilitate placement of the needle microelectrode. From this entry point, the tip of microelectrode was introduced directly into the muscle incision. For the paraspinous and hindpaw incisions, the 16-gauge needle was again used to pierce the outer part of the incision, and then the electrode needle tip was introduced approximately 8 mm subcutaneously directly into the wound. In the gastrocnemius incisions, we could place the tip of the microelectrode into muscle. For the hindpaw incision, of which pain-related behaviors have been best characterized, skin and fascia from the underlying flexor muscle were inseparable. It was likely that the microelectrode reading was a mixed output from subcutaneous tissue and skeletal muscle. In the paraspinal incision, the tip of the microelectrode could be placed into the subcutaneous layer.
Measurements were recorded after a 10-min stabilization period (see Fig. 1). The corresponding contralateral side was used for control, and measurements were made only once. When the oxygen tension from two sides was measured, the side measured first was randomized: the incised side was measured first in approximately 50% of the rats and after the control side in approximately 50% of the rats. Also, in animals with both a gastrocnemius and paraspinal incision, the measurements were taken in a randomized order. This prevented the duration of anesthesia or ventilatory status from influencing the tissue oxygen measurement in incised tissue more than control sites.
Immunohistochemistry
Tissue hypoxia was evaluated immunohistochemically using pimonidazole (Hypoxyprobe™-1, HPI, Inc., Burlington, MA) staining. After being injected systemically, pimonidazole is distributed to all tissue via the circulation and forms irreversible adducts with intracellular and extracellular proteins under hypoxic conditions: the formation of proteins adducts was shown to increase steeply at oxygen partial pressure less than 10 mmHg (16). Using this method, regions of hypoxia can be identified immunohistochemically. Tissues were harvested for immunohistochemistry against pimonidazole adducts 1, 2, 4 and 10 days following gastrocnemius incision or hindpaw incision. The corresponding contralateral site was used for control.
Two hours before the tissue harvest, 3 rats from each group were injected intraperitoneally (IP) with 100 mg/kg of pimonidazole HCl (100 mg/ml in 0.9% saline). Then the animals were anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardiacally perfused with 0.1 M phosphate-buffered saline, followed by 4% paraformaldehyde. In rats with gastrocnemius incision, incised gastrocnemius muscle samples were harvested, and control muscle samples were removed from contralateral hindlimb. In rats with paw incision, the glabrous plantar skin samples together with deep tissue and flexor muscle were removed, and control samples were harvested from contralateral paws. Samples were then postfixed in 4% paraformaldehyde for 4 h. After dehydrating in gradually increasing concentrations of sucrose, samples were rapidly frozen in 2-methylbutane chilled at −80 °C. Consecutive sections (12 μm) were prepared and frozen at −80 °C until usage.
Endogenous peroxidases were inactivated by incubating the sections with 30% methanol containing 0.3% H2O2 (v/v) for 30 min. To block non-specific reactions, sections were incubated in serum free protein blocker (DAKO Corp, Carpinteria, CA) for 10 min. Tissue sections were then incubated with a primary monoclonal mouse antibody against Hypoxyprobe-1 (1:100, HPI, Inc., Burlington, MA) for 1 h at room temperature, followed by incubation with biotinylated rabbit anti-mouse secondary antibody (1:500, ABD Serotec, Raleigh, NC) for 20 min at room temperature. Peroxidase-conjugated streptavidin (DAKO Corp, Carpinteria, CA) was applied for 15 min, and the signal was visualized with 3,3′-diaminobenzidine reagent (DAB, DAKO Corp, Carpinteria, CA). Sections were counterstained with hematoxylin for 30 sec and mounted with aqueous mounting media (Paramount, DAKO Corp, Carpinteria, CA). One section of each set of 3 consecutive sections was stained for pimonidazole, and another section underwent hematoxylin and eosin (H & E) staining. The other section of the set was processed without the primary antibody as a control. For other controls, sections from the animals that were not injected with pimonidazole Cl underwent the staining protocol for pimonidazole. All sections from the same animal were processed simultaneously. To assess tissue hypoxia, pimonidazole labeling was evaluated qualitatively and semi-quantitatively by an experienced pathologist. Qualitative analyses were performed by determining the presence or absence of labeling and by characterizing the types of cells and tissues with positive labeling. Intensity of labeling was evaluated and described using semi-quantitative labeling intensity scores of 0–3+ (0 negative, 1+ weak, 2+ intermediate, and 3+ strong intensity).
Statistics
For the direct measurement of tissue oxygen tension, an a priori power analysis was conducted to determine the sample size. A sample size of 5 in each group was required to achieve 80% power to detect a difference of 20 mmHg in tissue oxygen tension with an estimated standard deviation (SD) of 10 mmHg and with a significance level of 0.05 using a paired t-test. The estimate of the SD was obtained from a pilot experiment. Since measurements at each site were taken only once in each rat, 9 groups (6 time points for the gastrocnemius/paraspinal incision and 3 time points for the hindpaw incision) of 5 rats each were used.
All data sets were tested for normality of distribution by Shapiro-Wilk W and Kolmogorov Smirnov tests. Tissue oxygen tension values were normally distributed and were compared using parametric statistics. Differences in tissue oxygen tension levels between incision and control were examined by two-way ANOVA with repeated measures on one factor; significant main effects of incision or interactions were followed by Bonferroni post hoc tests. Tissue oxygen tension levels in the control tissue and the arterial oxygen tension (PaO2)2 values at the different time points were compared by one-way ANOVA. Comparison of the PaO2 values between the first and second blood gas analyses at each time point was made using the paired t-test. Comparison of tissue oxygen tension between skin and muscle was made by paired t-test in control tissues, and by two-way ANOVA with repeated measures on one factor in incised tissues. Data are presented as the mean ± SD. p < 0.05 was considered significant. Statistical analysis was performed using NCSS 2007 program (NCSS Statistical Software, Kaysville, UT) and Prism 4.0 (Graphpad Software, Inc., San Diego, CA).
RESULTS
Direct measurement of tissue oxygen tension
Of the 46 rats used for the experiments, 1 rat was excluded for wound infection after hindpaw incision.
Insertion of the oxygen microelectrode into normal or incised tissue produced stable readings within 10 min. A real time digitalized output of tissue oxygen tension measured from the unincised (Fig. 2A) and incised (Fig. 2B) gastrocnemius muscle from the same rat 1 hour after incision is shown.
Figure 2.

Example oxygen tension recordings in control gastrocnemius muscle (A) and 1 hour after gastrocnemius incision (B). The recording was started immediately after the insertion of the needle microelectrode into gastrocnemius muscle. Readings were obtained 10 min after insertion, indicated by arrows.
Tissue oxygen tension of incised gastrocnemius muscle (Fig. 1A) was significantly decreased compared to that of control, unincised muscle (Fig. 1B; p < 0.0001). One hour after gastrocnemius incision, tissue oxygen tension in the incised muscle was 11.3 ± 10.6 mmHg (p < 0.001 vs. control) and in the control muscle was 47.9 ± 10.5 mmHg (Fig. 1B). Tissues oxygen tension in the incised gastrocnemius muscle was lowest on POD1 (5.5 ± 6.6 mmHg) and remained significantly decreased through POD7. By POD10, tissue oxygen tension of incised muscle recovered to that of the control. There was no difference in the tissue oxygen tension in the control, unincised muscle at the different time points after incision was made.
Tissue oxygen tension was significantly less in the incised paraspinal skin (Fig. 1C) compared to that in the control, unincised skin (Fig. 1D; p < 0.0001). On the day of paraspinal skin incision, tissue oxygen tension in the incised side was 32.6 ± 23.4 mmHg (p < 0.01 vs. control) and in the control side was 63.1 ± 12.8 mmHg (Fig. 1D). Tissue oxygen tension remained decreased in the incised tissue through POD7, and recovered to that of the control by POD10. There was no difference in the tissue oxygen tension in the control tissues at different time points.
Compared to control, unincised hindpaw, tissue oxygen tension was significantly decreased in the incised hindpaw (Figs. 1E, F; p < 0.0001). One day after plantar incision (Fig. 1E), the oxygen tension in the incised hindpaw was 18.0 ± 15.1 mmHg (p < 0.001 vs. control) and in the control hindpaw was 64.4 ± 8.5 mmHg (Fig. 1F). Seven days after surgery, oxygen tension in the incision was not different from that in the contralateral paw. Again, there was no difference in the tissue oxygen tension in the control hindpaws at the different time points after incision was made.
The PaO2 values of the gastrocnemius/paraspinal incision group were 86.6 ± 12.2, 85.7 ± 5.9, 85.9 ± 5.4, 89.6 ± 8.2, 90.4 ± 8.2, and 88.1 ± 8.3 mmHg 1 h, 1, 2, 4, 7, and 10 days after incision, respectively. The PaO2 values of the hindpaw incision group were 87.1 ± 11.5, 89.6 ± 9.7, and 89.2 ± 6.9 mmHg 1,2, and 7 days after incision, respectively. There was no difference in the PaO2 values among different time points. Two blood gas analyses were performed in each animal, and there was no difference in the PaO2 values between the first and second measurements at each time point.
Immunohistochemistry
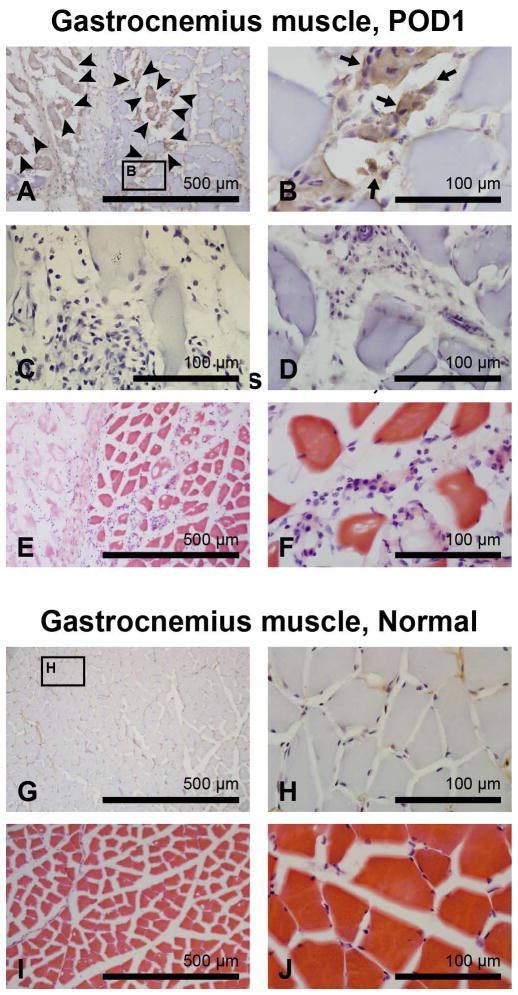
Fig. 3 shows representative images of pimonidazole and H & E staining of incised and contralateral control gastrocnemius muscle tissue on POD1. A hypoxic area was identified along the incision line, showing 2–3+ pimonidazole labeling (Figs. 3A, B). The negative controls that omitted primary antibody (Fig. 3C) or omitted the pimonidazole injection (Fig. 3D) did not show any DAB labeling. H & E staining of the consecutive sections showed that the myocytes in the hypoxic region lost their normal polygonal shape (Figs. 3E, F) compared to control (Figs. 3I, J), indicating muscle fiber damage. Infiltration of polymorphonuclear neutrophils (PMNs)3, lymphocytes, and mesenchymal cells was observed at the incision border in incised muscle (Figs. 3E, F). Tissue edema, as demonstrated by a widening of the interstitial space between myocytes, was also noted in incised gastrocnemius muscle. There was no pimonidazole labeling detected in control muscle (Figs. 3G, H).
Figure 3.
Immunohistochemistry for pimonidazole adducts in incised and control gastrocnemius muscle on postoperative day 1 (POD1). (A, B): Pimonidazole staining of incised gastrocnemius muscle. Pimonidazole labeling is noted in the incision site, indicated by arrowheads in (A) and by arrows in (B). (C, D): The negative control with omitted primary antibody (C) or omitted pimonidazole administration (D) does not show any DAB labeling. (E, F): Hematoxylin and eosin staining of incised gastrocnemius muscle. (G–J): Pimonidazole (G, H) and hematoxylin and eosin staining (I, J) of contralateral control gastrocnemius muscle. For (A–D), (G), and (H), hematoxylin was used for counterstaining.
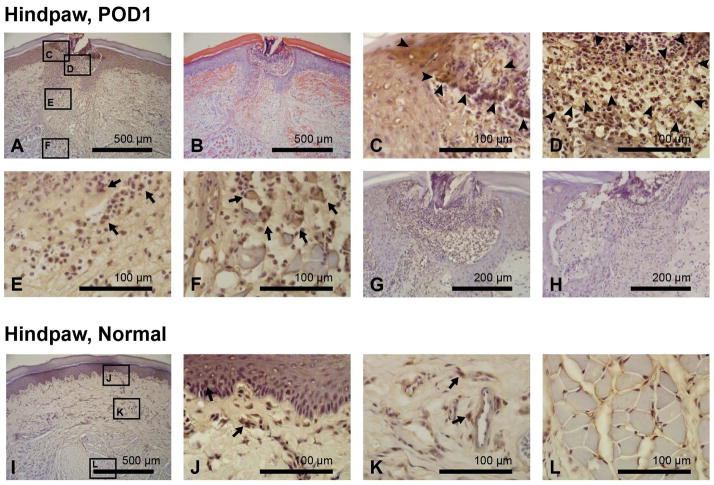
The immunohistochemical staining for pimonidazole adducts in incised and control hindpaw tissue on POD1 is shown in Fig. 4. In incised skin, pimonidazole labeling localized hypoxic areas in epidermis (2–3+ staining; Fig. 4C), dermis (2+ staining; Fig. 4E), and wound exudate (2–3+ staining; Fig. 4D). Intense infiltration of PMNs was observed in the wound exudate, and ~2+ pimonidazole staining was noted in these cells (Fig. 4D). Pimonidazole labeling also identified hypoxic areas in incised flexor digitorum brevis muscle on POD1 (Figs. 4A, F). Consistent with the findings observed in the incised gastrocnemius muscle (Figs. 3A–F), loss of normal polygonal shape of myocytes, infiltration of inflammatory cells, and a widening of the interstitial space between muscle fibers were noted in the hypoxic area of incised flexor digitorum brevis muscle (Fig. 4F). The negative controls that omitted primary antibody (Fig. 4G) or omitted the pimonidazole injection (Fig. 4H) did not show any DAB labeling. Contralateral control hindpaw skin showed focal staining of pimonidazole in the epidermis (Fig. 4J) and the perivascular area of the dermis (Fig. 4K). There was no pimonidazole labeling detected in the unincised flexor digitorum brevis muscle (Fig. 4L).
Figure 4.
Immunohistochemistry for pimonidazole adducts in incised and control plantar skin and flexor digitorum brevis muscle on postoperative day 1 (POD1). (A–F): Pimonidazole (A, C–F) and hematoxylin and eosin (B) of incised hindpaw. (C–F): Higher magnification photographs identify hypoxic area in epidermis (C), wound exudates (D), dermis (E), and flexor muscle fibers (F). Pimonidazole labeling is indicated by arrowheads and arrows. (G, H): The negative control with omitted primary antibody (G) and omitted pimonidazole administration (H) does not show any DAB labeling. (I–L): Pimonidazole staining of contralateral control skin and flexor digitorum brevis muscles. Pimonidazole labeling is indicated by arrows. In the unincised epidermis and dermis, focal, single cell uptakes were observed, suggesting background staining variability. For (A) and (C–L), hematoxylin was used for counterstaining.
In Fig. 5A, the changes in pimonidazole and H & E staining of gastrocnemius muscle at different times after incision are shown. On POD1, 2–3+ pimonidazole labeling was noted in myocytes and interstitium in the incision site (Figs. 5Ab, Ag). On POD2, 1–2+ pimonidazole staining was observed in the interstitial space of the incised gastrocnemius muscle, especially in infiltrated PMNs and mesenchymal cells (Figs. 5Ac, Ah). On POD4, markedly diminished PMNs and moderately increased mesenchymal filling was observed in the incision (Fig. 5Ai). Diffuse 1+ pimonidazole labeling was noted in this area (Fig. 5Ad). By POD10, denser granulation tissue had formed in the incised gastrocnemius muscle, and hypoxia labeling had largely resolved (Figs. 5Ae, Aj).
Figure 5.
The time course of changes in immunohistochemistry for pimonidazole adducts in incised gastrocnemius muscle (A), plantar skin (B) and flexor digitorum brevis muscle (C). The upper panels of (A), (B), and (C) show pimonidazole staining (hematoxylin counterstain) and the lower panels show hematoxylin and eosin staining. The leftmost lane of (A), (B), and (C) shows staining of unincised, control tissues. After incision, immunohistochemical staining for pimonidazole adducts revealed hypoxia in the incision site. Pimonidazole labeling is indicated by arrows. Intensity of staining peaks at postoperative day (POD) 1 and POD 2 when hypoxia is most pronounced. On POD 10, staining for hypoxia had largely resolved. See text for details.
Figs. 5B and C shows the changes in pimonidazole and H & E staining of plantar skin and flexor digitorum brevis muscle at different times after incision. On POD1 and POD2, pimonidazole labeling was observed in epidermis, dermis, wound exudates and inflammatory cells of the incised hindpaw skin (Figs. 5Bb, Bc). On POD4, re-epithelialization had occurred, and diffuse 1+ pimonidazole labeling was seen in granulation tissue, dermal perivascular areas, and epidermis (Figs. 5Bd, Bi). By POD10, hypoxia labeling had largely resolved in the incised hindpaw skin except in the epidermis of prior scar line (~1+ staining) (Fig. 5Be). Changes in pimonidazole staining of the incised flexor digitorum brevis muscle followed a similar time course to those of the incised gastrocnemius muscle (Fig. 5C).
Table 1 shows the overall intensity of pimonidazole labeling (median labeling intensity score from three animals) observed in gastrocnemius muscle, plantar skin, and flexor digitorum brevis muscle at different times after incision.
Table 1.
Overall intensity of pimonidazole labeling at different time points after incision
| Control | POD1 | POD2 | POD4 | POD10 | |
|---|---|---|---|---|---|
| Gastrocnemius muscle incision | 0 | 3+ | 2+ | 1+ | 0 |
| Hindpaw skin incision | 1+ | 3+ | 3+ | 1+ | 1+ |
| Hindpaw muscle incision | 0 | 3+ | 1+ | 1+ | 0 |
Each value is the median labeling intensity score from three animals. POD, Postoperative day.
DISCUSSION
While a decrease in cutaneous oxygen tension after incision has been demonstrated in both animal and human studies (9, 10), the literature is sparse regarding the tissue oxygen tension of incised deeper tissue like skeletal muscle after surgery. The present study demonstrated that tissue oxygen tension was decreased in both skeletal muscle and cutaneous tissues compared to the unincised contralateral control tissues, for several days after incision: when measured directly using an oxygen-sensitive microelectrode, tissue oxygen tension decreased immediately and remained low for several days after incisions of the gastrocnemius muscle, paraspinal skin and the plantar hindpaw. In addition, immunohistochemical staining for pimonidazole adducts revealed hypoxic areas in incised muscle and skin for several days after incision. By POD10, tissue oxygen tension recovered to that of control tissue, and hypoxia labeling in the incised tissue largely resolved.
The measurements of the oxygen tension in the uninjured skeletal muscle have been reported in both animal (17) and human studies (18). Oxygen tension values in normal skeletal muscle were comparable but slightly lower than our result (43.9 ± 14. 9 mmHg): when measured using a microelectrode, it was 34 ± 2.6 mmHg (mean ± SEM) in normal gastrocnemius muscle of unanesthetized rats (17), and 27.3 ± 12.1 mmHg in tibialis anterior muscle of awake human subjects (18). Considering that the measurements were done in awake subjects in these previous studies, and in anesthetized rats in the present study, this difference in the recording conditions could have contributed to comparably higher oxygen tension in the present study. That is, general anesthesia with isoflurane could have increased tissue oxygen tension by causing vasodilation, and thus increasing blood flow to peripheral tissue (19). In addition, potential mild hypothermia during measurements might also have affected the oxygen tension. While reported average body temperature of rats is around 37.5 °C, body temperature was maintained at 36 ± 1 °C in the present study. Hypothermia could potentially affect tissue oxygen tension by altering the oxygen delivery/consumption relationship. However, it is unlikely that factors such as anesthesia or hypothermia significantly contributed to differences in tissue oxygen tension levels observed between the incision and control sites. Since we used the corresponding site contralateral to the incision as a control, oxygen tension from both incision and control sites were both affected by these factors. It is possible that these factors influence blood flow and oxygen tension differently in incised and unincised tissue.
Similar to the marked decrease in the oxygen tension in the incised skeletal muscle observed in the present study (Fig. 1B), hypoxia was reported in the crush-injury model of skeletal muscle in rats (20). The oxygen tension of the crushed gastrocnemius muscle decreased from baseline (42–47 mmHg) to a value of 8–15 mmHg as soon as 2 hours after crush-injury, and remained low (8–18 mmHg) during the first 4 days. Afterwards, the oxygen tension began to increase, and reached the level of the intact muscle 10 days after crush injury. Immunohistochemical demonstration of the hypoxic regions in the skeletal muscle wounds after surgery or trauma, using hypoxia markers such as pimonidazole, has not been reported previously.
The development of tissue hypoxia in the cutaneous wound tissues has been previously examined in experimental animals (10, 21, 22). When a viscose cellulose sponge was implanted in the subcutaneous layer of the back skin of rats to create a dead space wound, the oxygen level decreased to 20–30 mmHg on the first day of wounding (22). The oxygen tension further declined to reach a minimum of 6–7 mmHg by 5 days after injury, and remained low at least for about 2 weeks. Oxygen tension was lower and hypoxia was present for a longer period of time, compared with our measurement values from back skin (Fig. 1D); however, this may be due to differences between a dead space wound and a closed incision. This was also true for lactate: compared to our previous study demonstrating an increase in wound lactate level for 7–8 days after incision (7), lactate in aspirates from dead space wound was increased for longer period of time (for at least 10–14 days) (23).
Other investigators demonstrated tissue hypoxia in the cutaneous wound immunohistochemically using pimonidazole staining; hypoxia was evident between 3 and 7 days in the full-thickness groin incisional wound in rats (10). However, because the earliest time point evaluated was 3 days after incision, information on the tissue hypoxia early after incision was unavailable from this study. Pimonidazole staining has been examined in punch wounds, also called excision wounds. In contrast to our results showing intense pimonidazole staining on POD1 and POD2, little labeling was observed in the excisions on the back skin of rats 1 day after wounding (21). These wounds started to exhibit some pimonidazole staining on day 2, and the most intense staining pattern was observed on day 4. This discrepancy is thought to reflect the differences in the type of wounds, incision versus excision. That is, the depth of punch-biopsy wounds was limited only to the dermis while our incisional wounds involved cutting through all layers of the skin. Also, the punch-biopsy wound was an open wound, while the incisional wound was closed with sutures. Therefore, oxygen could diffuse from the air through the disrupted epithelial barrier into the punch-biopsy wounds, preventing hypoxia until reepithelialization or scab formation occurred (21). Decreases in subcutaneous oxygen tension in the wound were also demonstrated in mastectomy wounds (9). A significant decrease in the oxygen tension from baseline (60.3 ± 2.5 mmHg) to approximately 30 to 40 mmHg was present through POD5.
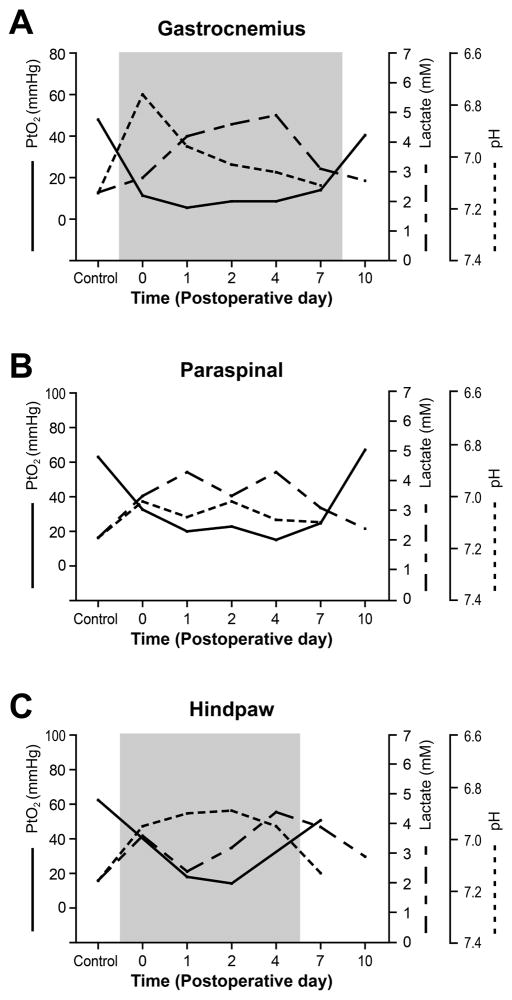
In our previous studies, using the same tissue in which we demonstrated wound hypoxia, we also demonstrated increased lactate level (7) and decreased pH (8). Tissue lactate levels were elevated from the day of incision and remained increased through 4–8 days (7). Tissue pH levels were decreased immediately after incision and were sustained for at least 4 days. The superimposed graphs in Fig. 6 are showing that these changes in tissue lactate level and pH occur concurrently with the decrease in tissue oxygen tension after incision. By POD10, oxygen tension, lactate, and tissue pH have returned to that of the control. Increased lactate may not necessarily solely indicate an oxygen limitation, because other various factors in the wound environment, such as aerobic glycolysis and activated leukocytes, can be sources of lactate, even without hypoxia (24). However, presence of significant hypoxia in the incisional wounds and its temporal relationship with tissue lactate and pH (Fig. 6) suggest that these changes in lactate level and pH in the incisional wounds could be at least partially attributed to the oxygen limitation and all occur concomitantly. The reduction in tissue oxygen tension could also reflect high neutrophil/macrophage activity in the wound environment, since these cells produce significant amount of lactate while consuming oxygen at a high rate. Future studies to further examine the correlation between tissue oxygen tension and pain-related behaviors after incision would be interesting. It would be also useful for future studies to investigate the effect of increased fraction of inspired oxygen on tissue oxygen tension and pain-related behaviors.
Figure 6.
Time course changes in tissue pH, lactate concentration, and tissue oxygen tension after gastrocnemius muscle (A), paraspinal skin (B) and plantar (C) incision. A decrease in tissue oxygen tension occurs concurrently with a decrease in pH and an increase in lactate after incision. By postoperative day 10, tissue oxygen tension, lactate, and pH have returned to that of the control. The area shaded in gray represents the time after incision when pain-related behaviors are observed. Control is the value for the contralateral unincised site. Error bars were omitted for simplicity. (Adapted and modified from reference 11 & 12)
Previously, we have characterized pain-related behaviors after hindpaw incision (2) and gastrocnemius incision (Fig. 6) (3). The findings of our present and previous studies suggest that postsurgical pain and wound healing may share common mechanisms. That is, lactate contributes to wound healing by promoting regeneration of new vessels and by stimulating the proliferation of fibroblasts (25). On the other hand, increased lactate and decrease pH together with significant hypoxia in the incised skeletal muscle suggest that these mediators in the wound environment may also contribute postoperative pain through ischemic-like mechanisms. Ischemic pain is characteristic of muscle rather than skin, and ischemic muscle has been proposed as the primary mechanism underlying various painful conditions, such as acute stage of complex regional pain syndrome type 1 (26) and myofascial pain syndromes (27). Our previous studies, using behavioral and in vivo electrophysiological approaches, also demonstrated that deep muscle tissue rather than skin causes ongoing pain and spontaneous activity in nociceptive pathways after incision (28, 29).
Acid-sensing ion channel 3 (ASIC3)4 has been proposed as a candidate channel for transducing ischemic pain due to its channel property and distribution (14, 15). In an in vitro study using muscle sensory neurons, it has been shown that rat ASIC3 produces a sustained current during a pH decrease from 7.4 to 6.7, which mimics the extracellular acidification that accompanies muscle ischemia (30). Furthermore, lactate enhances ASIC3 sensitivity to protons (14), allowing better detection of acidosis caused by ischemia. It has also been shown that small muscle afferents are more likely to express ASIC3 than are small skin afferents (13). In addition to lactate and protons, the incisional wound environment contains other mediators that might affect the ASIC3-mediated pain signal pathways. For example, we have demonstrated upregulation of NGF, which can induce ASIC3 overexpression in pathological conditions, in the incised skeletal muscle (31). In addition, injured skeletal muscle could be expected to release adenosine triphosphate (ATP) through various mechanisms, including passive release from damaged cells, release from muscle fibers during contraction (32), and hypoxia-induced release from mesenchymal cells and PMNs (33). Previous studies have shown that extracellular ATP increases acid sensitivity of ASIC channels, thus rendering a sensory neuron more sensitive to ischemic acidosis (15). In a study using the rat in vitro muscle-nerve preparation, we have shown that lactic acid (15 mM, pH 6.5–5.5) activated more nociceptors in incised muscle compared to control, unincised muscle (34).
Various etiological and contributing factors can be considered in the development of hypoxia in the incised skeletal muscle and skin tissue. Initially, hypoxia occurring in the wounds is in part due to inadequate perfusion caused by damage to vasculature, and the consequent coagulation which widens the area of diminished oxygen supply (35, 36). Tissue edema in the interstitial space can also decrease oxygen supply by increasing diffusion distance of oxygen and by decreasing perfusion pressure (37). Furthermore, the regeneration process of skeletal muscle following acute injury normally involves initial degeneration or breakdown of the damaged muscle fibers, further contributing to the wound hypoxia (38). Indeed, in the present study, damaged muscle fibers in the incised gastrocnemius (Figs. 3A, B) and flexor digitorum brevis muscles (Fig. 4F) on POD1 showed pimonidazole labeling. Hypoxia can be caused not only by decreased oxygen supply, but also by consumption of oxygen by metabolically-active inflammatory cells and highly proliferative fibroblasts in the wound (21). Previous studies have shown that sympathetic nervous system activation and consequent vasoconstriction caused by pain and surgical stress can decrease wound oxygen tension. (39) Therefore, in the present study, postoperative pain could also have contributed to wound hypoxia.
In summary, our experiments demonstrate that tissue oxygen tension decreases after incision especially in deep muscle tissue and remains low through POD7. These results support the evidence that a hypoxic condition is present in incisions and that an ischemic-like mechanism may contribute to postoperative pain.
Acknowledgments
Funding: The authors are grateful for the support by the Department of Anesthesia at the University of Iowa and by the National Institutes of Health, Bethesda, Maryland grant GM067762 to T.J.B.
ABBREVIATIONS
- POD
Postoperative day
- PaO2
arterial oxygen tension
- PMNs
polymorphonuclear neutrophils
- ASIC3
acid-sensing ion channel 3
Footnotes
Disclosure statement: None of the authors has declared any actual or potential conflict of interest including any financial, personal or other relationship with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, our work.
References
- 1.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94(1):130–7. doi: 10.1097/00000539-200201000-00025. table of contents. [DOI] [PubMed] [Google Scholar]
- 2.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 3.Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain. 2002;6(4):295–305. doi: 10.1053/eujp.2002.0339. [DOI] [PubMed] [Google Scholar]
- 4.Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nishikawa K, Narimatsu E, et al. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain. 2002;100(1–2):77–89. doi: 10.1016/s0304-3959(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99(26):17179–84. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology. 1998;88(1):143–56. doi: 10.1097/00000542-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8(1):59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101(2):468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Chang N, Goodson WH, 3rd, Gottrup F, Hunt TK. Direct measurement of wound and tissue oxygen tension in postoperative patients. Ann Surg. 1983;197(4):470–8. doi: 10.1097/00000658-198304000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokmic Z, Darby IA, Thompson EW, Mitchell GM. Time course analysis of hypoxia, granulation tissue and blood vessel growth, and remodeling in healing rat cutaneous incisional primary intention wounds. Wound Repair Regen. 2006;14(3):277–88. doi: 10.1111/j.1743-6109.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 11.Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L, et al. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur J Pain. 2003;7(1):93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 12.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208(3):191–4. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 13.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4(9):869–70. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 15.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38(11):1561–9. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- 16.Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res. 1999;151(5):580–9. [PubMed] [Google Scholar]
- 17.Bernshtein VA, Berezovskii VA. Oxygen tension in skeletal muscle of unanesthetized rats during hypothermia. Bull Exp Biol Med. 1966;65(2):152–3. [PubMed] [Google Scholar]
- 18.Jung F, Kessler H, Pindur G, Sternitzky R, Franke RP. Intramuscular oxygen partial pressure in the healthy during exercise. Clin Hemorheol Microcirc. 1999;21(1):25–33. [PubMed] [Google Scholar]
- 19.Stevens WC, Cromwell TH, Halsey MJ, Eger EI, 2nd, Shakespeare TF, Bahlman SH. The cardiovascular effects of a new inhalation anesthetic, Forane, in human volunteers at constant arterial carbon dioxide tension. Anesthesiology. 1971;35(1):8–16. doi: 10.1097/00000542-197107000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Jozsa L, Reffy A, Demel S, Szilagyi I. Alterations of oxygen and carbon dioxide tensions in crush-injured calf muscles of rat. Z Exp Chir. 1980;13(2):91–4. [PubMed] [Google Scholar]
- 21.Haroon ZA, Raleigh JA, Greenberg CS, Dewhirst MW. Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann Surg. 2000;231(1):137–47. doi: 10.1097/00000658-200001000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninikoski J, Heughan C, Hunt TK. Oxygen and carbon dioxide tensions in experimental wounds. Surg Gynecol Obstet. 1971;133(6):1003–7. [PubMed] [Google Scholar]
- 23.Hunt TK, Conolly WB, Aronson SB, Goldstein P. Anaerobic metabolism and wound healing: an hypothesis for the initiation and cessation of collagen synthesis in wounds. Am J Surg. 1978;135(3):328–32. doi: 10.1016/0002-9610(78)90061-2. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell MD, Shearer J, Morris A, Mastrofrancesco B, Henry W, Albina JE. Evidence for aerobic glycolysis in lambda-carrageenan-wounded skeletal muscle. The Journal of surgical research. 1984;37(1):63–8. doi: 10.1016/0022-4804(84)90162-8. [DOI] [PubMed] [Google Scholar]
- 25.Hunt TK, Hussain MZ. Can wound healing be a paradigm for tissue repair? Med Sci Sports Exerc. 1994;26(6):755–8. doi: 10.1249/00005768-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Schattschneider J, Binder A, Siebrecht D, Wasner G, Baron R. Complex regional pain syndromes: the influence of cutaneous and deep somatic sympathetic innervation on pain. The Clinical journal of pain. 2006;22(3):240–4. doi: 10.1097/01.ajp.0000169672.49438.67. [DOI] [PubMed] [Google Scholar]
- 27.Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Archives of physical medicine and rehabilitation. 2009;90(11):1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144(3):329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112(1):153–64. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99(5):501–9. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009;110(1):140–9. doi: 10.1097/ALN.0b013e318190bc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. Journal of applied physiology (Bethesda, Md : 1985) 2003;95(2):577–83. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 33.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends in cardiovascular medicine. 2008;18(3):103–7. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Gu H, Brennan TJ. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain. 2010;151(3):744–55. doi: 10.1016/j.pain.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70(4):478–90. [PubMed] [Google Scholar]
- 36.Hunt TK, Zederfeldt B, Goldstick TK. Oxygen and healing. Am J Surg. 1969;118(4):521–5. doi: 10.1016/0002-9610(69)90174-3. [DOI] [PubMed] [Google Scholar]
- 37.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ. 1998;317(7169):1370–3. doi: 10.1136/bmj.317.7169.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodine-Fowler S. Skeletal muscle regeneration after injury: an overview. J Voice. 1994;8(1):53–62. doi: 10.1016/s0892-1997(05)80319-4. [DOI] [PubMed] [Google Scholar]
- 39.Akca O, Melischek M, Scheck T, Hellwagner K, Arkilic CF, Kurz A, et al. Postoperative pain and subcutaneous oxygen tension. Lancet. 1999;354(9172):41–2. doi: 10.1016/S0140-6736(99)00874-0. [DOI] [PubMed] [Google Scholar]