Abstract
Endogenous memory CD8 T cells infiltrate MHC-mismatched cardiac allografts within 12–24 hours post-transplant in mice and are activated to proliferate and produce IFN-γ. To more accurately assess the graft injury directly imposed by these endogenous memory CD8 T cells, we took advantage of the ability of anti-LFA-1 mAb given to allograft recipients on days 3 and 4 post-transplant to inhibit the generation of primary effector T cells. When compared to grafts from IgG treated recipients on day 7 post-transplant, allografts from anti-LFA-1 mAb treated recipients had increased numbers of CD8 T cells but these grafts had marked decreases in expression levels of mRNA encoding effector mediators associated with graft injury and decreases in donor-reactive CD8 T cells producing IFN-γ. Despite this decreased activity within the allograft, CD8 T cells in allografts from recipients treated with anti-LFA-1 mAb continued to proliferate up to day 7 post-transplant and did not upregulate expression of the exhaustion marker LAG-3 but did have decreased expression of ICOS. These results indicate that endogenous memory CD8 T cells infiltrate and proliferate in cardiac allografts in mice but do not express sufficient levels of functions to mediate overt graft injury and acute rejection.
Keywords: memory, cardiac allograft, T cell graft infiltration, T cell activation/inactivation
Introduction
The high frequency of T cells directly reactive to allogeneic MHC molecules underlies the potent alloimmune response to MHC-mismatched allografts that necessitates the need for immunosuppression to inhibit development of the primary donor-reactive T cell response. Many transplant patients harbor a repertoire of memory T cells that are reactive to allogeneic MHC molecules, generating an additional problem for increased risk of rejection and limiting the available donor pool for a transplant (1–4). Such patients can become sensitized to allogeneic MHC molecules through prior transplants, blood transfusions, and for female patients, multiple pregnancies (5, 6). When compared to primary effector T cells that develop in response to allograft-derived antigen-presenting cells, memory T cells reactive to donor MHC molecules present a substantial problem in transplantation as they have a lower threshold for activation, are not dependent on CD28-mediated costimulation, and traffic more rapidly to the allograft (5–7).
It is now recognized that memory T cells reactive to allogeneic MHC molecules also develop during immune responses to various antigens to which the transplant candidate has been previously exposed. Such heterologous immunity can be induced by viral and bacterial infection as well as to other exogenous antigen exposure, including vaccination, resulting in repertoires of foreign peptide/self MHC-specific memory T cells that cross-react with various allogeneic peptide/MHC complexes (7, 8). Seminal studies by Adams and coworkers (9) used a mouse model to demonstrate that viral infection generated memory CD8 T cells that reacted with allogeneic class I MHC molecules and the presence of these memory T cells in heart allograft recipients expressing those allogeneic class I MHC molecules promoted graft rejection despite recipient conditioning with a tolerance-inducing strategy. Many examples of the impact of memory CD8 T cells induced by viral infection on graft outcome in rodent models have now been reported (10–12). In clinical renal transplant patients and in non-human primate recipients of renal allografts, the presence of endogenous memory CD8 T cells with reactivity to allogeneic class I MHC molecules has also been shown to correlate with more frequent acute rejection episodes and poorer graft outcome (1, 3, 13, 14). While the problem of endogenous memory CD8 T cells reactive to donor class I alloantigens is now well appreciated, mechanisms underlying graft injury directly mediated by endogenous memory CD8 T cells remain poorly understood.
Previous studies from this laboratory indicated endogenous memory CD8 T cell infiltration into MHC-mismatched heart allografts in mice within 12–24 hours after reperfusion of the graft (15, 16). These endogenous memory CD8 T cells are reactive to allogeneic class I MHC molecules of the graft and are rapidly activated to proliferate and to produce IFN-γ within the allograft. This endogenous memory CD8 T cell infiltration and activation within the allograft is not dependent on CD28- or CD154-mediated costimulation or on the activity of CD4 T cells. The impact of endogenous memory CD8 T cell activation within the allograft is increased neutrophil infiltration and activation with increased graft tissue inflammation that promotes the infiltration of effector CD4 and CD8 T cells into the allograft following their priming to donor antigens in the recipient spleen on days 4–5 post-transplant. It is important to note our detection of CD4 T cells infiltrating the allografts at early times post-transplant as well, but these CD4 T cells are not responsible for the IFN-γ production or increased neutrophil infiltration observed for the first 1–3 days after graft reperfusion (15–17). During a recent study investigating potential strategies to inhibit endogenous memory CD8 T cell infiltration into MHC-mismatched allografts, we observed that peri-transplant treatment with anti-LFA-1 mAb was particularly effective in inhibiting endogenous memory CD8 T cell and neutrophil infiltration into complete MHC-mismatched cardiac allografts (18). This treatment also inhibited the priming of donor-reactive effector CD4 and CD8 T cells until after day 20 post-transplant resulting in extended allograft survival from day 8 in control treated recipients to 25–40 days. Delay of anti-LFA-1 mAb administration to days 3 and 4 post-transplant delayed the functional development of primary effector T cells to IFN-γ producing cells until day 14 post-transplant and the allografts were rejected around this time (18). These results led us to reason that this delayed administration of anti-LFA-1 mAb strategy would allow us to test the impact of endogenous memory CD8 T cells infiltrating the allograft prior to initiation of anti-LFA-1 mAb administration without the complication of primary effector T cell activity.
Materials and Methods
Mice
Colonies of C57BL/6 (H-2b) and A/J (H-2a) mice were purchased from Charles River Laboratories (Wilmington, MA). Male mice, 8–10 weeks of age, were used in all experiments and all procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Cardiac transplantation and harvest
Heterotopic, intra-abdominal cardiac transplantation was performed following the method of Corry and coworkers (19). Total operative times averaged 40 min and hearts resumed spontaneous contraction immediately upon reperfusion. Graft survival was monitored by abdominal palpation of the graft and rejection was confirmed by laparotomy. At the time of graft harvest, the circulatory system was drained prior to removal of the heterotopic graft, which was immediately snap-frozen in liquid nitrogen or placed in media for digestion and purification of graft-infiltrating cells.
In vivo antibody treatments
Animals were treated either on days −1 and 0 or on days 3 and 4 i.p. with 200 μg anti-LFA-1 mAb (rat IgG2b, clone FD441.8 from Bio X Cell, West Lebanon, NH) or control rat IgG (Sigma-Aldrich, St. Louis, MO). The dose of FD441.8 used was based on results from a limited preliminary allograft survival study and a previously reported study (18). Groups of mice were treated with 1 mg anti-CD4 mAb GK1.5 or 250 μg anti-CD154 mAb MR1 on days 0 and +1.
Flow cytometry
Flow cytometric detection of graft-infiltrating cells was performed using a modification of the method published by Afanasyev and colleagues (20). Briefly, harvested graft tissues were weighed and incubated for 1 h at 37°C in RPMI with Type II collagenase (Sigma-Aldrich). After incubation, tissue was pressed through a 40 μm filter and the collected cells were washed twice in RPMI, counted using a hemocytometer, and stained for common phenotypic surface markers (CD45, CD4, CD8, CD44, CD62L, Gr1, and F4/80) using commercially available antibodies (BD Bioscience, San Jose, CA; eBioscience, San Diego, CA). Flow cytometry was performed using a FACSCalibur (BD Biosciences) cytometer and FlowJo analysis software (Tree Star Inc., Ashland OR). The forward scatter and FL1 (CD45+) channels were used to gate the leukocytes in the graft tissue followed by analysis of specific leukocyte populations. For each sample, 200,000 gated events were accumulated. Total numbers of each leukocyte population were calculated by: (the total number of leukocytes in the sample counted using the hemocytometer) x (% of the leukocyte population in the CD45+ cells by flow cytometry)/100. The data are reported as number of each leukocyte population/mg graft tissue.
Aliquots of graft infiltrating cells were stained with antibodies to CD8 and to LAG-3 or ICOS (both eBioscience) to assess expression of these markers on the gated CD8 T cells. To assess proliferation of the graft infiltrating cells, graft recipients were injected with 1 mg BrdU (Sigma Aldrich) i.p. on the day before graft harvest. Following preparation of the graft infiltrating cell suspensions, aliquots were stained with anti-CD8 and anti-BrdU antibody (BD Bioscience) and incorporation of the BrdU was tested on gated CD8 T cells.
RNA purification and qRT-PCR
Snap-frozen grafts were crushed, homogenized, and RNA was isolated using fibrous tissue kits (Qiagen, Valencia, CA). Reverse transcription and real-time PCR were performed using commercially available reagents, probes, and a 7500 fast real-time thermocycler, all from Applied Biosystems (Foster City, CA).
ELISPOT
Donor-reactive T cells producing IFN-γ in cardiac allografts were enumerated by ELISPOT assay as previously detailed (15, 16). Briefly, harvested graft tissues were digested as above and graft-infiltrating CD8 T cells were positively selected using magnetic beads (Stem Cell Technologies, Vancouver, BC). Aliquots of the isolated CD8 T cells were cultured with mitomycin C-treated donor stimulator cells for 24 h at 37°C in serum-free HL-1 media in 96 well plates coated with capture anti-IFN-γ mAb. After culture, the cells were washed from the plate and detecting biotinylated anti-IFN-γ mAb was added, followed by anti-biotin alkaline phosphatase, and development with the chromagen. The total number of spots per well was quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Immunohistochemistry
A midventricular portion of the cardiac graft was embedded in OCT compound (Sakura Finetek USA) and immediately frozen in liquid nitrogen after harvest, and 6 μm thick sections were prepared as previously described. Slides were stained with 10 μg/mL anti-CD8 mAb (53–6.7) or anti-Ly-6G mAb (RB6–8C5) in PBS with 1% BSA for 1 h at room temperature and then with biotinylated rat anti-rat IgG (Dako, Carpinteria, CA) diluted 1:100 in PBS with 1% BSA for 20 minutes at room temperature. The slides were developed with DAB for color change and counterstained with hematoxylin. Images were captured and analyzed with a Nikon Eclipse 55i and NIS Elements BR (Nikkon, Melville, NY). C4d staining was performed on acetic methanol (60% methanol, 10% acetic acid) fixed tissue using polyclonal rabbit antibody to C4d and a secondary peroxidase-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) (46).
Statistics
Graft survival between experimental groups was compared using Kaplan-Meier survival curves and Log-rank statistics. For other experiments statistical analyses were performed using the Mann–Whitney nonparametric test to analyze differences between experimental groups. A p value < 0.05 was considered significant. Error bars reflect Standard Error from the Mean (SEM) for each group.
Results
Delayed treatment with anti-LFA-1 mAb does not inhibit memory CD8 T cell infiltration into cardiac allografts
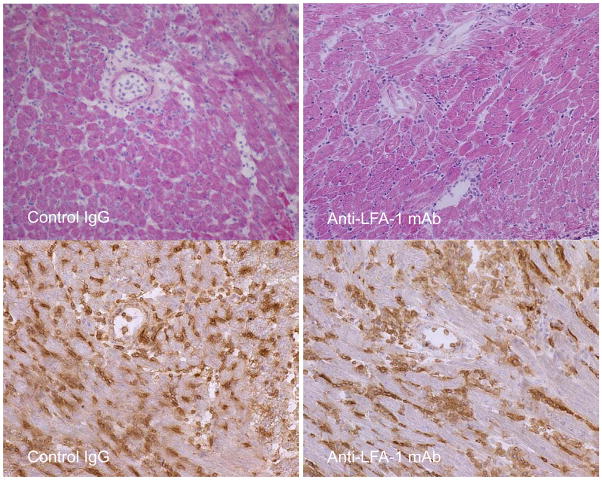
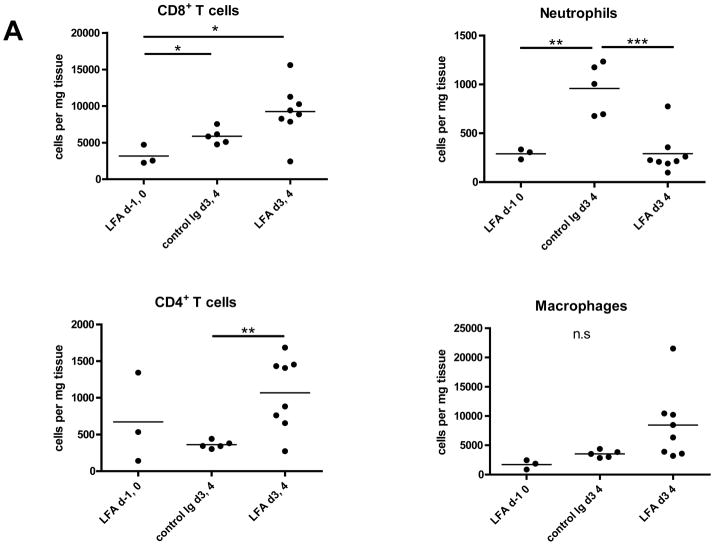
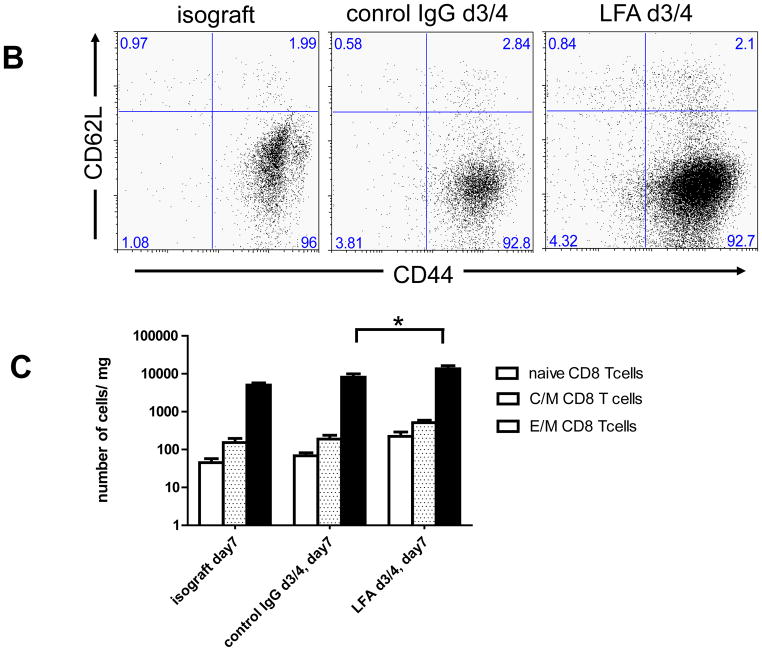
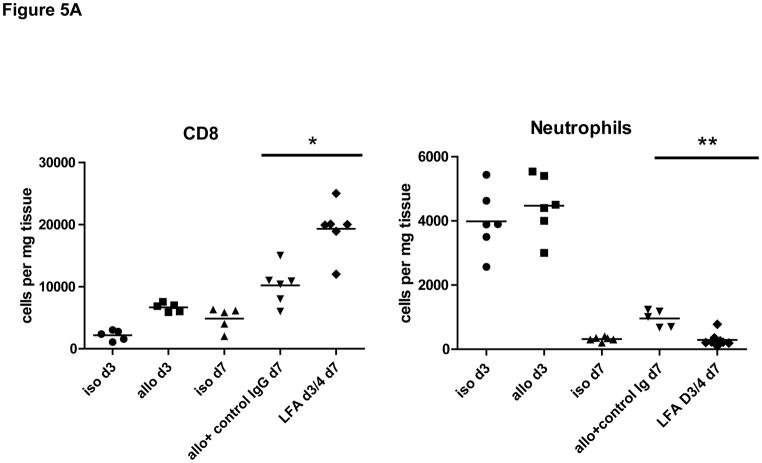
Peri-transplant treatment with anti-LFA-1 mAb on days −1 and 0 extended complete MHC-mismatched cardiac allograft survival from day 7–8 in control IgG-treated recipients to day 20–40, whereas delayed treatment with anti-LFA-1 mAb on days 3 and 4 post-transplant had a more modest effect in prolonging allograft survival to day 13–17 (18). Despite strong beating at day 7 post-transplant, allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4 had intense infiltration of CD8 T cells similar to that observed in rejecting allografts from the control IgG-treated recipients (Figure 1). The infiltration of various leukocyte populations into isografts and allografts on day 7 post-transplant in recipients treated with control IgG or anti-LFA-1 mAb was directly assessed by digesting harvested grafts to prepare single cell suspensions and staining aliquots of the cells and flow cytometry to determine the numbers of infiltrating cell populations per mg of graft tissue. As previously observed (18), administration of anti-LFA-1 mAb on days −1 and 0 caused marked decreases in the infiltration of neutrophils, macrophages and CD8 T cells into complete MHC-mismatched cardiac allografts when assessed at day 7 post-transplant (Figure 2A). In contrast, administration of anti-LFA-1 mAb on days 3 and 4 post-transplant consistently resulted in slightly higher numbers of CD8 T cells in allografts on day 7 post-transplant than were observed in allografts from control IgG-treated recipients. There were also increases in CD4 T cells and macrophages infiltrating the allografts in recipients treated with anti-LFA-1 mAb on days 3 and 4 post-transplant when compared to allografts from control IgG-treated recipients. However, a striking decrease of neutrophils in allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4 was observed when compared to allografts from the control treated recipients and these decreased numbers were similar to those observed in allografts from recipients treated with anti-LFA-1 mAb on days −1 and 0. The graft infiltrating CD8 T cells were also stained with antibodies to assess expression of CD44 and CD62L to distinguish naïve (CD62LhighCD44low) from central memory (CD62LhighCD44high) and effector/memory (CD62LlowCD44high) T cell phenotypes. More than 90% of the CD8 T cells in the allografts from recipients treated with control IgG or anti-LFA-1 mAb on days 3 and 4 post-transplant were of the effector/memory phenotype and less than 1.0% were of a naïve phenotype (Figure 2B and C). In addition, there were low numbers of CD62LhighCD44high central memory phenotype CD8 T cells in the allografts from each group.
Figure 1. CD8 T cells in complete MHC-mismatched cardiac allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4 post-transplant.
C57BL/6 mice were treated with 200 μg control rat IgG or with anti-LFA-1 mAb on days 3 and 4 after an A/J cardiac transplant. On day 7, grafts were harvested and formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin (upper panels) or frozen sections were stained by immunohistochemistry to detect CD8 T cells (lower panels). Images are representative of 5 or more separate allografts for each treatment group. Magnification, x200.
Figure 2. Memory CD8 T cell infiltration into cardiac allografts from recipients treated with anti-LFA-1 mAb.
Groups of C57BL/6 mice were treated with 200 μg anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4 or with control rat IgG on days 3 and 4. The mice received complete MHC mismatched A/J cardiac allografts on day 0. (A) Grafts were harvested on day 7 post-transplant, weighed, digested to prepare single cell suspensions, and graft-infiltrating cells were analyzed by antibody staining and flow cytometry to determine the numbers of graft-infiltrating CD4 and CD8 T cells, neutrophils and macrophages. *p ≤ 0.02; **p < 0.01; ***p < 0.002; n.s. no significant differences. (B) Aliquots of the iso- and allo-graft infiltrating cells from each group were stained with antibodies to detect the expression of CD44 and CD62L on CD8 T cells. A representative example of 5 grafts/group is shown. (C) The number of graft infiltrating CD8 T cells expressing each of the indicated naïve (CD62LhighCD44low) and memory (central memory, C/M CD62LhighCD44high and effector memory E/M CD62LlowCD44high) phenotypes for groups of 5 grafts ± SEM is shown on a log scale. *p < 0.03.
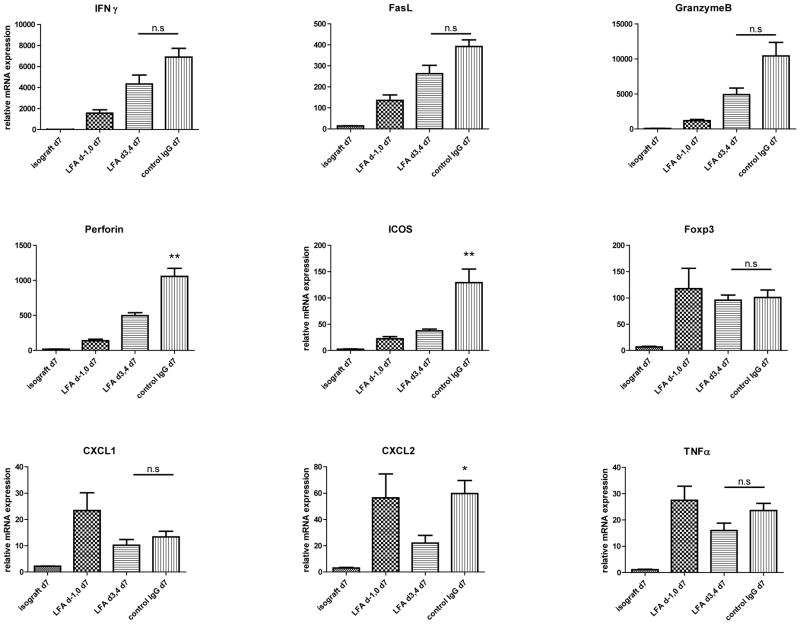
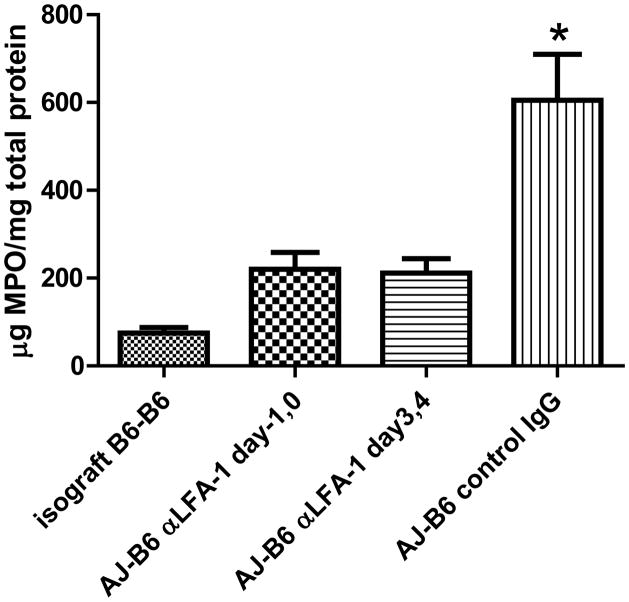
Despite the increased numbers of CD8 T cells in allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4, there was decreased expression of mRNA encoding inflammatory mediators and effector memory T cell functions in the allografts on day 7 post-transplant (Figure 3). When compared to levels expressed during acute cell mediated rejection of allografts by control IgG-treated recipients, there were significant decreases in the expression of the neutrophil chemoattractant CXCL2 (p ≤ 0.02) and the T cell effector molecules perforin, and ICOS (p ≤ 0.01), as well as marked but statistically insignificant decreases in IFN-γ, FasL, and granzyme B. Expression levels of CXCL1, TNFα and FoxP3 were not significantly different from the levels detected in allografts from control IgG treated recipients. The low number of neutrophils in the allografts of recipients treated with anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4 was reflected by the low amount of myeloperoxidase protein in the allografts on day 7 post-transplant (Figure 4).
Figure 3. Intragraft cytokine and chemokine mRNA expression in cardiac isografts and in allografts from anti-LFA-1 mAb treated recipients.
Groups of C57BL/6 mice were treated with 200 μg anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4 or with control rat IgG on days 3 and 4. The mice received isografts or complete MHC mismatched A/J cardiac allografts on day 0. Grafts were harvested on day 7 post-transplant, whole cell RNA was isolated from graft homogenates, and quantitative real-time PCR was used to measure expression levels of mRNA encoding the indicated cytokines, chemokines and phenotypic markers. *p ≤ 0.02; **p < 0.01; n.s. not significantly different.
Figure 4. Neutrophil activation in cardiac allografts from recipients treated with anti-LFA-1 mAb.
Groups of C57BL/6 mice were treated with 200 μg anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4 or with control rat IgG on days 3 and 4. The mice received isografts or complete MHC mismatched A/J cardiac allografts on day 0. Grafts were harvested on day 7 post-transplant, soluble lysates were prepared and the total protein in the lysates determined. Aliquots of the lysate were tested by ELISA for quantity of myeloperoxidase (MPO) protein as a measure of neutrophil activation in the graft. *p ≤ 0.02.
Endogenous memory CD8 T cells within allografts of recipients treated with anti-LFA-1 mAb do not express ICOS
Our previous studies indicated the activation and proliferation of endogenous memory CD8 T cells within MHC-mismatched cardiac allografts between days 1–3 post-transplant (15), a time prior to the administration of anti-LFA-1 mAb on days 3 and 4. Therefore, increases in CD8 T cells within the allograft from day 3 to day 7 were tested as an indication of memory CD8 T cell expansion within the graft. On day 3 post-transplant the numbers of CD8 T cells in the allografts were 3–4 fold higher than those observed in isografts (Figure 5a). By day 7 post-transplant, however, CD8 T cell numbers in allografts from anti-LFA-1 mAb-treated recipients were increased further than those observed in allografts from control IgG treated recipients. In contrast to the allograft infiltrating CD8 T cells, the numbers of neutrophils in allografts in anti-LFA-1 mAb treated recipients had decreased to background levels by day 7 when compared to the numbers observed infiltrating the allografts on day 3, prior to anti-LFA-1 mAb treatment on days 3 and 4.
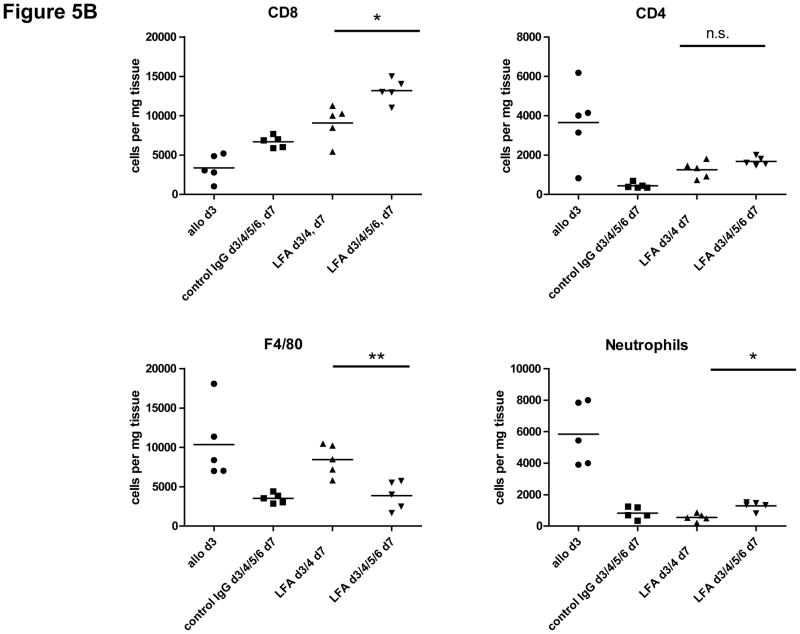
Figure 5. Temporal increase in CD8 T cell numbers in cardiac allografts from recipients treated with anti-LFA-1 mAb.
(A) C57BL/6 mice were treated with 200 μg anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4 or with control rat IgG on days 3 and 4. The mice received complete MHC mismatched A/J cardiac allografts on day 0. Groups of grafts were harvested on day 3 or on 7 post-transplant, digested to prepare single cell suspensions, and graft-infiltrating cells were analyzed by antibody staining and flow cytometry to determine the numbers of graft-infiltrating CD8 T cells and neutrophils. *p ≤ 0.002; **p < 0.001. (B) Recipients were also treated with 200 μg control rat IgG or anti-LFA-1 mAb each day from day 3 to 6 post-transplant. Grafts were harvested on day 7 post-transplant, digested to prepare single cells suspensions, and graft-infiltrating cells were analyzed by antibody staining and flow cytometry to determine the numbers of graft-infiltrating CD4 and CD8 T cells, neutrophils and macrophages. *p ≤ 0.0; **p ≤ 0.008; n.s. not significantly different.
Our previous studies reported that allograft recipient treatment with anti-LFA-1 mAb on days 3 and 4 inhibited the development of donor-reactive CD4 and CD8 T cells to IFN-γ–but not to IL-2-producing cells when assessed on day 7 post-transplant (18). This raised the possibility that the increased number of CD8 T cells observed in the allografts from these recipients might be due to the infiltration of IL-2-producing CD8 T cells that developed from donor-reactive naïve precursor cells. Therefore, we repeated the experiment but included an additional group of recipients treated with anti-LFA-1 mAb on each day from day 3 to 6 post-transplant in order to inhibit graft infiltration of any leukocyte population within this treatment time frame. The results indicated that treatment with anti-LFA-1 antibody every day from days 3 to 6 resulted in further increases in CD8 T cell numbers in the allografts when compared to numbers in allografts from recipients treated with the antibody on days 3 and 4 (Figure 5B). As an indication of the efficacy of the approach, treatment with anti-LFA-1 mAb on days 3–6 resulted in decreased F4/80+ cell (i.e. macrophage) infiltration into the allograft when compared to the numbers in allografts from recipients treated with anti-LFA-1 mAb only on days 3 and 4.
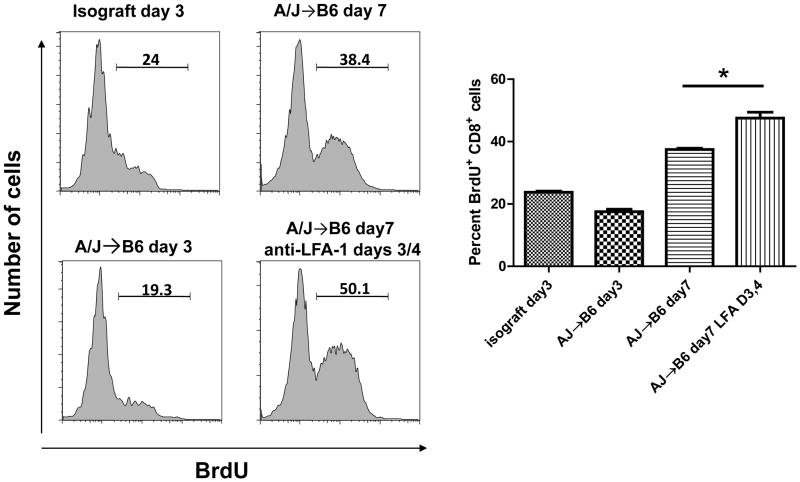
Groups of iso- and allo-graft recipients were injected with BrdU on either day 2 or 6 and the day after the injection the grafts were harvested, digested and single cell suspensions were prepared and aliquots stained with anti-BrdU and anti-CD8 antibodies for flow cytometry analysis to assess CD8 T cell uptake of BrdU as a measure of proliferation within the graft. On day 3 post-transplant, the level of CD8 T cell proliferation was similar in iso- and in MHC-mismatched allografts (Figure 6A and 6B). On day 7 post-transplant the numbers of proliferating CD8 T cells were increased from that observed on day 3 and were not diminished by the administration of anti-LFA-1 mAb. Furthermore, the numbers of BrdU incorporating CD8 T was significantly greater in allografts from the anti-LFA-1 mAb-treated recipients than those observed in allografts from control IgG treated recipients (Figure 6B).
Figure 6. Proliferation of CD8 T cells in cardiac allografts from recipients treated with anti-LFA-1 mAb.
Groups of C57BL/6 mice were treated with 200 μg anti-LFA-1 mAb either on days −1 and 0 or on days 3 and 4. The mice received isografts or complete MHC mismatched A/J cardiac allografts on day 0. The day before graft harvest, day 2 or day 6, recipients were injected i.p. with BrdU. Grafts were harvested on day 3 or 7 post-transplant, digested to prepare single cells suspensions, and aliquots stained with anti-BrdU and anti-CD8 mAb and analyzed by flow cytometry to assess BrdU incorporation by the graft infiltrating CD8 T cells. Representative histograms of BrdU incorporation by gated CD8 T cells in grafts from each group is shown. The mean percentage of BrdU+CD8+ cells in 4 grafts from each group ± SEM is shown. *p < 0.03.
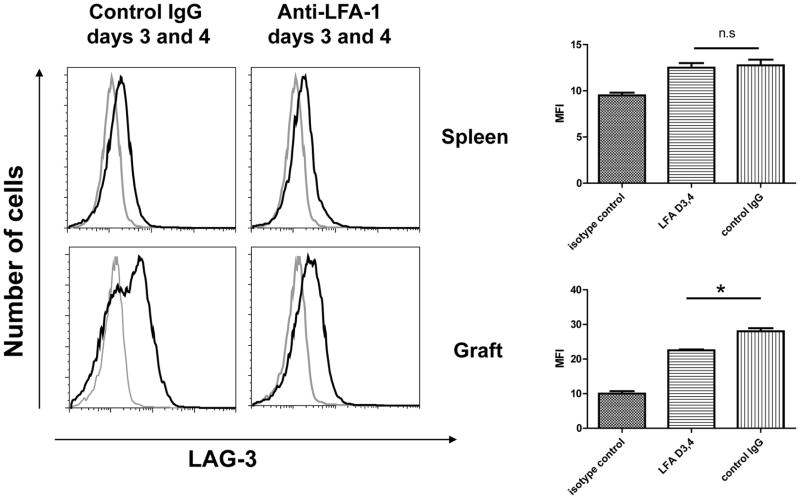
Staining CD8 T cells from the spleens of control IgG- and anti-LFA-1 mAb treated allograft recipients with antibody to LAG-3, a marker of T cell exhaustion, indicated no difference in cell surface expression of LAG-3 on day 7 post-transplant (Figure 7). However, allograft infiltrating CD8 T cells from recipients treated with anti-LFA-1 mAb on days 3 and 4 had decreased LAG-3 expression when compared to allograft infiltrating CD8 T cells from control IgG-treated recipients. Similar results were observed with expression of PD1 in that CD8 T cells within allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4 did not express higher levels of PD1 than CD8 T cells infiltrating allografts in control Ig treated recipients (S. Iida, data not shown).
Figure 7. Expression of LAG-3 on CD8 T cells in spleens and cardiac allografts from recipients treated with anti-LFA-1 mAb.
Groups of C57BL/6 mice were treated with 200 μg control rat IgG or anti-LFA-1 mAb on days 3 and 4. The mice received complete MHC mismatched A/J cardiac allografts on day 0. Recipient spleens (top rows) and allografts (bottom rows) were harvested on 7 post-transplant, digested to prepare single cells suspensions, and aliquots stained with anti-LAG-3 (solid line) or control IgG (shaded line) and anti-CD8 mAb and analyzed by flow cytometry to assess LAG-3 expression by CD8 T cells in the recipient spleen and by the graft infiltrating CD8 T cells. Representative histograms of LAG-3 expression by gated CD8 T cells in grafts from each group is shown. The mean change in Mean Fluorescence Intensity (MFI) of LAG-3 staining for the gated CD8 T cells in 4 grafts from each group SEM is shown. *p ≤ 0.03; n.s. not significantly different.
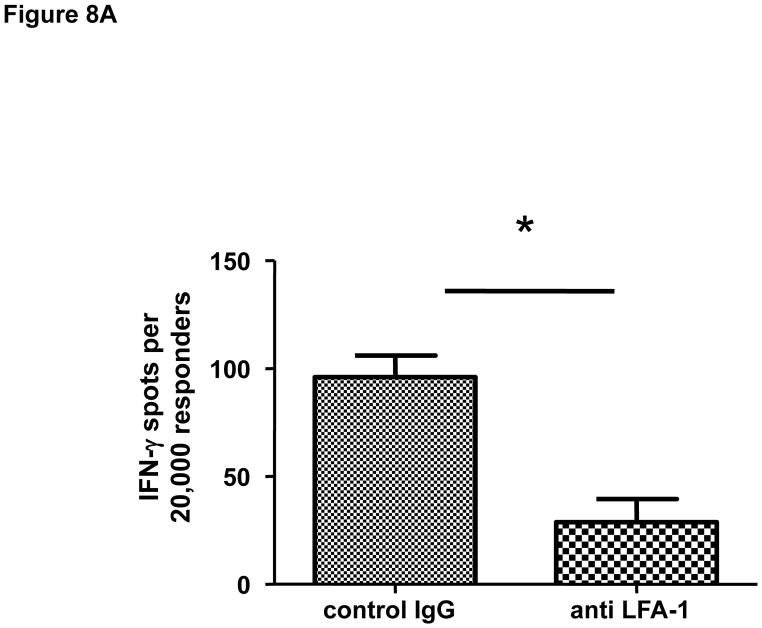
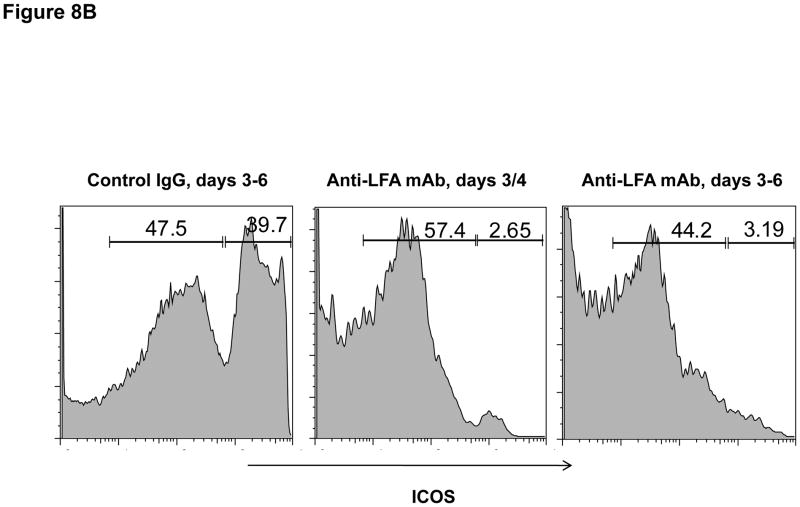
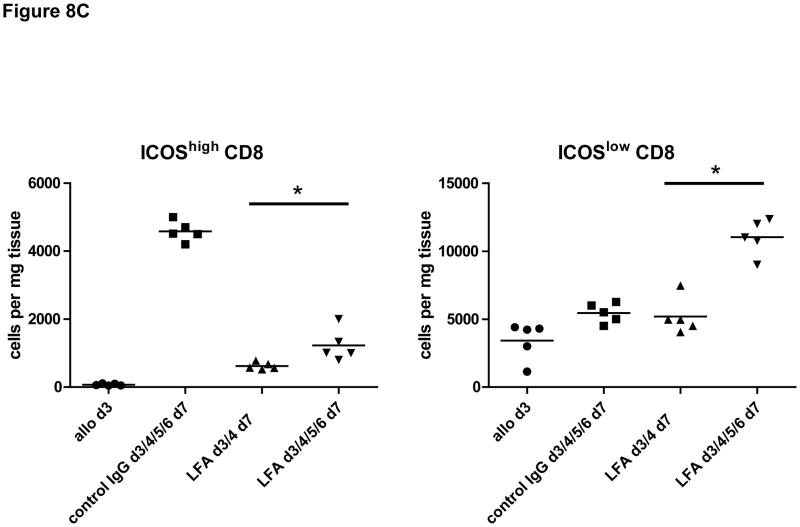
Finally, the effector functions of CD8 T cells within allografts of recipients treated with anti-LFA-1 mAb on days 3 and 4 post-transplant were directly tested by enumerating donor-reactive CD8 T cells producing IFN-γ. When compared to the number of donor-reactive CD8 T cells producing IFN-γ in allografts from control IgG treated recipients, the number infiltrating allografts in recipients treated with anti-LFA-1 mAb was reduced by approximately 60% (Figure 8A). This decrease correlated with a marked decrease in the expression of ICOS on infiltrating CD8 T cells in allografts from the anti-LFA-1 mAb treated recipients (Figure 8B and 8C).
Figure 8. Activation of CD8 T cells in cardiac allografts from recipients treated with anti-LFA-1 mAb.
Groups of C57BL/6 mice were treated with 200 μg control rat IgG or anti-LFA-1 on days 3 and 4 and received complete MHC mismatched A/J cardiac allografts on day 0. (A) On day 7, grafts were harvested, weighed, digested to prepare single cell suspensions, and the allograft infiltrating CD8 T cells were isolated by positive selection. Aliquots of the CD8 T cells were tested by ELISPOT assay to enumerate donor-reactive CD8 cells producing IFN-γ. Data are representative of two independent experiments and the data indicate mean number of donor-reactive CD8 T cells producing IFN-γ ± SEM. *p ≤ 0.004. (B) Aliquots of the graft infiltrating cells from recipients treated with control IgG or with anti-LFA-1 mAb as indicated were stained with anti-ICOS mAb and anti-CD8 mAb and analyzed by flow cytometry to assess ICOS expression by the graft infiltrating CD8 T cells. Representative histograms of ICOS expression by gated CD8 T cells in grafts from each group are shown. (C) Individual numbers of graft infiltrating CD8 T cells expressing high vs. low ICOS is shown for groups of 5 grafts, with the mean number indicated by the vertical bar. *p < 0.01.
Endogenous memory CD8 T cells within allografts of recipients treated with anti-CD4 or anti-CD154 mAb do not express functional activity
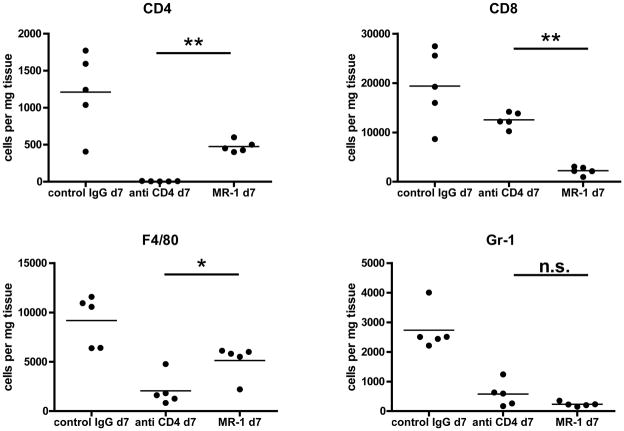
The results indicating the inability of allograft infiltrating memory CD8 T cells to mediate rejection in anti-LFA-1 mAb treated recipients prompted investigation into other models where strategies that promoted long-term allograft survival did not inhibit early endogenous memory CD8 T cell infiltration into the allografts (16). Groups of heart allograft recipients were treated with anti-CD4 or with anti-CD154 mAb on days 0 and +1 and then on day +7 post-transplant the allografts were retrieved and the presence of infiltrating CD8 T cells was assessed. First, the infiltration of various cell populations into the allografts from the different treatment groups on day 7 post-transplant was directly assessed by antibody staining of cell aliquots prepared from digested graft tissue and flow cytometry analysis. Equivalent CD8 T cell numbers were observed in allografts treated with control IgG or CD4-depleting antibodies on day 7 post-transplant whereas these numbers were significantly reduced in allografts from recipients treated with the anti-CD154 mAb (Figure 9). Neutrophil numbers were high in allografts from control IgG treated recipients but were low-absent in allografts from recipients treated with either anti-CD4 or anti-CD154 mAb. Recipient treatment with anti-CD4 mAb but not with anti-CD154 mAb also resulted in a significant decrease in the number of macrophages in the allografts on day 7 post-transplant.
Figure 9. Memory CD8 T cell infiltration into cardiac allografts from recipients treated with anti-CD4 or anti-CD154 mAb.
Groups of C57BL/6 mice were treated with 200 μg control rat IgG or with anti-CD4 or anti-CD154 mAb either on days −1 and 0. The mice received complete MHC mismatched A/J cardiac allografts on day 0. Grafts were harvested on day 7 post-transplant, weighed, digested to prepare single cell suspensions, and aliquots stained with antibodies to detect graft-infiltrating CD4 and CD8 T cells, neutrophils and macrophages by flow cytometry. *p < 0.02; **p < 0.0001; n.s. not significantly different.
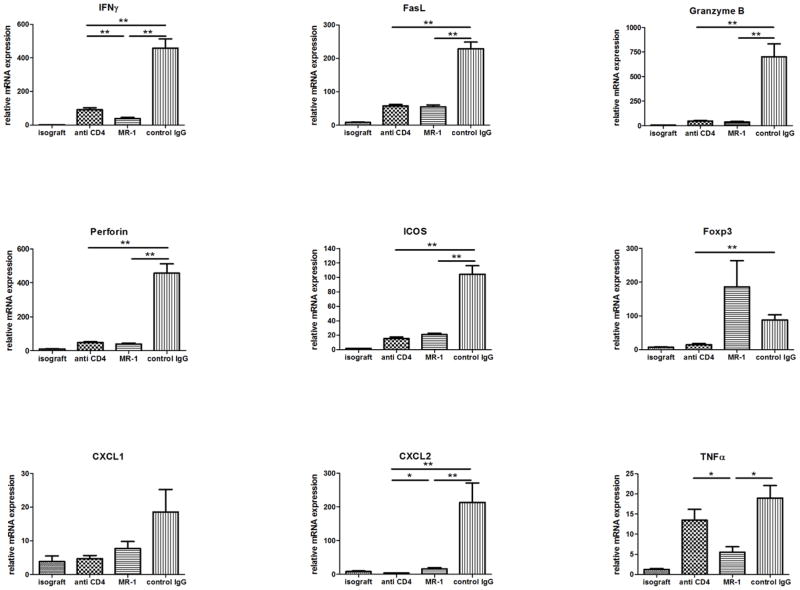
Finally, the expression levels of mRNA encoding innate inflammatory and effector T cell molecules in allografts harvested on day 7 post-transplant from each of the recipient treatment groups were determined by qRT/PCR (Figure 10). Recipient treatment with anti-CD4 mAb resulted in marked decreases in expression of all of the markers tested in the allografts on day 7 post-transplant with the exception of TNFα. Treatment with anti-CD154 mAb also resulted in marked decreases in all of the markers including TNFα with the exception of Foxp3 which was increased more than two-fold.
Figure 10. Intragraft cytokine and chemokine mRNA expression in cardiac isografts and in allografts from anti-LFA-1 mAb treated recipients.
Groups of C57BL/6 mice were treated with 200 μg control rat IgG or with anti-CD4 or anti-CD154 mAb either on days −1 and 0. The mice received isografts or complete MHC mismatched A/J cardiac allografts on day 0. Grafts were harvested on day 7 post-transplant, whole cell RNA was isolated from graft homogenates, and quantitative real-time PCR was used to measure expression levels of mRNA encoding the indicated cytokines, chemokines and phenotypic markers. *p ≤ 0.05; **p < 0.01; n.s. not significantly different.
Discussion
This investigation was prompted by clinical studies indicating that the detection of high levels of endogenous memory T cells with donor reactivity prior to transplant portends poor renal graft outcomes and pre-clinical studies indicating that memory CD8 T cells induced to viral infections confer costimulatory blockade resistant rejection of allografts (1, 3, 14, 18). We had previously reported the rapid infiltration of endogenous memory CD8 T cells into MHC-mismatched cardiac allografts in naive mice and their activation to proliferate and produce IFN-γ prior to the appearance of donor antigen-primed effector T cells in the recipient spleen (16). Although these early memory CD8 T cell mediated inflammatory events promoted the subsequent trafficking of the donor-antigen primed effector T cells into the graft, it remained unclear whether the activation and proliferation of these endogenous memory CD8 T cells provoked sufficient injury to cause rejection independently of the primary effector T cells. To test this we took advantage of the early infiltration of the memory CD8 T cells into the allograft and the ability of anti-LFA-1 mAb given on days 3 and 4 to delay the development of the primary effector T cells in the recipient spleen until day 12–15 post-transplant. Although higher numbers of CD8 T cells were observed on day 7 in the allografts of the anti-LFA-1 mAb- vs. from control-treated recipients, the T cells did not mediate sufficient injury to provoke graft rejection. Two immediate questions arise from this result: first, are endogenous memory CD8 T cells that infiltrate the allograft during the first 1–2 days after graft reperfusion the source of the high numbers of CD8 T cells observed at day 7 post-transplant; and second, what restricts the expression of effector function by these cells within the allograft?
The results of this report indicate it is likely that the high numbers of CD8 T cells observed in the allografts from recipients treated with anti-LFA-1 mAb on days 3 and 4 arise from the endogenous memory CD8 T cells that infiltrate the allografts prior to the initiation of anti-LFA-1 mAb treatment. We have previously reported that the endogenous memory CD8 T cells as well as transferred memory CD8 T cells primed to donor antigens proliferate following infiltration into the allograft (15) and the results in the current report indicate that they continue to do so, at least up to day 7 post-transplant. We have also reported the ability of the anti-LFA-1 mAb to inhibit virtually all recipient leukocyte infiltration into allografts for several days after administration of the antibody (18). This would suggest that little leukocyte infiltration into the allograft occurs after administration of the antibody on days 3 and 4 including infiltration of primary effector T cells that develop in the recipient lymphoid organs. Consistent with this is the marked decreases in macrophage infiltration into allografts from recipients treated with the anti-LFA-1 mAb every day from day 3 to 6 whereas the number of CD8 T cells within the allograft continued to increase. Although not yet tested, it is possible that cytokines such as IL-7 and/or IL-15 that drive the proliferation of memory CD8 T cells may be present in sufficient quantity in the allograft to sustain this proliferation.
These results raise questions about the functional capability of the endogenous memory CD8 T cells in mediating graft tissue injury and rejection. Studies by Adams and colleagues (9) had indicated that a threshold number of viral-induced memory CD8 T cells is required to overcome costimulation blockade and contribute to rejection of skin and heart allografts in recipients treated with CTLA-4-Ig and anti-CD154 mAb that promotes long-term allograft survival in recipients not receiving the memory CD8 T cells. In human adults humans have 104 more T cells than an adult mouse and in humans more of these are memory T cells, particularly as the human ages (21–23). Thus, this increase in human memory T cells could achieve the threshold numbers required to overcome costimulation blockade and could account for the differences in efficacy of many strategies that promote tolerance in mouse transplant models yet fail in human transplant patients. With regards to the activity of endogenous memory CD8 T cells responding to heart allografts in mice, either an insufficient number of endogenous memory CD8 T cells may not infiltrate the allografts or a threshold number of these T cells may not be achieved during proliferation within the allograft to provoke the injury needed to reject the graft. However, when assessed on day 7 post-transplant there were more CD8 T cells in the allografts from the anti-LFA-1 mAb treated recipients than the control treated recipients that were rejecting their grafts at that time. This suggests that it is absent or decreased memory CD8 T cell function rather than sufficient numbers that underlies the absence of rejection in these recipients.
The decreases in CD8 T cells within the allograft that produce IFN-γ in response to donor cells indicate that the proliferating cells lose the ability to express effector functions with time in the allograft. Many studies have documented the transition of primary effector and memory CD8 T cells into a state of exhaustion during responses to allografts, tumors and during chronic viral infections (24–27). However, we did not observe a difference in expression of markers associated with the transition to an exhausted phenotype on the CD8 T cells in the allografts including PD-1 (S. Iida, data not shown) and LAG-3. Our previous studies had indicated that IFN-γ production by endogenous memory CD8 T cells infiltrating cardiac allografts was dependent on ICOS-mediated costimulation (15). ICOS expression is induced on the endogenous memory CD8 T cells and on donor antigen primed memory CD8 T cells during proliferation. As discussed above, the CD8 T cells in allografts from anti-LFA-1 mAb treated recipients are clearly proliferating in the allograft but do not express high levels of ICOS and the absence of this costimulatory pathway may underlie their inability to express sufficient effector function to mediate overt graft injury.
It was also apparent that in addition to decreased numbers of CD8 T cells producing IFN-γ, there was a marked decrease in the number of neutrophils in the allografts from the anti-LFA-1 mAb treated recipients. Results from this and other laboratories have demonstrated a key role for neutrophil activation in the tissue injury induced following reperfusion of ischemic organs (28–34). Furthermore, the presence of neutrophils and their activation products is often observed during T cell- and/or antibody-mediated acute rejection of organ allografts (17, 35–43). These studies implicate a key role for neutrophils in mediating tissue injury during allograft rejection and their absence in allografts heavily infiltrated with endogenous memory CD8 T cells may underlie the absence of overt injury in anti-LFA-1 mAb treated recipients. Neutrophil infiltration into allografts early after graft reperfusion is directed by production of CXCL1 and CXCL2 and neutrophil numbers in the allograft are sustained by the activities of the endogenous memory CD8 T cells (17). Interestingly, we observed decreased numbers of neutrophils in allografts following recipient treatment with anti-LFA-1 mAb on days 3 and 4 that correlated with decreased levels of CXCL1 mRNA but high levels of CXCL2 mRNA in the allograft, suggesting that CXCL1 may be important for promoting neutrophil infiltration and viability in the allograft. It is also worth noting the increased numbers of macrophages in these allografts raising questions about their ability or inability to contribute to graft tissue injury. It has now become clear that macrophages can assume pro- or anti-inflammatory functions that is dependent on the tissue environment they enter (44–46), and the attenuation of endogenous memory CD8 T cell mediated inflammation may direct graft infiltrating macrophage to express anti-inflammatory functions.
We had previously reported that peri-transplant treatment with either anti-CD4 or anti-CD154 mAb did not inhibit endogenous memory CD8 T cell infiltration or activation in cardiac allografts when assessed on days 2 and 3 post-transplant (16). Yet peri-transplant treatment with these mAb results in marked prolongation in complete MHC mismatched cardiac allograft survival. Elegant studies from the Bishop laboratory have indicated that peri-transplant administration of anti-CD4 mAb promotes prolonged survival of cardiac allografts although the grafts eventually develop occlusive vasculopathy and interstitial fibrosis whereas anti-CD154 mAb promotes prolonged survival without evidence of this chronic injury (47–49). The current studies indicate that treatment with anti-CD4 mAb does not inhibit the accumulation of CD8 T cells in the allograft on day 7 but the CD8 T cells do not reject the graft and it is also worth noting the absence of neutrophils in these grafts. In contrast, recipient treatment with anti-CD154 mAb results in the disappearance of the memory CD8 T cells from the allograft sometime between days 3 to 7 post-transplant. It is quite noticeable that on day 7 there are high numbers of CD4 T cells and mRNA expression of FoxP3 suggesting either infiltration of CD4+FoxP3+ Tregs or the conversion of CD4 T cells to Tregs within the allograft. Studies from many laboratories have reported the induction of CD4 T regulatory cells by strategies including anti-CD154 mAb (50–52). Despite the origin of the Tregs on day 7 post-transplant, their presence in the graft is associated with loss of the memory CD8 T cells that infiltrate shortly after reperfusion. The induction of these Tregs and/or the absence of endogenous memory CD8 T cells may very well account for the absence of chronic allograft injury in anti-CD154 mAb-treated vs. anti-CD4 mAb-treated recipients (47–49). Whether this is due to the disappearance of the endogenous memory CD8 T cells and/or neutrophil activity at early times post-transplant as well as the mechanisms underlying the absence of ICOS on these CD8 T cells and the decreased expression of effector functions within the allograft remain undefined at this time but certainly warrant rigorous investigation to more completely understand this component of the early immune response to the allograft.
Acknowledgments
We thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study. The authors thank Drs. Joshua Rosenblum and Anna Valujskikh for helpful comments during the course of this work.
This work was supported by grants from the NIH (RO1 AI40459 and PO1 AI087586) to RLF.
Footnotes
Disclosure
The authors have no financial conflict of interest to declare.
References
- 1.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5:1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 2.Cherkassky L, Lanning M, Lalli PN, Czerr J, Siegel H, Danzinger-Isakov L, Srinivasa Valujskikh T, Shoskes DA, Baldwin W, Fairchild RL, Poggio ED. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL-2 receptor blockade. Am J Transplant. 2011;11:1388–1396. doi: 10.1111/j.1600-6143.2011.03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphcytes is a manifestation of immunologic memory and correlates with the risk of post-transplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 4.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, Hricik DE, Heeger PS. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83:847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 5.Krummey SM, Ford ML. Heterogeneity within T cell memory: implications for transplant tolerance. Front Immunol. 2012;3:1–15. doi: 10.3389/fimmu.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 7.Valujskikh A, Baldwin WM, III, Fairchild RL. Recent progress and new perspectives in studying T cell responses to allografts. Am J Transplant. 2010;10:1117–1125. doi: 10.1111/j.1600-6143.2010.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams AB, Williams MR, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 11.Kitchens WH, Haridas D, Wagener ME, Song M, Ford ML. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous memory alloresponses. Transplantation. 2012;93:997–1005. doi: 10.1097/TP.0b013e31824e75d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh RM, markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, Greiner DL, Rossini AA. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadazdin O, Boskovic S, Murakami T, O’Conner DH, Wiseman RW, Karl JA, Tuscher JJ, Sachs DH, Madsen JC, Tocco G, Kawai T, Cosimi AB, Benichou G. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T, Cosimi AB, Benichou G. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002093. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9:64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate mediated inflammation and graft necrosis. Am J Pathol. 2004;165:147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, III, Tanabe K, Fairchild RL. LFA-1 antagonism inhibits early infiltration of endgenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am J Transplant. 2011;11:923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 22.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 23.Nikolich-Zugich J, Sifka MK, Messaoudi I. The many important facets of T-cell repertoire divesity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth responsse gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med. 2012;209:2157–2163. doi: 10.1084/jem.20120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cll exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2012.12.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valujskikh A, Li XC. Memory T cells and their exhaustive differentiation in allograft tolerance and rejection. Curr Opin Organ Transplant. 2012;17:15–19. doi: 10.1097/MOT.0b013e32834ee443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belperio JA, Keane MP, Burdick MD, Gomperts BN, Xue YY, Hong K, Mestas J, Zisman D, Ardehali A, Saggar R, Lynch JP, 3rd, Ross DJ, Strieter RM. CXCR2/CXCR2 ligand biology during lung transplant ischemia/reperfusion injury. J Immunol. 2005;175:6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 29.De Greef KE, Ysebaert DK, Ghielli M, Vercauteren S, Nouwen EJ, Eyskens EJ, De Broe ME. Neutrophils and acute ischemia-reperfusion injury. J Nephrol. 1998;11:110–122. [PubMed] [Google Scholar]
- 30.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. The role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 31.Iwahori Y, Ishiguro N, Shimizu T, Kondo S, Yabe Y, Oshima T, Iwata H, Sendo F. Selective neutrophil depletion with monoclonal antibodies attenuates ischemia/reperfusion injury in skeletal muscle. J Reconstr Microsurg. 1998;14:109–116. doi: 10.1055/s-2007-1000152. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke H, Farhood A, Smith C. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3364. [PubMed] [Google Scholar]
- 33.Kreisel D, Sugimoto S, Tietjens J, Zhu J, Yamamoto S, Krupnick AS, Carmody RJ, Gelman AE. Bcl3 prevents acute inflammtory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121:265–276. doi: 10.1172/JCI42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Groα and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citro A, Cantarelli E, maffi P, Nano R, Melzi F, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, Ruffini PA, Allegretti M, Secchi A, Bonifacio E, Piemonti L. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–3651. doi: 10.1172/JCI63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denicola MM, Weigt SS, Belperio JA, Reed EF, Ross DJ, Wallace WD. Pathologic findings in lung allografts with anti-HLA antibodies. J Heart Lung Transplant. 2013 doi: 10.1016/j.healun.2012.11.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devitt JJ, King CL, Lee TDG, Friesen CLH. Early innate immune events induced by prolonged cold ischemia exacerbate allograft vaasculopathy. J Cardiothorac Surg. 2011;6:2–10. doi: 10.1186/1749-8090-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 39.Healy DG, Watson RWG, O’Mahony U, Egan JJ, Wood AE. Neutrophil immunosurveillance for heart transplant rejection: a prospective study. Transplant Proc. 2010;42:1788–1792. doi: 10.1016/j.transproceed.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger K, Rauschulte H, Heine J, Scheinichen D, Leuwer M, Winkler M, Kuse ER. Neutrophil respiratory burst following liver transplantation: in vitro effects of granulocyte colony-stimulating factor. Transplant Intect Dis. 1999;1:153–156. doi: 10.1034/j.1399-3062.1999.010303.x. [DOI] [PubMed] [Google Scholar]
- 41.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-γ. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soo A, Maher B, McCarthy J, Nolke L, Woo0d A, Watson RWG. Pre-operative determination of an individual’s neutrophil response: a potential predictor of early cardiac transplant cellular rejection. J Heart Lung Transplant. 2009;28:1198–1205. doi: 10.1016/j.healun.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Swirski FK, Wildgruber M, Ueno T, Figueiredo J-L, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Mnahrendorf Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas SK, Mantovani A. Macrophage plasicity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Biswas SK, Faldiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodeling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 46.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csencsits K, Wood SC, Lu G, Faust SM, Brigstock D, Eichwald EJ, Orosz CG, Bishop DK. Transforming growth factor beta-induced connective tissue growth factor and chronic allograft rejection. Am J Transplant. 2006;6:959–966. doi: 10.1111/j.1600-6143.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 48.Nathan MJ, Yin D, Eichwald EJ, Bishop DK. The immunobiology of inductive anti-CD40L therapy in transplantation: allograft acceptance is not dependent upon the deletion of graft-reactive T cells. Am J Transplant. 2002;2:323–332. doi: 10.1034/j.1600-6143.2002.20406.x. [DOI] [PubMed] [Google Scholar]
- 49.Piccotti JR, Li K, Cahn SY, Eichwald EJ, Bishop DK. Cytokine regulation of chronic cardiac allograft rejection: evidence against a role for th1 in the disease process. Transplantation. 1999;67:1548–1555. doi: 10.1097/00007890-199906270-00008. [DOI] [PubMed] [Google Scholar]
- 50.Muller YD, Mai G, Morel P, Serre-Beinier V, Gonelle-Sispert C, Yung GP, Ehirchiou D, Wyss J-C, Bigenzahn S, Iria M, Heusser C, Golshayan D, Seebach JD, Wekerle T, Buhler LH. Anti-CD154 mAb and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigby MR, Trexler AM, Pearson TC, Lasen CP. CD28/CD154 blockade prevents autoimmune dabetes by inducing nondeletional tolerance after effector T-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57:2672–2683. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamazaki M, Pearson T, Brehm MA, Miller DM, Mangada JA, Markees TG, Shultz LD, Mordes JP, Rossini AA, Greiner DL. Different mechanisms control peripheral and central tolerance in hematopoietic chimeric mice. Am J Transplant. 2007;7:1710–1721. doi: 10.1111/j.1600-6143.2007.01839.x. [DOI] [PubMed] [Google Scholar]