Abstract
Background
Prenatal alcohol exposure can contribute to a wide range of neurodevelopmental impairments in children and adults including behavioral and neuropsychiatric disorders. In rhesus monkeys we examined whether moderate level prenatal alcohol exposure would alter acoustic startle responses and prepulse inhibition of the acoustic startle (PPI). PPI is a highly quantifiable measure of inhibitory neural processes or sensorimotor gating associated with neuropsychiatric disorders.
Methods
Acoustic startle and PPI of the acoustic startle was tested in 37 adult rhesus monkeys (Macaca mulatta) from four experimental conditions: (a) moderate level prenatal alcohol-exposed, (b) prenatally-stressed, (c) moderate level prenatal alcohol-exposed + prenatally-stressed, and (d) sucrose controls.
Results
Prenatal alcohol-exposed monkeys showed a higher magnitude of acoustic startle response and disrupted PPI compared with monkeys not exposed to alcohol prenatally. Monkeys in all conditions showed higher HPA-axis responses after undergoing the startle procedure, but HPA responses were unrelated to startle response magnitude, latency, or PPI.
Conclusion
Finding altered PPI in monkeys prenatally exposed to a moderate dose of alcohol suggests that reduced sensorimotor gating is one effect of prenatal alcohol exposure. Because reduced sensorimotor gating is observed in many neuropsychiatric disorders, sensorimotor gating deficits could be an aspect of the co-morbidity between FASD and mental health conditions.
Keywords: prepulse inhibition, fetal alcohol spectrum disorder (FASD), mental health, inhibitory control, acoustic startle response
INTRODUCTION
Moderate level prenatal alcohol exposure is associated with adverse developmental effects on cognitive and behavioral function (Riley and McGee, 2005). There is a high prevalence of prenatal alcohol exposure; over 50% of women in the U.S. of childbearing age report using alcohol in the previous month, and many fetuses are exposed to alcohol before pregnancy is detected (Rasmussen et al., 2009). The term fetal alcohol spectrum disorder (FASD) is used to describe a range of impairments related to prenatal alcohol exposure including not just fetal alcohol syndrome (FAS) (Mattson et al., 1998) but also “alcohol-related birth defects (ARBD)” and “alcohol-related neurodevelopmental disorder (ARND)”. ARND includes conditions in which children prenatally exposed to alcohol show neurobehavioral problems including reduced inhibitory control, impulsivity, attention deficits, problems in regulation of arousal, and impaired information processing without the full-blown FAS syndrome (see Mattson et al., 2011, for a review).
Individuals with FASD are also at high risk for mental health conditions (O’Connor and Kasari, 2000; Streissguth et al., 2004). For example, Mattson and Riley (2000) found that 90% of prenatally alcohol-exposed children had clinically significant scores on several externalizing domains, including social problems, attention problems and aggressive behavior. Prenatal alcohol-exposed children are also at risk for major depressive disorder of childhood (O’Connor and Kasari, 2000), ADHD (Herman et al., 2008), conduct disorder (Fryer et al., 2007), substance abuse problems (Baer et al., 2003) and elevated risk for suicide (Baldwin, 2007).
The underlying neural mechanisms of mental health conditions in FASD are not well understood, and this hinders development of selective and effective treatment. One useful way to study possible neural mechanisms underlying mental health problems in prenatal alcohol-exposed individuals is with the acoustic startle response and pre-pulse inhibition (PPI) of acoustic startle. Prepulse inhibition (PPI) of acoustic startle refers to the reduction in response to a high intensity stimulus when that stimulus is shortly preceded by a detectible but lower intensity stimulus, the prepulse (Graham, 1975). PPI of the acoustic startle response functions as a measure of sensorimotor gating or the inhibitory neural processes involved in filtering information. Thus, the weak prepulse stimulus activates inhibitory neural processes that reduce or inhibit the motor (startle) response to the intense stimulus (Graham 1975). PPI of startle shows close similarity between humans and experimental animals (Swerdlow et al., 1999), and also shows test-retest reliability indicating that it may have trait-like characteristics (Cadenhead et al., 1999).
In humans PPI deficits are found in several neuropsychiatric conditions including anxiety disorder, major depressive disorder, schizophrenia, Tourette’s syndrome, obsessive-compulsive disorder, Huntington’s disease, as well as in children of alcoholics (Braff et al., 2001; Grillon et al., 2005; Ludewig et al., 2003; Sutherland Owens et al., 2011; Swerdlow et al., 1995; Swerdlow and Geyer, 1998). Because of such associations in humans, PPI has been used in animal studies to examine neural and genetic underpinnings of neuropsychiatric disorders.
It is not known, however, whether PPI deficits are found in individuals with FASD. We found only one study of PPI in prenatal alcohol-exposed rats. The pregnant dams consumed a large dose of alcohol throughout pregnancy (12.36 g/kg/day) and the offspring were developmentally delayed, showed increased reactivity to the acoustic startle stimulus, but they did not show a disruption of PPI (Potter and Berntson, 1987). A study of early postnatal alcohol administration in rats (5.25 g/kg PD 4-9) also failed to find disrupted PPI (Woolfrey et al., 2005). No studies to date have examined PPI as a function of prenatal alcohol exposure in nonhuman primates.
Primary PPI–regulatory circuits include projections from the ventral temporal lobe (particularly the ventral hippocampus) to the nucleus accumbens and medial prefrontal cortex via the fornix (Swerdlow et al., 2001). Human neuroimaging studies have shown that PPI is modulated by the cortico-striatal-pallido-thalamic circuitry (Hazlett et al., 2001; Kumari et al., 2003). Moreover, the dopamine (DA) system plays a key role, with activation of dopamine D2 receptors, particularly in the ventral striatum, blocking PPI in rats (Peng et al., 1990). Co-activation of D2 receptors with D1 and D3 receptors disrupts PPI in the rat; however D2 receptor activation is also sufficient to disrupt PPI (Weber et al., 2010). DA receptor antagonists have been shown to enhance PPI (Swerdlow, 2006). Given current knowledge of PPI regulatory circuits, examining PPI in a nonhuman primate model of prenatal alcohol exposure could contribute to understanding the neural systems underlying neuropsychiatric disorders associated with FASD.
Only a few prior studies have characterized either acoustic startle or PPI of startle in nonhuman primates, but many parameters of both startle and PPI appear to be similar to both humans and rodents. For example, one study that measured startle without physical restraint of the animals found that startle magnitude habituated with repeated exposures, even though ACTH and cortisol increased above baseline following the startle procedure (Parker et al., 2010). Habituation of startle has also been found in other primate studies (Linn and Javitt, 2000; Parr et al., 2002; Winslow et al., 2002).
Two other primate laboratories have demonstrated PPI of startle magnitude and a decrease in latency with increasing interval of the prepulse stimulus (Linn and Javitt, 2000; Winslow et al., 2002). Using eye-blink EMG in chair- and head-restrained capuchins (Cebus apella), Linn and Javitt (2000) showed that dosing with phencyclidine (PCP) prior to the startle procedure produced no change in startle to a stimulus by itself, but there was a significant decrease in PPI compared to both saline and baseline. These findings were replicated in crab-eating macaques (Macaca fascicularis) (Javitt and Lindsley, 2001). The disruption of PPI by PCP was reversed by administering clozapine (Linn et al., 2003). These findings bolster the use of startle and PPI in nonhuman primates as an animal model of sensory gating phenomena applicable to humans.
There are two studies of the effects of early experience on startle or PPI in nonhuman primates. First, nursery-rearing, considered to be an early life stressor, compared with mother rearing induced higher motor startle responses (recorded from a platform under the animal’s feet) in chair-restrained rhesus monkeys (Parr et al., 2002). However, the nursery-reared animals showed lower cardiac acceleration to the startle stimulus, a finding interpreted as autonomic blunting. Second, maternal exposure to moderate bacterial infection during pregnancy disrupted PPI in infant rhesus monkeys measured without restraint in the same apparatus that we report here (Willette et al., 2011).
The rhesus monkeys in the present study are part of a longitudinal experiment that independently manipulated prenatal alcohol and prenatal stress exposure. Previous findings from this study that are relevant to PPI include prenatal alcohol-induced impairments in neonatal orienting (an early indicator of sensory gating) (Schneider et al., 1997), higher cortisol and ACTH response to a stressor (separation from the mother for weaning) (Schneider et al., 2004), increased tactile sensitivity to repeated stimulation (an animal model of ‘sensory defensiveness’, conceptualized as an aspect of impaired sensory gating) (Schneider et al., 2008) and lower striatal dopamine D2 receptor availability that depended on the gestational timing of alcohol exposure (Schneider et al., 2005). In prenatally-stressed animals we have found reduced neonatal orienting, higher D2 receptor availability in striatum, and blunted HPA responses to stress (Roberts et al., 2004; Schneider et al., 2004).
We tested the hypothesis that moderate prenatal alcohol exposure would disrupt PPI of acoustic startle in adult rhesus monkeys. Given the importance of stress responsivity in startle and PPI of startle, the higher stress responsivity of the prenatally alcohol-exposed animals (Schneider et al., 2004) may also predispose them to disrupted PPI. However, in rhesus monkeys Parr et al. (2002) observed a disconnect between an autonomic measure of startle (cardiac acceleration) and motor startle, with blunted autonomic responses apparently associated with higher magnitude of motor startle. In addition, because we have found blunted HPA-axis responses as a function of prenatal stress, (Schneider et al., 2004) and because early life stress in humans is also associated with blunted HPA responses (Gunnar et al., 2006) we tested the hypothesis that prenatal stress will also disrupt PPI of acoustic startle.
METHODS
Maternal Alcohol and Stress Treatments
As described previously (Schneider et al., 1997) healthy adult female rhesus monkeys in the breeding colony were screened for two weeks for daily voluntary consumption of a 0.6 g/kg of a 6% volume/volume (v/v) alcohol solution sweetened with NutraSweet (300 mg/100 ml) (Equal Sweetener, Merisant US, Inc., Chicago, IL). Before breeding, blood samples were obtained 60 min after consumption of 0.6g/kg alcohol, which produced average blood alcohol concentrations of 20 to 50 mg/dl. This dosage is comparable to an average-size woman consuming two drinks daily. Females that reliably consumed alcohol were randomly assigned to the control group or one of three experimental groups prior to breeding: alcohol only, prenatal stress only, or alcohol + stress. In the alcohol conditions mothers voluntarily consumed the alcohol solution daily at 1600 hours. Blood samples were not collected during pregnancy because a goal of the experiment was to unconfound prenatal alcohol and prenatal stress. The control mothers consumed a sucrose solution that was designed to be approximately equivolemic and equicaloric (8g/100 ml water) to the alcohol solution. The stress treatment was administered five times per week at approximately 1530 hours during mid-to-late gestation (Day 90 through Day 145 post-conception). This gestational period was chosen to avoid the risks of either early fetal loss (miscarriage) or early parturition (prematurity), as well as to cover the period of the brain growth spurt and the time during which previous studies had shown hippocampal damage from exposure to glucocorticoids (see Schneider et al., 1998). The treatment involved transfer of the pregnant female to a transport cage and taking her to a darkened room where three noise bursts (1300 Hz, 115 dB intensity at 1 m) were randomly administered over a 10-min period.
All females were housed individually and identically, undisturbed except for necessary routine animal husbandry. These studies were conducted in accordance with the Institutional Animal Care and Use Committee.
Subjects
The offspring subjects in this study were 37 rhesus monkeys (Macaca mulatta) that resulted from one of the 4 pregnancy conditions described above. There were 13 controls (10 F, 3 M), 10 prenatal alcohol offspring (7 F, 3 M), 6 prenatal-stressed offspring (1 F, 5 M), and 8 alcohol + stress offspring (3 F, 5 M). The study initially had 41 monkeys. At the time of this assessment 39 animals were available for testing. Data from the startle procedure were lost for 2 animals due to computer malfunction.
The rearing conditions and previous testing of these subjects were described in detail elsewhere (Schneider et al., 1997). Briefly, all infant monkeys were housed with their mothers in individual cages during the first 6 months of life except for brief weekly separations for neurobehavioral testing during the first month of life. At 6 months of age, they were separated permanently from their mothers and reared in mixed-sex peer groups consisting of 5-6 monkeys from similar prenatal conditions. At 30 months of age they were removed from their social groups and individually housed for 3 days for assessments, followed by pair-housing with same-sex peers. They were maintained on a diet of Purina Monkey Chow supplemented 3 times weekly with fresh fruit. Housing conditions were 8 hours dark and 16 hours light and temperature (21 ± 0.5 degree C) was controlled. They were approximately 13 years old at the time of this study.
PPI testing apparatus and procedure
Startle testing was conducted in a custom built system designed at our laboratory specifically for use with rhesus monkeys without the need for physical restraint. The PPI testing took place in a sound-attenuated, dimly lit, and ventilated chamber in a cage measuring 46cm x 61cm x 46cm. The size of the cage allows for normal movement, walking and sitting.
The startle apparatus uses a 6-channel AMTI MC3A-6-1000 force transducer that has a single cylindrical strain element that outputs forces in all three dimensions (X, Y and Z). The transducer is center mounted underneath the aluminum splash-guard and cage. Signals from the transducer are amplified by an AMTI DSA-6 amplifier and collected by computer with a 1 ms sample rate. The transducer, splash-guard, and cage are mounted to a heavy metal table.
The startle procedure consisted of the following events. First, a monkey was placed in the startle cage for a 15-minute acclimation period with a 65-dB background masking noise. The initial sequence consisted of a blank trial (one minute with no event) followed by two presentations of the startle stimulus at each of two-decibel levels (white noise at 105 dB and 115 dB). The 4 initial startle trials were to acclimate the animal to the startle stimulus to reduce variability in the startle response, a standard procedure in startle research (Alsene et al., 2010). The remainder of the experiment consisted of 3 blocks of 10 trials each. The 40 ms white noise startle probe was presented at one of two intensities (105 and 115 dB), preceded at 45, 120 or 500 ms by a 20 ms white noise prepulse stimulus at 80 dB, or by no pre-pulse stimulus (startle stimulus only). In addition, each block included a presentation of the pre-pulse stimulus by itself, and a “blank” trial during which no event occurred. Hence, each block consisted of 8 startle stimulus presentations, six with pre-pulse and 2 without and 2 time periods with no startle stimulus (the “blank” and the prepulse-only).
HPA measures
Approximately 1 week prior to startle testing (baseline) and immediately following the startle procedure (post-startle) a blood draw was taken between 10:50 hr and 11:00am hr and assayed for both cortisol and adrenocorticotrophic hormone (ACTH). Samples (1 ml) were collected by femoral venipuncture into ethylenediamine tetra-acetic acid (EDTA) treated vacutainers within 2 to 3 min of room entry by experimenters. Following centrifuging, plasma was harvested and stored at −70 degrees C until assay.
Cortisol radioimmunoassay (RIA). Rhesus serum cortisol levels were measured using an antibody coated I125 RIA kit, Cat. CA-1549 from DiaSorin, Stillwater, MN, in the Assay Services Unit of the National Primate Research Center (NPRC), University of Wisconsin-Madison. The kit protocol was modified to extend the range of the standard curve 2 points higher by pipetting 20 mL and 25 mL of the highest standard vial. The interassay coefficient of variation for eight assays (32 quality control tubes) was 9.43% and the mean intra-assay coefficient of variation was 3.44%. RIA tubes were counted in a TmAnalytic gamma counter model 1290. RIA data reduction was computed by weighted least squares regression analysis (Rodbard and Lewald, 1970).
ACTH
Rhesus serum ACTH levels were measured using an in-house WRPRC assay. Standards were purchased from Bachem Corporation, Torrance, CA; primary and secondary antibodies were purchased from IgG Corporation, Nashville, TN; and the I-125 trace and precipitating solution was purchased from Diagnostic Products Corporation, Los Angeles, CA. The interassay coefficient of variation for six assays (36 quality control tubes) was 21.17% and the mean intra-assay coefficient of variation was 6.77%.
Startle data processing
The 2 seconds prior to and following each trial event were analyzed using Matlab (Version 7.0.5.338, The Mathworks, 2007). First the data series was centered by removing the mean of the entire interval and was rectified by taking absolute values. Graphical tracings of the centered data of each trial were visually examined for motion artifacts. A total of 44 trials with movement were removed from analysis, or 3.4 percent of total trials. Seventeen monkeys (45%) had at least one trial with motion. Only 3 monkeys had 5 or more motion trials removed from analysis, and one monkey had 11 trials removed. There was no association between presence of trials with motion and prenatal exposure condition.
The baseline response for each trial was defined as the mean of the 3 highest values on each dimension in a 300 ms window ending 100 ms prior to the trial event. The startle onset was defined by the initial time point at which the response value exceeded the baseline by 2 standard deviations for 15 ms. The latency to startle was then the median post-event time point across the 3 dimensions recorded by the apparatus.
The startle magnitude in each dimension was the mean of the 3 highest values in a 300ms window following the startle onset. The final startle magnitude was then calculated as the median of the 3 dimensions post-onset minus the median of the 3 dimensions during baseline. The median magnitudes and latencies were then calculated across blocks and the two-decibel values for the available trials, yielding 4 values each of startle magnitude and latency (startle-only, 45 ms prepulse, 120 ms prepulse, and 500 ms prepulse).
Statistical analysis of startle magnitude scores
The percentage PPI (ratio of the pre-pulse startle magnitudes to the startle only magnitudes) yielded data that severely violated homogeneity of variance (ratio of largest to smallest standard deviation > 10). Therefore the startle magnitudes were analyzed with startle-only included as a level of the pre-pulse factor in a 2 (Prenatal Alcohol exposure or not) x 2 (Prenatal stress exposure or not) x 4 (Pre-pulse interval: none, 45, 120, and 500 ms) analysis of variance with repeated measures on the Prepulse variable. We also tested the orthogonal trend components on the 3 pre-pulse trial types (45, 120 and 500 ms). Hunyh-Feldt adjusted p-levels are reported to account for any violations of sphericity in the repeated measures factor. The latency data were analyzed in the same way. The magnitudes and latencies of the 4 initial startle-only trials that preceded the main PPI procedure were analyzed in a 2 (Prenatal Alcohol exposure or not) x 2 (Prenatal stress exposure or not) x 4 (Trials) ANOVA with repeated measures on trials to test for possible differences in habituation as a function of treatment.
ACTH and cortisol
The ACTH values were log(10) transformed to stabilize the variances and reduce skew. Both ACTH and cortisol were analyzed in a 2 (Prenatal Alcohol exposure or not) x 2 (Prenatal stress exposure or not) x 2 (time: baseline and post-startle) analysis of variance with repeated measures on the Time variable. For all analyses the main effects of sex of animal were examined and are reported where significant.
RESULTS
Initial Startle-Only Trials
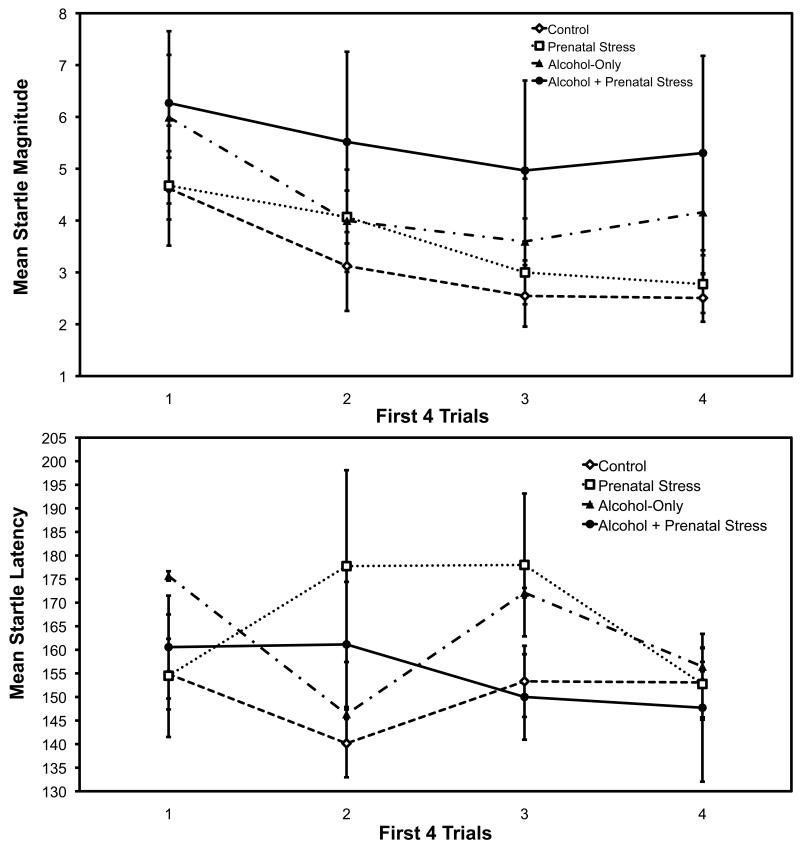
The first 4 trials showed significant habituation of startle magnitude, as is normally found: trials main effect, F (3, 87) = 5.18, p < .003. There was no change in startle latency over trials, F (3, 87) = 1.62, p = .190. These results are presented in Figure 1 and establish that startle can be reliably measured without restraint in the rhesus monkey. There were no treatment effects for either startle magnitude or latency on the first 4 trials, and no main effects of sex of animal (all ps > .10).
Fig. 1.
Mean startle magnitudes (top panel) and latencies (bottom panel) for the first four trials with a separate curve for each prenatal treatment group. Bars are +/− 1 se of the mean.
PPI results: Startle magnitude
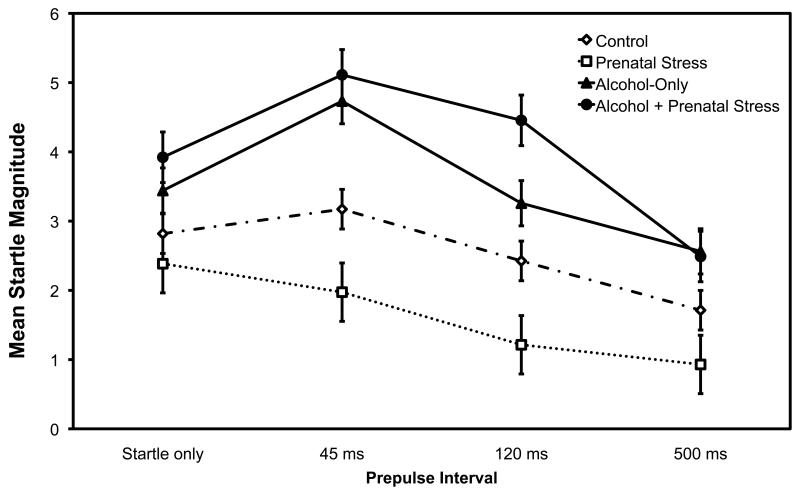
PPI was disrupted by prenatal alcohol exposure as shown by significant effects of alcohol exposure, F (1, 33) = 4.76, p = 0.036, and an interaction of Prenatal Alcohol x Prepulse interval, F (3, 99) = 3.10, p = .03 (see Figure 2 and, for comparison with other studies, Table 1 presents the percentage PPI versus baseline). The effect of prepulse interval was also significant, as expected (no prepulse, 45, 120, and 500 ms), F (3, 99) = 18.50, p < 0.001. Over all groups, startle magnitude decreased at the longer prepulse intervals (mean startle magnitude with no prepulse, 45, 120, and 500 ms = 3.14, 3.75, 2.81 and 1.96, respectively). The prenatal alcohol conditions (prenatal alcohol-only and prenatal alcohol + prenatal stress groups) had higher startle magnitude across all trials (M = 3.75, se = .53) compared with monkeys not exposed to alcohol during gestation (Control and Prenatal-stress groups, M = 2.08, se = .51). However, the pattern of startle magnitudes across prepulse intervals was altered by prenatal alcohol exposure; the Alcohol and Alcohol + Stress conditions showed startle facilitation at the 45 ms prepulse interval, whereas this did not occur for the Control and Stress-only conditions. The Prepulse interval quadratic trend x Alcohol interaction was significant, F (1, 33) = 6.43, p = .016. There were no significant effects involving Prenatal Stress (Fs < 1).
Fig. 2.
Mean startle magnitudes as a function of prepulse intervals (none, 45, 120 and 500ms) with a separate curve for each prenatal treatment group. Bars are +/− 1 se of the mean.
Table 1.
Mean percentage pre-pulse inhibition vs baseline
| Pre-pulse Interval |
Control | Prenatal Stress |
Alcohol- Only |
Prenatal Stress + Alcohol |
|---|---|---|---|---|
| 45 ms | −11 (13) | −34 (42) | −48 (24) | −99 (53) |
| 120 ms | 19 (10) | 54 (13) | 10 (11) | −20 (21) |
| 500 ms | 40 (6) | 61 (2) | 22 (9) | 22 (11) |
Note: Percentage pre-pulse inhibition calculated as 100 * {1 – (prepulse startle magnitude / no pre-pulse startle magnitude)}. Standard errors are given in parentheses.
PPI results: Startle Latency
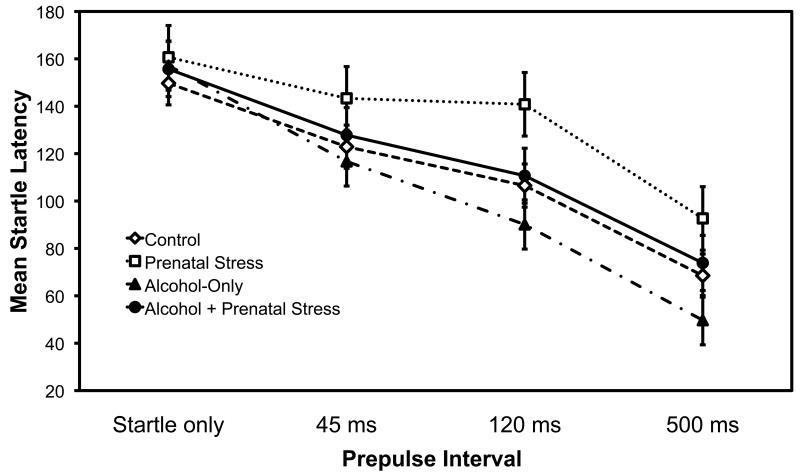
The latencies showed a significant effect of prepulse interval, F (3,99) = 39.51, p < .001, with a shorter latencies at longer prepulse intervals (see Figure 3). The prepulse latency means were 82, 72, and 46% of the mean latency to the startle pulse alone, a considerable decline. There was a tendency for a longer latency to startle for the prenatal stress conditions (prenatal stress only and prenatal alcohol + stress) (M = 125.70, SE = 7.59) compared to no stress conditions (control and prenatal alcohol-only) (M = 107.67, SE = 5.92), but it did not reach significance, F (1, 33) = 3.44, p = 0.07. There was no significant main effect for alcohol exposure, F (1, 33) = 1.77, p = 0.19, and no significant interactions, all p’s > .15).
Fig. 3.
Mean startle latencies as a function of prepulse intervals (none, 45, 120 and 500 ms) with a separate curve for each prenatal treatment group. Bars are +/− 1 se of the mean.
Relationships among startle parameters
The first 4 startle-only trials correlated .80 (p < .01) with the startle-only trials embedded in the PPI procedures, indicating high reliability of our procedure. Similarly, startle magnitudes for the three PPI intervals (45, 120 and 500 ms) correlated highly with startle-only magnitude, r’s = .81, .91, .90, respectively. Latency of the first 4 startle-only trials correlated significantly with latency for the embedded startle-only trials, r = .45, p < .01.
HPA responses
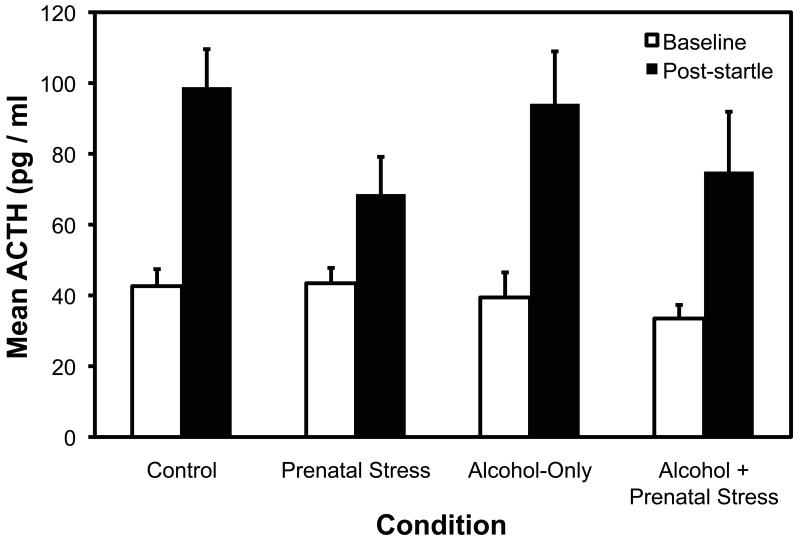
There was a significant increase from baseline to post-startle for both log-ACTH, F (1, 34) = 128.49, p < .001, and cortisol, F (1, 35) = 228.10, p < .001. For ACTH, there was also a significant Prenatal Stress x Time interaction, F (1, 34) = 4.38, p = .044, indicating that the animals in the Prenatal Stress conditions (Prenatal Stress only, Prenatal Alcohol + Stress) showed a blunted ACTH response compared to the no stress conditions (Control, Prenatal Alcohol only) (see Figure 4). The main effect of sex showed that female animals had higher ACTH overall (Ms = 71.9, 50.2, SE = 5.01, 5.70, for females and males respectively), F (1, 34) = 8.04, p < .01). For cortisol, there was a trend toward a lower response in the Prenatal Stress conditions compared to the No Prenatal Stress conditions (Ms = 38.73, 35.73, SE = 1.56, 1.88, respectively), but it did not reach significance, F (1, 35) = 2.98, p = .093. The main effect of sex was nonsignificant for cortisol (p > .10).
Fig.4.
Mean plasma ACTH (pg/ml) at baseline (1 week before startle testing) and after startle testing (immediately following the 45 min startle session) as a function of prenatal treatment group. Bars are +/− 1 se of the mean. ACTH, adrenocorticotrophic hormone.
There were significant correlations between baseline and post-test startle for cortisol and ACTH, as expected (see Table 2). Interestingly, as seen in the lower part of Table 2, startle magnitude and startle latency were uncorrelated with ACTH and cortisol measures.
Table 2.
Correlations Among HPA-Axis Measures and Startle Variables
| Baseline Cortisol |
Log Baseline ACTH |
Post PPI Cortisol |
Post PPI Log ACTH |
|
|---|---|---|---|---|
| Log baseline ACTH | 0.44** | |||
| Post PPI Cortisol | 0.35* | 0.30 | ||
| Post PPI Log ACTH | 0.19 | 0.58*** | 0.39* | |
| Startle Magnitude | −0.09 | −0.17 | 0.11 | 0.17 |
| Startle Latency | 0.20 | 0.25 | 0.24 | 0.19 |
p<.05,
p <.01,
p<.001
DISCUSSION
This is the first study to our knowledge of PPI in nonhuman primates exposed prenatally to moderate level alcohol and/or stress. There were two important findings: 1) moderate level prenatal alcohol exposure increased startle magnitude and disrupted PPI, and 2) undergoing the startle procedure elevated HPA axis responses (plasma cortisol and ACTH) compared with baseline, but the HPA responses were unrelated to startle parameters.
The first finding, that moderate level prenatal alcohol exposure increased startle magnitude and disrupted PPI, suggests that one important effect of prenatal alcohol may be disruption of fundamental sensory gating mechanisms in the brain. Our finding of increased magnitude of startle in the prenatal alcohol conditions concurs in part with rodent findings of increased startle as a function of prenatal alcohol-exposure. There was, however, no apparent disruption of PPI in the prenatal alcohol-exposed rat offspring compared with controls (Potter and Berntson, 1987), whereas we found disrupted PPI. Other animal studies have shown that adverse prenatal conditions can reduce PPI. Restricted uteroplacental blood flow during midgestation disrupted PPI in guinea pigs (Rehn et al., 2004) and prenatal exposure to infectious agents produced lasting deficits in PPI in rats (Fortier et al., 2007).
The facilitation of startle magnitude at the 45 ms prepulse interval in prenatal alcohol-exposed monkeys in the present study may reflect altered sensorimotor gating. Because the two sounds (prepulse, startle stimulus) occur close together in the 45 ms condition, the prepulse may have activated a summation process in prenatal alcohol-exposed monkeys but not in non-prenatally alcohol-exposed monkeys (Wynn et al., 2000). It appears that inhibitory neural processes were activated in the 500 ms prepulse interval whereas summation was activated in the 45 ms prepulse interval for the alcohol-exposed animals. In other words, a substantial gap between the prepulse and the startle onset was necessary to activate inhibitory processes in the prenatal alcohol-exposed conditions.
Although PPI has not been evaluated in individuals with FASD, the impaired inhibitory control reported in such individuals (including inattention, impulsivity, and hyperactivity, as well as the high incidence of alcohol use problems) has often been thought to be related to disruptions of inhibitory mechanisms in the brain. Because PPI deficiency is considered to reflect difficulty filtering out rapidly presented information, resulting in sensory flooding or “sensory overload”, our findings are consistent with that interpretation. It is also interesting that children exposed to alcohol during pregnancy, compared with typically-developing peers, are three times more likely to be classified as having clinically significant sensory processing problems (Jirikowic, et al., 2008). Sensory processing disorder is characterized by atypical (usually exaggerated) responses to non-noxious sensory stimulation (Ayres, 1972). Further research could clarify whether sensory processing problems in children with FASD could result from a sensory summation process as described above. Thus, our findings in monkeys provide experimental evidence relevant to the literature in humans suggesting that prenatal alcohol exposure may induce long-term impairments in inhibitory neural circuits.
While the brain mechanisms mediating PPI are highly complex, current research indicates that PPI is regulated by a network of midbrain and brainstem mechanisms involving the ventral hippocampus, amygdala, and projections to ventral striatum (nucleus accumbens) and medial prefrontal cortex (PFC) (Swerdlow et al., 2001). Interestingly, these neural regions that regulate PPI are areas that have been shown to be particularly vulnerable to prenatal alcohol exposure (see Lebel et al., 2011 for a review).
Our second finding is that overall, the monkeys showed a significant increase from baseline to post-startle condition for ACTH and cortisol but that it was unrelated to startle magnitude or PPI. This finding is similar to that reported by Parr and colleagues (2002) who found a disconnect between autonomic (cardiac) and motor startle. In the present study, the prenatal stress conditions (prenatal stress-only, prenatal stress + alcohol) yielded animals with blunted post-startle ACTH compared with the no prenatal stress conditions (control, prenatal alcohol-only). Other studies have shown that exposure to adversity during sensitive periods in development can reprogram the fetal HPA axis (Gunnar and Vazquez, 2006).
In summary, our finding of disrupted PPI in adult monkeys from prenatal alcohol-exposed pregnancies, even at a moderate dose, underscores the potential role of prenatal alcohol exposure on the development of inhibitory circuits. Our findings not only raise questions about whether reduced sensorimotor gating could contribute to mental health problems in individuals with FASD but also suggest that neural circuits vulnerable to prenatal alcohol exposure, such as those involving the hippocampus, amygdala, striatum, and PFC, which underlie PPI, could be critical for some of the most deleterious neuropsychiatric disorders related to prenatal alcohol exposure. For example, Grillon and colleagues (2000) argued that reduced PPI is a risk factor for alcoholism and Cloninger (1987) reported that lack of inhibitory control is associated with certain types of alcoholism. If prenatal alcohol-exposed individuals with sensorimotor gating dysregulation can be identified early in life, targeted and effective intervention may be possible.
Acknowledgement
This study was supported by AA10079 and AA12277 from the National Institute of Alcoholism and Alcohol Abuse and Wallace Research Foundation grant to M.L. Schneider. We thank Vishali Bakshi for consultation on the PPI paradigm. We also thank UW-Madison undergraduate engineering students Mike Keller and Mike Mussallem for their contribution with the construction of the PPI apparatus.
REFERENCES
- Alsene KM, Fallace K, Bakshi VP. Ventral striatal noradrenergic mechanisms contribute to sensorimotor gating deficits induced by amphetamine. Neuropsychopharmacology. 2010;35:2346–2356. doi: 10.1038/npp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres AJ. Sensory integration and learning disorders. Western Psychological Services; Los Angeles: 1972. [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10:73–84. doi: 10.1017/S1461145705006358. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45:362–364. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Fortier M-E, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Graham FK, Putnam LE, Leavitt LA. Lead-stimulation effects of human cardiac orienting and blink reflexes. J Exp Psychol Hum Percept Perform. 1975;104:175–182. [PubMed] [Google Scholar]
- Grillon C, Sinha R, Ameli R, O’Malley SS. Effects of alcohol on baseline startle and prepulse inhibition in young men at risk for alcoholism and/or anxiety disorders. J Stud Alcohol. 2000;61:46–54. doi: 10.15288/jsa.2000.61.46. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, Merkangas KR, Bruder GE, Tenke CE, Nomura Y, Leite P, Weissman MM. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry. 2005;57:953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Developmental neuroscience. 2nd ed Vol. 2. Wiley; New York: 2006. pp. 533–577. [Google Scholar]
- Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MB, Wei T-C, Byne W, Haznedar MM. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry. 2001;50:281–291. doi: 10.1016/s0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a Fetal Alcohol Spectrum Disorder. Can J Clin Pharmacol. 2008;15:411–419. [PubMed] [Google Scholar]
- Javitt DC, Lindsley RW. Effects of phencyclidine on prepulse inhibition of acoustic startle response in the macaque. Psychopharmacology (Berl) 2001;156:165–168. doi: 10.1007/s002130100758. [DOI] [PubMed] [Google Scholar]
- Jirikowic T, Kartin D, Olson HC. Children with fetal alcohol spectrum disorders: A descriptive profile of adaptive function. Can J Occup Ther. 2008;75:238–248. doi: 10.1177/000841740807500411. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, Ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SCR, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsycol Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn GS, Javitt DC. Phencyclidine (PCP)-induced deficits of prepulse inhibition in monkeys. Neuroreport. 2000;12:117–120. doi: 10.1097/00001756-200101220-00031. [DOI] [PubMed] [Google Scholar]
- Linn GS, Negi SS, Gerum SV, Javitt DC. Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology. 2003;169:234–239. doi: 10.1007/s00213-003-1533-8. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Matlab, computer program. Version 7.0.5.338 The Mathworks; 2007. [Google Scholar]
- Mattson S, Crocker N, Nguyen T. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res. 2000;24:226–231. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis L, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin Exp Res. 2000;24:1084–1092. [PubMed] [Google Scholar]
- Parker KJ, Hyde SA, Buckmaster CL, Tanaka SM, Brewster KK, Schatzberg AF, Lyons DM, Woodward SH. Somatic and neuroendocrine responses to standard and biologically salient acoustic startle stimuli in monkeys. Psychoneuroendocrinology. 2010;36:547–556. doi: 10.1016/j.psyneuen.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2002;116:378–386. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats. Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- Potter BM, Berntson GG. Prenatal alcohol exposure: effects on acoustic startle and prepulse inhibition. Neurotoxicol Teratol. 1987;9:17–21. doi: 10.1016/0892-0362(87)90064-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Erickson JD, Reef SE, Ross DS. Teratology: From science to birth defects prevention. Birth Defects Res A Clin Mol Teratol. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- Rehn AE, Van Den Buuse M, Copolov D, Briscoe T, Lambert G, Rees S. An animal model of chronic placental insufficiency: Relevance to neurodevelopmental disorders including schizophrenia. Neuroscience. 2004;129:381–391. doi: 10.1016/j.neuroscience.2004.07.047. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, Nickles RJ, Schueller MJ, Shelton SE, Schneider ML. Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicol Teratol. 2004;26:169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Lewald JE. Computer analysis of radioligand assay and radioimmunoasssay data. Acta Endocrinol Suppl. 1970;147:79–103. doi: 10.1530/acta.0.065s079. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res. 2005;29:1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Gajewski LL, Larson JA, Roberts AD, Converse AK, DeJesus OT. Sensory processing disorder in a primate model: evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008;79:100–113. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child Dev. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induces attention and neuromotor impairments in primate infants. Child Dev. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton Scale. Child Dev. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sutherland Owens AN, Miguel EC, Swerdlow NR. Sensory gaiting scales and premonitory urges in Tourette Syndrome. ScientificWorldJournal. 2011;11:736–741. doi: 10.1100/tsw.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci. 1999;877:202–216. doi: 10.1111/j.1749-6632.1999.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters NS, Geyer MA, Swenson R. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington”s disease. J Neurol Neurosurg Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Platten A, Kim YK, Gaudet I, Shoemaker J, Pitcher L, Auerbach P. Sensitivity to the dopaminergic regulation of prepulse inhibition in rats: evidence for genetic, but not environmental determinants. Pharmacol Biochem Behav. 2001;70:219–226. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Braff DL. Prepulse-elicited motor reactions do not differ between schizophrenia patients and control subjects. Behav Neurosci. 2006;120:224–227. doi: 10.1037/0735-7044.120.1.224. [DOI] [PubMed] [Google Scholar]
- Weber M, Chang W-L, Breier MR, Yang A, Millan MJ, Swerdlow NR. The effects of the dopamine D2 agonist sumanirole on prepulse inhibition in rats. Eur Neuropsychopharmacol. 2010;20:421–425. doi: 10.1016/j.euroneuro.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res. 2011;219:108–115. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured rhesus monkeys. Biol Psychiatry. 2002;51:859–866. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Musisca NJ, Hunt PS, Burk JA. Early postnatal ethanol administration does not affect prepulse inhibition in rats. Physiol Behav. 2005;84:747–752. doi: 10.1016/j.physbeh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Dawson ME, Schell AM. Discrete and continuous prepulses have differential effects on startle prepulse inhibition and skin conductance orienting. Psychophysiology. 2000;37:224–230. [PubMed] [Google Scholar]