Abstract
Objective
To compare commonly-available tests for antibodies to citrullinated protein antigens (ACPAs) for diagnostic accuracy and assay agreement in established rheumatoid arthritis (RA) and subjects at elevated risk for RA.
Methods
ELISA testing for anti-cyclic citrullinated peptide (anti-CCP) antibodies was performed using CCP2 (Axis-Shield) and CCP3.1 (IgA/IgG INOVA) in the following subjects: 1) probands with established RA (N=340) from the Studies of the Etiology of RA (SERA), 2) first degree relatives (FDRs) without RA (family members of SERA RA probands; N=681), 3) Department of Defense Serum Repository (DoDSR) RA cases with pre-diagnosis samples (N=83; 47/83 also had post-diagnosis samples), and 4) blood-donor and DoDSR controls (N=283).
Results
In established RA, CCP2 was more specific (99.2% vs. 93.1%, p<0.01), but less sensitive (58.7% vs. 67.4%, p=0.01) than CCP3.1, with specificity of CCP3.1 increasing to 97.2% if levels ≥3 times the standard cut-off level were considered. In all subjects, at standard cut-off levels, CCP3.1 positivity was more prevalent. In DoDSR cases, CCP2 was more specific than CCP3.1 for a future diagnosis of RA, and higher CCP levels trended towards greater specificity for disease onset within 2 years. At standard cut-off levels, assay agreement was good in established RA (kappa=0.76), but poor in FDRs without inflammatory arthritis (kappa=0.25).
Conclusion
Anti-CCP assays differ to an extent that may be meaningful in diagnosing RA in patients with inflammatory arthritis, and in evaluating the natural history of RA development in subjects at-risk for future RA. Mechanisms underlying these differences in test performance need further investigation.
Keywords: Rheumatoid arthritis, autoantibodies, CCP, ACPA, preclinical
Seropositive rheumatoid arthritis (RA) is characterized by abnormal elevations of circulating RA-related autoantibodies including antibodies to citrullinated protein antigens (ACPAs), which are highly specific for RA in the setting of inflammatory arthritis1,2. Multiple studies have also demonstrated that these ACPA elevations are present years prior to arthritis onset in RA, and in case-control studies, ACPAs are highly specific for the future development of arthritis that is classifiable as RA3–5. Therefore, there is hope that ACPA testing can be used to identify currently asymptomatic individuals with elevated risk for the future development of clinically-apparent RA who can be studied to understand the natural history of RA as well as be utilized to develop predictive and preventive approaches for disease6.
However, there are several limitations to applying ACPA testing to identify individuals who are at elevated risk for clinically-apparent RA which include: 1) there are multiple commercial ACPA assays that differ in antigen and autoantibody isotype reactivities, 2) there are limited data comparing the accuracy of each of these assays in established RA, and 3) there are limited data comparing the ability of individual ACPA assays to predict future clinically-apparent RA in currently asymptomatic individuals1. In addition, ACPA positivity is incorporated in the 2010 American College of Rheumatology (ACR)/ European League Against Rheumatism (EULAR) classification criteria for RA, yet there is not an established gold standard CCP assay - an issue that could have a clinically meaningful impact on classification and diagnosis of inflammatory arthritis if ACPA assays differ significantly in performance. As such, to better understand the significance of elevated ACPA levels in individuals with and without a current classification of RA, we evaluated commonly utilized commercially-available ACPA assays that test for autoantibodies to cyclic citrullinated peptides (anti-CCP) in subjects with established RA, subjects at elevated risk for development of classifiable RA, and controls.
PATIENTS AND METHODS
Study subjects
Studies of the Etiology of Rheumatoid Arthritis (SERA)
The SERA project is a multi-centered prospective cohort established to investigate the natural history of RA development in subjects at elevated risk for RA because of their family history of disease7,8. Briefly, the SERA project identifies probands with established RA (SERA RA Probands), and their first-degree relatives without inflammatory arthritis (SERA FDRs). SERA RA Probands undergo a single study visit, and SERA FDRs are followed prospectively every 1–2 years after a baseline visit. SERA RA Probands and SERA FDRs are recruited from multiple SERA study sites including Denver, CO; Los Angeles, CA; Omaha, NE (as the coordinating site of the Rheumatoid Arthritis Investigational Network (RAIN)); Seattle, WA; Chicago, IL; and Manhasset, NY.
SERA RA Probands
As of July 2012, SERA includes 1223 RA probands who fulfill 1987 ACR RA criteria or who have RA based on their treating rheumatologist’s diagnosis, confirmed through chart review by SERA study rheumatologists according to standardized procedures. All SERA RA Probands undergo a single research visit during which they complete a standardized questionnaire and blood collection. For this study, we randomly selected 340 SERA RA Probands with sufficient serum for CCP testing with multiple assays.
SERA FDRs
At each study visit, SERA FDRs complete a standardized questionnaire and interview including joint symptom assessment, 68-joint examination performed by a trained study physician or nurse, and serum testing. Of the 1755 FDRs followed in SERA, CCP testing with multiple assays was performed on 681 subjects including all SERA FDRs seen for a routine study visits after February 2010 (N=463) and 218 randomly selected SERA FDRs seen before February 2010 that had a sufficient serum available for testing. These 681 SERA FDRs were from 513 unique families (mean FDRs per family=1.7, range 1–7), and all FDRs were without clinical evidence of inflammatory arthritis per a 68-joint count examination by a trained examiner at the time of serum testing.
SERA blood donor controls
A Colorado-based blood donation center was utilized to obtain 200 blood donor serum samples to serve as controls for SERA RA Probands. Anonymous samples were randomly selected with no demographic information available for these subjects; therefore, they are a random sampling of the population, but are not specifically screened to exclude RA.
Department of Defense Serum Repository (DoDSR)
Full details of this cohort are published elsewhere9,10, but briefly the DoDSR is a repository of samples collected from members of the United States Military at entry and intervals during their military service. This repository allows for evaluation of serum samples from prior to and after a clinical diagnosis of RA.
DoDSR RA cases
83 military subjects with established RA were identified through the Walter Reed Army Medical Center Rheumatology Clinic, and confirmation of their diagnosis (and date of diagnosis) by 1987 ACR RA criteria or by diagnosis from a treating rheumatologist was established through chart review by military rheumatologists through standardized procedures. All 83 subjects had ≥1 serum sample available prior to the diagnosis of RA, and 47/83 (57%) had a post-diagnosis sample available.
DoDSR controls
83 military subjects without RA were also identified in the DoDSR to serve as controls. These DoDSR controls were matched to cases for age at RA diagnosis, sex, race, number of samples available, duration of sample storage and enlistment region, and they were without RA by chart review at the time of inclusion.
Biomarker and genetic analyses
Serum samples from all subjects were tested for anti-CCP autoantibodies in the University of Colorado Division of Rheumatology Clinical and Research Laboratory that is a College of American Pathologists/Clinical Laboratory Improvement Amendments (CAP/CLIA) certified facility using ELISA-based tests that included CCP2 (Diastat, Axis-Shield Diagnostics, Ltd.) and CCP3.1 (IgA/IgG INOVA Diagnostics, Inc.). A subset of SERA RA Probands and SERA FDRs had additional testing with the CCP3 ELISA assay (IgG INOVA Diagnostics, Inc). DoDSR subjects were not tested with the CCP3 assay due to limited sera. All kits were purchased by the investigators, and standard kit cut-off levels were used for CCP positivity (CCP2 >5 units; CCP3 and CCP3.1 ≥20 units). Serum samples were also tested for rheumatoid factor (RF) by nephelometry (Dade-Behring), and RF positivity was established based on levels that are positive in <5% of 491 randomly selected blood donors (these blood donor samples are separate from the SERA blood donor controls). DNA from SERA RA Probands and SERA FDRs was analyzed for the presence of alleles containing the shared epitope (SE). The methodologies for SE testing are described elsewhere7, but briefly subtypes considered SE positive included: DR4 alleles (DRB1*0401, 0404, 0405, 0408, 0409, 0410, 0413, 0416, 0419 and 0421) and DR1 alleles (DR1*0101, 0102, 0104, 0105, 0107, 0108 and 0111).
Statistical analysis
Sensitivity and specificity were performed using 2×2 table analyses in which SERA blood donor controls served as controls for SERA RA Probands, and the DoDSR case-matched controls served as controls for DoDSR RA cases. Differences in accuracy between assays, prevalence of positivity, and the effect of sex, race, SE status, and history of ever smoking were calculated using chi-squared or Fisher’s exact testing as appropriate. Median CCP levels were compared using the Wilcoxon rank sum test. Demographics were compared using non-parametric, one-way ANOVA, and chi-squared testing as appropriate. Assay agreement was compared using Cohen’s kappa statistic.
Ethical considerations
The study protocol and analyses were approved by the Institutional Review Boards at participating institutions.
RESULTS
Subject demographics are presented in Table 1. Overall, SERA RA Probands were older and had a higher proportion of women compared to SERA FDRs and DoDSR subjects (p<0.01). Also, a higher proportion of SERA RA Probands were SE positive (p<0.01) and ever smokers (p=0.04) compared to SERA FDRs.
Table 1.
Subject demographics by cohort
| SERA RA Pro bands (N=340) |
SERA FDRs (N=681) |
DoDSR RA Cases (N=83)** |
P-value* | |
|---|---|---|---|---|
| Age, (mean ± SD) | 57.2 ± 13.7 | 49.7 ± 16.5 | 40.0 ± 10.0 | <0.01 |
| % Female | 84.1 | 73.6 | 41.0 | <0.01 |
| % Non-Hispanic White | 76.0 | 78.4 | 68.7 | 0.10 |
| % ≥1 allele containing the ‘shared epitope’ | 64.3 | 53.2 | - | <0.01 |
| % Ever smokers | 45.9 | 38.8 | - | 0.04 |
Comparison using non-parametric testing for age, ANOVA testing for % female and non-Hispanic white, and chi-squared testing for % ≥1 allele containing the ‘shared epitope’ and ever smokers.
DoDSR controls (N=83) were matched to DoDSR RA cases and demographics included: mean age 40.0 (± 9.9), 41.0% female, and 68.7% Non-Hispanic white
CCP results in established RA
In subjects with established RA (SERA RA Probands [N=340] and DoDSR cases post-diagnosis [N=47]), at standard cut-off levels, CCP2 was more specific (99.2% vs. 93.1%, p<0.01) but less sensitive (58.7% vs. 67.4%, p=0.01) than CCP3.1 (Table 2). Combined testing for positivity of CCP2 and/or CCP3.1 resulted in a sensitivity and specificity of 68.7% and 92.7%, respectively. Furthermore, as cut-off levels for positivity were increased, specificity increased for CCP3.1 (standard cut-off 93.1%, ≥3x standard cut-off 97.2%, p=0.06); however, specificity for CCP2 remained largely unchanged with increasing cut-off levels (standard cut-off 99.2%, >3x standard cut-off 99.6%, p=1.0). When CCP2 cut-off levels were reduced by half (standard cut-off >5 units reduced to >2.5 units), specificity was 97.5% and sensitivity was 62.4%.
Table 2.
Diagnostic accuracy of anti-CCP assays in subjects with established rheumatoid arthritis
| Standard kit cut-off | Cut-off ≥2x standard | Cut-off ≥3x standard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CCP 2 | CCP3.1 | p-value§ | CCP2 | CCP3.1 | p-value§ | CCP2 | CCP3.1 | p-value§ | |
| Established RA* | |||||||||
| (N=387) | |||||||||
| Sensitivity | 58.7‡ | 67.4 | 0.01 | 55.6 | 62.8 | 0.05 | 53.7 | 60.5 | 0.07 |
| Specificity | 99.2‡ | 93.1 | <0.01 | 99.6 | 96.8 | 0.04 | 99.6 | 97.2 | 0.07 |
| Stratification by Cohort | |||||||||
| SERA RA Probands | |||||||||
| (N=340) | |||||||||
| Sensitivity | 57.4 | 66.2 | 0.02 | 54.1 | 61.8 | 0.05 | 52.4 | 59.1 | 0.09 |
| Specificity | 99.0 | 94.0 | 0.01 | 99.5 | 96.5 | 0.07 | 99.5 | 97.0 | 0.12 |
| DoDSR (N=47) | |||||||||
| Post-diagnosis** | |||||||||
| Sensitivity | 68.1 | 76.6 | 0.49 | 66.0 | 70.2 | 0.82 | 63.8 | 70.2 | 0.66 |
| Specificity | 100 | 89.4 | 0.06 | 100 | 97.9 | 1.00 | 100 | 97.9 | 1.00 |
| DoDSR (N=83) | |||||||||
| Pre-diagnosis*** | |||||||||
| Sensitivity | 61.4 | 63.9 | 0.87 | 57.8 | 59.0 | 0.87 | 51.8 | 56.6 | 0.64 |
| Specificity | 100 | 91.6 | 0.01 | 100 | 95.2 | 0.12 | 100 | 98.8 | 1.00 |
Comparing proportions of true positives (sensitivity) and true negatives (specificity) with chi-squared or Fischer’s exact testing when appropriate
Calculated for SERA RA Probands (N=340) and DoDSR RA cases post-diagnosis (N=47) compared to SERA blood donor controls (N=200) and DoDSR matched controls (N=47)
Using CCP2 cut-off level >2.5 units (1/2 the standard cut-off level), sensitivity 62.4%, specificity 97.5%
Calculated for DoDSR RA cases compared to DoDSR matched controls. 47/83 DoDSR cases had a post-diagnosis sample available; median 2.2 years after RA diagnosis
243 pre-diagnosis samples were available for 83 DoDSR RA cases. Calculated using 1 sample per case, and if ≥1 sample available, the positive and/or sample closest to the time of diagnosis was used for analysis; median 1.4 years prior to RA diagnosis. Cases were compared to matched controls
In CCP2 positive subjects, the median CCP2 level was non-significantly higher in established RA (N=227; 84.2 units) as compared to CCP2 positive SERA FDRs (N=15; 57.0 units; p=0.59) and CCP2 positive SERA blood donor controls (N=2; 28.5 units, p=0.48). In CCP3.1 positive subjects, the median CCP3.1 level was significantly higher in established RA (N=261, 304.0 units) as compared to SERA FDRs (N=65; 35.0 units, p<0.01) and SERA blood donor controls (N=12; 60.5 units, p<0.01).
In SERA RA Probands and DoDSR RA cases post-diagnosis, at standard cut-off levels, the prevalence of positivity was higher for CCP3.1 than CCP2 (Table 3), and the higher prevalence of positivity of CCP3.1 compared to CCP2 remained when subjects were stratified by sex, race, presence of the SE and history of ever smoking (data not shown). Positivity for an individual CCP assay was not affected by sex or race in established RA; however, in SERA RA Probands, CCP2 and CCP3.1 positivity rates were significantly higher in subjects with ≥1 SE allele compared to subjects without the SE (CCP2 67.1% vs. 38.9%, p<0.01; CCP3.1 76.5% vs. 46.9%, p<0.01). Additionally, there was a non-significant trend toward higher rates of CCP positivity in ever smokers compared to never smokers (CCP2 60.3% vs. 53.9%, p=0.30; CCP3.1 69.5% vs. 61.8%, p=0.18).
Table 3.
Percent prevalence of positivity for CCP assays and RF in subjects with and without rheumatoid arthritis‡
| SERA RA Probands |
DoDSR post-RA diagnosis |
DoDSR pre RA diagnosis‡ |
SERA FDRs‡ |
SERA blood donors |
|
|---|---|---|---|---|---|
| (N=340) | (N=47) | (N=83) | (N=681) | (N=200) | |
| CCP2 | 57.4* | 68.1 | 61.4 | 2.2* | 1.0* |
| CCP3.1 | 66.2* | 76.6 | 63.9 | 9.5* | 6.0* |
| RF | 62.4 | 83.0 | 55.4 | 7.6 | 5.0 |
| Stratification by RF status | |||||
| RF(+) and | |||||
| CCP2 and CCP3.1 (+) | 46.5 | 59.6 | 49.4 | 1.0 | 0 |
| CCP2 and CCP3.1 (−) | 9.4 | 17.0 | 4.8 | 6.0 | 5.0 |
| CCP2 or CCP3.1 (+) | 52.9 | 66.0 | 50.6 | 1.6 | 0 |
| RF(−) and | |||||
| CCP2 and CCP3.1 (+) | 9.4 | 8.5 | 9.6 | 0.6 | 0.5 |
| CCP2 and CCP3.1 (−) | 22.9 | 6.4 | 28.9 | 83.8 | 88.5 |
| CCP2 or CCP3.1 (+) | 14.7 | 10.6 | 15.7 | 8.5 | 6.5 |
All results reported in the table are percentiles
In subjects tested ≥1 time, calculated using ever positive result
Comparing prevalence of positivity for CCP2 and CCP3.1 with chi-squared/Fisher’s exact testing; SERA RA p=0.02, SERA FDRs p<0.01, blood donors p=0.01
CCP results in pre-diagnosis testing from DoDSR RA cases
In DoDSR RA cases tested prior to diagnosis, CCP2 was more specific for future disease than CCP3.1 at standard cut-off levels (100% vs. 91.6%, p=0.01; Table 2). As CCP3.1 cut-off levels for positivity were increased, there was a non-significant trend for increased specificity for future RA (≥2x standard cut-off 95.2%; ≥3x standard cut-off 98.8%; Table 2). Also, as the time to RA diagnosis decreased, mean CCP levels increased (data not shown), and sensitivity for both CCP assays significantly increased, although specificity did not significantly change for either CCP assay (Table 4).
Table 4.
Diagnostic accuracy of CCP assays at various time intervals prior to the diagnosis of rheumatoid arthritis in DoDSR cases
| Time interval prior to RA | CCP2# | CCP3.1# | p-value † |
|---|---|---|---|
| 0–1 years (N=31) * | |||
| Sensitivity | 67.1 | 71.0 | 0.78 |
| Specificity | 100 | 93.5 | 0.49 |
| 1–3 years (N=50) * | |||
| Sensitivity | 56.0 | 62.0 | 0.68 |
| Specificity | 100 | 88.0 | 0.03 |
| 3–5 years (N=44) * | |||
| Sensitivity | 31.8 | 34.1 | 0.82 |
| Specificity | 100 | 88.6 | 0.06 |
| > 5 years (N=50) * | |||
| Sensitivity | 28.0 | 30.0 | 0.83 |
| Specificity | 100 | 94.0 | 0.24 |
The increase in sensitivity as time to diagnosis approached was statistically significant for CCP2 (p<0.01) and CCP3.1 (p<0.01)
Comparing proportions of true positives (sensitivity) and true negatives (specificity) with chi-squared/ Fischer’s exact testing
Calculated for DoDSR RA cases using 1 sample per case per time interval compared to matched controls. If ≥1 sample available per interval, the positive and/or sample closest to the time of diagnosis was used for analysis. Median time before RA diagnosis by time interval: 0–1 years = 0.5 years; 1–3 yrs = 1.8 years; 3–5 yrs = 3.9 yrs; >5 years = 6.5 years
Furthermore, in the DoDSR cases that were ever CCP positive (56/83 [67%] for CCP2, 60/83 [72%] for CCP3.1), as pre-diagnosis CCP cut-off levels of positivity were increased, there was a non-significant trend for increasing specificity for the development of RA within 2 years. For CCP2, specificity for development of RA within 2 years increased from 45.7% at standard cut-off levels, to 50.0% for levels >2x the standard cut-off, and 54.3% for levels >3x the standard cut-off. Similarly for CCP3.1, specificity for development of RA within 2 years increased from 46.0% at standard cut-off levels, to 50.0% for levels ≥2x the standard cut-off, and 54.0% for levels ≥3x the standard cut-off. Finally, in DoDSR cases that were ever positive for both CCP2 and CCP3.1 (N=55), there was a non-significant trend for CCP3.1 to be positive first in pre-diagnosis testing: CCP3.1 was positive before CCP2 in 6 cases (10%) compared to only 1 case (1.8%) that was initially CCP2 positive (p=0.11).
CCP results in SERA FDRs
The prevalence of CCP3.1 positivity was higher than CCP2 in SERA FDRs (9.5% vs. 2.2%, p<0.01; Table 3). While not statistically significant, at standard cut-off levels, the prevalence of CCP positivity was higher in SERA FDRs than SERA blood donor controls (for CCP2, 2.2% vs. 1.0%, p=0.39; for CCP3.1, 9.5% vs. 6.0%, p=0.16; Table 3). Individual CCP assay positivity rates were not affected by sex or race (data not shown), but there was a non-significant higher rate of CCP2 and CCP3.1 positivity in SERA FDRs that were never smokers compared to ever smokers (CCP2 2.7% vs. 1.1%, p=0.20; CCP3.1 10.1% vs. 8.7%, p=0.68) and in SERA FDRs with ≥1 SE allele (CCP2 3.1% vs. 1.3%, p=0.13; CCP3.1 9.6% vs. 8.9%, p=0.89).
CCP3 assay
We additionally tested the CCP3 assay in a randomly selected subset of SERA RA Probands (N=215), SERA FDRs (N=266), and all SERA blood donor controls (N=200). In SERA RA Probands, CCP3 had a sensitivity (63.3%) and specificity (95.0%) that was intermediate to CCP2 and CCP3.1 assays (Table 2). In this subset, as CCP3 cut-off levels for positivity were increased, specificity increased (≥2x standard cut-off 100%), while sensitivity decreased (≥2X standard cut-off 60.9%; ≥3x standard cut-off 57.7%). Prevalence of positivity of CCP3 in SERA FDRs was 4.5%; this was non-significantly different in these subjects than the prevalence of CCP2 (2.3%, p=0.23), but lower and significantly different than CCP3.1 (4.5% vs. 9.8%, p=0.03). CCP3 was positive in 5.0% of SERA blood donor controls - significantly different than CCP2 (1.0%, p=0.04) but similar to CCP3.1 (6.0%, p=0.83). There was no significant difference between the prevalence of CCP3 positivity between SERA FDRs and SERA blood donor controls (4.5% vs. 5.0%, p=0.98).
Assay agreement
At standard cut-off levels, the overall CCP2 and CCP3.1 assay agreement in subjects with established RA was kappa (k)=0.76 (SERA RA Probands k=0.75, DoDSR cases post-diagnosis k=0.79) (Table 5); however, the agreement between these assays was lower in SERA FDRs (k=0.25). CCP2 and CCP3.1 assay agreement in SERA FDRs improved with increasing CCP3.1 cut-off levels of positivity suggesting the low range positive CCP3.1 levels (i.e. 20–60 units) encompass most of the discordant samples in SERA FDRs (k=0.53). In contrast, increasing CCP3.1 cut-off levels in established RA had minimal effect on assay agreement (Table 5). The overlap of CCP2 and CCP3.1 assay positivity in SERA RA Probands and SERA FDRs is shown in Figure 1. In addition, CCP2 and CCP3 assay agreement was k=0.74 in SERA RA Probands and k=0.20 in SERA FDRs, whereas CCP3 and CCP3.1 assay agreement was k=0.95 in SERA RA Probands and k=0.55 in SERA FDRs.
Table 5.
Agreement of CCP2 and CCP3.1 assays in subjects with and without rheumatoid arthritis
| Overall agreement† | Agreement by RF status† | ||
|---|---|---|---|
| Standard kit cut-offs‡ | RF positive | RF negative | |
| Subjects with RA | |||
|
SERA RA Probands (N=340) |
0.75 | 0.68 | 0.68 |
|
DoDSR post-diagnosis N=47) |
0.79 | 0.79 | 0.75 |
|
DoDSR pre-diagnosis (N=83)§ |
0.85 | 0.88 | 0.67 |
| SERA FDRs (N=681) | 0.25 | 0.73** | 0.11** |
| Blood donors (N=200) | 0.13 | n/a* | 0.13 |
Agreement calculated as Cohen’s kappa
Agreement using CCP2 positivity at standard kit cut-offs and CCP3.1 positivity at ≥3x standard kit cut-offs included: SERA RA Probands k=0.79; DoDSR post-diagnosis k=0.85, DoDSR pre-diagnosis k=0.85; SERA FDRs k=0.53; Blood donors k=(−)0.02. Agreement using CCP2 and CCP3.1 positivity ≥3x standard kit cut-offs included: SERA RA Probands k=0.79; DoDSR post-diagnosis k=0.86, DoDSR pre-diagnosis k=0.81; SERA FDRs k=0.55; Blood donors k=(−)0.01
Includes 1 sample per case, and if ≥1 pre-diagnosis sample available, the positive and/or sample closest to the time of diagnosis was used. Median time before diagnosis = 1.4 years
Percent agreement in SERA FDRs that were positive for CCP2 and/or CCP3.1, 63.6% in RF (+) SERA FDRS, 6.9% in RF (−) SERA FDRs, p<0.01
Unable to calculate kappa as there were no positive results in either CCP assay
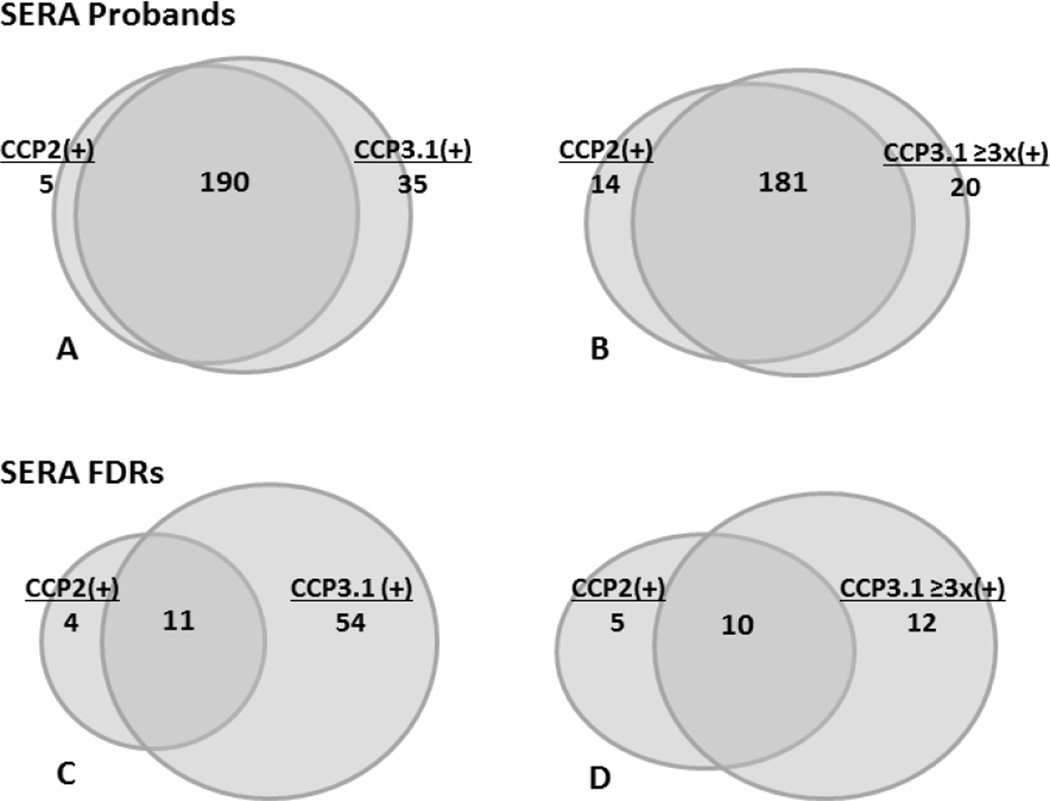
Figure 1. Overlap of CCP2 and CCP3.1 positivity in subjects with and without RA.
Diagrams depicting the overlap of CCP positivity in subjects that were CCP2 and/or CCP3.1 positive including positivity of CCP2 and CCP3.1 at standard kit cut-off levels (A, SERA RA Probands; C, SERA FDRs) as well as positivity of CCP2 at standard cut-off levels and CCP3.1 at ≥3x standard cut-off levels (B, SERA RA Probands; D, SERA FDRs).
Rheumatoid factor
RF positivity in absence of CCP positivity was present in 9.4% of SERA RA Probands and 17.0% of DoDSR RA cases tested post-diagnosis (Table 3); conversely, CCP2 and/or CCP3.1 positivity in absence of RF was present in 14.7% of SERA RA Probands and 10.6% of DoDSR RA cases post-diagnosis (Table 3). Furthermore, RF status did not significantly affect CCP assay agreement in subjects with established RA, but in analyses of SERA FDRs stratified by RF positivity, the agreement between CCP assays was greater in RF positive compared to RF negative subjects (k=0.73 vs. k=0.25; percent agreement in SERA FDRs that were positive for ≥1 CCP assay 63.6% vs. 6.9%, p<0.01) (Table 5).
DISCUSSION
Anti-CCP assays differ in established RA and subjects without inflammatory arthritis that are at elevated risk for development of RA. Notably, if applying CCP assays at their standard cut-off levels, CCP2 is more specific although less sensitive for established RA compared to CCP3.1.Given the 2010 ACR/EULAR RA classification criteria include ACPA testing without specification for a particular assay11, the results presented herein suggest that CCP assays differ to an extent that may have a clinically meaningful impact on classification of synovitis as RA. Therefore, in clinical practice, it is essential to understand these differences when interpreting CCP testing results, as well as to recognize what specific CCP assay is used in the particular clinical laboratory conducting the test, especially since commonly-utilized clinical laboratories in the United States are using different CCP assays including CCP2, CCP3 or CCP3.112,13.
Several studies have found results similar to those presented herein that CCP2 is more specific but less sensitive than other ACPA assays in patients with established RA1,14–16, and several studies have also evaluated ACPA assays in FDRs of patients with RA7,17–19. However, we present the first comparison of multiple commercial anti-CCP assays in subjects with established RA prior to diagnosis as well as in FDRs at-risk for future RA based on family history. We found that similar to established RA, at standard cut-off levels, CCP2 is more specific than CCP3.1 for the development of future RA in our DoDSR cohort, although the sensitivity of CCP3.1 was higher. In addition, we found that SERA FDRs demonstrate a trend toward a higher prevalence of CCP positivity compared to SERA blood donor controls. Finally, similar to other studies20–22, we found higher ACPA positivity rates in subjects with established RA who had the SE allele and a trend toward higher rates in smokers. In SERA FDRs, there was also a trend toward higher ACPA positivity rates in subjects with the SE but a trend toward an inverse relationship with smoking; this seemingly contradictory relationship in subjects without RA needs further exploration in larger studies.
An important consideration when evaluating each CCP assay is to examine the CCP level. As we describe above, higher levels of CCP3.1 positivity (≥3x standard cut-off levels) resulted in higher specificity for established RA. It is of interest that the CCP3.1 package insert23 acknowledges a level ≥60 units (i.e., ≥3x standard cut-off) as a ‘strong positive’ result. Furthermore, similar to other studies, we found that regardless of the specific test, the highest CCP levels were in subjects with established RA, although these findings are difficult to interpret for CCP2 given the small number of CCP2 positive SERA FDRs and SERA blood donor controls. In addition, in DoDSR subjects that developed RA, CCP levels increased prior to RA diagnosis, and levels of CCP2 and CCP3.1 at ≥2 or 3x the standard cut-off were associated with a higher specificity than standard cut-offs for a clinical diagnosis of RA within 2 years4,10,24–26. Finally, higher ACPA levels carry increased weight for the 2010 ACR/EULAR classification criteria for RA11, appear to predict imminent onset of RA in other studies of preclinical disease3,4,27, and carry increased weight in the prediction of which subjects with ‘arthralgias’ in absence of clinical inflammatory arthritis may progress to classifiable RA26. Overall, these data support that CCP2 at standard levels or higher CCP3.1 levels are more specific for established RA, and higher CCP levels in general may be useful for predicting both the likelihood and timing of future clinically-apparent RA, a finding that may be important to identify subjects at high-risk for future classifiable RA based on CCP positivity for future prevention trials in RA. Going forward, it will be important to validate the ability of CCP levels to predict imminent RA in our ongoing prospective study of SERA FDRs and similar studies.
While we have identified several differences in CCP assay performance in subjects with and without RA, it remains unclear that a single CCP assay is optimal for all situations. Specifically, the application of CCP results may vary in clinical settings of synovitis compared to research settings investigating the development of autoimmunity. Many prior ACPA testing studies consider the presence of inflammatory arthritis that is classifiable as RA to be the gold standard in the determination of diagnostic accuracy of ACPA testing, and this may be appropriate for clinical applications. However, such a gold standard is inadequate to identify asymptomatic subjects at-risk for future RA who may be evaluated to understand the natural history of RA. Even low-titer elevations of these autoantibodies may be important to the biology of developing RA-related autoimmunity. Related to this issue, herein, in SERA FDRs at risk for future RA, we found higher rates of CCP3 and CCP3.1 positivity compared to CCP2. These findings lead to an important question – are these differences related to test accuracy that is, as discussed above, to some extent related to cut-off levels of positivity, or are the CCP3 and CCP3.1 assays, even at low titer elevations, detecting the presence of unique and meaningful factors that may be important to understand the biology of developing RA-related autoimmunity even in absence of clinically apparent synovitis?
In our study, the overall high specificity (>89%) of CCP3 and CCP3.1 in established RA, even at standard cut-off levels, suggests elevations of these autoantibodies are related to meaningful RA-related autoimmunity, although the ~6–8% prevalence of positivity of these assays in SERA blood donor and DoDSR controls may suggest the possibility of false positivity. However, in this setting, the term ‘false positive’ relates only to the presence or development of clinically apparent RA. This distinction is important because elevations of anti-CCP autoantibodies, even if such elevations are positive at low levels and in absence of clinically apparent RA, may still indicate that RA-related autoimmunity has occurred. As such, identifying subjects with these biomarkers may be important to understand the earliest steps in the development of RA. Further studies including longitudinal follow-up to identify the risk for development of clinically-apparent RA in asymptomatic CCP positive individuals, as well as studies of the biologic mechanisms that result in the generation of CCP positivity even in absence of inflammatory arthritis are necessary to evaluate these issues.
There are several caveats to our results including the small number of DoDSR cases available, and the variability in the number and temporal distribution of their samples that limited our ability to assess the diagnostic accuracy of CCP testing for timing of future RA. Also, while we chose to test three commonly utilized CCP assays, there are other ACPA assays commercially available including anti-mutated/modified citrullinated vimentin that may have their own unique differences2. These issues will need to be addressed in future studies.
In addition, we have not explored in detail the exact etiology of the CCP assay differences described herein. The finding that diagnostic accuracy in established RA and test agreement in SERA FDRs improves if the positive cut-off level for CCP3.1 is raised suggests that the assay cut-off level may be an important part of the differences in these assays. However, if these assay differences were completely the result of a CCP3.1 cut-off level for positivity set too low, one would expect that raising the CCP3.1 cut-off level would lead to strong agreement of CCP3.1 and CCP2 results, yet this did not occur in SERA FDRs (Table 5). Furthermore, one would expect that lowering the CCP2 cut-off below its standard cut-off level would result in similar CCP2 and CCP3.1 results; however, when we reduced the CCP2 cut-off level by half, CCP2 specificity for RA still exceeded the CCP3.1 test at the standard cut-off.
These data suggest that the levels of these CCP assays alone are likely insufficient to explain the differences in results. As such, going forward, it will be important to identify potential mechanisms underlying these differences including differences in antigen detection. It is of interest that in a recent study by Sokolove et al24, increasing CCP2 levels prior to RA diagnosis were associated with epitope spreading measured by increasing numbers of autoantibodies to specific citrullinated peptides. This suggests that a rising CCP titer may be in part a surrogate for expansion of epitope reactivity of autoantibodies. Therefore, the breadth and type of epitope reactivity, rather than the actual levels of the CCP assays, may explain why the agreement between these assays improves at higher levels. At present, a growing number of ACPA tests for detection of specific citrullinated antigens are under investigation24,28,29, and while these are not currently commercially-available, these types of assays will be of interest to directly compare differences in antigen detection between commercial CCP assays as a potential source of assay differences. Furthermore, given that CCP2 and CCP3 detect an IgG antibody whereas CCP3.1 detects IgG and IgA antibodies, differing isotype detection is a potential explanation for CCP assay differences. Notably, a study by Barra and colleagues found higher rates of IgA-ACPA positivity compared to other isotypes in FDRs without inflammatory arthritis suggesting that IgA may be an important component of RA-related autoimmunity in preclinical disease19. Furthermore, in subjects with established RA, IgG- and IgA-CCP2 autoantibodies have been associated with high specificity for RA, including retrospective testing of preclinical samples (IgG-CCP2 >98% specific, IgA-CCP2 >97% specific)25,30. As such, the importance of specific isotypes of RA-related autoantibodies, and in particular IgA, needs further study.
Finally, in DoDSR cases tested prior to RA diagnosis and in SERA FDRs, we found that, despite the level of CCP assays, the agreement between CCP2 and CCP3.1 improved in subjects that were also RF positive. As such, it may be that RF positivity in these subjects is a marker of expansion of underlying autoimmunity and epitope spreading that has resulted in an increased likelihood to detect RA-related autoimmunity with multiple unique CCP assays. Importantly, based on published literature, individuals that are positive for both RF and CCP are at higher risk for future clinically-apparent RA compared to individuals that are positive for either autoantibody alone – a finding that further suggests that expansion of autoimmunity to multiple autoantibody systems is more likely to be related to RA-related autoimmunity that progresses to clinically-apparent disease24. These issues will need further evaluation in prospective studies of incident disease as it could have a major impact on interpretation of individual CCP assay results as well as future screening and preventive efforts in RA.
CONCLUSION
Commonly used CCP assays differ in subjects with and without established RA to an extent that may be meaningful in clinically diagnosing RA in subjects with inflammatory arthritis and in evaluating the natural history of RA development in subjects at-risk for future RA. These findings as well as potential mechanisms underlying these differences need further investigation as an improved understanding of CCP assay differences will improve our ability to diagnose RA in patients with synovitis, understand the overall pathogenesis of RA, and improve our ability to predict development of clinically apparent disease.
Acknowledgments
Grant Funding: This project was supported by funding from the NIH AI050864, AR051394, AI61479, AR07534 and AR051461, the Arthritis Foundation, and the Walter S. and Lucienne Driskill Foundation.
Footnotes
Disclaimer: The views expressed in this publication are those of the authors and do not reflect the official policy of the Department of the Army, Department of the Navy, Department of Defense, or U.S. Government.
Disclosure: No authors have financial relationships with CCP assay manufacturers.
REFERENCES
- 1.Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med. 2010;152:456–464. doi: 10.7326/0003-4819-152-7-201004060-00010. W155-66. [DOI] [PubMed] [Google Scholar]
- 2.Demoruelle MK, Deane K. Antibodies to citrullinated protein antigens (ACPAs): clinical and pathophysiologic significance. Curr Rheumatol Rep. 2011;13:421–430. doi: 10.1007/s11926-011-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 4.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 5.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36:213–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demoruelle MK, Deane KD. Treatment Strategies in Early Rheumatoid Arthritis and Prevention of Rheumatoid Arthritis. Curr Rheumatol Rep. 2012 doi: 10.1007/s11926-012-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61:1735–1742. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60:661–668. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 9.Majka DS, Deane KD, Parrish LA, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane KD, O'Donnell CI, Hueber W, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age dependent manner. Arthritis & Rheumatism. 2010;62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 12.Cyclic Citrullinated Peptide (CCP) Antibodies, IgG/IgA, ELISA. (Accessed at https://www.labcorp.com/wps/portal/!ut/p/c1/04_SB8K8xLLM9MSSzPy8xBz9CP0os_hACzO_QCM_IwMLXyM3AyNjMycDU2dXQwN3M6B8JG55AwMCuv088nNT9SP1o8zjQ11Ngg09LY0N_N2DjQw8g439TfyM_MzMLAz0Q_QjfYCKIvEqKsiNKDfUDVQEALvGFag!/dl2/d1/L0lJWmltbUEhL3dQRUJGUUFoTlFBaERhQUVBWEtHL1lJNXlsdyEhLzdfVUU0UzFJOTMwT0dTMjBJUzNPNE4yTjY2ODAvdmlld1Rlc3Q!/?testId=1978238.)
- 13.Cyclic Citrullinated Peptide (CCP) Antibody IgG. (Accessed at http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=11173).
- 14.Wiik AS, van Venrooij WJ, Pruijn GJ. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10:90–93. doi: 10.1016/j.autrev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 15.dos Anjos LM, Pereira IA, d 'Orsi E, Seaman AP, Burlingame RW, Morato EF. A comparative study of IgG second- and third-generation anti-cyclic citrullinated peptide (CCP) ELISAs and their combination with IgA third-generation CCP ELISA for the diagnosis of rheumatoid arthritis. Clin Rheumatol. 2009;28:153–158. doi: 10.1007/s10067-008-0999-5. [DOI] [PubMed] [Google Scholar]
- 16.van der v MP, van der Woude D, Ioan-Facsinay A, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009;60:2232–2241. doi: 10.1002/art.24716. [DOI] [PubMed] [Google Scholar]
- 17.El-Gabalawy HS, Robinson DB, Hart D, et al. Immunogenetic risks of anti-cyclical citrullinated peptide antibodies in a North American Native population with rheumatoid arthritis and their first-degree relatives. J Rheumatol. 2009;36:1130–1135. doi: 10.3899/jrheum.080855. [DOI] [PubMed] [Google Scholar]
- 18.El-Gabalawy HS, Robinson DB, Smolik I, et al. Familial clustering of the serum cytokine profile in the relatives of rheumatoid arthritis patients. Arthritis Rheum. 2012;64:1720–1729. doi: 10.1002/art.34449. [DOI] [PubMed] [Google Scholar]
- 19.Barra L, Scinocca M, Saunders S, et al. Anti-citrullinated protein antibodies (ACPA) in unaffected first degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013 doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 20.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 21.Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willemze A, van der Woude D, Ghidey W, et al. The interaction between HLA shared epitope alleles and smoking and its contribution to autoimmunity against several citrullinated antigens. Arthritis Rheum. 2011;63:1823–1832. doi: 10.1002/art.30409. [DOI] [PubMed] [Google Scholar]
- 23.QUANTA Lite CCP3.1 IgG/IgA ELISA [package insert, revision 2] (Accessed at www.inovadx.com/pdf/di/704550_en.pdf.)
- 24.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PloS one. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2012-202127. [DOI] [PubMed] [Google Scholar]
- 27.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36:706–711. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snir O, Widhe M, Hermansson M, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62:44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 29.Hansson M, Mathsson L, Schlederer T, et al. Validation of a multiplex chip-based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res Ther. 2012;14:R201. doi: 10.1186/ar4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svard A, Kastbom A, Soderlin MK, Reckner-Olsson A, Skogh T. A comparison between IgG- and IgA-class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J Rheumatol. 2011;38:1265–1272. doi: 10.3899/jrheum.101086. [DOI] [PubMed] [Google Scholar]