Abstract
Background:
Positive surgical margins (PSM) are an important determinant of biochemical recurrence after radical prostatectomy (RP). We use a population-based cancer registry to evaluate PSM by stage, Gleason and prostate-specific antigen (PSA).
Methods:
We identified men undergoing RP from the Surveillance, Epidemiology and End Results (SEER) database between 2004 and 2007. Differences between those with and without PSM were compared with chi-squared tests. The proportion of cases with PSM were stratified by PSA and Gleason sum for both pT2 and pT3a tumours. Factors associated with PSM were analyzed using chi square and multivariate logistic regression analysis. A composite variable was used in a second multivariate analysis to display the odds ratio (OR) for a PSM for each discrete combination of PSA, Gleason score and pT stage
Results:
In total, 28 461 RP patients were identified and a PSM was present in 19.5%. PSM were 42% in pT3a and 16% in pT2 cases. Higher PSAs (<4.0, 4–9.9, >10) were associated with higher proportions of PSM (12%, 20% and 28%, p < 0.001). Similarly, higher Gleason scores (≤6, 3+4, 4+3, ≥8) were associated with higher PSM (12%, 22%, 27% and 33%, p < 0.001). For pT2 tumours, the proportion of PSM ranged from 8% (Gleason ≤6, PSA <4.0) to 28% (Gleason 8–10, PSA ≥10). For pT3a tumours, the PSM was higher in each Gleason/PSA strata compared to those with pT2 tumours, reaching 63% for those with pT3a, Gleason 8–10, PSA >10 disease. On multivariate analysis, stage was the largest predictor for PSM (OR 3.05, 95% confidence interval 2.81–3.30), although Gleason score and PSA remained statistically significant.
Conclusion:
In this population-based study of PSM after RP, the proportion of PSM vary significantly within different PSA and Gleason strata for organ-confined and extracapsular disease. These data can be used as a reference for urologist self-assessment.
Introduction
Positive surgical margins (PSM) at the time of radical prostatectomy (RP) are independent predictors of biochemical recurrence, local recurrence, distant metastasis and, in some series, have been shown to predict for prostate cancer specific mortality.1–6 The occurrence of PSM often prompts adjuvant treatments, such as radiotherapy, which has been shown to improve biochemical recurrence-free survival and overall survival in this population.7–9 In fact, a PSM may be the strongest predictor of the utility of radiotherapy in the adjuvant setting.10 As such, avoiding a PSM is a clear goal of surgery; a PSM is perhaps the only risk factor for poor outcomes that can be affected by the surgeon.
Recent quality improvement initiatives in Canada have focused on accurate reporting of PSM rates. A publication by Cancer Care Ontario has mandated that less than a 25% PSM rate for T2 disease should be achieved.11 Yet, a previously published report indicated that the median PSM rate in the province was 33%.12 The most recent meeting of the Canadian Urologic Association contained numerous presentations showing highly variable rates of PSM between presenters.
Rates of PSM vary significantly in the literature and are dependent on a number of factors, including surgical expertise, pathological stage, Gleason grade, percent of positive cores, serum PSA, prostate volume and the interobserver variability between pathologists.13–18 However, much of the data used to derive rates of PSM are based on information from single institutions and tertiary care centres of excellence. They are, therefore, of limited utility to the practicing urologist who is seeking to compare his or her own results against a normative sample. Additionally, not all tumours are created equally even within the same pathological stage. Rates of PSM may be profoundly affected by other risk factors generating varying PSM rates within each pathological stage.
We explore the rates of PSM within PSA, Gleason and pathologic stage strata for its occurrence in a large population-based study. This was done to generate a tool to be used by urologists for their own self-assessment.
Methods
Data source
The cohort was identified from the Surveillance, Epidemiology, and End Results (SEER) Program database. SEER collects cancer incidence, primary treatment and other variables from 17 population-based cancer registries in the United States accounting for about 26% of the population.19 Data from 2004 to 2007 from 13 SEER registries were used (metropolitan areas of San Francisco-Oakland, San Jose-Monterey, Los Angeles, Atlanta, Detroit, Seattle-Puget Sound and the states of Connecticut, Hawaii, New Mexico, Utah, Iowa). Cases before 2004 were excluded since PSA and Gleason scores was not reported prior to that date. The Alaska and Rural Georgia registries were also excluded since they provided less the 0.3% of the total cases.
Study population
Subjects were identified using the International Classification of Diseases for Oncology (ICD-O-3) site codes for the prostate (C61.9) and ICD-O-3 histology codes for adenocarcinoma (8550) and acinar cell carcinoma (8140). There were 33 758 eligible cases undergoing RP during the study period. Margin status is not reported for pathologic stage pT3b (seminal vesicle invasion) or pT4 (adjacent organ invasion) and therefore were excluded (pT3b: 1,612 (4.6%); pT4: 425 (1.2%)). Those with missing Gleason score (n = 85 [0.3%]) or PSA (n = 3692 [11.6%]) were also excluded.
Statistical analysis
Clinical and pathologic characteristics were compared between those with and without PSM with chi-squared tests. The proportion of PSM were determined within each strata of Gleason sum (2–6, 3+4, 4+3, 8–10) and PSA (<4.0, 4.0–9.9, 10+) for both organ-confined (pT2) and extracapsular (pT3a) tumours. Chi-squared tests were used to test for differences within these strata. Multivariate logistic regression analysis was used to determine factors which significantly predicted for PSM. Included in the model were Gleason score, preoperative PSA, age, race, registry site and year of diagnosis. A composite variable composed of PSA, Gleason score and pathological T stage was then developed. This was added to our multivariate model to display the odds ratio for a PSM for each discrete combination of PSA, Gleason score and T stage. All statistical analyses were conducted using Stata software version 11.0 (StataCorp LP, College Station, TX).
Results
The analytic cohort consisted of 28 459 men who underwent RP with complete data available between 2004 and 2007. PSM were reported in 19.5%. We tallied the clinical and pathological characteristics of the men who underwent RP (Table 1). Pathologic tumour stage was highly associated with PSM, as 15.8% of men with pT2 tumours, and 41.8% of men with pT3a tumours (p < 0.001) had PSM (Table 1). The proportion of PSM declined annually during the study period.
Table 1.
Distribution of clinical and pathological characteristics of 28 459 radical prostatectomy patients by surgical margin status
|
Negative margin N (%) |
Positive margin N (%) |
p value | |
|---|---|---|---|
| Overall | 22 921 (80.5) | 5538 (19.5) | |
| Age (years) | |||
| <55 | 4514 (80.7) | 1082 (19.3) | 0.003 |
| 55–59 | 5408 (81.6) | 1219 (18.4) | |
| 60–64 | 5744 (81.1) | 1341 (18.9) | |
| 65–70 | 4649 (79.5) | 1201 (20.5) | |
| 70+ | 2605 (78.9) | 695 (20.1) | |
| Race | |||
| Caucasian | 19 019 (80.7) | 4552 (19.3) | 0.3 |
| African-American | 2216 (79.5) | 572 (20.5) | |
| Other | 1686 (80.3) | 414 (19.7) | |
| Year of diagnosis | |||
| 2004 | 5667 (77.7) | 1629 (22.3) | <0.001 |
| 2005 | 5180 (79.5) | 1325 (20.4) | |
| 2006 | 5734 (81.9) | 1267 (18.1) | |
| 2007 | 6340 (82.8) | 1317 (17.2) | |
| Clinical stage | |||
| T1c | 13 031 (81.1) | 3031 (18.9) | <0.001 |
| cT2 | 9293 (79.5) | 2400 (20.5) | |
| cT3 | 234 (75.7) | 75 (24.3) | |
| Pathologic stage | |||
| pT2 | 20 608 (84.2) | 3871 (15.8) | <0.001 |
| pT3 | 2313 (58.1) | 1667 (41.8) | |
| PSA | |||
| <4.0 ng/mL | 4690 (87.6) | 662 (12.4) | <0.001 |
| 4–9.9 | 15 160 (80.5) | 3674 (19.5) | |
| 10+ | 3071 (71.9) | 1202 (28.1) | |
| Gleason sum | |||
| 2–6 | 10 426 (87.7) | 1465 (12.3) | <0.001 |
| 3+4 | 8705(77.7) | 2492 (22.3) | |
| 4+3 | 2154 (73.1) | 790 (26.8) | |
| 8–10 | 1636 (67.4) | 791 (32.6) |
PSM were more commonly observed with higher PSA levels (Table 1). The proportion of men with a PSM rose for each level of PSA, from 12.4% to 19.5% to 29.1% for PSAs <4.0 ng/mL, 4–9.9, and ≥10, respectively (p < 0.001). Similarly, higher Gleason scores were associated with PSM (Table 1). PSM were observed in 12.3%, 22.3%, 26.8% and 32.6% of men with Gleason ≤6, 3+4, 4+3 and 8–10 disease, respectively (p < 0.001)
To determine the role of preoperative PSA and pathologic Gleason score by pathologic stage, we grouped PSM into PSA and Gleason sum strata within each pTstage (Table 2). For pT2 tumours, the lowest PSM were seen in those with PSAs <4.0 and Gleason 2–6 cancer (7.9%). The proportion of PSM rose with increasing PSA levels and Gleason aggressiveness to a high of 28.4% for those with Gleason 8–10 tumours with PSAs ≥10. A similar trend was seen for pT3 tumours, although for each respective strata, the corresponding PSM proportion was higher than observed for pT2 tumours.
Table 2.
Radical prostatectomy positive margin rate by pathologic stage by PSA and Gleason sum
|
Organ confined (pT2)
|
|||||||||
| PSA level | Gleason 2–6 | Gleason 3+4 | Gleason 4+3 | Gleason 8–10 | p value | ||||
| N | % Positive margin | N | % Positive margin | N | % Positive margin | N | % Positive margin | ||
|
| |||||||||
| <4.0 | 2903 | 7.9 | 1529 | 14.1 | 278 | 15.1 | 169 | 16.6 | <0.001 |
| 4–9.9 | 7305 | 12.6 | 6681 | 19.4 | 1416 | 19.4 | 942 | 20.2 | <0.001 |
| 10+ | 1207 | 12.1 | 1283 | 24.7 | 379 | 26.7 | 387 | 28.4 | <0.001 |
| p value | <0.001 | <0.001 | 0.002 | <0.001 | |||||
|
| |||||||||
|
Extra-capsular extension (pT3a)
|
|||||||||
| PSA level | Gleason 2–6 | Gleason 3+4 | Gleason 4+3 | Gleason 8–10 | p value | ||||
| N | % Positive margin | N | % Positive margin | N | % Positive margin | N | % Positive margin | ||
|
| |||||||||
| <4.0 | 78 | 28.2 | 211 | 28.9 | 87 | 34.5 | 97 | 36.1 | 0.50 |
| 4–9.9 | 334 | 37.7 | 1132 | 38.7 | 507 | 38.7 | 517 | 44.7 | 0.09 |
| 10+ | 64 | 34.4 | 361 | 44.9 | 277 | 53.1 | 315 | 62.5 | <0.001 |
| p value | 0.28 | 0.001 | <0.001 | <0.001 | |||||
PSA: prostate-specific antigen.
Multivariate logistic regression analysis showed that pathological stage was the strongest predictor for a PSM (odds ratio 3.04, 95% confidence interval 2.81–3.30). PSA and Gleason score maintained statistical power in the multivariate model as did tumour registry location and year of diagnosis (data not shown). Our composite variable showed an increase risk of PSM with increasing PSA, Gleason and pathological T stage (Fig. 1).
Fig. 1.
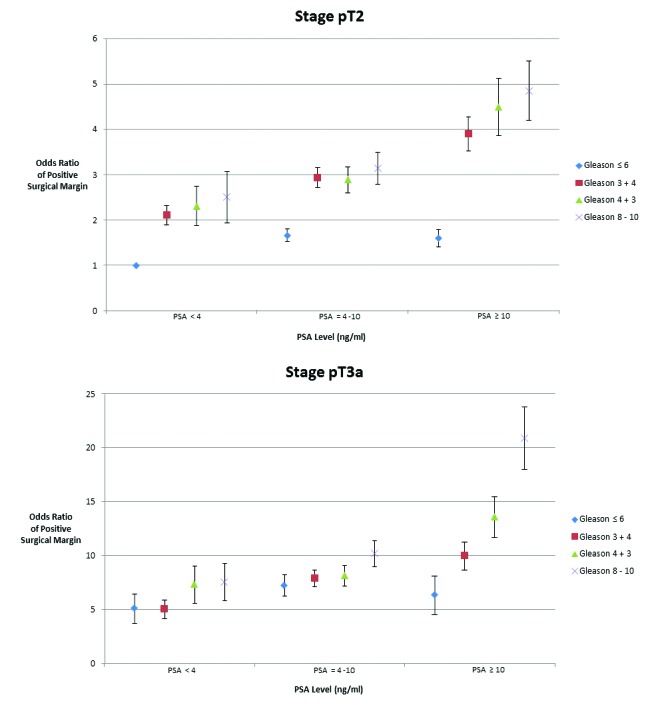
Odds ratio of a positive surgical margin stratified by stage, grade and Gleason sum. Data were derived using multivariate logistic regression analysis adjusting for age, race, stage, Gleason sum, prostate-specific antigen (PSA), registry site and year of diagnosis. Data are displayed as odd ratios with standard errors of a positive surgical margin using Gleason 6, PSA <4 ng/mL and pathological stage T2 as the referent.
Discussion
Our study augments previous studies that have linked grade, PSA and pathological stage to PSM by stratifying patient cohorts by these parameters. While these results may seem intuitive, we present the data as a benchmarking tool for urologists to assess their operative results as a quality measure.
A limitation of the existing literature on PSM is that the work comes from single institution series or pooled results from a few centres of prostate cancer excellence which may not reflect the average urologist’s experience. A recent review article of PSM reported rates from 11% to 38% over-all with organ-confined and non-organ-confined disease, with ranges of 3% to 18% and 17% to 53%, respectively.20 In our study, organ-confined tumours had PSM in 16% of cases compared to 42% in tumours that extended through the capsule. Recently, Patel and colleagues analyzed the records of 8418 patients with pathological organ-confined disease undergoing robotic RP from 7 institutions.21 Their PSM rates were 9.45% and 37.2% for pT2 and pT3a disease, respectively. They found on multivariate analysis that pathological T stage, preoperative PSA, elevated body mass index and smaller prostates were predictive of PSM. In those with organ-confined disease, preoperative PSA was the most important predictor of PSM.
Other studies have specifically examined PSM in organ-confined disease. Ahyai and colleagues studied 932 men with pathological T2 disease undergoing RPs over a 12-year span at a German institution. They found that preoperative PSA was not predictive of PSM.22 Their series had an overall rate of PSM of 12.9%, which was similar to the 15.8% seen in the organ-confined subset of our study. On their univariate and multivariate analysis, predictors of a PSM were tumour volume, nerve-sparing procedure and surgeon volume. Interestingly, their study showed neither Gleason score nor PSA level predictive of PSM. Both Gleason score and preoperative PSA predicted biochemical recurrence in univariate models, but not multivariable models where tumour volume, percent high-grade tumour volume and surgical margin status were the only predictors of biochemical recurrence.
Lawrenschuk and colleagues established a population-based assessment of PSM in organ-confined disease in a Canadian cohort.12 The Ontario province-wide PSM rate in pT2 disease was 33%, which is considerably higher than in our series. PSA and Gleason grade, however, were not included in the analysis. Surgical volume fell short of predicting for PSM in a statistically significant manner, although this association has been shown in other series.13,23 Information regarding surgeon volume is not available within the SEER dataset and could not be included in our analysis. However, tumour registry location significantly affected PSM rates even after controlling for all other factors in the multivariate model, indicating significant geographic variability among providers.
In our study, PSM rates declined over the 4-year interval. Other groups have noted similar decreases over time. Han and colleagues analyzed the changes in pathologic surgical margin status over a 20-year period at a single institution.24 They noted a significant decrease in PSM overall, but explained this phenomenon by a corresponding increase in the percentage of patients with organ-confined disease. Their data included prostatectomies performed up to 2001 and may, therefore, represent a different underlying cause for the change in PSM seen in our study. In our analysis, there was no statistical difference in the number of patients presenting with lower stage disease over the 4-year period (data not shown). It is possible that this decrease may be the result of refinement in surgical technique over time or may represent a change in surgical approach with the increasing utilization of robot assisted techniques. Unfortunately, information on surgical approach is not available in the SEER registry.
Williams and colleagues recently analyzed the SEER-Medicare dataset and found that individual surgical volume did not predict PSM in a dataset of 4247 men.25 To produce a meaningful predictive and quality control tool for urologists, our study had several advantages over the Williams study. First, our dataset contained the results of over 28 000 RPs which may have given it power to detect differences that were not statistically significant given their small sample size limited to the Medicare population. Second, they provide estimated thresholds for PSM within pathologic stage strata based on surgeon volume. Our analysis is different because we report on the actual rates of PSM within pathologic stages stratified by Gleason and PSA, which both strongly predict margin status. This distinction will allow urologists to stratify their patients with respect to risk factors for PSM to more accurately judge and monitor their own quality control.
There are several limitations associated with our analysis. First, recent work from one of the SEER sites has suggested that the SEER data may underreport PSM.26 Information regarding comorbidities was not available and several groups have reported that increased body mass index is associated with an increased risk of PSM.21,27,28 The location and number of positive cores from transrectal ultrasound (TRUS) guided biopsies of the prostate guide surgeons with surgical planning. They have been shown in various series to affect surgical margin status.29,30 Patient information regarding the results from TRUS biopsies are not contained within the SEER dataset. Not all PSM may convey the same risk of biochemical recurrence.31,32 Our analysis does not stratify patients based on location, size or multifocality of surgical margins. Finally, surgical details, such as robotic or open approach, nerve-sparing status and surgeon volume, are not available in SEER.
Despite these limitations, we believe we have developed an important tool to assist urologists in assessing surgical quality within their own practice. While it does not incorporate all factors associated with PSM, the strength of our study lies in the simplicity of the tool and its ability to stratify patients into easily discernible categories for quick comparisons of surgical outcomes.
Conclusion
We report on the rates of PSM from a contemporary population-based cohort of over 28 000 men with prostate cancer treated with RP. We developed a simple table that stratified the risk of PSM based on PSA, Gleason score and pathological T stage. These data provide valuable information regarding population-based rates of PSM, which can serve as a quality benchmark for urologists to compare their results.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–50. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 2.Wright JL, Dalkin BL, True LD, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–8. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2008;179:S47–51. doi: 10.1016/j.juro.2008.03.137. [DOI] [PubMed] [Google Scholar]
- 4.Pfitzenmaier J, Pahernik S, Tremmel T, et al. Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int. 2008;102:1413–8. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 5.Boorjian SA, Karnes RJ, Crispen PL, et al. The impact of positive surgical margins on mortality following radical prostatectomy during the prostate specific antigen era. J Urol. 2010;183:1003–9. doi: 10.1016/j.juro.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Alkhateeb S, Alibhai S, Fleshner N, et al. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. 2010;183:145–50. doi: 10.1016/j.juro.2009.08.132. [DOI] [PubMed] [Google Scholar]
- 7.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossfeld GD, Chang JJ, Broering JM, et al. Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: data from the CaPSURE database. J Urol. 2000;163:1171–7. doi: 10.1016/S0022-5347(05)67716-6. quiz 1295. [DOI] [PubMed] [Google Scholar]
- 10.Van der Kwast TH, Bolla M, Van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25:4178–86. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 11.Chin JL, Srigley J, Mayhew LA, et al. Guideline for optimization of surgical and pathological quality performance for radical prostatectomy in prostate cancer management: evidentiary base. Can Urol Assoc J. 2010;4:13–25. doi: 10.5489/cuaj.08105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrentschuk N, Evans A, Srigley J, et al. Surgical margin status among men with organ-confined (pT2) prostate cancer: a population-based study. Can Urol Assoc J. 2011;5:161–6. doi: 10.5489/cuaj.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292–5. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Aronson WJ, Terris MK, et al. Percent of prostate needle biopsy cores with cancer is significant independent predictor of prostate specific antigen recurrence following radical prostatectomy: results from SEARCH database. J Urol. 2003;169:2136–41. doi: 10.1097/01.ju.0000065588.82511.06. [DOI] [PubMed] [Google Scholar]
- 15.Evans AJ, Henry PC, Van der Kwast TH, et al. Interobserver variability between expert: Urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol. 2008;32:1503–12. doi: 10.1097/PAS.0b013e31817fb3a0. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti PE, Shikanov S, Razmaria AA, et al. Impact of prostate weight on probability of positive surgical margins in patients with low-risk prostate cancer after robotic-assisted laparoscopic radical prostatectomy. Urology. 2011;77:677–81. doi: 10.1016/j.urology.2010.07.512. [DOI] [PubMed] [Google Scholar]
- 17.Pettus JA, Masterson T, Sokol A, et al. Prostate size is associated with surgical difficulty but not functional outcome at 1 year after radical prostatectomy. J Urol. 2009;182:949–55. doi: 10.1016/j.juro.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coelho RF, Chauhan S, Orvieto MA, et al. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2010;57:1022–9. doi: 10.1016/j.eururo.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 19. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2005) ( www.seercancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch.
- 20.Yossepowitch O, Bjartell A, Eastham JA, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol. 2009;55:87–99. doi: 10.1016/j.eururo.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 21.Patel VR, Coelho RF, Rocco B, et al. Positive surgical margins after robotic assisted radical prostatectomy: a multi-institutional study. J Urol. 2011;186:511–6. doi: 10.1016/j.juro.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 22.Ahyai SA, Zacharias M, Isbarn H, et al. Prognostic significance of a positive surgical margin in pathologically organ-confined prostate cancer. BJU Int. 2010;106:478–83. doi: 10.1111/j.1464-410X.2009.09162.x. [DOI] [PubMed] [Google Scholar]
- 23.Chun FK, Briganti A, Antebi E, et al. Surgical volume is related to the rate of positive surgical margins at radical prostatectomy in European patients. BJU Int. 2006;98:1204–9. doi: 10.1111/j.1464-410X.2006.06442.x. [DOI] [PubMed] [Google Scholar]
- 24.Han M, Partin AW, Chan DY, et al. An evaluation of the decreasing incidence of positive surgical margins in a large retropubic prostatectomy series. J Urol. 2004;171:23–6. doi: 10.1097/01.ju.0000098604.09395.27. [DOI] [PubMed] [Google Scholar]
- 25.Williams SB, D’Amico AV, Weinberg AC, et al. Population-based determinants of radical prostatec-tomy surgical margin positivity. BJU Int. 2011;107:1734–40. doi: 10.1111/j.1464-410X.2010.09662.x. [DOI] [PubMed] [Google Scholar]
- 26.Shah SK, Fleet TM, Williams V, et al. SEER coding standards result in underestimation of positive surgical margin incidence at radical prostatectomy: results of a systematic audit. J Urol. 2011;186:855–9. doi: 10.1016/j.juro.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 27.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 28.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174:919–22. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Slezak J, Bergstralh EJ, et al. Preoperative prediction of surgical margin status in patients with prostate cancer treated by radical prostatectomy. J Clin Oncol. 2000;18:2862–8. doi: 10.1200/JCO.2000.18.15.2862. [DOI] [PubMed] [Google Scholar]
- 30.Quinn DI, Henshall SM, Brenner PC, et al. Prognostic significance of preoperative factors in localized prostate carcinoma treated with radical prostatectomy: importance of percentage of biopsies that contain tumor and the presence of biopsy perineural invasion. Cancer. 2003;97:1884–93. doi: 10.1002/cncr.11263. [DOI] [PubMed] [Google Scholar]
- 31.Kordan Y, Salem S, Chang SS, et al. Impact of positive apical surgical margins on likelihood of biochemical recurrence after radical prostatectomy. J Urol. 2009;182:2695–701. doi: 10.1016/j.juro.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Emerson RE, Koch MO, Jones TD, et al. The influence of extent of surgical margin positivity on prostate specific antigen recurrence. J Clin Pathol. 2005;58:1028–32. doi: 10.1136/jcp.2005.025882. [DOI] [PMC free article] [PubMed] [Google Scholar]


