Abstract
Malignant peripheral nerve sheath tumours (MPNST) of the kidney are very rare, with only 3 cases reported in the English and French literature. However, we report the first case of fast growing atypical renal cyst where a magnetic resonance imaging was an interesting adjunct to the computed tomography scan in imaging this rare tumour.
Introduction
Malignant peripheral nerve sheath tumours (MPNST), also known as malignant schwannoma, arise from Schwann cells which are derived from the neural crest. These tumours are often associated with Von Recklinghausen’s disease.1–3 Preoperative diagnosis remains challenging and is based on histology and positive immunohistochemical studies for S-100 protein. Occurrence is usually in the head and neck region or in the limbs.2–5 Renal or perirenal MPNST are exceptionally rare findings. We report a case of renal malignant peripheral nerve sheath tumour that highlights the importance of a magnetic resonance imaging (MRI) in such situation.
Case report
A 70-year-old ex-smoking male known for hypertension, diabetes mellitus, dyslipidemia, obesity (body mass index 40) and chronic renal failure (estimated glomerular filtration rate 45.4 mL/min/1.73 m2) was referred to our institution for a growing renal cyst. The patient was asymptomatic and physical examination was unremarkable. Personal and familial history was negative for von Recklinghausen’s disease. Laboratory studies, including complete blood count, electrolytes, urinalysis, glycemia and hepatic profile, were normal except for a high level of serum creatinine and normocytic anemia secondary to chronic renal disease. A computed tomography (CT) scan confirmed multiple simple cysts on both kidneys, except for an 8-cm left mid-upper pole dense cyst. A controlled CT scan 6 months later showed a slight growth and peripheral rim enhancement of this cyst with subtle changes in its content (Fig. 1, part A). An ultrasound confirmed some internal echoes compatible with cellular content. A MRI was recommended by our radiologist 2 months later, and showed a slightly hypointense left renal lesion (Fig. 1, part B) with heterogeneous enhancement following gadolinium administration on T1-weighted images (Fig. 1, part C). Lesion also appeared heterogeneously hyperintense on T2-weighted images. (Fig. 1, part D). Considering the differences between the CT and the MRI images, our radiologist suggested a repeat abdominal CT scan 3 months later which showed significant growth of the left renal mass to 14 cm with heterogeneous internal enhancement (Fig. 1, part E) suggesting a renal tumour. There was focal fat stranding involving the perirenal space. The renal vein remained permeable and without hydronephrosis.
Fig. 1.
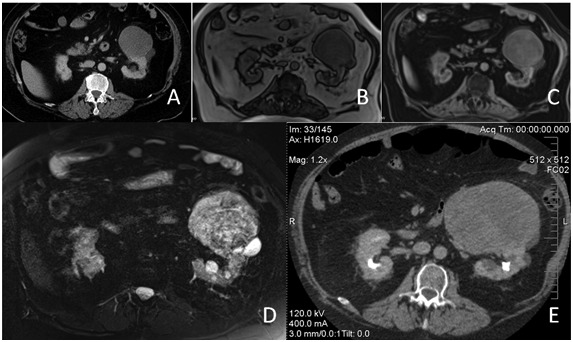
(A) Contrast enhanced computed tomography (CT) scan shows a 8-cm left renal lesion with a density of 13 Hounsfield units and slight peripheral rim enhancement. (B) T1-weighted magnetic resonance image reveals a slightly hypointense left renal lesion (C) which shows peripheral and heterogeneous internal enhancement following gadolinium administration. (D) On T2-weighted image, the left renal lesion appears heterogeneously hyperintense, with a small and very hyperintense (cystic) posterior component. (E) There is a significant growth to 14 cm of the left renal lesion on a follow-up contrast enhanced CT scan done 3 months later. The lesion shows heterogeneous internal enhancement and displaces the kidney posteriorly.
The patient underwent a transperitoneal laparoscopic radical nephrectomy. Lymph nodes were explored during surgery and none were found. Ipsilateral adrenal gland was not removed because it was radiologically normal.
Gross examination of the specimen showed a 17 × 14 × 10-cm kidney weighting about 3 kg. The tumour was gray-white, 15 cm in length and occupied almost all renal parenchyma (Fig. 2). Macroscopically, the tumour infiltrated perirenal fat and was in proximity to the fascia of Gerota without reaching it.
Fig. 2.
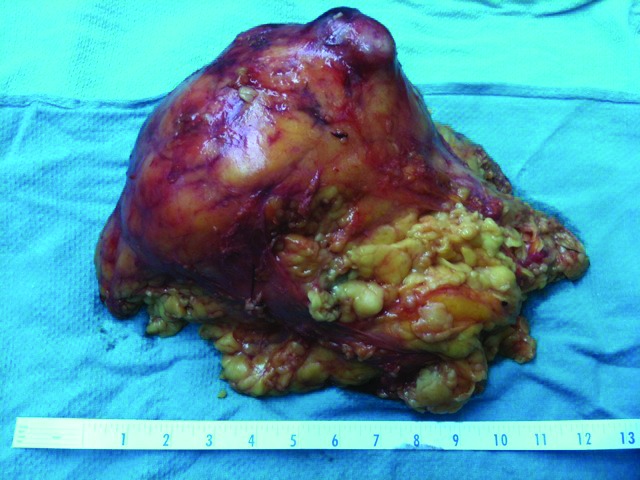
Gross pathology specimen removed by a transperitoneal laparoscopic approach. Ruler graduated in inches.
Histological examination (Fig. 3, part A) revealed a sarcoma with fusiform cell. Mitotic count showed multiple zones with >10 mitosis per high field power. Multiple areas of tumour necrosis were found. Adjacent renal parenchyma was normal, except for glomerular hyperfiltration related to chronic renal failure. No vascular emboli were present and surgical margins were negative. Immunohistochemical studies of the tumour showed strong S-100 immunoreactivity (Fig. 3, part B) consistent with MPNST and 40% to 50% of tumour cells were positive for Ki-67 (Fig. 3, part C).
Fig. 3.
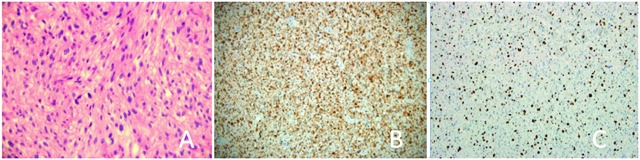
(A) Microscopic imaging (hematoxylin and eosin stain; 40×) of the tumoural proliferation composed of spindle cells with moderate cellularity and nuclear atypia (B) Immunohistochemical study demonstrating strong S-100 immunoreactivity consistent with the diagnosis of malignant peripheral nerve sheath tumours. (C) 40% to 50% of the tumour cells were positive for Ki-67. Tumour cells were negative for muscle markers.
Six months after surgery, the CT scan revealed a 7-cm ipsilateral renal fossa tumour recurrence, as well as a metastasis to the ipsilateral adrenal and pancreas. The patient died 10 months after his surgical procedure.
Discussion
MPNST are exceptionally rare tumours with an incidence of 1 to 10 per 1 000 000, representing between 3% and 12% of all soft tissue sarcomas.1,2 These tumours are usually found in patients between 20 to 50 years old, in the head and neck region, trunk or in the limbs.2–5 MPNST show poor prognosis, with reported rates of 5-year disease specific survival of 16% to 60%.1–5 The most effective treatment remains surgical resection and margin status is of primary importance.2–4,6 Conflicting evidence suggests that adjuvant radiotherapy might play a role in the management of MPNST1–3,6 However, no studies found that chemotherapy improves survival.1–3,5,7
We report the fourth case of MPNST in the renal parenchyma (Table 1).8–10 Martinot and colleagues8 reported the first case in 1960 of a 70-year-old male whose left kidney was almost totally replaced by tumour tissue; this patient died on postoperative day 5. Naslund and colleagues9 described an aggressive case of MPNST invading the diaphragm and lung. Surgical margins were positive and the patient declined radiation and chemotherapy. Four months later a 7-cm lesion was found in the renal fossa and the patient eventually died 15 months after surgery. Most recently, a case of renal MPNST with metastasis to the scalp, lung and shoulder was treated with neoadjuvant doxorubicin and surgical resection of all lesions.10 This patient is free from recurrence at the 2-year follow-up.
Table 1.
Summary of renal and perirenal MPSNT in English and French literature
| First author | Age/ Sex | Presentation | Size/side, site (cm) | Main treatment | Follow-up |
|---|---|---|---|---|---|
| Present case | 70/M | Incidental finding | 15/left, parenchyma | Laparoscopic radical nephrectomy | Died at 10 mo |
| Martinot8 | 70/M | Lower back and flank pain, weight loss, fatigue, fever | NR/left, parenchyma | Radical nephrectomy | Died 5 days postoperative |
| Naslund9 | 50/F | Flank pain, weight loss, palpable mass | 14/left, parenchyma | Radical nephrectomy | Died at 15 mo with metastasis |
| Williams10 | 75/F | Scalp lesion (metastasis) | 8/left, parenchyma | Radical nephrectomy | 24 mo |
| Khandelwal11 | 40/M | Lower back discomfort, palpable mass | NR/left, renal capsule | Radical nephrectomy | NR |
| Jankulovski12 | 65/F | Flank pain, weight loss, palpable mass | 13/left, renal capsule | Radical nephrectomy | 6 mo |
| Romics13 | 52/M | Flank pain, fever | NR/right, renal capsule | Radical nephrectomy | Died at 3 mo with metastasis |
| Voznesensky15 | 46/F | Gross hematuria | 4/right, renal pelvis | Laparoscopic nephroureterectomy | 36 mo |
| Fein14 | 51/F | Flank pain, recurrent pyelonephritis, palpable mass | 6/right, renal pelvis | Radical nephrectomy | 24 mo |
| Pantuck16 | 50/F | Palpable mass | 28/right, perirenal | Radical nephrectomy | Died at 42 mo |
| Parfitt17 | 63/M | Weakness, fatigue, weight loss, palpable mass | 11 and 6/left, perirenal | Radical nephrectomy | 9 mo |
| Bair18 | 56/M | Microscopic hematuria | 7/right, perirenal | Radical nephrectomy | 5 mo |
| Deming19 | 33/F | Abdominal pain, palpable mass | NR/left, perirenal | Tumour excision without nephrectomy | Died at 11 mo with metastasis |
| Cachay20 | 74/F | Follow-up for malignant subcutaneous schwannoma | 9/right, metastasis | Radical nephrectomy | 5 mo |
| García Figueiras21 | 73/F | Follow-up for malignant soft-tissue MPNST | 8/right, metastasis | Radical nephrectomy | Died at 5 mo |
MPNST: malignant peripheral nerve sheath tumours; mo: months, NR: not reported.
Cases of MPNST of the renal capsule were also reported.11–13 Romics and colleagues13 reported a case of MPSNT arising from the right renal capsule invading liver and extending up to diaphragm. Surgical margins were positive and the patient developed pulmonary metastasis and died at 3 months following surgical resection. Longer survivals of 2414 and 3615 months were reported in 2 cases of renal pelvis MPSNT. Primary16–19 and metastatic20,21 perirenal MPNST have also been reported and tend to present as large renal masses.
Despite the fact that most MPNST are diagnosed in patients with von Recklinghausen’s disease,1–3 no renal or perirenal cases were reported. The diagnosis of renal or perirenal MPNST remains challenging and was established by pathologic examination in all reported cases. As known, the renal CT scan is the gold standard for delineating the nature of a renal mass and MRI remains an alternate modality. However, some MRI findings were reported to be suggestive of benign peripheral nerve sheath tumour (PNST).22 They included isointensity on T1-weighted images, a high signal intensity on T2-weighted images22,23 and strong and homogeneous gadolinium-enhancement on T1-weighted images.24,25 We report, however, the first case of malignant tumor with MRI. Although our case had heterogeneous enhancement following gadolinium administration, it shared some features of PNST, such as enhancing outer rim on T1-weighted images and hyperintensity on T2-weighted images,22,23 that should raise suspicion for MPNST. Finally, the rapid evolution of the radiological features reported in this case and the poor survival shows the aggressiveness of MPNST.
In this case, the discrepancies between CT scan and MRI images might be due to the superiority of MRI for detecting such tumours or to actual tumour growth as images were taken 2 months apart. However, it shows that MRI might help in the differential diagnosis of renal cyst when ultra-sonography and CT scan cannot provide a definitive diagnosis. It also highlights the importance of MRI and serial radiological follow-up for atypical renal cyst with rapid growth that might carry poor prognosis and should prompt surgical management.
Conclusion
We reported a rare case of MPNST presenting as an atypical renal cyst where MRI provided important information along with the CT scan.
Acknowledgments
We would like to thank Tania Fayad, PhD, for the critical reading of this article.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Kar M, Deo SV, Shukla NK, et al. Malignant peripheral nerve sheath tumors (MPNST) – Clinicopathological study and treatment outcome of twenty-four Cases. World J Surg Oncol. 2006;4:55. doi: 10.1186/1477-7819-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–21. doi: 10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Wanebo JE, Malik JM, VandenBerg SR, et al. Malignant peripheral nerve sheath tumors. A clinico-pathologic study of 28 cases. Cancer. 1993;71:1247–53. doi: 10.1002/1097-0142(19930215)71:4<1247::AID-CNCR2820710413>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Lafemina J, Qin LX, Moraco NH, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013;20:66–72. doi: 10.1245/s10434-012-2573-2. . Epub 2012 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–85. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 6.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–74. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 7.Moretti VM, Crawford EA, Staddon AP, et al. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol. 2011;34:417–21. doi: 10.1097/COC.0b013e3181e9c08a. [DOI] [PubMed] [Google Scholar]
- 8.Martinot M, Dupont A, Demaille A. Malignant schwannoma of the kidney. J Urol Medicale Chir. 1960;66:748–52. [PubMed] [Google Scholar]
- 9.Naslund MJ, Dement S, Marshall FF. Malignant renal schwannoma. Urology. 1991;38:477–9. doi: 10.1016/0090-4295(91)80243-Z. [DOI] [PubMed] [Google Scholar]
- 10.Williams SB, Szlyk GR, Manyak MJ. Malignant peripheral nerve sheath tumor of the kidney. Int J Urol. 2006;13:74–5. doi: 10.1111/j.1442-2042.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 11.Khandelwal A, Gupta A, Khandelwal K. Malignant peripheral nerve sheath tumor of the kidney. Iran J Kidney Dis. 2011;5:373. [PubMed] [Google Scholar]
- 12.Jankulovski N, Stankov O, Banev S, et al. Isolated malignant peripheral nerve sheath tumor of kidney capsule. Prilozi. 2008;29:361–9. [PubMed] [Google Scholar]
- 13.Romics I, Bach D, Beutler W. Malignant schwannoma of kidney capsule. Urology. 1992;40:453–5. doi: 10.1016/0090-4295(92)90463-7. [DOI] [PubMed] [Google Scholar]
- 14.Fein RL, Hamm FC. Malignant schwannoma of the renal pelvis: a review of the literature and a case report. J Urol. 1965;94:356–61. doi: 10.1016/S0022-5347(17)63631-0. [DOI] [PubMed] [Google Scholar]
- 15.Voznesensky MA, Yamase H, Taylor JA. Malignant peripheral nerve sheath tumor of the renal pelvis. Urol Int. 2009;83:370–2. doi: 10.1159/000241687. [DOI] [PubMed] [Google Scholar]
- 16.Pantuck AJ, Barone JG, Amenta PS, et al. Diagnosis and management of malignant perirenal schwannoma. Am Surg. 1996;62:1024–7. [PubMed] [Google Scholar]
- 17.Parfitt HE, Jr, Hammond ME, Middletin AW., Jr Perirenal malignant schwannoma: a case report and review of the literature. J Urol. 1982;128:1299–301. doi: 10.1016/s0022-5347(17)53471-0. [DOI] [PubMed] [Google Scholar]
- 18.Bair ED, Woodside JR, Williams WL, et al. Perirenal malignant schwannoma presenting as renal cell carcinoma. Urology. 1978;11:510–2. doi: 10.1016/0090-4295(78)90173-5. [DOI] [PubMed] [Google Scholar]
- 19.Deming CL, Newman HR. Schwannomas. J Urol. 1954;72:316–23. doi: 10.1016/S0022-5347(17)67587-6. [DOI] [PubMed] [Google Scholar]
- 20.Cachay M, Sousa-Escandón A, Gibernau R, et al. Malignant metastatic perirenal schwannoma. Scand J Urol Nephrol. 2003;37:443–5. doi: 10.1080/00365590310019026. [DOI] [PubMed] [Google Scholar]
- 21.García Figueiras R, Cachay Ayala ME, et al. Solitary perirenal metastasis from a malignant peripheral nerve sheath tumour mimicking a primary renal tumour. Australas Radiol. 2003;47:188–9. doi: 10.1046/j.0004-8461.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 22.Hung SF, Chung SD, Lai MK, et al. Renal schwannoma: case report and literature review. Urology. 2008;72:716e3–6. doi: 10.1016/j.urology.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Anders KH. Neurilemoma of the kidney. Urology. 1996;47:575–81. doi: 10.1016/S0090-4295(99)80500-7. [DOI] [PubMed] [Google Scholar]
- 24.Tsurusaki M, Mimura F, Yasui N, et al. Neurilemoma of the renal capsule: MR imaging and pathologic correlation. Eur Radiol. 2011;11:1824–37. doi: 10.1007/s003300000767. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa K, Yamahana T, Hirano S, et al. MR imaging of neurilemoma arising from the renal hilus. J Comput Assist Tomogr. 1990;14:830–2. doi: 10.1097/00004728-199009000-00034. [DOI] [PubMed] [Google Scholar]


