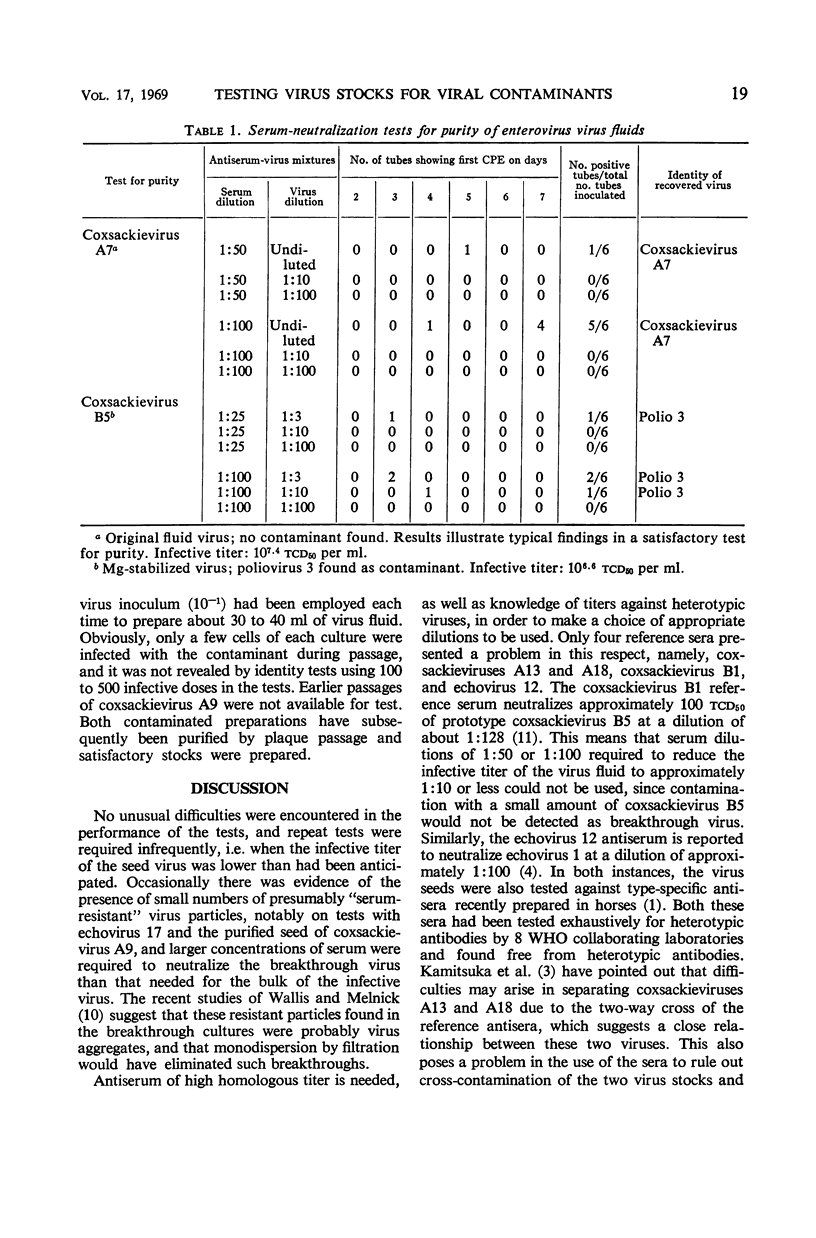
Abstract
A practical method for testing the purity of virus stocks has been developed and applied to reference stocks of enteroviruses. The method requires the use of a reference antiserum that is substantially free from heterotypic antibody. When selected dilutions of this antiserum are reacted with high concentrations of virus, virus intentionally allowed to escape neutralization is recovered and then identified. A contaminating virus present as a minor component of the population has a far greater probability of being revealed under the conditions of this “breakthrough” test than under the commonly used virus identity tests which customarily employ approximately 100 TCD50 of virus and, therefore, only identify the major component of the virus population. The breakthrough test described has been used for tests of 104 reference stocks of all the enteroviruses that propagate in monkey kidney cells and human amnion cells. Although all the materials had been previously tested and approved by the commonly employed virus identity test, the breakthrough test in two instances revealed contaminating heterotypic enteroviruses present at a very low titer in the stocks. Without resorting to the stringent, elaborate, and expensive tests for absolute purity, such as those that are required to assure safety of vaccines, the use of the breakthrough test described provides reasonable assurance of purity for stock viruses to be employed as diagnostic reagents or for general laboratory research purposes where a multiplicity of viral agents and antisera are required.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hampil B., Melnick J. L. WHO collaborative studies on enterovirus reference antisera: second report. Bull World Health Organ. 1968;38(4):577–593. [PMC free article] [PubMed] [Google Scholar]
- Hara M., Komatsu T., Sasaki M., Tagaya I. Lack of serological relationship of ECHO virus types 1 and 12. Proc Soc Exp Biol Med. 1968 Jul;128(3):683–687. doi: 10.3181/00379727-128-33099. [DOI] [PubMed] [Google Scholar]
- KAMITSUKA P. S., LOU T. Y., FABIYI A., WENNER H. A. PREPARATION AND STANDARDIZATION OF COXSACKIEVIRUS REFERENCE ANTISERA. I. FOR TWENTY-FOUR GROUP A VIRUSES. Am J Epidemiol. 1965 May;81:283–306. doi: 10.1093/oxfordjournals.aje.a120516. [DOI] [PubMed] [Google Scholar]
- KAMITSUKA P. S., SOERGEL M. E., WENNER H. A. Production and standardization of ECHO reference antisera. I. For 25 prototypic ECHO viruses. Am J Hyg. 1961 Jul;74:7–25. doi: 10.1093/oxfordjournals.aje.a120203. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Hampil B. WHO collaborative studies on enterovirus reference antisera. Bull World Health Organ. 1965;33(6):761–772. [PMC free article] [PubMed] [Google Scholar]
- OCAMPO A. R., MELNICK J. L. PRODUCTION OF HIGH-TITER ENTEROVIRUS ANTISERA IN BABOONS. Am J Hyg. 1964 May;79:349–356. doi: 10.1093/oxfordjournals.aje.a120389. [DOI] [PubMed] [Google Scholar]
- ROSEN L., BEHBEHANI A. M., KAMITSUKA P. S., KERN J., LENNETTE E. H., MELNICK J. L., SCHMIDT N. J., WENNER H. A. ON THE ALLEGED ANTIGENIC RELATION BETWEEN ECHO VIRUS TYPES 29 AND 32. Proc Soc Exp Biol Med. 1965 Jul;119:908–910. doi: 10.3181/00379727-119-30333. [DOI] [PubMed] [Google Scholar]
- ROSEN L., KERN J. TOLUCA-3, A NEWLY RECOGNIZED ENTEROVIRUS. Proc Soc Exp Biol Med. 1965 Feb;118:389–391. doi: 10.3181/00379727-118-29852. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Cationic inactivation of vacuolating virus (SV40) in poliovirus suspensions. Tex Rep Biol Med. 1961;19:701–705. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967 Jun;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



