Abstract
Objective
To determine the relationship between mortality risk and the CD4 cell response to antiretroviral therapy (ART).
Design
Observational community-based ART cohort in South Africa.
Methods
CD4 cell counts were measured 4 monthly, and deaths were prospectively ascertained. Cumulative person-time accrued within a range of updated CD4 cell count strata (CD4 cell-strata) was calculated and used to derive CD4 cell-stratified mortality rates.
Results
Patients (2423) (median baseline CD4 cell count of 105 cells/ml) were observed for up to 5 years of ART. One hundred and ninety-seven patients died during 3155 person years of observation. In multivariate analysis, mortality rate ratios associated with 0–49, 50–99, 100–199, 200–299, 300– 399, 400–499 and at least 500 cells/ml updated CD4 cell-strata were 11.6, 4.9, 2.6, 1.7, 1.5, 1.4 and 1.0, respectively. Analysis of CD4 cell count recovery permitted calculations of person-time accrued within these CD4 cell strata. Despite rapid immune recovery, high mortality in the first year of ART was related to the large proportion of person-time accrued within CD4 cell-strata less than 200 cells/ml. Moreover, patients with baseline CD4 cell counts less than 100 cells/ml had much higher cumulative mortality estimates at 1 and 4 years (11.6 and 16.7%) compared with those of patients with baseline counts of at least 100 cells/ml (5.2 and 9.5%) largely because of greater cumulative person-time at CD4 cell counts less than 200 cells/ml. Conclusion: Updated CD4 cell counts are the variable most strongly associated with mortality risk during ART. High cumulative mortality risk is associated with person-time accrued at low CD4 cell counts. National HIV programmes in resource-limited settings should be designed to minimize the time patients spend with CD4 cell counts less than 200 cells/ml both before and during ART.
Keywords: Africa, antiretroviral, antiretroviral therapy, CD4, death, HIV, immune reconstitution, immune recovery, mortality
Introduction
The countries of sub-Saharan Africa have been hit hardest by the HIV/AIDS epidemic with an estimated 22.5 million adults and children living with the infection in 2007 [1]. Of these, 1.6 million died, representing 76% of global AIDS deaths that year. Expansion of access to antiretroviral therapy (ART) in recent years, however, has offered hope of averting hundreds of thousands of deaths [2]. By April 2007, approximately 1.3 million people in the region were receiving treatment [3], and early pessimism concerning effective delivery of ART on a large scale using a simplified public health approach has largely proven unfounded. However, early mortality risk is much higher among patients treated in resource-limited settings compared with those treated in high-income countries [4,5].
Between 8 and 26% of patients receiving ART in sub-Saharan Africa die within the first year of treatment, with most deaths occurring in the first few months [4]. Baseline patient characteristics independently associated with early mortality include low CD4 cell count, advanced World Health Organization (WHO) clinical stage of disease, low body mass index, anaemia and male sex. In many programmes in Africa, median baseline CD4 cell counts are low (approximately 100–150 cells/μl) [4,6–11], and this has been highlighted as a key problem [4].
Although most existing studies from sub-Saharan Africa have examined short-term mortality outcomes and their association with baseline patient characteristics [4], some data suggest that risks of morbidity and mortality change according to the response to ART [11–13]. Data are now needed on longer term outcomes and, in particular, how mortality risk changes in association with CD4 cell count recovery. We hypothesized that even in those with rapid immune recovery, many patients with advanced immunodeficiency at baseline accrue considerable time with low CD4 cell counts, resulting in a high cumulative mortality risk. To examine these issues, we analysed prospectively collected data from patients with up to 5 years of follow-up in a large ART cohort in Cape Town, South Africa.
Methods
Antiretroviral treatment service
The ART service established in Gugulethu township in Cape Town in 2002 has previously been described in detail [6,12–14]. The district has a predominantly African population of over 300 000, the vast majority of whom live in conditions of low socioeconomic status. Ambulatory patients eligible for ART are referred to this service from a series of other primary care clinics in the township. During this study, the median time between enrolment of a patient in the service and initiation of ART was approximately 1 month. This permitted thorough preparation of patients for ART and screening and management of opportunistic infections and other comorbidities. The vast majority of patients with tuberculosis (TB) in this service have received at least 1 month of TB treatment prior to ART [15].
National ART guidelines were based on the WHO 2002 recommendations [16], providing treatment for those with a prior AIDS diagnosis (WHO stage 4 disease) or a blood CD4 cell count of less than 200 cells/μl. First-line ART comprised stavudine, lamivudine and a nonnucleoside reverse transcriptase inhibitor (predominantly efavirenz) and was supplied free of charge to patients. Treatment compliance was high, and the virological failure rate is approximately only 2% of patients per year [17]. All patients routinely received prophylaxis with trimethoprim-sulphamethoxazole both before and during ART.
Data collection
Blood CD4 cell count and plasma viral load measurements were done routinely at baseline and 4 monthly during ART within accredited laboratories. Detailed structured clinical and laboratory records were maintained for every patient visit from the time of enrolment in the programme. Data were transferred on a weekly basis to an electronic database. Patients requiring in-patient care were referred to a nearby 200-bed secondary hospital. Information on in-patient care was gained from discharge letters, hospital and laboratory records and postmortem examinations. Additional deaths and losses to follow-up were ascertained by active community-based follow-up by peer counsellors as previously described [13]. Patients were classified as lost to follow-up if they did not attend the clinic for a consultation or to collect medication for a period of 12 weeks. Collection of data on this study population for research purposes was approved by the Research Ethics Committee of the University of Cape Town; all patients enrolled gave written informed consent.
Data analysis
Data were analysed using STATA (StataCorp LP, College Station, Texas, USA). Data from all patients who initiated ART within the programme between September 2002 and June 2007 were included. Person-time of observation was accrued from the date of enrolment in the programme until either occurrence of death, loss to follow-up, transfer to another ART programme or censoring of observation in September 2007.
Person-time on ART was subdivided into 4-month intervals, each of which was defined by the CD4 cell count measurement at the start of the interval. In the event of missing CD4 cell data from the start of the interval (<5% of all intervals), we used the mean of the CD4 cell values immediately before and after the interval. Intervals were categorized into CD4 cell count strata (CD4cell-strata): 0–49, 50–99, 100–199, 200–299, 300–399, 400–499 and at least 500 cells/μl. Total person-time accrued within each of these CD4 cell-strata during follow-up was calculated.
Overall mortality rates and rates within CD4 cell-strata were calculated. Kaplan–Meier (product-limit) analyses were used to estimate cumulative mortality risk both in the overall cohort and stratified by baseline CD4 cell count. We estimated the association between baseline risk factors, updated CD4 cell counts and viral load and the incidence of mortality using univariate and multivariate-mixed effect Poisson regression models.
The relationship between CD4 cell counts and duration of ART was illustrated using lowest smooth curves obtained from a series of locally weighted regressions with bandwidth of 0.8. Poisson base confidence intervals (CI) were calculated for per person-year incidence rates and binomial exact CIs for cumulative mortality rates. In other analyses, Wilcoxon rank-sum tests were used to compare medians, and all statistical tests are two-sided at alpha value of 0.05.
Results
Cohort characteristics and follow-up
During the study period, 2878 patients were enrolled in the programme. At the time data were censored, 2423 (84%) patients had started ART, and their baseline characteristics showed that most had advanced immunodeficiency (Table 1). Of 455 patients who did not receive ART, 298 (10%) had been deferred from the programme (due to ineligibility for ART, treatment refusal or loss to follow-up), 52 (2%) were currently preparing to start treatment and 105 (4%) had died before starting ART.
Table 1.
Characteristics of the patients starting antiretroviral treatment and of the patients who subsequently died during treatment.
| Patient characteristics | Patients starting ART (n = 2423) | Survivors (n = 2231) | Deaths (n = 192) |
|---|---|---|---|
| Median (IQR) age | 33 (28–39) | 32 (28–38) | 36 (30–43) |
| Female patients | 1624 (67) | 1512 (68) | 112 (58) |
| Baseline CD4 cells (cells/μl) | |||
| Median (IQR) | 101 (48–157) | 104 (52–159) | 57 (16–111) |
| 0–49 | 618(21) | 531 (24) | 87 (45) |
| 50–99 | 574 (20) | 531 (24) | 43 (22) |
| 100–149 | 549 (1 9) | 516(23) | 33 (17) |
| 150–200 | 423 (17) | 406 (18) | 17(9) |
| ≥200 | 259 (11) | 247 (11) | 12(6) |
| Baseline log viral load | |||
| ≥5.0 log copies/ml | 1052 (43) | 946 (42) | 106 (55) |
| WHO stage | |||
| 1 and 2 | 575 (24) | 560 (25) | 15(8) |
| 3 | 1395(53) | 1 300 (58) | 95 (50) |
| 4 | 552 (23) | 470(21) | 82 (43) |
| Updated CD4 cell count prior to death (cells/μl) | |||
| Median (IQR) | – | – | 78(25–171) |
| 0–99 | – | – | 107(56) |
| 100–199 | – | – | 45 (23) |
| ≥200 | – | – | 40(21) |
| Updated viral load prior to death | |||
| ≥400 copies/ml | – | – | 1 28 (67) |
| Months ART at time of death | |||
| Median (IQR) | – | – | 7.8(3.3–22.8) |
Values show numbers (%) unless otherwise stated. ART, antiretroviral therapy; IQR, interquartile range.
Individuals were followed up for up to 5 years, and a total of 3155 person-years of follow-up accrued during ART. With cohort expansion over time, the numbers of patients alive and receiving treatment after 1, 2, 3 and 4 years of treatment were 1426, 681, 262 and 104, respectively. Of those who started ART, 1856 (77%) were still alive and receiving treatment at data censorship, 232 (10%) had been lost to follow-up, 143 (6%) had been transferred out to another ART service and 192 (8%) had died. Those who died had low CD4 cell counts; at baseline, a median CD4 cell count of 78 cells/μl [interquartile range (IQR) 25–171; range 1–966)] at the time of death (Table 1). The proportions of patients with viral load suppression of less than 400 copies/ml at 12, 24, 36 and 48 months of ART were 90.5, 90.3, 93.3 and 90.5%, respectively. One-third of those who died had an undetectable viral load just prior to death (Table 1).
Mortality rates and cumulative mortality estimates
The mortality rate during the 1-month period prior to starting ART was very high [26.6 deaths/100 person-years; 95% CI 21.8–32.3]. The rate during months 0–4 of ART was also high (16.3 deaths/100 person-years; 95% CI 13.3–19.7) but decreased steeply thereafter, reaching a rate of 4.4 (2.8–6.4) deaths/100 person-years during 8–12 months of treatment. Mortality rates in the second, third and fourth years of ART were 2.6 (1.6–3.9), 0.7 (0.2–1.6) and 0.4 (0.1–1.6) deaths/100 person-years, respectively.
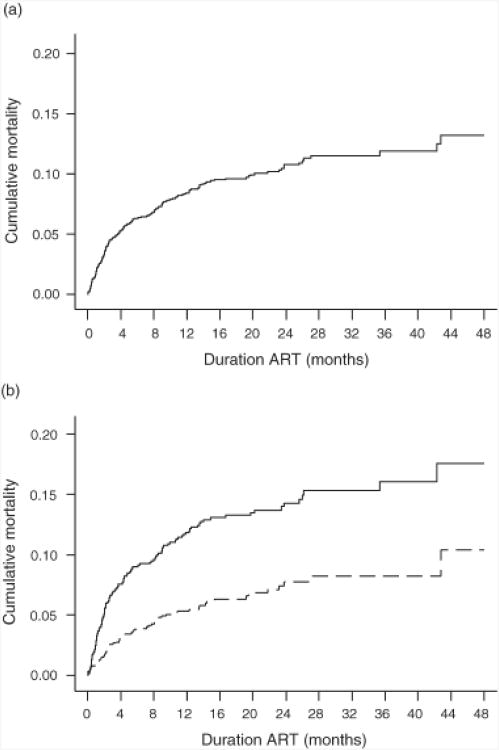
Cumulative mortality estimates over 48 months of ART were derived using Kaplan-Meier analyses (Fig. 1a). The mortality estimate at 12 months (8.4%) was approximately two-thirds of the overall estimate at 48 months (13.2%). Cumulative mortality at 48 months in patients with baseline CD4 cell counts less than 100 cells/μl was approximately double that of patients with counts more than 100 cells/μl (P < 0.001) (Fig. 1b).
Fig. 1. Kaplan–Meier plots showing cumulative mortality risk over 48 months.
(a) Cumulative mortality estimates for the whole cohort at 1 2 and 48 months of ART were 8.4 and 1 3.2%, respectively, (b) Cumulative mortality estimates al 12 and 48 months from enrolment were 11.6 and 16.7%, respectively, in those with baseline CD4 cell counts of less than 100 cells/μl (―) compared with 5.2 and 9.5% in those wilth baseline counts more than 100 cells/μl (----).
To permit comparison with data from other cohorts, mortality estimates after 48 months of ART were also determined for subgroups of patients. For those with WHO stages 1–3 and baseline CD4 cell counts of more than 100 cells/μl or less than 100 cells/μl, estimates were 6.3 and 12.6%, respectively. For those with WHO stage 4 disease and baseline CD4 cell counts of more than 100 cells/μl or less than 100 cells/μl, estimates were 20.1 and 24.8%, respectively.
Mortality rates and CD4 cell response to antiretroviral therapy
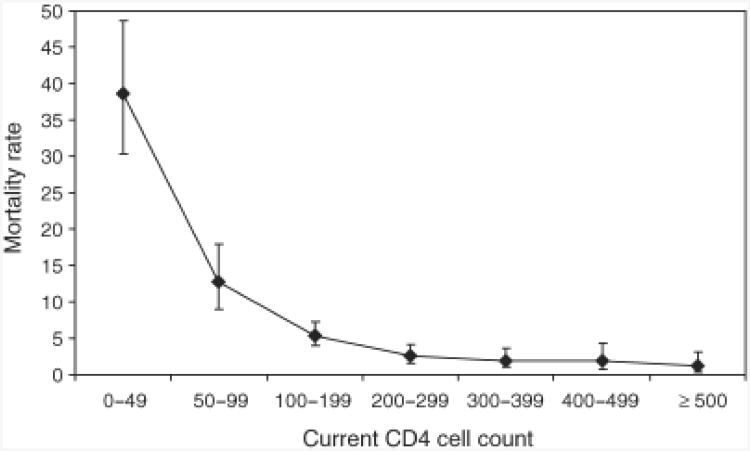
We next examined how mortality rates changed in relation to the CD4 cell response to ART. We categorized person-time of observation according to the CD4 cell count at the start of each 4-month follow-up interval. We then calculated cumulative person-time accrued within a series of CD4 cell-strata and used these as denominators to calculate mortality rates stratified by updated CD4 cell counts. The relationship between updated CD4 cell counts and mortality rates was extremely strong (Fig. 2).
Fig. 2. Graph showing the mortality rates (95% confidence interval, deaths/100 person-years) according to updated CD4 cell counts during antiretroviral therapy.
CD4 cell counts (cells/μl) were measured at baseline and 4 monthly during ART. Follow-up time intervals were defined by the CD4 cell count at the start of each interval and categorized into one of six different CD4 cell count strata. Cumulative person-time accruing within those strata was determined and used to derive the CD4 cell-stratum-specific mortality rates. Unadjusted mortality rates during person-time accrued within the 0–49, 50–99, 100–199, 200–299, 300–399, 400–499 and at least 500 cells/μl CD4 cell-strata were 38.6 (30.3–48.6), 12.8 (8.9–17.9), 5.4 (3.9–7.2), 2.7(1.6–4.1), 2.0(1.0–3.6), 2.0(0.7–4.3) and 1.2 (0.3–3.2) deaths/100 person-years, respectively.
Risk factors for mortality during antiretroviral therapy
We examined crude and adjusted associations between mortality and patient baseline characteristics and updated viral load and CD4 cell counts. In crude analyses, older age, male sex, WHO stage 4 disease, updated CD4 cell counts and detectable updated viral load measurements were all significantly associated with mortality risk (Table 2). With the exception of male sex, each of these variables remained significantly associated in the multivariate model. However, updated CD4 cell counts were the variable most strongly associated with death. Those CD4 cell-strata lying below a threshold of 200 cells/μl were each associated with a more than two-fold greater adjusted mortality risk compared with the more than 500 cells/μl CD4 cell-stratum. Above a threshold of 200 cells/μl, small cumulative reductions in mortality rates occurred as CD4 cell counts increased further, but these differences did not reach statistical significance.
Table 2.
Risk factors for mortality among patients (n = 2423) receiving antiretroviral therapy.
| Crude association | Multivariate model | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Patient characteristics | IRR | 95% CI | P-value | IRR | 95% CI | P-value |
| Age | 1.03 | 1.02–1.05 | <0.001 | 1.04 | 1.02–1.05 | < 0.001 |
| Male sex | 1.72 | 129–2.29 | <0.001 | 1.10 | 0.82–1.49 | 0.524 |
| WHO stage | ||||||
| 1–3 | 1 | 1 | ||||
| 4 | 2.31 | 1.73–3.08 | <0.001 | 2.28 | 1.70–3.07 | < 0.001 |
| Cohort enrolment year | ||||||
| 1 | 1 | 1 | ||||
| 2 | 0.59 | 0.25–1.39 | 0.227 | 0.58 | 0.25–1.36 | 0.211 |
| 3 | 0.86 | 0.41–1. HI | 0.689 | 0.82 | 0.39–1.75 | 0.615 |
| 4 | 1.38 | 0.69–2.76 | 0.360 | 1.06 | 0.52–2.16 | 0.862 |
| 5 | 2.26 | 1.12–4.55 | 0.022 | 1.27 | 0.62–2.61 | 0.513 |
| Updated CD4 cell count (cells/μl) | ||||||
| >500 | 1 | 1 | ||||
| 400–499 | 1.59 | 0.45–5.63 | 0.460 | 1.41 | 0.40–5.01 | 0.597 |
| 300–399 | 1.60 | 0.50–5.11 | 0.425 | 1.45 | 0.45–4.64 | 0.533 |
| 200–299 | 2.16 | 0.74–6.34 | 0.158 | 1.66 | 0.56–4.91 | 0.358 |
| 100–199 | 4.37 | 1.57–12.17 | 0.005 | 2.59 | 0.90–7.42 | 0.076 |
| 50–99 | 10.44 | 3.71–29.43 | <0.001 | 4.93 | 1.66–14.70 | 0.004 |
| 0–49 | 31.46 | 11.50–86.08 | <0.001 | 11.63 | 3.95–34.29 | < 0.001 |
| Updated viral load (copies/ml) | ||||||
| <400 | 1 | |||||
| >400 | 5.76 | 414–7.81 | <0.001 | 2.06 | 1.37–3.09 | < 0.001 |
Age included as a continuous variable. CI, confidence interval; IRR, incidence rate ratio.
In another model, we excluded the first 4 months of observation during ART, so that baseline CD4 cell counts could be included as a separate variable in addition to updated CD4 cell counts. In this model, updated but not baseline CD4 cell counts were significantly associated with mortality risk. Together, these models indicate that the absolute CD4 cell count at any given time point is a key determinant of mortality risk.
CD4 cell count recovery during antiretroviral therapy
We next examined how the overall distribution of CD4 cell counts in the cohort changed over time. Figure 3 shows changes in the proportions of patients with CD4 cell counts lying below a series of thresholds during ART. The proportion of patients with a CD4 cell count less than 100 cells/μl decreased rapidly from 41% at baseline, reaching a plateau of approximately 2% after 12–18 months of ART. The proportion of patients with a CD4 cell count of less than 200 cells/μl also decreased from 89% at baseline and reached a plateau of approximately 10% of patients after 24–36 months of ART. Conversely, the proportion of patients with CD4 cell counts of more than 500 cells/μl steadily increased from 0% at baseline to approximately 50% after 48 months (Fig. 3). The slope of the 500 cells/μl contour indicates substantial immune recovery was ongoing after 4 years of treatment.
Fig. 3. Graph showing smoothed changes in distribution of CD4 cell counts in the cohort over time during 48 months of antiretroviral therapy.
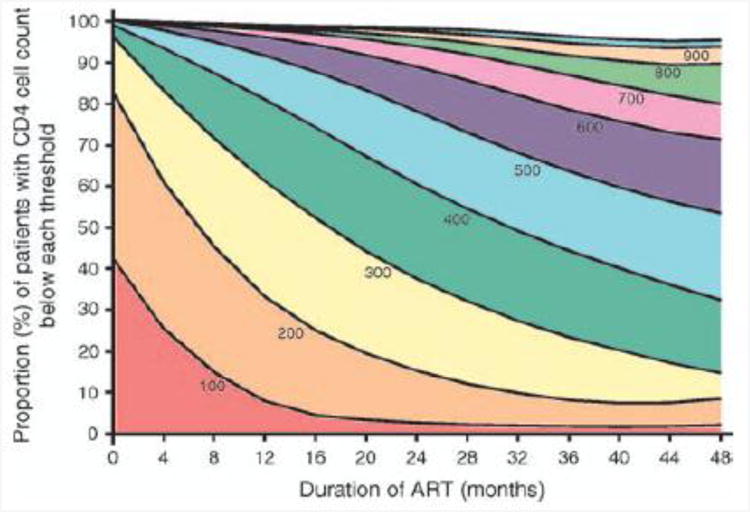
The proportions (percentage) of patients with CD4 cell counts lying below thresholds of 100, 200, 300, 400, 500, 600, 700, 800, 900 and 1000 cells/μl are shown. The proportions of patients with CD4 cell counts less than 100 and 200cells/μl decreased steeply over the 1st and 2nd years of ART, respectively. The proportion of patients with CD4 cell counts more than 500 cells/μl steadily increased throughout follow-up, indicating ongoing immune recovery. Median CD4 cell counts at 0, 12,24,36 and 48 months of ART were 101,261, 355,417 and 483 cells/μl, respectively.
Cumulative person-time within low CD4 cell-strata and mortality risk
Having established the relationship between mortality risk and updated CD4 cell counts and having characterized CD4 cell recovery over time, we reasoned that mortality rates in the overall cohort would be strongly related to the cumulative person-time accrued within low CD4 cell-strata. For each sequential year of ART, we calculated the proportions of total person-time that were accrued within different CD4 cell-strata. During years 1, 2, 3 and 4 of ART, the proportions of person-time accrued at CD4 cell counts less than 100 cells/μl were 26, 4, 1 and 1%, respectively, and the proportions of person-time accrued at CD4 cell counts less than 200 cells/μl were 63, 25, 12 and 8%, respectively. These observations provide insight into the very high mortality rates in the first year of ART, which rapidly decreased to long-term rates that were much lower.
Baseline CD4 cell counts were strongly predictive of the cumulative person-time accrued within the lowest CD4 cell-strata. Those with the lowest baseline counts (<100 cells/μl) accrued 28 and 53% of person-time with CD4 cell counts of less than 100 and 200 cells/μl, respectively, compared with 1 and 28% of person-time for those with higher baseline counts (>100 cells/μl). Thus, patients with lower baseline CD4 cell counts had a much higher cumulative mortality risk (Fig. 1b).
Discussion
In this analysis, we examined the relationship between mortality risk and CD4 cell counts measured at baseline and 4 monthly during ART (updated CD4 cell counts) in a large cohort. Mortality risk decreased markedly in association with ART-induced CD4 cell recovery, but patients remained at substantially greater mortality risk during person-time accrued at CD4 cell counts below a threshold of 200 cells/μl. Compared with patients with the highest updated CD4 cell counts, patients with updated CD4 cell counts of less than 200 cells/μl had a more than two-fold greater adjusted mortality risk and those with updated counts of less than 50 cells/μl had a more than 10-fold greater adjusted mortality risk. Cumulative person-time accrued within CD4 cell-strata lying below 200 cells/μl explains both the high mortality observed during the first year of ART and the high long-term cumulative mortality risk among those with the lowest baseline CD4 cell counts. Early HIV diagnosis and timely initiation of ART before CD4 cell counts fall 200 cells/μl are required.
Despite virological suppression rates of more than 90% and excellent CD4 cell count recovery in this cohort (Fig. 3), cumulative mortality estimates after 48 months of ART were far greater than those observed in ART cohorts in high-income countries [18,19]. Similarly aged patients in the Dutch AIDS Therapy Evaluation Project (ATHENA) cohort, for example, who have sexually acquired HIV and baseline CD4 cell counts of less than 100 cells/μl have 5-year cumulative mortality estimates of 8% (AIDS patients) or 4% (non-AIDS patients) [18]. These compare to 4-year estimates of 24.8 and 12.6%, respectively, among similarly stratified patients in our cohort. Corresponding estimates from the ATHENA cohort for patients with baseline CD4 cell counts of 100–200 cells/μl were 4% (AIDS patients) and 2% (non-AIDS patients) compared with estimates of 20.1 and 6.3% in our cohort. These findings indicate that long-term cumulative mortality estimates are over three-fold higher in our cohort.
Our data extend the findings of the Antiretroviral Treatment in Lower Income Countries (ART-LINC) collaboration, which reported that after adjustment for baseline patient characteristics, mortality risk in the first year of ART was higher among patients treated in resource-limited settings compared with those treated in high-income settings despite similar immunological and virological responses to treatment [5,20]. Reasons underlying this have yet to be defined but may include differences in the spectrum of opportunistic pathogens and access to healthcare. The most common causes of early deaths in our cohort are TB, acute sepsis, cryptococcal meningitis, wasting syndrome and Kaposi's sarcoma [6,15,21]. Immune reconstitution disease has been speculated to contribute to early mortality in this setting, and our experience is that cryptococcal disease rather than TB is more important in this regard [6,22].
Existing studies from both high-income and resource-limited settings have largely focused on the relationship between mortality risk and baseline patient characteristics [4,6–11,13,18,19]. We extended these findings, showing that mortality risk changed markedly in relationship with CD4 cell count recovery during ART (Fig. 2) and that the absolute CD4 cell count at any given time point was the key determinant of mortality risk. However, above a threshold of 200 cells/μl, further increments in CD4 cell counts were associated with only minor additional reductions in mortality rates that did not reach statistical significance in this analysis.
Immune recovery in this cohort compares very favourably with that observed in ART cohorts in high-income countries [23]. The proportion of patients with CD4 cell counts less than 200 cells/μl decreased steeply to reach a plateau of approximately 10% after 24–36 months of ART (Fig. 3). In the long term, the minority of patients with updated counts less than 200 cells/μl contributed disproportionately to overall mortality rates, and this proportion may be an important factor underlying differences in mortality rates between cohorts. Such patients may have immunological nonresponse (approximately 10% of this cohort at 48 weeks [24]) or primary or secondary virological failure. A detectable viral load during follow-up was also an independent risk factor for death, highlighting the importance of maintaining virological suppression.
The slope of the 500 cells/μl CD4 cell count contour in Fig. 3 indicates that substantial immune recovery was ongoing after 48 months of ART. However, as mortality risk varied relatively little above a CD4 cell count threshold of 200 cells/μl, the steadily increasing proportion of patients with counts more than 500 cells/μl is unlikely to impact overall mortality rates substantially. This may be more important, however, with regard to reducing risk of morbidity due, for example, to TB, which persists at high rates during ART in this setting [25].
Cumulative mortality risk was strongly related to the proportions of person-time accrued within different CD4 cell-strata. During the first year of ART, the proportions of person-time accrued within CD4 cell-strata lying below 200 cells/μl were large, and the mortality that accrued within this period was correspondingly high. Beyond 1 year of treatment, these proportions rapidly decreased, accounting for much lower mortality rates thereafter.
In multivariate analyses, mortality risk at any given time point was associated with updated CD4 cell count rather than the baseline value. Lower baseline counts were, however, predictive of greater proportions of person-time accrued within low CD4 cell-strata with high mortality risk. Thus, cumulative mortality estimates at 48 months were much higher among patients with baseline CD4 cell counts less than 100 cells/μl compared with those with higher baseline counts (Fig. 1b). Median baseline CD4 cell counts are low in most ART programmes in sub-Saharan Africa, and so many patients remain at high mortality risk for considerable periods.
These data indicate that patients should start ART before their CD4 cell counts fall below 200 cells/μl, though this only occurs in a small minority of patients in Africa at present. Earlier initiation of ART will require early HIV diagnosis and strengthening of longitudinal HIV care, with serial assessments of CD4 cell counts and clinical status to trigger initiation of ART at an appropriate time point. Eligibility criteria for ART in national programmes in the region need to be reconsidered. The policy in South Africa, for example, restricts ART to patients with WHO stage 4 disease (AIDS) or a CD4 cell count less than 200 cells/μl [26], which is not in keeping with current WHO guidelines [27]. Thus, current South African policy restricts the potential survival benefits that could be derived from ART.
Strengths of this study include the completeness of data, with less than 5% of CD4 cell count data-points missing, good ascertainment of outcomes and a low loss to follow-up rate. Previous data indicate that inadvertent misclassification of deaths as losses to follow-up in this cohort is infrequent [13]. This analysis is novel with inclusion of updated CD4 cell counts and viral load measurements as time-dependent covariates. A much larger cohort would be needed to detect any significant differences in mortality risk in higher CD4 cell-strata, however. Baseline characteristics of patients were typical of those accessing ART in other public sector programmes across sub-Saharan Africa. However, mortality and loss to follow-up rates were lower than those observed in many other programmes, and absolute CD4 cell-stratified mortality rates may not be representative of all programmes [4,28].
In summary, long-term cumulative mortality estimates in this South African cohort were approximately three-fold higher than those in cohorts in high-income countries despite excellent immunological and virological responses to ART. Mortality risk during ART was strongly associated with updated CD4 cell counts, and overall mortality was related to the proportions of person-time accrued within low CD4 cell-strata. Person-time accrued at CD4 cell counts less than 200 cells/μl must be minimized before and during ART. National HIV programmes should take measures to promote early diagnosis of HIV and initiation of ART before CD4 cell counts fall below 200 cells/μl.
Acknowledgments
We acknowledge the dedicated work of the staff at the Hannan Crusaid ART clinic in Gugulethu, Cape Town and the Desmond Tutu HIV Centre at the University of Cape Town, which made this research possible. The analyses were designed by S.D.L., F.L. and R.W. and carried out by F.L., L.-G.B., R.K., C.O. and R.W. were responsible for the study cohort. R.K., E.C. and C.O. acquired and managed the data. S.D.L. wrote the manuscript with critical input from all the authors who approved the final version. S.D.L. is funded by the Wellcome Trust, London, UK with grant number 074641. R.W. and L.-G.B. are funded in part by the National Institutes of Health through a CIPRA grant 1U19AI53217-01 and RO1 grant (A1058736-01A1). These funding agencies played no role in the conducting the research or the preparation of this manuscript.
Footnotes
There were no conflicts of interest.
References
- 1.UNAIDS/WHO. AIDS epidemic update. 2007 http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.Walensky RP, Wood R, Weinstein MC, Martinson NA, Losina E, Fofana MO, et al. Scaling up antiretroviral therapy in South Africa: the impact of speed on survival. J Infect Dis. 2008;197:1324–1332. doi: 10.1086/587184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector:progress report. 2007 http://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf.
- 4.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 7.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 8.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 9.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 10.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 11.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 14.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact within an antiretroviral treatment programme in sub-Saharan Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation. Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach; Executive Summary. Geneva: World Health Organisation, Geneva; 2002. [PubMed] [Google Scholar]
- 17.Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker LG, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12:83–88. [PubMed] [Google Scholar]
- 18.van Sighem AI, van de Wiel MA, Ghani AC, Jambroes M, Reiss P, Gyssens IC, et al. Mortality and progression to AIDS after starting highly active antiretroviral therapy. AIDS. 2003;17:2227–2236. doi: 10.1097/00002030-200310170-00011. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 20.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2252. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, French MA. Immune reconstitution disease: recent developments and implications for antiretroviral treatment in resource-limited settings. Curr Opin HIV/AIDS. 2007;2:339–345. doi: 10.1097/COH.0b013e3281a3c0a6. [DOI] [PubMed] [Google Scholar]
- 23.Battegay M, Nuesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Wood R. National adult antiretroviral therapy guidelines in South Africa: concordance with 2003 WHO guidelines? AIDS. 2007;21:121–122. doi: 10.1097/QAD.0b013e3280117fa5. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organisation. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. WHO; Geneva: 2006. revision. [PubMed] [Google Scholar]
- 28.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]