Abstract
The possible interaction between brain hypoperfusion related to aging and/or vascular disease, vascular parkinsonism and Parkinson’s disease, as well as the possible contribution of aging-related chronic brain hypoperfusion in the development or severity of Parkinson’s disease are largely unknown. We used a rat model of chronic cerebral hypoperfusion to study the long-term effects of hypoperfusion on dopaminergic neurons and the possible synergistic effects between chronic hypoperfusion and factors that are deleterious to dopaminergic neurons, such as the dopaminergic neurotoxin 6-hydroxydopamine. Chronic hypoperfusion induced significant loss of dopaminergic neurons and striatal dopaminergic terminals and a reduction in striatal dopamine levels. Furthermore, intrastriatal administration of 6-hydroxydopamine in rats subjected to chronic hypoperfusion induced a significantly greater loss of dopaminergic neurons than in sham-operated control rats. The dopaminergic neuron loss was significantly reduced by oral treatment with angiotensin type 1 receptor antagonist candesartan (3 mg/kg/day). The levels of angiotensin type 2 receptors were lower and the levels of angiotensin type 1 receptors, interleukin-1 β and nicotinamide adenine dinucleotide phosphate oxidase activity were higher in the substantia nigra of rats subjected to chronic hypoperfusion than in control rats; this was significantly reduced by treatment with candesartan. The results suggest that early treatment of vascular disease should be considered in the treatment of aged Parkinson’s disease patients and Parkinson’s disease patients with cerebrovascular risk factors. The findings also suggest that inhibition of brain renin–angiotensin activity may be useful as a neuroprotective strategy.
Keywords: Aging, Renin–angiotensin system, Cerebrovascular disease, Ischemia, Parkinson, Vascular parkinsonism
Introduction
Vascular parkinsonism (VP) is an aging-related heterogeneous clinical entity caused by cerebrovascular disease, which is generally considered distinct from idiopathic Parkinson’s disease (PD). However, the literature is somewhat conflicting and this simple dichotomy may need revision. It remains largely unknown whether there is an interaction between vascular pathology and PD and whether aging-related vascular pathology contributes to PD development or severity. An increase in the severity of PD may be merely the result of an additive effect of two independent but convergent diseases; PD and cerebrovascular disease increase in prevalence with age, and therefore may occur concomitantly and overlap in their syntomatology (Bohnen and Albin 2011; Jellinger 2006; Papapetropoulos et al. 2004). However, both diseases may also be interdependent; aging-related chronic cerebral hypoperfusion may induce dopaminergic cell death or act synergistically with other factors to induce or exacerbate dopaminergic degeneration. It is known that aging and cerebrovascular disease are accompanied by a reduced cerebral blood flow (Farkas and Luiten 2001), and we have recently shown an age-dependent decrease in density of nigral microvessels (Villar-Cheda et al. 2009). As dopaminergic neurons are highly sensitive to metabolic stress and more vulnerable to damage than those in other brain regions (Mercuri et al. 1994; Singh et al. 2007), modifications of the vascular microenvironment may affect the vulnerability of the dopaminergic neurons, and contribute to development or exacerbation of PD. Consistent with this, recent studies have observed dopaminergic cell loss and parkinsonian signs in elders without PD (Buchman et al. 2012), and reduction in presynaptic dopaminergic function in majority of patients with VP (Zijlmans et al. 2007). However, the contributions of cerebrovascular disease and chronic hypoperfusion to dopaminergic neuron degeneration have not been demonstrated experimentally. Furthermore, identification of potential factors and mechanisms linking hypoperfusion and neurodegeneration may lead to neuroprotective strategies.
The brain renin–angiotensin system (RAS) may play a major role in linking vascular disease and dopaminergic degeneration. Local AII, via angiotensin receptors type 1 (AT1) receptors, is one of the most important known inducers of inflammation and oxidative stress, and the link between arterial wall RAS and the pathophysiology of the vascular disease is well known (Griendling et al. 2000; Münzel and Keany 2001; Ruiz-Ortega et al. 2001). Furthermore, numerous studies in different tissues have shown that local RAS is involved in age-related degenerative changes and longevity (Basso et al. 2005; Benigni et al. 2009, 2012). Finally, in recent studies, we have shown that brain AII, via AT1 receptors, exacerbates dopaminergic cell death and may play a synergistic role in the pathogenesis and progression of PD (Joglar et al. 2009; Rodriguez-Pallares et al. 2008), and that aging decreases nigral microvascularization (Villar-Cheda et al. 2009) and enhances nigral RAS activity (Rodriguez-Perez et al. 2012; Villar-Cheda et al. 2012). In the present study, we used a well-known rat model of chronic cerebral hypoperfusion (CHP; Farkas et al. 2007) to study the long-term effects of hypoperfusion on dopaminergic neurons and the possible synergistic effects between hypoperfusion and factors that are deleterious to dopaminergic neurons (low doses of the dopaminergic neurotoxin 6-hydroxydopamine), which may lead to increased dopaminergic cell death. Furthermore, we studied the involvement of the nigral RAS, and considered whether the blockage of AT1 receptors may inhibit the hypoperfusion-derived dopaminergic degeneration.
Methods
Experimental design
Male Sprague–Dawley rats (10 weeks old at the beginning of the experiments; 18 weeks old when the brains were analyzed) were used in the present study. All experiments were carried out in accordance with Directive 2010/63/EU and the Directive 86/609/CEE and approved by the corresponding committee at the University of Santiago de Compostela. The first set of experiments (Table 1) was carried out to investigate the effects of chronic cerebral hypoperfusion on dopaminergic neuron degeneration and the possible role of nigral RAS activation in this effect. CHP was induced by permanent bilateral common carotid artery occlusion under ketamine/medetomidine (0.5/75 mg/kg) anesthesia. In some mammal species, carotid occlusion leads to severe forebrain ischemia. In contrast, the complete circle of Willis in the rat affords incessant (but reduced) blood flow from the vertebral arteries after the onset of occlusion, so that cerebral hypoperfusion rather than stroke occurs (see for details on this animal model Farkas et al. 2007). The rats were divided into three groups. Rats in group A1 were sham-operated controls (n = 24). Rats in group B1 were subjected to carotid artery occlusion (n = 24). Rats in group C1 (n = 24) were subjected to carotid artery occlusion and oral treatment with the AT1 receptor antagonist candesartan (Astra-Zeneca; 3 mg/kg/day) from 20 days postsurgery until they were killed (56 days postsurgery). The powdered drug was administered orally to the rats mixed with peanut butter; animals in control groups were given only peanut butter. Some rats from different groups (n = 5 per group) were perfused and processed for immunohistochemistry (see below). The remaining rats were killed by decapitation and the brains were rapidly removed. The area of the substantia nigra in the ventral mesencephalon and the striatum were carefully dissected on an ice-cold plate, frozen on dry ice, and stored at −80 °C until analysis. The samples were processed for high-performance liquid chromatography (HPLC), real-time reverse transcription polymerase chain reaction (RT-PCR), western blot or chemiluminescence to determine the levels of dopamine and metabolites, expression of AT1 and angiotensin receptors type 2 (AT2) receptors, levels of the pro-inflammatory cytokine interleukin-1 β (IL-1β), expression of the nicotinamide adenine dinucleotide phosphate (NADPH) cytosolic subunit p47 (as an indicator of the level of activation of the NADPH complex) and levels of NADPH oxidase activity. In a second series of experiments, rats in the different groups (control rats, group A2; CHP rats, group B2; rats with CHP and treated with candesartan, group C2) were injected with low doses of the dopaminergic neurotoxin 6-hydroxydopamine (6-OHDA) or vehicle (CHP + vehicle, group B1) in the right striatum (25 days after the bilateral common carotid occlusion; n = 6 per group). Rats in group C2 were treated with candesartan from 10 days before 6-OHDA lesion until they were killed as above (56 days after carotid artery occlusion or sham surgery) and processed for immunohistochemistry to investigate the possible synergistic effect of chronic cerebral hypoperfusion and other possible deleterious factors acting on dopaminergic neurons.
Table 1.
Experimental design

Chronic cerebral hypoperfusion surgery and intrastriatal injection of 6-OHDA
A midline ventral cervical incision was made to expose both common carotid arteries. The carotid arteries were then carefully isolated from the carotid sheath and vagus nerve, and each isolated artery was ligated with a 5/0 silk suture. The mortality rate was 15–20 %. Careful isolation of the carotid arteries and in particular very careful handling of the vagus nerve by a researcher with experience in surgical procedures is essential to minimize mortality. Animals in the sham-operated group (group A) were subjected to the same surgical procedure, except that the arteries were not ligated. During the surgical procedure, the rectal temperature of the rats was maintained at 37 ± 0.5 °C by using a temperature-maintained platform. As postsurgical care, each rat was kept in a separate cage until recovery.
Rats in the second series of experiments were injected intrastriatally with 6-OHDA or vehicle (Przedborski et al. 1995; Sauer and Oertel 1994). Thirty minutes prior to intrastriatal injection with 6-OHDA or vehicle, rats were treated with desipramine (Sigma, 25 mg/kg i.p.), a selective inhibitor of the norepinephrine transporter, to prevent uptake of 6-OHDA by noradrenergic terminals. The rats were injected in the right striatum with 7 μg of 6-OHDA (in 3 μl of saline containing 0.2 % ascorbic acid; Sigma, USA). Stereotaxic coordinates were 1 mm anterior to bregma, 3.0 mm right of midline, and 5.5 mm ventral to the dura, tooth bar at −3.3. Control animals were injected with 3 μl of sterile ascorbate saline alone. Finally, rats were killed by an overdose of chloral hydrate (400 mg/kg), and then perfused and processed for histology (see below). The location of the 6-OHDA injection site was confirmed in the immunostained striatal sections.
Immunohistochemistry cresyl violet staining and dopaminergic neuron quantification
The animals used for immunohistochemistry were perfused, firstly with 0.9 % saline, and then with cold 4 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed, washed, and cryoprotected in the same buffer containing 20 % sucrose, and finally cut on a freezing microtome (30 μm thick). Sections were processed for tyrosine hydroxylase (TH) immunohistochemistry as follows. The sections were incubated for 1 h in 10 % normal swine serum with 0.25 % Triton X-100 in 20 mM potassium phosphate-buffered saline containing 1 % bovine serum albumin (KPBS-BSA) and then incubated overnight at 4 °C with a mouse monoclonal antiserum to TH (1:10,000; Sigma). The sections were subsequently incubated; first, with the corresponding biotinylated secondary antibody (horse antimouse, 1:200), and then with avidin–biotin–peroxidase complex (ABC, 1:100, Vector). Finally, the labeling was revealed by treatment with 0.04 % hydrogen peroxide and 0.05 % 3-3′diaminobenzidine (Sigma).
The total number of TH-immunoreactive (TH-ir) neurons in the substantia nigra compacta (SNc) was estimated by an unbiased stereological method (i.e., the optical fractionator). The stereological analysis was carried out with the Olympus CAST-Grid system (Computer-Assisted Stereological Toolbox; Olympus, Denmark). Uniform randomly chosen sections through the substantia nigra (i.e., every fourth section) were analyzed for the total number of TH-ir cells by means of a stereological grid (fractionator), and the nigral volume was estimated according to Cavalieri’s method (Gundersen et al. 1988). To confirm that 6-OHDA induces neuron death (i.e., the loss of TH-ir is not due to phenotypic downregulation of TH activity) series of sections through the entire substantia nigra of control rats and rats treated with 6-OHDA were counterstained with cresyl violet, and the total number of neurons in the SNc was estimated by the unbiased stereology method described above for TH-ir cells (see Rey et al. 2007 for details).
The density of striatal dopaminergic terminals was estimated as the optical density of the striatal TH-ir with the aid of NIH-Image 1.55 image analysis software (Wayne Rasband, MIMH) in a personal computer coupled to a video camera (CCD-72, MTI) and a constant illumination light table (Northern Light, St. Catharines, Canada). At least four sections, through the central striatum of each animal, were measured (both the right and left striatum) and for each section, the optical densities were corrected by subtraction of background, as observed in the corpus callosum.
RNA extraction and real-time quantitative RT-PCR
Total RNA from the nigral region was extracted with Trizol (Invitrogen), according to the manufacturer’s instructions. Total RNA (2.5 μg) was reverse-transcribed to cDNA with dNTPs, random primers, and Moloney murine leukemia virus reverse transcriptase (M-MLV; 200U; Invitrogen). Real-time PCR was used to examine the relative levels of IL-1β, p47, angiotensin receptors type 1 (AT1a) and type 2 (AT2) mRNA. Experiments were performed with a real-time iCyclerTM PCR platform (BioRad). β-Actin was used as a housekeeping gene and was amplified in parallel with the genes of interest. The comparative Ct method was used to examine the relative mRNA expression. The expression of each gene was obtained as relative to the housekeeping transcripts. The data were then normalized to the values of the control group (group A) of the same batch (100 %), to counteract any possible variability among batches. The specifity of the RT-PCR data used to identify RAS components has been confirmed by negative controls, omitting reverse transcriptase (Fig. 1). Finally, the results were expressed as means ± SEM. Primer sequences were as follows: for IL-1β forward 5′-ATCTCACAGCAGCATCTC, reverse 5′-TAGCAGGTCGTCATCATC; for AT1a, forward 5′-TTCAACCTCTACGCCAGTGTG-3′, reverse 5′-GCCAAGCCAGCCATCAGC-3′; for AT2, forward 5′-AACATCTGCTGAAGACCAATAG-3′, reverse 5′-AGAAGGTCAGAACATGGAAGG-3; for p47phox, forward 5′-CCACACCTCTTGAACTTCTTC-3′, reverse 5′-CTCGTAGTCAGCGATGGC-3′; for β-actin, forward 5′-TCGTGCGTGACATTAAAGAG-3′, reverse 5′-TGCCACAGGATTCCATACC-3′.
Fig. 1.
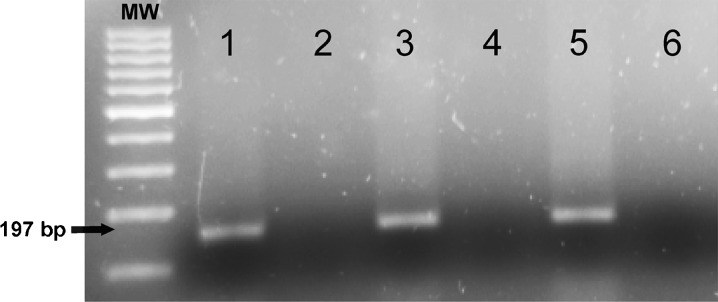
AT1 receptor gene amplification in 2 % agarose gel stained with Sybr®Safe (Invitrogen). 1, 3, and 5 Positive amplification of cDNA from control (sham operated) rats, and rats subjected to CHP and chronic cerebral hypoperfusion + candesartan (CHP+cand), respectively. 2, 4, and 6 The corresponding negative controls without reverse transcription, which show negative amplification
Western blot analysis and NADPH oxidase activity
Tissue was homogenized in RIPA buffer containing protease inhibitor cocktail (P8340, Sigma) and PMSF (P7626, Sigma). Homogenates were centrifuged and protein concentrations were determined with the Bradford protein assay. Equal amounts of protein were separated by 5–10 % Bis-Tris polyacrylamide gel, and transferred to nitrocellulose membranes. The membranes were incubated overnight with primary antibodies (1:200) against AT1 receptor (sc-31181), AT2 receptor (sc-9040), p47-phox (sc-7660), and IL-1β (sc-1252; all obtained from Santa Cruz Biotechnology). The specifity of the antibodies used to identify RAS components has been established in our previous studies (Rodriguez-Perez et al. 2010; Valenzuela et al. 2010). The horseradish peroxidase (HRP)-conjugated secondary antibodies used were Protein A (NA9120V, GE Healthcare) and Protein G (18–161, Upstate-Millipore). Immunoreactivity was detected with an Immun-Star HRP Chemiluminescent Kit (170–5044, BioRad) and imaged with a chemiluminescence detection system (Molecular Imager ChemiDoc XRS System, BioRad). Blots were stripped and reprobed for anti-GAPDH (G9545, Sigma; 1:25,000) as loading control. In each animal, protein expression was measured by densitometry of the corresponding band and expressed as relative to the GAPDH band value. The data were then normalized to the values of the control group of the same batch (i.e., expressed relative to the value obtained for the control rats; 100 %) to counteract possible variability among batches. Finally, the results were expressed as means ± SEM.
NADPH oxidase activity in ventral mesencephalic tissue was measured by lucigenin-enhanced chemiluminescence with an Infinite M200 multiwell plate reader (TECAN), as described by Griendling et al. (2000); Griendling et al. (1994) and Hong et al. (2006). Chemiluminescence was expressed as relative light units (RLU; RLU per minute per milligram protein). The level of activation of the NADPH oxidase complex was confirmed by determination of the p47phox expression (see above). NADPH oxidase complex is composed of membrane-bound subunits and cytosolic subunits such as p47phox, which is considered a key subunit for NADPH activation (Li and Shah 2003). The translocation of cytosolic subunits to the membrane is necessary for NADPH activation, which leads to ROS generation. The level of the NADPH oxidase subunit p47phox is correlated with NADPH activity and NADPH-derived superoxide formation. (Rueckschloss et al. 2002; Touyz et al. 2002).
High-performance liquid chromatography
The striata were dissected on an ice-cold plate and the striatal tissue frozen on dry ice and stored at −80 °C until analysis. Striatal tissue was homogenized and then centrifuged at 14,000×g for 20 min at 4 °C. The supernatant fractions were decanted, filtered (0.22 μm), and injected (20 μl/injection) into the HPLC system (Shimadzu LC prominence). Dopamine and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid were separated with a reverse phase analytical column (Waters Symmetry300 C18; 150 × 3.9 mm, 5 μm particle size; Waters). The mobile phase (70 mM KH2PO4, 1 mM octanesulfonic acid, 1 mM EDTA, 10 % MeOH, pH 4) was delivered at a rate of 1 ml/min. Detection was performed with a coulometric electrochemical detector (ESA Coulochem III). The first and second electrodes of the analytical cell were set at +50 and +350 mV, respectively; the guard cell was set at −100 mV. Data were acquired and processed with Shimadzu LCsolution software. Results were expressed in nanograms per microgram of tissue (wet weight) and presented as means ± SEM (n = 5 per group).
Statistical analysis
All data were obtained from at least three independent experiments and were expressed as means ± SEM. Two-group comparisons were analyzed by a Student’s t test and multiple comparisons were analyzed by one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test. The normality of populations and homogeneity of variances were tested before each ANOVA. Differences were considered significant at p < 0.05. Statistical analyses were carried out with SigmaStat 3.0 from Jandel Scientific (San Rafael, CA, USA).
Results
Effect of chronic cerebral hypoperfusion on dopaminergic degeneration and dopamine levels. Neuroprotective effect of the AT1 antagonist candesartan
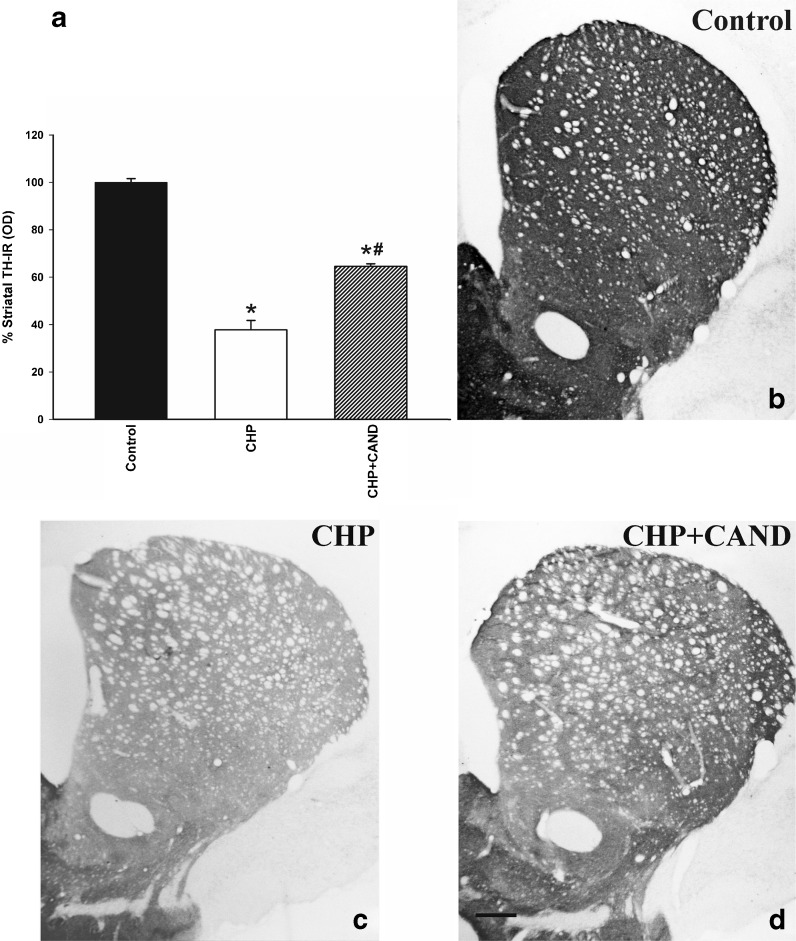
In control rats (group A1), the dopaminergic neurons in the substantia nigra compacta were intensely immunoreactive to TH, and dense, evenly distributed TH-ir was observed throughout the striatum, indicating the presence of a dense network of nigrostriatal dopaminergic terminals. A loss of about 33 % of nigral TH-ir neurons was observed in the SNc of rats subjected to chronic cerebral hypoperfusion (group B1) in comparison with the SNc of control rats (group A1; Fig. 2). In control rats (group A1), the number of neurons counted in cresyl violet-stained sections (22,792 ± 335) was slightly higher than the number of TH-ir (Fig. 2a) neurons since some nondopaminergic neurons located in the SNc were also included. However, sections from group B1 rats showed a significant reduction in the number of cresyl violet-stained neurons in the SNc (16,087 ± 988), which confirms that chronic cerebral hypoperfusion induces cell death and not just phenotypic downregulation in TH activity. Rats subjected to chronic cerebral hypoperfusion and treated with the AT1 antagonist candesartan (group C1) showed a significant loss of dopaminergic neurons (about 18 % decrease). However, the loss was significantly lower than in untreated rats. Similarly, the density of striatal TH-ir terminals, relative to control rats, was significantly lower in rats subjected to chronic hypoperfusion than in control rats, as a consequence of the loss of striatal dopaminergic terminals (Fig. 3). However, the reduction in the density of striatal TH-ir terminals was significantly lower in group C1 rats treated with candesartan. No striatal infarcts or ischemic lesions, which could induce significant degeneration of dopaminergic neurons, were observed (Fig. 3c–d).
Fig. 2.
Dopaminergic (TH-ir) neurons (a–d) in the SNc of controls (sham-operated rats), rats subjected to CHP or chronic cerebral hypoperfusion and treatment with candesartan (CHP + cand). Representative photomicrographs of the SNc of different groups of rats are shown in b–d. The dopaminergic neurons were quantified as the total number of TH-ir neurons in the right + left SNc (a). Data are means ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test). Scale bar 500 μm
Fig. 3.
Striatal TH-ir in controls (sham-operated rats) and rats subjected to CHP or chronic cerebral hypoperfusion and treatment with candesartan (CHP + cand). TH-ir (i.e., spared dopaminergic terminals) is higher in the treated group than in the rats that did not receive candesartan (a). Representative photomicrographs of the striatum of different groups of rats are shown in b–d. Density of striatal dopaminergic terminals was estimated as optical density and expressed as a percentage of the value obtained in the control group. Data are means ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test). Scale bar 250 μm
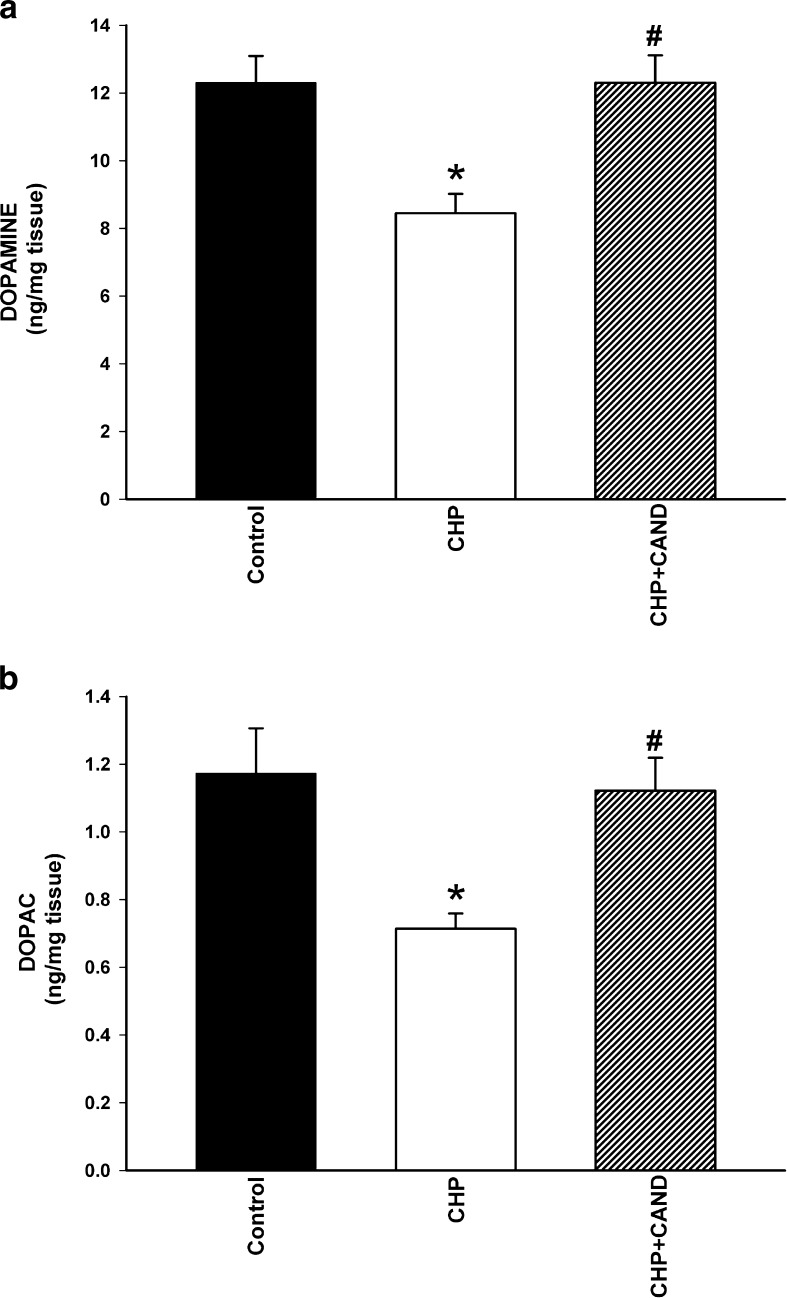
The functional effects of chronic cerebral hypoperfusion were confirmed by determination of the striatal levels of dopamine and its metabolites by HPLC (Fig. 4). Levels (nanogram per milligram wet weight of tissue) of dopamine and DOPAC in control rats (group A1) were significantly higher than those observed in rats with chronic cerebral hypoperfusion (group B1), but not significantly different to those observed in rats with chronic cerebral hypoperfusion treated with candesartan (group C1). However, the DOPAC/dopamine ratio values were not significantly different between control rats (0.094 ± 0.021), rats with chronic hypoperfusion (0.089 ± 0.024) and rats subjected to chronic hypoperfusion and treated with candesartan (0.092 ± 0.019).
Fig. 4.
Striatal levels (nanogram per milligram wet weight of tissue) of dopamine (a) and its metabolite DOPAC (b) in control rats as compared with rats subjected to CHP or chronic cerebral hypoperfusion and treatment with the AT1 blocker candesartan (CHP + cand). Data are means ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test)
Effect of chronic cerebral hypoperfusion on angiotensin AT1 and AT2 receptors in the substantia nigra. Effect of treatment with candesartan
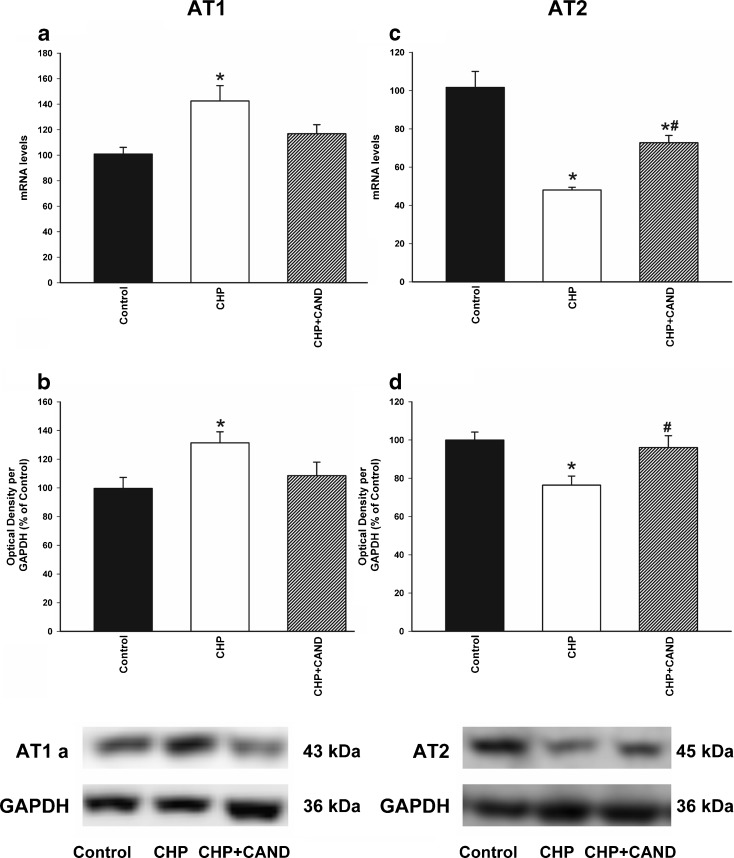
Real-time RT-PCR analysis revealed significantly higher expression of AT1 receptor mRNA and lower levels of AT2 mRNA in rats with chronic cerebral hypoperfusion than in control rats (Fig. 5a, c). Similarly, Western blot (WB) studies revealed significantly higher expression of AT1 receptors in rats with chronic cerebral hypoperfusion than in control rats (Fig. 5b), and the expression of AT2 receptors was significantly lower in rats with chronic cerebral hypoperfusion (Fig. 5d). However, treatment of rats with chronic cerebral hypoperfusion with the AT1 receptor blocker candesartan induced a significant increase in the levels of AT2 receptor mRNA and protein (Fig. 5c, d).
Fig. 5.
Real-time quantitative RT-PCR (a, c) and Western blot (WB; b, d) analysis of AT1, AT2 receptor expression in control rats as compared with rats subjected to CHP or chronic cerebral hypoperfusion and treatment with candesartan (CHP + cand). Protein expression was obtained relative to the GAPDH band value and the expression of each gene was obtained relative to the housekeeping transcripts (β-Actin). Note that AT1 receptors are blocked in the CHP + cand group. The results were then normalized to values for control rats (100 %). Data are mean values ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test)
Effect of chronic cerebral hypoperfusion on NADPH complex activation and IL-1β expression in the substantia nigra. Effect of treatment with candesartan
Rats with chronic cerebral hypoperfusion showed a significantly greater activation of the NADPH complex than control rats, as determined by p47 subunit expression and NADPH oxidase activity. Real-time RT-PCR analysis revealed higher mRNA levels of p47phox in rats with chronic cerebral hypoperfusion than in control rats (Fig. 6a), and WB also revealed a significant increase in the expression of p47phox in rats with chronic cerebral hypoperfusion (Fig. 6b). Treatment with candesartan induced a significant decrease in levels of NADPH activity and expression of p47 (Fig. 6).
Fig. 6.
Real-time quantitative RT-PCR (a), western blot (b), and Lucigenin enhanced chemiluminescence (c) analysis of the NADPH complex activation in the ventral mesencephalon of control rats as compared with rats subjected to CHP or chronic cerebral hypoperfusion and treatment with the AT1 blocker candesartan (CHP + cand). Protein expression was obtained relative to the GAPDH band value and the expression of each gene was obtained relative to the housekeeping transcripts (β-actin). The results were then normalized to values for control rats (100 %). NADPH oxidase activity (c) was expressed as relative light units (RLU/min/mg protein). Data are means ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test)
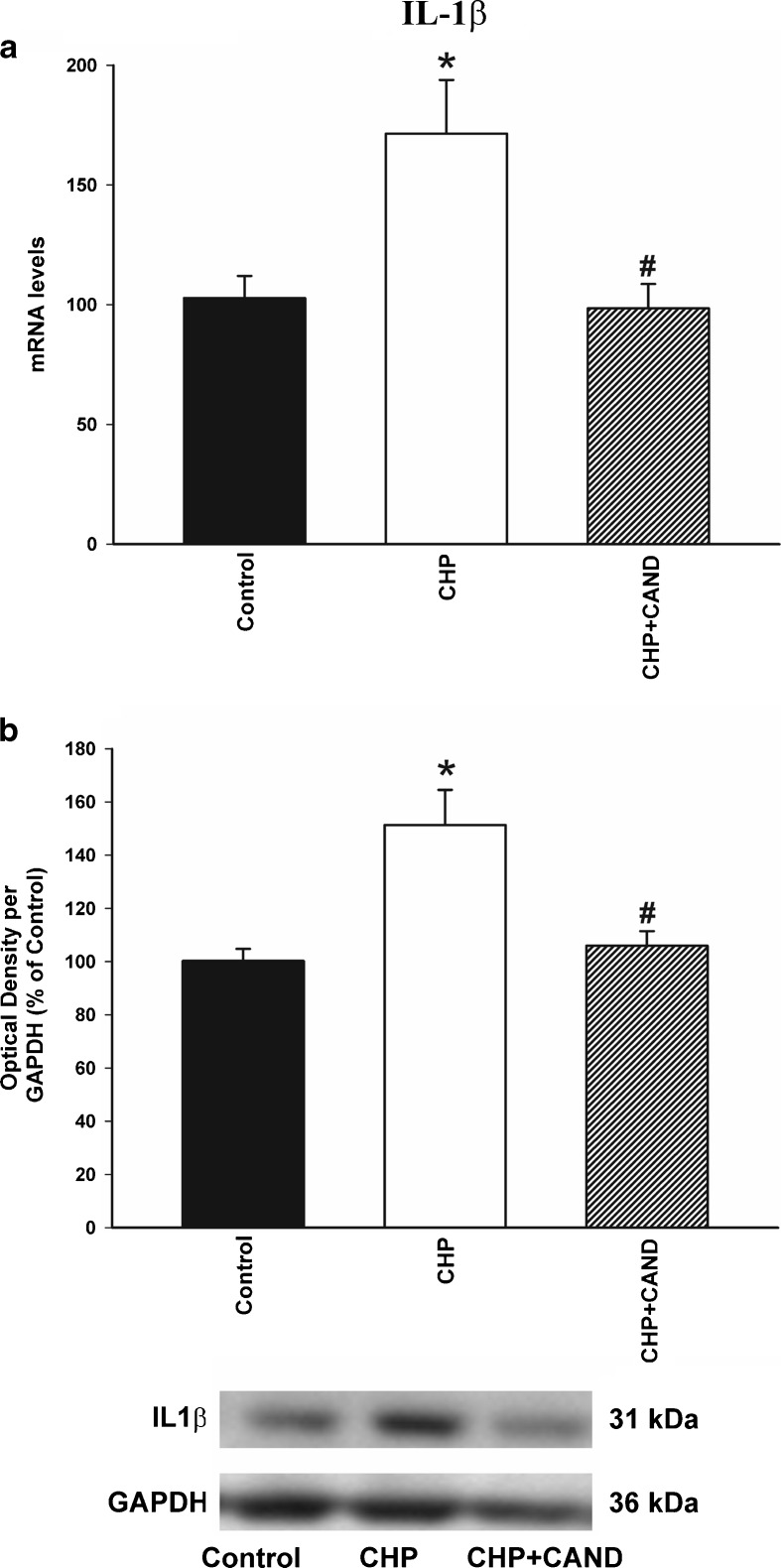
In addition, levels of the pro-inflammatory cytokine IL-1β were determined by real-time RT-PCR analysis and WB, and were significantly higher in rats with chronic cerebral hypoperfusion than in control rats. Treatment with candesartan induced a significant decrease in the expression of IL-1β mRNA and protein (Fig. 7a, b).
Fig. 7.
Real-time quantitative RT-PCR (a) and Western blot (WB; b) analysis of interleukin-1 β (IL-1β) expression in control rats as compared with rats subjected to CHP or chronic cerebral hypoperfusion and treatment with the AT1 blocker candesartan (CHP + cand). Protein expression was obtained relative to the GAPDH band value and the expression of each gene was obtained relative to the housekeeping transcripts (β-actin). The results were then normalized to values for control group (100 %). Data are mean values ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the group subjected to chronic hypoperfusion (one-way ANOVA and Bonferroni post hoc test)
Synergistic effect of chronic cerebral hypoperfusion and 6-OHDA on dopaminergic degeneration. Neuroprotective effect of the AT1 antagonist candesartan
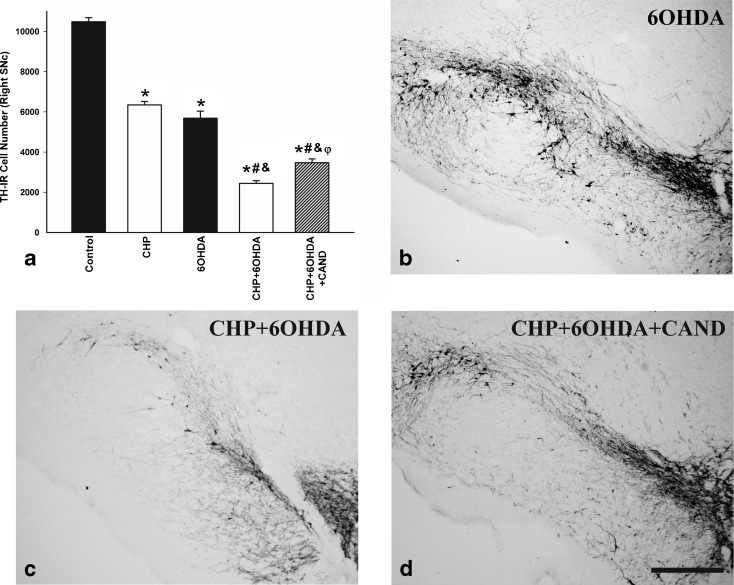
In order to investigate the possible synergistic effect of chronic cerebral hypoperfusion and other possible deleterious factors causing dopaminergic neuron degeneration, rats in the different groups (A2, B2, C2) were injected with low doses of the dopaminergic neurotoxin 6-OHDA (Fig. 8). As described above, rats subjected to chronic hypoperfusion (without 6-OHDA injection) showed a significant loss of TH-ir neurons. Intrastriatal administration of 6-OHDA in control rats also induced a significant loss of TH-ir neurons in the ipsilateral SNc (group A2). However, intrastriatal administration of 6-OHDA in rats subjected to chronic hypoperfusion (group B2) induced a significantly higher loss of TH-ir neurons than in control rats (group B1). The difference in 6-OHDA-induced loss of TH-ir neurons between control rats and rats subjected to chronic hypoperfusion was significantly reduced by treatment of the latter rats with the AT1 receptor antagonist candesartan (group C2; Fig. 8).
Fig. 8.
Dopaminergic (TH-ir) neurons in the SNc of control (sham-operated) rats and rats subjected to CHP alone, intrastriatal injection of 6-OHDA alone, chronic cerebral hypoperfusion and intrastriatal injection of 6-OHDA (CHP + 6-OHDA), or chronic cerebral hypoperfusion, intrastriatal injection of 6-OHDA and treatment with candesartan (CHP + 6-OHDA + cand). Representative photomicrographs of the SNc of different groups of rats are shown in b–d. The SNc of control rats and rats subjected to CHP alone is shown in Fig. 2b, c. The dopaminergic neurons were quantified as the total number of TH-ir neurons in the ipsilateral SNc (a). Data are means ± SEM. *p < 0.05 relative to the control group, #p < 0.05 relative to the CHP group, &p < 0.05 relative to the group treated with 6-OHDA alone, φp < 0.05 relative to the group subjected to CHP and 6-OHDA (one-way ANOVA and Bonferroni post hoc test). Scale bar 500 μm
Discussion
Chronic cerebral hypoperfusion is directly responsible for dopaminergic degeneration. Chronic cerebral hypoperfusion can act synergistically with other factors to induce progression of dopaminergic neuron loss
Data from several clinical studies suggest an interaction between aging-related cerebrovascular disease/brain hypoperfusion and dopaminergic degeneration. Dopaminergic cell loss and parkinsonian signs have been observed in elders without PD (Buchman et al. 2012), presynaptic dopaminergic function is reduced in the majority of patients with VP (Zijlmans et al. 2007), and a subset of patients with clinically suspected VP were found to have a good therapeutic response to l-dopa (Thanvi et al. 2005; Zijlmans et al. 2004a, b). The present model of chronic brain hypoperfusion shows that chronic hypoperfusion induces a significant loss of dopaminergic neurons and a significant decrease in striatal dopaminergic terminals and striatal dopamine levels. Furthermore, the loss of dopaminergic neurons and striatal TH and dopamine levels were significantly reduced by treatment with candesartan, which was initiated 20 days after the bilateral occlusion of the carotid arteries, thus excluding the possibility that loss of dopaminergic neurons was produced in the hypoxic–ischemic acute phase and confirming the long-term loss of dopaminergic neurons in the chronic phase.
A second important question addressed in the present study is whether the hypoperfusion derived from aging and/or vascular disease, acting synergistically with factors that induce PD, may increase the risk of development of PD (i.e., accelerate the onset of a latent PD) or exacerbate the progression and severity of already established PD. It is usually recognized that the presence of age-associated white matter lesions or a subclinical vascular impairment may contribute to dementia and possibly other symptoms such as postural stability and gait dysfunctions in PD as a consequence of additive or overlapping mechanisms (Bohnen and Albin 2011; Rektor et al. 2006, 2009). However, the present study shows that hypoperfusion may also lead to increased dopaminergic cell death by enhancing the deleterious effects of other factors (such as the low doses of the dopaminergic neurotoxin 6-OHDA in the present experiments).
Aging, cerebral hypoperfusion, and Parkinson’s disease
It is well known that advancing age itself is one of the most significant risk factors for the development of neurodegenerative diseases such as PD and that prevalence of both PD and cerebrovascular disease increases with age. A recent study revealed that nigral pathology is common (almost 40 %) in elders without PD (Buchman et al. 2012), and we have recently observed an age-dependent decrease in nigral vascularization and nigral vascular endothelial growth factor levels (Villar-Cheda et al. 2009). The present results suggest that aging-related cerebrovascular disease and hypoperfusion contribute to the degenerative changes in dopaminergic neurons, and the subsequent neuron death. Interestingly, rats subjected to hypoperfusion showed a significant decrease in striatal levels of dopamine and DOPAC with no significant change in dopamine turnover (DOPAC/dopamine ratio). It has been observed that the compensatory increase in dopamine turnover observed in young animals after acute dopaminergic lesions is completely absent in aged animals (Collier et al. 2007), and the present results suggest that the aging-related chronic hypoperfusion may also be involved in this effect. Hypoperfusion may affect the compensatory response in the remaining DA neurons; however, other mechanisms may be involved in the lack of compensatory DA turnover observed after chronic hypoperfusion (Ben et al. 1999; Nandhagopal et al. 2011).
Role of the brain renin–angiotensin system as a potential link between cerebrovascular disease and Parkinson’s disease
The mechanistic links between hypoperfusion/vascular disease and neurodegeneration are unknown. In the present study chronic hypoperfusion led to dopaminergic cell death as well as increased expression of inflammatory markers such as IL-1β and increased levels of oxidative stress markers such as NADPH activity, which have been shown to be involved in progression of dopaminergic cell death in animal models of PD and PD patients (Koprich et al. 2008; Wu et al. 2003). NADPH oxidases are a major source of superoxide (O−2) and are upregulated in major aging-related diseases such as hypertension, diabetes and atherosclerosis (Griendling et al. 2000; Münzel and Keany 2001). Hypoperfusion-induced dopaminergic cell death, IL-1β expression, and NADPH activity were reduced by treatment with the AT1 antagonist candesartan, which shows that the nigral RAS is involved in this process. In animals subjected to chronic hypoperfusion, the increased RAS activity in the substantia nigra was confirmed by observation of increased expression of AT1 receptors and decreased expression of AT2 receptors. AT1 and AT2 receptors have opposing effects and AT2 receptors counterbalance the deleterious effect of AT1 receptor stimulation, so that functional interactions between the two receptor subtypes and their specific distribution determine the AII-induced effects (Sohn et al. 2000). AT1 receptor activation upregulates NADPH oxidase activity, which is inhibited by AT2 receptor activation. In addition to these major components of the RAS, several emerging components of the RAS such as angiotensin (1–7) (Clark et al. 2001) or prorenin receptors (Valenzuela et al. 2010) may also modulate brain RAS activity (Labandeira-Garcia et al. 2011). In the case of rats with chronic hypoperfusion, changes in local RAS activity creates a pro-oxidative and pro-inflammatory state, as suggested by the increased NADPH activity and IL-1β expression relative to control rats.
Recent studies have shown that the local brain RAS is involved in several brain functions and disorders (Labandeira-Garcia et al. 2011; Phillips and de Oliveira 2008; Wright and Harding 2011). In several animal models of PD, we have previously shown that there is a local RAS in the substantia nigra and that dopaminergic cell loss is enhanced by AII via AT1 receptors, activation of the microglial NADPH oxidase complex (Joglar et al. 2009; Rey et al. 2007; Rodriguez-Pallares et al. 2008) and subsequent mitochondrial-derived oxidative stress (Rodriguez-Pallares et al. 2012). Interestingly, we have also observed increased RAS activity (including increased AT1 expression, decreased AT2 expression, increased NADPH activity and increased IL-1β levels) in the nigra of aged rats together with increased vulnerability of dopaminergic neurons to neurotoxins, which were inhibited by treatment with the AT1 antagonist candesartan (Rodriguez-Perez et al. 2012; Villar-Cheda et al. 2012). The mechanism responsible for the increased RAS activity in the nigra of aged animals has not been clarified. However, the present results suggest that the increase may be at least partially due to the chronic hypoperfusion observed in the substantia nigra of aged animals (Villar-Cheda et al. 2012).
In conclusion, this study details the effects of brain hypoperfusion on dopaminergic neurons in the rat. Brain hypoperfusion induces dopaminergic neuron loss and enhances the effect of the dopaminergic neurotoxin 6-hydroxydopamine. The AT1 receptor antagonist candesartan has a neuroprotective effect, which suggests that increased angiotensin II activity participates in dopaminergic degeneration during brain hypoperfusion. The present results suggest that the presence of vascular disease or aging-derived hypoperfusion may not only increase the severity of PD by aggravating some symptoms (i.e., an additive or overlapping effect of PD and VP), but may lead to significant loss of dopaminergic neurons and terminals and, particularly, act synergistically with other factors to accelerate the onset of PD or exacerbate the progression and severity of the already established PD. Therefore, early treatment of vascular disease should be considered for the treatment of aged PD patients and PD patients with cerebrovascular risk factors. The results also suggest that inhibition of RAS activity with low doses of AT1 antagonists such as candesartan may constitute a useful neuroprotective strategy as may inhibit nigral inflammation and oxidative stress and also improve hypoperfusion by acting on cerebral microvessels.
Acknowledgments
The authors thank Pilar Aldrey, Iria Novoa, and Jose A. Trillo for their excellent technical assistance. The authors are thankful to Astra Zeneca for providing candesartan for the experiments. Funding: Spanish Ministry of Science and Innovation, Spanish Ministry of Health (RD06/0010/0013 and CIBERNED), Galician Government (XUGA) and European Regional Development Fund (FEDER).
References
- Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores Arnaiz M, Inserta F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Ben V, Blin O, Bruguerolle B. Time-dependent striatal dopamine depletion after injection of 6-hydroxydopamine in the rat. Comparison of single bilateral and double bilateral lesions. J Pharm Pharmacol. 1999;51:1405–1408. doi: 10.1211/0022357991777038. [DOI] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Orisio S, Noris M, Iatropoulos P, Castaldi D, Kamide K, Rakugi H, Arai Y, Todeschini M, Ogliari G, Imai E, Gondo Y, Hirose N, Mari D, Remuzzi G (2012) Variations of the angiotensin II type 1 receptor gene are associated with extreme human longevity. Age (Dordr), in press [DOI] [PMC free article] [PubMed]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, Schneider JA, Bennett DA. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–266. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Diz DI, Tallant EA. Angiotensin-(1–7) downregulates the angiotensin II type 1 receptor in vascular smooth muscle cells. Hypertension. 2001;37:1141–1146. doi: 10.1161/01.HYP.37.4.1141. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NADPH oxidase. Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bendsen TF, Korbo L, Marcussen N, Moller A, Nielsen K. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APIMS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hong H, Zeng JS, Kreulen DL, Kaufman DI, Chen AF. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291:H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- Jellinger K. A response to The effects of vascular disease on late onset Parkinson’s disease (Papapetropoulos et al.) Eur J Neurol. 2006;13(10):e1. doi: 10.1111/j.1468-1331.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Joglar B, Rodriguez-Pallares J, Rodríguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, Rodriguez-Pallares J, Villar-Cheda B, Rodríguez-Perez AI, Garrido-Gil P, Guerra MJ. Aging, angiotensin system and dopaminergic degeneration in the substantia Nigra. Aging Dis. 2011;2:257–274. [PMC free article] [PubMed] [Google Scholar]
- Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Johnson SW, Stratta F, Calabresi P, Bernardi G. Effects of anoxia on rat midbrain dopamine neurons. J Neurophysiol. 1994;71:1165–1173. doi: 10.1152/jn.1994.71.3.1165. [DOI] [PubMed] [Google Scholar]
- Münzel T, Keany JF. Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation. 2001;104:1571–1577. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, McCormick S, Ruth TJ, Sossi V, de la Fuente-Fernandez R, Stoessl AJ. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain. 2011;134:3290–3298. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, Ellul J, Argyriou AA, Talelli P, Chroni E, Papapetropoulos T. The effect of vascular disease on late onset Parkinson’s disease. Eur J Neurol. 2004;11:231–235. doi: 10.1046/j.1468-1331.2003.00748.x. [DOI] [PubMed] [Google Scholar]
- Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-R. [DOI] [PubMed] [Google Scholar]
- Rektor I, Rektorová I, Kubová D. Vascular parkinsonism–an update. J Neurol Sci. 2006;248:185–191. doi: 10.1016/j.jns.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Rektor I, Goldemund D, Sheardová K, Rektorová I, Michálková Z, Dufek M. Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:24–29. doi: 10.1016/j.parkreldis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Rey P, Lopez-Real A, Sanchez-Iglesias S, Muñoz A, Soto-Otero R, Labandeira-Garcia JL. Angiotensin type-1-receptor antagonists reduce 6-hydroxydopamine toxicity for dopaminergic neurons. Neurobiol Aging. 2007;28:555–567. doi: 10.1016/j.neurobiolaging.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Rey P, Parga JA, Muñoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira-Garcia JL (2012) Mitochondrial ATP-sensitive potassium channels enhance angiotensin-induced oxidative damage and dopaminergic neuron degeneration. Relevance for aging-associated susceptibility to Parkinson’s disease. Age (Dordr) 34:863–80 [DOI] [PMC free article] [PubMed]
- Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Lanciego JL, Labandeira-Garcia JL. Estrogen and angiotensin interaction in the substantia nigra. Relevance to postmenopausal Parkinson’s disease. Exp Neurol. 2010;224:517–526. doi: 10.1016/j.expneurol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Labandeira-Garcia JL. Different dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats. Role of the brain angiotensin system. Brain. 2012;135:124–138. doi: 10.1093/brain/awr320. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.ATV.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases. Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunohistochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-X. [DOI] [PubMed] [Google Scholar]
- Singh V, Carman M, Roeper J, Bonci A. Brief ischemia causes long-term depression in midbrain dopamine neurons. Eur J Neurosci. 2007;26(6):1489–1499. doi: 10.1111/j.1460-9568.2007.05781.x. [DOI] [PubMed] [Google Scholar]
- Sohn HY, Raff U, Hoffmann A, Gloe T, Heermeier K, Galle J, Pohl U. Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br J Pharmacol. 2000;131:667–672. doi: 10.1038/sj.bjp.0703566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanvi B, Lo N, Robinson T. Vascular parkinsonism–an important cause of parkinsonism in older people. Age Ageing. 2005;34:114–119. doi: 10.1093/ageing/afi025. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries, regulation by angiotensin II. Circ Res. 2002;14:1205–1213. doi: 10.1161/01.RES.0000020404.01971.2F. [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Barroso-Chinea P, Muñoz A, Joglar B, Villar-Cheda B, Lanciego JL, Labandeira-Garcia JL. Location of prorenin receptors in primate substantia nigra: effects on dopaminergic cell death. J Neuropathol Exp Neurol. 2010;69:1130–1142. doi: 10.1097/NEN.0b013e3181fa0308. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Sousa-Ribeiro D, Rodriguez-Pallares J, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J Cereb Blood Flow Metab. 2009;29:230–234. doi: 10.1038/jcbfm.2008.127. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Valenzuela R, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol Aging. 2012;33:204.e1-11. doi: 10.1016/j.neurobiolaging.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Brain renin-angiotensin–a new look at an old system. Prog Neurobiol. 2011;95:49–67. doi: 10.1016/j.pneurobio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Wu D, Teisman P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson¨s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans JC, Daniel SE, Hughes AJ, Révész T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- Zijlmans JC, Katzenschlager R, Daniel SE, Lees AJ. The L-dopa response in vascular parkinsonism. J Neurol Neurosurg Psychiatry. 2004;75:545–547. doi: 10.1136/jnnp.2003.018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans J, Evans A, Fontes F, Katzenschlager R, Gacinovic S, Lees AJ, Costa D. [123I] FP-CIT spect study in vascular parkinsonism and Parkinson’s disease. Mov Disord. 2007;22:1278–1285. doi: 10.1002/mds.21479. [DOI] [PubMed] [Google Scholar]