Abstract
High-fat (HF) diet regular intake along life highly contributes to vascular dysfunction and to an increment in prevalence of metabolic syndrome (MetS) and erectile dysfunction (ED), a surrogate symptom of occult vascular disease, in the elderly. However, little is known about the effects of energy restriction (ER) alone/or after an HF-feeding period. We show here that in male Sprague–Dawley rats, 16 months of HF-diet consumption led to an increase in body adiposity, blood pressure, lipidemia, C-reactive protein, and insulin resistance and to hypoadiponectinemia, conditions that cluster in MetS. In addition, this treatment strongly favored collagen deposition in cavernous tissue and myocardium. Conversely, for the same time period, the ingestion of 75 % of ad libitum energy intake by controls (ER) extensively counteracted these outcomes. The impact of 6-month ER after 10-month HF period was also analyzed, and despite the decrease in body weight, adiposity, blood pressure, lipidemia, and C-reactive protein and improvement of insulin sensitivity, no differences were observed either in adiponectin blood levels or in retroperitoneal fat pad mass. Moreover, this treatment led to a reduction in cavernous tissue collagen deposition, but not in the myocardium, and evidenced differential mobilization of adipose tissue accretions. The data show the ability of HF diet to cause MetS and produce unwanted effects on myocardium and corpora vascular structure. They also indicate that these consequences are preventable upon ER diet starting early, but not later, in life.
Keywords: Aging, Energy restriction, High-fat diet, Cavernous tissue, Adiponectin, Metabolic syndrome
Introduction
Human life expectancy increased enormously in the last century. Since normal aging results in compromised biological functions of the organism and enhanced susceptibility to disease, age-related health complications gained an increased importance during this period. In addition, the shift in demographic curves towards elderly population bound to the rise in regular intake of energy-rich diets tends to exacerbate the epidemic obesity that currently exists (Picard and Guarente 2005).
In fact, the increased prevalence of metabolic syndrome (MetS), the cluster of cardiometabolic risk factors, such as insulin resistance/diabetes, hypertension, dyslipidemia, and abdominal obesity, has been associated with increased cardiovascular disease (CVD) incidence and overall mortality in the elderly people (Grundy 2007; Lakka et al. 2002). In addition, aging was recently considered an independent risk factor for cardiovascular disorders (Camici et al. 2011).
Most of those CVD risk factors are shared by erectile dysfunction (ED) (Shin et al. 2011), a common entity defined as the consistent or recurrent inability to achieve and/or maintain a penile erection sufficient for satisfactory sexual performance (NIH Consensus Conference 1993). Since ED often precedes vascular disease diagnosis in patients, several authors consider ED as a surrogate symptom of occult vascular disease (Kirby et al. 2001) and as a predictive marker for CVD (Billups et al. 2008; Jackson et al. 2006; Kirby et al. 2001) and even acute cardiac events (Chew et al. 2010; Jackson 2008; Kostis et al. 2005). Recently, ED itself was also considered an independent risk factor of CVD (Dong et al. 2011).
Normal penile erectile function results from a complex interaction between vascular, hormonal, neurologic, and psychological factors. Erection attainment and maintenance require an adequate inflow of arterial blood and efficient trapping of venous outflow. Consequently, even small interferences in penile vascular flow could lead to any degree of ED. The early event shared by this condition and CVD is endothelial dysfunction (Marx and Grant 2007).
Endothelial dysfunction develops in the presence of the aforementioned cardiovascular risk factors. At the subcellular level, the underlying mechanism is based on the impaired bioavailability of nitric oxide (NO) that compromises normal NO-mediated vasodilatation. This condition favors vasoconstriction, deregulates intima proliferation, and promotes a proinflammatory environment that cause plaque destabilization (Rodriguez et al. 2005). Endothelial dysfunction is thus considered a basic initiator in the formation of atheroma plaque (Marx and Grant 2007), and therefore, it precedes the clinical manifestations of atherosclerosis (Boneti et al. 2003). In this setting, ED is a manifestation of endothelial dysfunction and also a part of the spectrum of atherosclerotic disease that culminates in arterial insufficiency (Jackson et al. 2006; Montorsi et al. 2006).
The artery size hypothesis proposes ED as a silent clinical marker for vascular disease (Montorsi et al. 2003). This theory states that, albeit atherosclerosis is a systemic condition, it affects all arteries in a time- and arterial diameter-dependent fashion. Thus, in the small penile vessels, the same level of plaque burden has a greater effect on blood flow compared with the coronary, carotid, or femoral arteries.
The vascular consequences of high-fat (HF) diet, a condition favoring CVD risk, have been a subject of intense research. However, focus has been put on large conduit arteries as the aorta of animal models with established obesity. Although these studies documented well the most dramatic end-effects of obesity in large vessels, the nature of vascular derangements in small diameter vessels, consequent to high-energy intake but prior to overt obesity, attracted less attention. They contrast well with the effects of energy restriction (ER). In fact, in rodents, in nonhuman primates, and possibly in humans too, the ER regimen extends lifespan, decreases inflammation (Ye and Keller 2010), and reduces incidence of CVD and other age-related diseases (Camici et al. 2011). However, despite the potential impact that changed diet patterns may have on human’s CVD, little research has been conducted examining vascular system effects of ER after an HF-feeding period.
HF-diet conditions highly favor adipose tissue accretion, which leads to metabolic dysregulation. In fact, adiponectin, an adipose tissue-derived peptide, plays an important role in glucose and lipid metabolism (Karbowska and Kochan 2006), and its secretion is markedly downregulated in obesity-related conditions, such as insulin resistance and diabetes (Kern et al. 2003). Furthermore, clinical observations have demonstrated that hypoadiponectinemia has a close relationship with endothelial dysfunction of peripheral arteries (Shimabukuro et al. 2003) and that plasma total adiponectin levels are inversely related to the risk of cardiovascular events (Kumada et al. 2003). Despite the wealth of knowledge on adiponectin in established obesity and CVD, long-term studies on the effect of diet are lacking.
Therefore, the present study was designed to examine the impact of diet-induced cardiometabolic risk on cavernous tissue, along aging. Our specific goals were: (1) to verify whether long-term HF-diet consumption leads to MetS, increment in adiposity, and changes in adiponectin plasma levels; (2) to evaluate in what extent ER counteracts the effects of HF-diet chronic intake on MetS onset; and (3) to study how such dietary conditions affect the cellular organization of cavernous tissue, in contrast with the myocardium, a usual target of MetS derangement.
Materials and methods
All animal procedures were undertaken according to the European Community Guidelines (86/609/EEC) and the Portuguese Act (129/92) for the use of experimental animals.
Experimental groups and diets
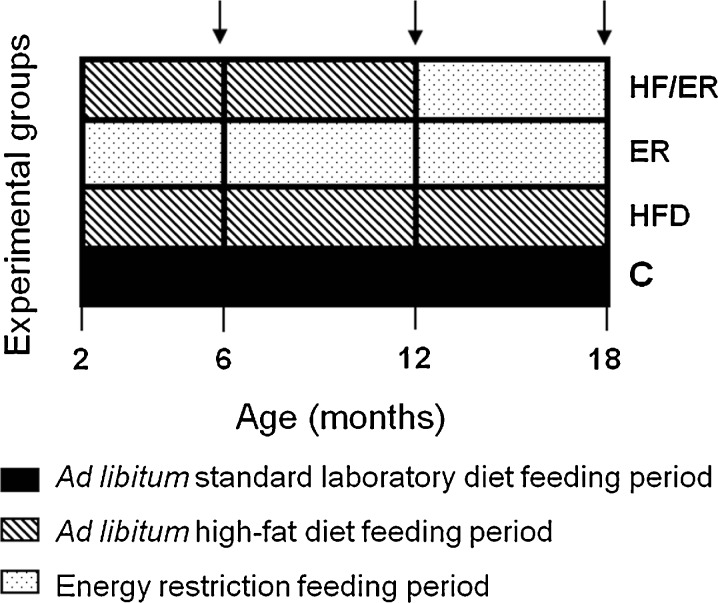
Two-month-old male Sprague–Dawley rats (n = 50), weighing 200–250 g (Charles River, Barcelona, Spain), were individually housed and maintained under controlled standard laboratory conditions (12:12 h light–dark cycle; 20–22 °C temperature; 40–60 % humidity). After acclimatization, rats were randomly divided into three experimental groups: control group (C, n = 15), rats with free access to a standard laboratory diet with 4 % of energy from fat mainly derived from fish (A04, Panlab® SL, Barcelona, Spain); high-fat diet group (HFD, n = 20), rats with free access to a purified rodent diet with 45 % of energy from fat derived from lard (#58V8 TestDiet®, Purina Mills®, LLC/PMI Nutrition International®, Richmond, USA); energy-restricted group (ER, n = 15), animals submitted to energy restriction, correspondent to 75 % of daily standard rodent chow individual intake of C rats. Food was restored every 2–3 days. All animals had free access to tap water and maintained the same diet pattern during experiments, except a group of five randomly selected rats which after 10 months of HF-diet was subjected to a 6-month ER period (HF/ER group, n = 5). Rats were sacrificed by decapitation, when they reached 6, 12, or 18 months (n = 5 for each subgroup of age). The experimental group design and the composition of the diets are shown in Fig. 1 and Table 1, respectively.
Fig. 1.
Time course of experimental procedures for studied animal groups. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months; arrows 6, 12, and 18 months endpoints
Table 1.
Major macronutrient constituents and total energy of high-fat and standard laboratory diets
| High-fat diet | Standard diet | |
|---|---|---|
| Protein (g 100 g−1) [%]a | 21.3 [18.3] | 14.6 [20.2] |
| Carbohydrate (g 100 g−1) [%]a | 41.9 [36.0] | 55.1 [76.0] |
| Lipid (g 100 g−1) [%]a | 23.6 [45.7] | 1.2 [3.8] |
| Fiber (g 100 g−1) | 5.8 | 3.9 |
| Total energy (kJ g−1)b | 19.5 | 12.1 |
aPercentage of energy content as protein, carbohydrate and fat (w/w)
bTotal energy (kilojoules per gram): sum of decimal fractions of protein, carbohydrate, and lipid, calculated on the basis of 16.7 kJ g−1 protein and carbohydrates and 37.7 kJ g−1 lipid
Rats’ body weight (BW) and food intake were monitored weekly to assess the effect of the diets on weight gain. The mean weekly energy intake (kilojoules per week) was calculated as follows: food intake (grams) × diet energy value (kilojoules per∙gram).
Blood pressure assessment
Heart rate and systolic (SBP) and diastolic blood pressures (DBP) were measured using the tail-cuff method in conscious rats (LE5008-05PL, Panlab S.I., Barcelona, Spain) at the beginning of treatment (2 months) and when rats completed 6, 12, and 18 months of age. Measurements were made 10–15 min after acclimatization under restraining conditions and were repeated in three consecutive days. Data from day 3 were considered valid.
Biochemical and hormonal determinations
Plasma glucose concentrations were determined using a glucose analyzer (OneTouch® Ultra™, Lifescan, Inc., Milpitas, CA, USA) at different time points (2, 6, 12, and 18 months) after a 16-h fasting. At the same time, rat tail vein or trunk blood samples were collected for biochemical and hormonal determinations. Plasma fractions were separated by centrifugation at 1,000 × g for 30 min at 4 °C and maintained at −80 °C until analysis. Plasma total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and triglyceride (TG) concentrations were determined by enzymatic colorimetry in an Olympus® auto-analyzer (Olympus America, Inc., NY, USA) employing commercial kits (OSR6516, OSR6587, and OSR61118, Olympus America, Inc., for TC, HDL-c, and TG, respectively). Low-density lipoprotein cholesterol (LDL-c) levels were calculated by Friedewald’s formula (LDL-c = TC − [HDL-c − 1/5∙TG], if serum TG is 400 mg dL−1 or less) (Friedewald et al. 1972).
C-reactive protein (CRP) levels in serum were analyzed with an immunoturbidimetric latex CRP assay, normal set (OSR6199, Olympus America, Inc.). Plasma testosterone levels were determined by electrochemiluminescence in an immunoassay analyzer (Cobas®, Roche Diagnosis GmbH, Mannheim, Germany) with a commercial kit (Testoterone II, #05200067, Roche Diagnosis GmbH). Insulin and adiponectin levels were evaluated using commercially available RIA kits (Sensitive Rat Insulin RIA Kit #SRI-13K, Millipore Co., Billerica, MA, USA, and Mouse Adiponectin RIA Kit #MADP-60HK, Linco Research, Mo, USA, respectively), according to the manufacturer’s instructions.
Insulin sensitivity was assessed by determination of homeostasis model assessment of insulin resistance (HOMA-IR) index for each animal. This index is based on the fact that the relationship between glucose and insulin in the basal state reflects the balance between hepatic glucose output and insulin secretion. HOMA index was calculated as follows: HOMA-IR index = G · I · 22.5−1, where G is fasting glucose (millimoles per∙liter) and I is fasting insulin (milliunits per liter) (Cacho et al. 2008).
Collection of samples
Rats were sacrificed after overnight fast and organ harvest initiated with complete removal of epididymal, mesenteric, and retroperitoneal fat pads as described by Hausman et al. (2003). Each fat pad was weighted, and the sum of mass of these three white adipose tissue (WAT) deposits calculated. Onwards, WAT refers to the sum of epididymal, mesenteric, and retroperitoneal fat pads. In order to assess the effects of the diet on body fat content, adiposity index (AI) was calculated by WAT · BW−1 · 100 and expressed as adiposity percentage (Fernandez et al. 2011).
The penises were removed after dissection from skin and surrounding fat, fixed in 10 % buffered formaldehyde, and paraffin-embedded, oriented along its transversal axis. Similar procedure was used to the proximal third of rat’s tail artery, considering that at this level the diameter of this segment is compatible with an NO-dependent conductance vessel (Souza et al. 2008). The heart was excised and cut halfway between the base and apex before fixation. Tissue sections of 4–6 μm in thickness were cut in a microtome (RM2145, Leica Microsystems GmbH, Germany) and placed on 0.1 % poly-l-lysine-covered microscopy slides for Masson’s trichrome staining and immunohistochemical detection of alpha-actin.
Histomorphometric evaluation of connective tissue content of rat myocardium and cavernous tissue
Two random selected slides of heart and penis of each age, and from all experimental groups, were stained by the laboratory routine method of Masson’s trichrome. After observation, the images were captured in a brightfield optical microscope connected to a digital camera (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) and further subjected to computer-assisted color histomorphometric analysis with ImageJ® software (NIH, Maryland, USA). This allowed the assessment of the connective tissue (CT, stained in blue) proportion to total myocardial or corpora area under analysis by pixel quantification. Areas belonging to tunica albuginea were excluded from the analysis.
Histomorphometric evaluation of perivascular smooth muscle layer density of rat cavernous tissue and tail artery
Perivascular smooth muscle layer (SML) density of rat cavernous tissue and tail artery was analyzed after immunodetection of alpha-actin, a specific smooth muscle cell (SMC) marker. Briefly, two penile and tail artery randomly selected slides of each age, and from all experimental groups, were deparaffinized, hydrated, treated with 3 % oxygen peroxide in methanol to block endogenous peroxidase activity, exposed to 1 M HCl for 30 min for epitope retrieval and neutralized with borax for 5 min, and incubated overnight with the monoclonal antibody mouse anti-alpha-actin (Chemicon International, Temecula, CA, USA). After 30 min of incubation with biotinylated secondary antibody, followed by 30 min with streptavidin–horseradish peroxidase complex (Vectastain, Vector, CA, USA), sections were simultaneously reacted with DAB/H2O2 and counterstained with hematoxylin. Color images of DAB-immunostained sections were captured in a brightfield optical microscope connected to a digital camera (Carl Zeiss MicroImaging) and then assessed by quantitative histomorphometry using ImageJ® software (NIH). Each image was analyzed after setting thresholds for automated DAB selection. This allowed the assessment of cavernous perivascular SML proportion to total cavernous tissue (areas belonging to tunica albuginea excluded) by pixel classification. Identical procedure was used to evaluate the SML of rat’s tail artery.
Statistical analysis
All descriptive data are expressed as mean value ± SEM. Analysis of variance, followed by Bonferroni multiple comparison test, was used to compare biometrical, biochemical, and hormonal data, as well as histomorphometrical results, within and between groups. Student’s t test was also used when appropriate. The correlation between serum adiponectin and total testosterone, HOMA-IR, WAT, epididymal, mesenteric and retroperitoneal mass, as well as between WAT and total testosterone, CRP, and HOMA-IR were done based on correlation analysis (Pearson coefficients) between pairs. Statistical analysis was performed using GraphPad Prism® software (GraphPad Software Inc., La Jolla, CA, USA). A probability value of P < 0.05 was accepted as statistically significant for all comparisons.
Results
Weight gain and food ingestion
Final BW, weight gain, food, and energy intake data from all experimental animal groups are summarized in Table 2. All the studied animals presented a linear increase in BW during the study. As expected and regardless of age, BW of HFD rats was significantly higher than that observed for ER groups (P < 0.001, for 6 and 12 months, and P = 0.002, for 18 months). Despite nutritional differences in diet, BW of HF-fed animals did not differ from that of age-matched controls (P = 0.232, P = 0.290, and P = 0.188, for 6, 12, and 18 months, respectively), nonetheless, HF-fed rats presented an increased fat accretion. In contrast, ER animals showed the lowest BW, when compared either with C (P < 0.001, P < 0.001, and P = 0.002, for 6, 12, and 18 months, respectively) or HFD groups (P < 0.001, P < 0.001, and P = 0.007, for 6, 12, and 18 months, respectively). In HF/ER rats, ER after HF-diet consumption led to the attenuation of total BW gain along the treatment (365.8 g) comparatively to age-matched HFD (605.6 g, P = 0.045) but not to C rats (416.2 g, P = 0.348). In general, the differences verified in BW gain were higher from 6 to 12 or 18 months than from 12 to 18 months (Table 2), regardless of the type of diet.
Table 2.
Final body weight, weight gain, adiposity index, and food and energy intake of rats from all experimental groups
| 6 months | 12 months | 18 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | HFD | ER | C | HFD | ER | C | HFD | ER | HF/ER | |
| Final BW (g) | 602.5 ± 13.5 | 656.1 ± 27.5 | 444.4 ± 5.1ab | 715.6 ± 27.2a | 763.1 ± 34.4b | 532.6 ± 11.6cde | 748.6 ± 33.8a | 888.8 ± 91.4b | 551.4 ± 20.6cfg | 681.6 ± 16.1h |
| BW gain (g) | 290.1 ± 18.2 | 288.7 ± 18.1 | 161.5 ± 10.8ab | 416.8 ± 32.8a | 474.7 ± 27.8b | 231.1 ± 13.0cde | 416.2 ± 38.6a | 605.6 ± 85.1b | 236.4 ± 17.0cfg | 365.8 ± 32.4gh |
| Adiposity indexj (%) | 6.2 ± 0.8 | 16.1 ± 1.8a | 4.9 ± 0.4b | 6.5 ± 0.5 | 12.2 ± 0.9d | 7.3 ± 0.4ce | 6.0 ± 0.8 | 11.8 ± 0.9f | 7.2 ± 0.7cg | 10.3 ± 0.7fh |
| Food intake (g week−1) | 199.8 ± 7.8 | 132.9 ± 10.6a | 147.0ia | 193.0 ± 10.0 | 139.8 ± 9.1d | 147.0id | 202.5 ± 7.5 | 138.5 ± 5.0f | 147.0if | 138.4 ± 8.8k, 147.0if |
| Energy intake (kJ week−1) | 2,417.4 ± 94.4 | 2,591.6 ± 206.7 | 1,778.7iab | 2,335.3 ± 121.0 | 2,726.1 ± 177.5 | 1,778.7ide | 2,450.3 ± 90.8 | 2,700.8 ± 97.5 | 1778.7ifg | 2,706.6 ± 171.6k, 1,778.7i |
Data are presented as mean ± SEM
BW body weight, C control group, HFD high-fat diet, ER energy-restricted group, HF/ER high-fat diet-fed group of rats that were energy-restricted from 12 to 18 months
aP < 0.05 vs. 6-month C; bP < 0.05 vs. 6-month HFD; cP < 0.05 vs. 6-month ER; dP < 0.05 vs. 12-month C; eP < 0.05 vs. 12-month HFD; fP < 0.05 vs. 18-month C; gP < 0.05 vs. 18-month HFD; hP < 0.05 vs. 18-month ER
iValues refers to fixed amount of food supplied (energy restriction)
jAdiposity index = [(∑ epididymal, mesenteric, retroperitoneal fat pads mass)∙(final body weight)−1]∙100
kHF diet ad libitum intake and energy intake from the beginning of the experiment to 12 months
The food intake of HFD rats was significantly lower than those observed for C groups (P = 0.001, P = 0.003, and P < 0.001, for 6, 12, and 18 months, respectively). Even though the energy value was higher in HF diet compared to standard rat chow (19.5 and 12.1 kJ g−1, respectively), the total energy intake of both groups was similar (Table 2). As expected, in ER groups, either the quantity or energy value of food was significantly lower than those observed in age-matched C groups; nevertheless, only energy intake differs from HFD animals. Note that the amount of food provided to ER animals was predetermined and fixed to achieve BW loss.
Blood pressure assessment
The highest heart rates were observed in HFD and HF/ER groups comparatively to ER and C animals (Table 3). Similarly, HFD groups presented the highest SBP values, significantly increased towards C groups at 12 and 18 months (P = 0.550, P = 0.006, and P < 0.001, for 6, 12, and 18 months, respectively). A consistent and significant raise in SBP and DBP along aging was observed in HF-treated animals (Table 3). Conversely, this age-related variation was not observed neither in C nor at ER rats, which presented lower values of SBP than C (P = 0.024, P = 0.038, and P = 0.054, for 6, 12, and 18 months, respectively) and HFD groups (P = 0.013, P < 0.001, and P = 0.019, for 6, 12, and 18 months, respectively). These rats also exhibited lower DBP than that observed in C and HFD rats. Concerning HF/ER rats, they presented higher SBP than C and ER at 18 months of age (P = 0.001 and P = 0.004, respectively). For this group, SBP do not differ from value observed in 18-month HFD (P = 0.245) despite the decrease in DBP (P = 0.008).
Table 3.
Blood pressure and fasting biochemical parameters of rats from all experimental groups
| Baseline | 6 months | 12 months | 18 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | HFD | ER | C | HFD | ER | C | HFD | ER | HF/ER | ||
| Blood pressure | |||||||||||
| Heart rate (bpm) | 374.4 ± 3.7 | 380.3 ± 5.2 | 383.1 ± 5.1 | 365.3 ± 5.0ab | 366.0 ± 6.5 | 384.0 ± 7.1 | 376.7 ± 6.3 | 348.0 ± 8.8a | 387.0 ± 4.5c | 352.4 ± 3.4d | 399.5 ± 1.3cde |
| Systolic (mmHg) | 113.6 ± 1.7 | 114.1 ± 1.7 | 115.9 ± 2.4 | 106.7 ± 2.5ab | 114.6 ± 2.3 | 124.7 ± 2.5bf | 104.9 ± 3.6fg | 104.2 ± 2.0af | 128.6 ± 1.5bc | 114.6 ± 3.9d | 133.5 ± 6.3ce |
| Diastolic (mmHg) | 86.5 ± 1.6 | 82.7 ± 1.3 | 92.1 ± 1.8a | 83.6 ± 3.7 | 90.2 ± 2.6a | 97.9 ± 2.0bf | 88.1 ± 3.1g | 90.8 ± 3.4 | 103.6 ± 2.2bc | 82.2 ± 2.3d | 83.5 ± 5.6d |
| Glucose metabolism | |||||||||||
| Glycemia (mg dL−1) | 108.9 ± 3.3 | 116.0 ± 5.6 | 115.6 ± 5.2 | 115.7 ± 8.0 | 127.8 ± 10.6 | 121.1 ± 5.9 | 111.1 ± 4.2 | 105.2 ± 12.4 | 135.8 ± 5.4b | 106.6 ± 4.8d | 111.0 ± 5.7d |
| Insulin (ng mL−1) | 1.83 ± 0.39 | 1.06 ± 0.18 | 1.12 ± 0.13 | 0.70 ± 0.04b | 0.93 ± 0.03 | 2.08 ± 0.30bf | 0.83 ± 0.07g | 0.86 ± 0.05 | 1.78 ± 0.08bc | 0.65 ± 0.03cd | 1.23 ± 0.44de |
| HOMA-IRi | 9.67 ± 1.93 | 7.92 ± 1.13 | 6.42 ± 0.74 | 4.76 ± 0.52a | 7.40 ± 1.04 | 14.8 ± 2.03bf | 5.68 ± 0.58g | 5.45 ± 0.75 | 14.3 ± 0.87bc | 4.12 ± 0.29d | 8.15 ± 1.16de |
| Lipid profile | |||||||||||
| TC (mg dL−1) | 89.8 ± 7.7 | 81.2 ± 6.2 | 148.2 ± 4.5a | 101.0 ± 15.2b | 107.8 ± 8.2a | 175.6 ± 20.0f | 136.7 ± 10.1 | 104.0 ± 13.6 | 167.4 ± 20.3c | 145.3 ± 15.3h | 161.5 ± 12.9c |
| HDL-c (mg dL−1) | 21.1 ± 1.4 | 39.8 ± 2.4 | 78.4 ± 0.6a | 51.6 ± 7.6b | 59.0 ± 4.8a | 95.0 ± 10.7f | 69.4 ± 8.3 | 62.4 ± 7.5a | 85.4 ± 8.6 | 66.8 ± 4.1 | 82.8 ± 4.3e |
| LDL-c (mg dL−1) | 44.1 ± 4.0 | 14.8 ± 2.3 | 38.2 ± 3.4a | 36.6 ± 7.4a | 18.3 ± 5.4 | 43.6 ± 4.9f | 38.8 ± 9.5 | 28.0 ± 11.0 | 40.6 ± 8.3 | 50.8 ± 6.3 | 51.8 ± 10.7 |
| Triglycerides (mg dL−1) | 56.8 ± 2.1 | 133.2 ± 19.5 | 158.0 ± 16.2 | 61.0 ± 10.1ab | 152.6 ± 11.2 | 185.2 ± 32.8 | 74.0 ± 22.6 | 155.3 ± 27.3 | 207.2 ± 40.8 | 88.6 ± 31.3d | 150.0 ± 16.4e |
Data are presented as mean ± SEM
TC total cholesterol, HDL-c high-density lipoprotein cholesterol, C control group, HFD high-fat diet, ER energy-restricted group, HF/ER high-fat diet-fed group of rats that were energy restricted from 12 to 18 months
aP < 0.05 vs. 6-month C; bP < 0.05 vs. 6-month HFD; cP < 0.05 vs. 18-month C; dP < 0.05 vs. 18-month HFD; eP < 0.05 vs. 18-month ER; fP < 0.05 vs. 12-month C; gP < 0.05 vs. 12-month HFD; hP < 0.05 vs. 6-month ER
iHOMA-IR = G∙(I∙22.5−1), where G is fasting glucose (millimoles per liter) and I is fasting insulin (milliunits per liter)
Glucose metabolism and lipid profile
Fasting biochemical parameters concerning glucose metabolism and lipid profile of rats from all experimental groups are depicted in Table 3. Plasma glucose levels presented low variations between groups throughout the study (P = 0.251) despite the significant increase observed from 6 to 18 months in HFD animals (P = 0.019). In addition, 18-month HFD rats had higher values of glycemia compared with ER and HF/ER of same age (P = 0.004 and P = 0.013, respectively), but no differences relatively to age-matched C were found.
All experimental groups presented serum insulin levels within the normal fasting range indicated by the manufacturer for the method employed (0.5–2.0 ng mL−1), excepting 12-month HFD rats (2.08 ng mL−1) (Table 3). Nevertheless, HF-diet feeding (HFD groups) led to an increase in insulin along aging, particularly evident at 12 months (6 vs. 12 months, P = 0.017; 6 vs. 18 months, P = 0.002; and 12 vs. 18 months, P = 0.320). Conversely, ER groups presented the lowest concentrations of insulin in blood, inferior to those observed in C rats, though significant difference was observed just at 18 months (P = 0.007). ER after HF-diet treatment (HF/ER group) led to a decrease in this hormone concentration when compared to 18-month HFD animals (P = 0.038); nevertheless, relative to 18-month ER rats, HF/ER group presents significantly higher values (P = 0.022).
HOMA-IR, a valid predictor of insulin sensibility (Cacho et al. 2008), was calculated individually for each animal (Table 3). As verified for serum insulin concentrations, HF diet led to an important elevation in this index particularly in 12- and 18-month rats (6 vs. 12 months, P = 0.006; 6 vs. 18 months, P < 0.001; and 12 vs. 18 months, P = 0.807). No differences with aging were verified either in ER or in C animals. ER rats presented the lowest values of HOMA-IR, comparatively either to age-matched HFD (P = 0.104, P = 0.004, and P < 0.001, for 6, 12, and 18 months, respectively) or C groups (P = 0.034, P = 0.172, and P = 0.113, for 6, 12, and 18 months, respectively). An important decrease was observed in this parameter when HF/ER animals were compared to the 18-month HFD group (P = 0.003); however, and accordingly to the observation for insulinemia, HF/ER present higher levels of HOMA-IR than 18-month ER group (P = 0.007).
In what concerns lipid profile (Table 3), TC levels were consistently elevated in HFD animals with aging, relatively to other experimental groups. Additionally, no differences were observed in HF/ER rats when compared with 18-mo HFD group (P = 0.814). ER and C animals presented similar TC levels at every age (P = 0.278, P = 0.083, and P = 0.071, for 6, 12, and 18 months, respectively). HDL-c levels progressively increased from the beginning of diet treatments in all experimental groups, which was just significant in C animals (6 vs. 12 months, P = 0.012; 6 vs. 18 months, P = 0.036; and 12 vs. 18 months, P = 0.713). No differences were observed between ER and C groups at any age. Although HFD animals presented the highest levels of HDL-c, the major differences were seen either between 6-month HFD animals and age-matched ER and C rats (P < 0.001 and P = 0.008, respectively) or between 12-month HFD and 12-month controls (P = 0.024). ER after HF-diet consumption apparently did not affect this lipoprotein concentration as no differences were verified between HF/ER group and 18-month HFD (P = 0.796). No differences were observed in LDL-c levels between groups, except for the increase in 6-month HFD and ER animals compared to age-matched C rats (P < 0.001 and P = 0.022, respectively).
As expected, plasma TG levels were high in HFD animals and, in opposition, low in ER rats (Table 3). However, due to inter-individual variation, significant differences were only seen between 6-month ER rats and age-matched HFD (P = 0.002) and C rats (P = 0.014), and 18-month ER and HFD (P = 0.041). HF/ER animals presented a decrease in TG levels comparatively to 18-month HFD (P = 0.247), similar to 18-month C (P = 0.876), but almost double that seen in ER (P = 0.022).
Total testosterone
Regarding plasma total testosterone mean values (Table 4), statistical significance was hard to establish due to the relatively high inter- and intra-individual variability. Nevertheless, our results suggest a physiological drop in this androgen concentration along rat’s aging, as reported before (Neves et al. 2006). Note the lower total testosterone levels evidenced by 6-month HFD animals in comparison to age-matched C rats (P = 0.049). Although no statistical significance was established, HF/ER animals presented an elevation of total testosterone levels comparatively to other 18-month experimental groups.
Table 4.
Plasma total testosterone levels, CRP, and adiponectin of rats from all experimental groups
| Baseline | 6 months | 12 months | 18 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | HFD | ER | C | HFD | ER | C | HFD | ER | HF/ER | ||
| Total testosterone (ng mL−1) | 0.80 ± 0.18 | 1.43 ± 0.60 | 0.17 ± 0.09a | 0.81 ± 0.29 | 0.34 ± 0.14 | 0.25 ± 0.06 | 0.45 ± 0.13 | 0.38 ± 0.15 | 0.17 ± 0.03 | 0.31 ± 0.09 | 0.72 ± 0.21 |
| CRP (mg dL−1) | 4.5 ± 0.6 | 5.4 ± 0.9 | 8.8 ± 1.2 | 9.6 ± 1.2a | 4.2 ± 1.1 | 6.0 ± 1.7 | 7.0 ± 1.0 | 3.6 ± 0.5 | 8.4 ± 1.0c | 7.8 ± 2.1 | 3.6 ± 1.1d |
| Adiponectin (μg mL−1) | 3.2 ± 0.4 | 6.0 ± 1.2 | 2.3 ± 0.3a | 10.5 ± 0.7ae | 4.8 ± 0.9 | 4.3 ± 0.6e | 7.1 ± 0.6bf | 4.3 ± 0.9 | 3.0 ± 0.3f | 7.5 ± 1.0cd | 2.8 ± 1.0g |
Data are presented as mean ± SEM
C control group, HFD high-fat diet, ER energy-restricted group, HF/ER high-fat diet-fed group of rats that were energy restricted from 12 to 18 months
aP < 0.05 vs. 6-month C; bP < 0.05 vs. 12-month HFD; cP < 0.05 vs. 18-month C; dP < 0.05 vs. 18-month HFD; eP < 0.05 vs. 6-month HFD; fP < 0.05 vs. 6-month ER; gP < 0.05 vs. 18-month ER
CRP and circulating adiponectinemia
No differences in serum CRP levels were verified along aging in any experimental group (Table 4). However, a potential association with diet may exist, considering the increase observed in 18-month HFD rats CRP levels compared to age-matched C group (P = 0.005). In addition, ER after HF feeding (HF/ER group) led to an important decrease comparatively to 18-month HFD animals (P = 0.013). Unexpectedly, the highest values of CRP were verified in ER rats, but only different from C animals at 6 months (P = 0.024).
Concerning the adiponectin serum concentrations (Table 4), ER groups presented the highest levels comparatively to either HFD animals (P < 0.000, P = 0.017, and P = 0.005, for 6, 12, and 18 months, respectively) or controls (P = 0.010, P = 0.086, and P = 0.040, for 6, 12, and 18 months, respectively). Indeed, the lowest adiponectinemia levels were associated to HF-diet consumption, but ER treatment after HFD failed to increase the levels of this adipokine (HF/ER vs. 18-month HFD, P = 0.465; HF/ER vs. 18-month ER, P = 0.021).
White adipose tissue mass and adiposity index
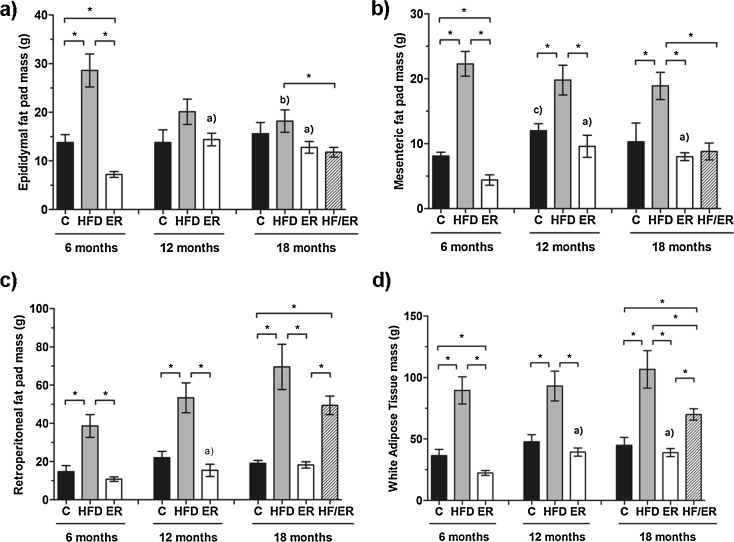
Immediately after euthanasia of each animal, epididymal (Fig. 2a), mesenteric (Fig. 2b), and retroperitoneal (Fig. 2c) fat pads were removed and weighted. The sum of these three fat deposits (WAT) is also depicted (Fig. 2d). HFD rats presented the highest values of each adipose tissue fraction and WAT, comparatively to the other experimental groups (ER, HF/ER, and C). In HFD animals, neither mesenteric nor retroperitoneal fat mass differed over the time of treatment, in opposition to epididymal fat that decreased significantly from 6 to 18 months (P = 0.008). Concerning ER animals, the lowest WAT and respective fractions were registered, and an age-related association was found, considering that epididymal, mesenteric, and retroperitoneal fat pads mass increased from 6 to 12 months (P = 0.001, P = 0.032, and P = 0.016, respectively), and stabilized until 18 months (P = 0.390, P = 0.408, and P = 0.463, respectively). With respect to HF/ER rats, ER treatment from 12 to 18 months led to a reduction in both epididymal (P = 0.046) and mesenteric fat pads (P = 0.005) to levels similar to those observed in 18-month ER rats. However, WAT mass in HF/ER group (70.0 ± 4.6 g) was largely superior to 18-month C (45.0 ± 6.3 g, P = 0.012) and ER rats (39.0 ± 3.2 g, P = 0.001), despite the decrease relative to 18-month HFD rats (106.6 ± 15.2 g, P = 0.049).
Fig. 2.
Graphical representation of adipose tissue fat pads mass from all experimental groups. Bars represent the mean in grams of epididymal (a), mesenteric (b), and retroperitoneal (c) fat pads, as well as white adipose tissue (d), and error bars indicate the standard error of the mean. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months. * P < 0.05, significant differences between groups; a P < 0.05 vs. 6-month ER, b P < 0.05 vs. 6-month HFD, c P < 0.05 vs. 6-month C
To further understand the effect of HF diet and ER on body adiposity, AI was determined as described by Fernandez et al. (2011). As summarized in Table 2, HFD animals presented the highest AI when compared either with C (P = 0.001, P < 0.001, and P = 0.001, for 6, 12, and 18 months, respectively) or ER rats (P = 0.003, P = 0.001, and P = 0.333, for 6, 12, and 18 months, respectively). A significant increment in ER rats adiposity was found between 6 and 12 months (P = 0.003), which further tended to stabilize (P = 0.880). Concerning ER after HF-diet feeding (HF/ER rats), it was observed a slight not significant decrease in AI comparatively to 18-month HFD rats (P = 0.212).
Correlation between serum adiponectin, HOMA-IR, CRP, total testosterone, fat pads mass, adiposity index, and insulin
Correlation coefficients determined for the parameters aforementioned are depicted in Table 5. Although ER animals presented increased levels of CRP and adiponectin, no correlation between these variables was found (r = +0.103, P = 0.291). Similarly, levels of total testosterone did not correlate with serum adiponectin (r = +0.162, P = 0.276) nor AI (r = −0.234, P = 0.114). On the other hand, it was found that this adipokine correlated inversely with HOMA-IR (r = +0.354, P = 0.015), with epididymal, mesenteric and retroperitoneal fat pads mass, WAT, and AI (r = −0.423, P = 0.003; r = −0.491, P < 0.001; r = −0.552, P < 0.001; r = −0.502, P < 0.001; and r = −0.504, P < 0.001, respectively), supporting that the increment of adiposity led to a diminution of circulating adiponectin.
Table 5.
Correlation coefficients of adiponectin, HOMA-IR, C-reactive protein, or plasma total testosterone with white adipose tissue mass, epididymal, mesenteric and retroperitoneal fat pads mass, and insulin
| Adiponectin | HOMA-IR | C-reactive protein | Total testosterone | |
|---|---|---|---|---|
| White adipose tissue mass | −0.502* | +0.420* | +0.331** | −0.039 |
| Epididymal fat pad mass | −0.427* | +0.460* | +0.084 | −0.178 |
| Mesenteric fat pad mass | −0.491* | +0.524* | +0.024 | −0.275** |
| Retroperitoneal fat pad mass | −0.552* | +0.353** | +0.251 | −0.144 |
| Adiposity indexa | −0.504* | +0.534* | −0.037 | −0.234 |
| Adiponectin | n.a. | −0.354** | +0.103 | +0.162 |
| Insulin | −0.362** | +0.452* | −0.142 | −0.275** |
| HOMA-IRb | −0.354** | n.a. | −0.067 | −0.194 |
| C-reactive protein | −0.103 | +0.067 | n.a. | −0.157 |
| Plasma total testosterone | +0.162 | −0.194 | −0.157 | n.a. |
n.a. not applicable
*P < 0.01; **P < 0.05
aAdiposity index = [(∑epididymal, mesenteric, retroperitoneal fat pads mass)∙(final body weight)−1]∙100
bHOMA-IR = G∙(I∙22.5−1), where G is fasting glucose (millimoles per liter) and I is fasting insulin (milliunits per liter)
Statistical analysis also evidenced that serum total testosterone correlated negatively with mesenteric fat (r = −0.275, P = 0.036), despite the absence of correlations with WAT, epididymal, or retroperitoneal fat pads mass (r = −0.039, P = 0.666; r = −0.178, P = 0.231; and, r = −0.144, P = 0.333, respectively).
It was verified that insulin resistance, evaluated by HOMA-IR index, was directly correlated with WAT (r = +0.420, P < 0.001), as well as with the different anatomic fat deposits (r = −0.460, P < 0.001; r = −0.524, P < 0.001; and r = −0.353, P = 0.03, for epididymal, mesenteric, and retroperitoneal, respectively) and AI (r = −0.543, P < 0.001). In addition, negative correlations between insulin levels and adiponectin or testosterone (r = −0.362, P = 0.01 and r = −0.275, P = 0.04, respectively), and positive correlation between CRP levels and WAT mass (r = +0.331, P = 0.018) were found.
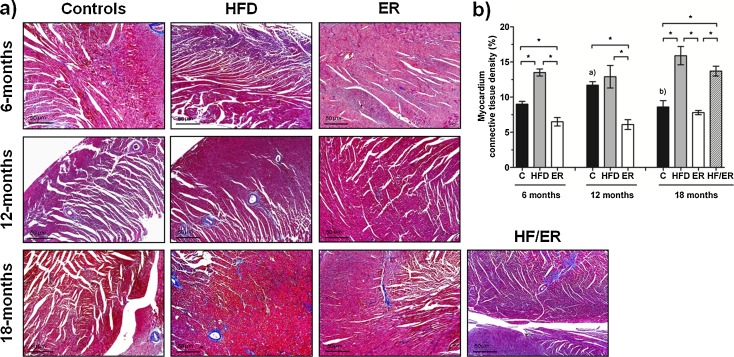
Connective tissue content in myocardium
Similarly to human tissue, rat myocardium is mainly composed by cardiomyocytes (striated cardiac muscle), with small vessels lined by endothelial cells and delimited by a thin SML. Surrounding myocytes, sparse CT is also present. Observation of Masson’s trichrome staining of myocardium sections (Fig. 3a) did not evidence marked differences between HFD and ER groups along aging or between 6 and 18-month C rats. On the other hand, CT content (stained blue) varied in accordance with rats’ diet composition (P < 0.000). Histomorphometric analysis (Fig. 3b) demonstrated an increase in CT content in myocardium of HFD groups, when compared with age-matched ER animals (P = 0.001, P = 0.004, and P = 0.003, for 6, 12, and 18 months, respectively) and C groups (P = 0.003, P = 0.464, and P = 0.011, for 6, 12, and 18 months, respectively). The percentage of CT present in the myocardium of ER animals was significantly inferior to that observed in C rats at 6 and 12 months (P = 0.023 and P < 0.001, respectively) but similar at 18 months (P = 0.468). ER after HF-diet consumption (HF/ER) did not produce changes in the heart CT content (P = 0.219 relative to 18-mo HFD). No correlations were found between CT content in myocardium neither with circulating levels of total testosterone, adiponectin, and CRP nor with HOMA-IR (data not shown).
Fig. 3.
Histomorphometric analysis of connective tissue content on rat myocardium. a Masson’s trichrome staining of rat myocardium sections from all experimental groups. Smooth muscle and connective tissues are stained in red and in blue, respectively. b Graphical presentation of quantitative changes in connective tissue content in all experimental groups, determined by computer-assisted histomorphometry. Bars represent the mean value in percentage of myocardium connective tissue content, and error bars indicate the standard error of the mean. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months. *P < 0.05, significant differences between groups; a P < 0.05 vs. 6-month C group, b P < 0.05 vs. 12-month C group
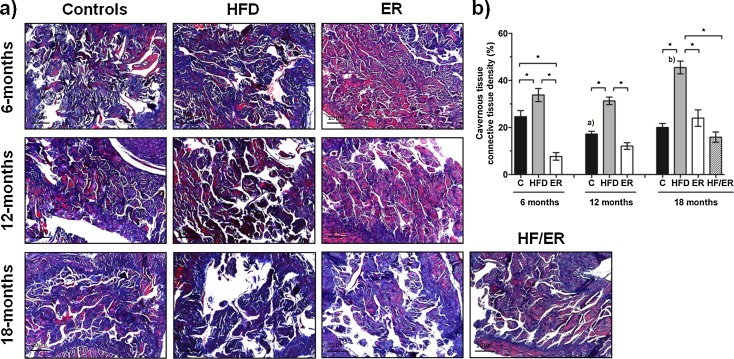
Connective tissue density in cavernous tissue
Rat cavernous tissue is a mesh of interconnected cavernous spaces, lined by endothelium surrounded by SML, and separated by trabecula mainly composed by CT (collagen fibers and fibroblasts), as previously reported (Neves et al. 2006, 2010). Masson’s trichrome staining (Fig. 4a) showed that CT content (stained blue) in cavernous tissue varied between groups (P < 0.000), as confirmed by quantitative computer-assisted histomorphometric analysis (Fig. 4b). HFD rats’ cavernous tissue presented higher CT density when compared to that of ER (P = 0.001, P = 0.036, and P = 0.001, for 6, 12, and 18 months, respectively), C (P = 0.032, P < 0.001, and P = 0.352, for 6, 12, and 18 months, respectively), and HF/ER groups (P < 0.000). In fact, ER treatment after HF diet led to CT values similar to those observed in 18-month ER and C rats (P = 0.091 and P = 0.152, respectively). In ER animals, although CT percentage almost doubled from 6 to 12 months, and from 12 to 18 months, no statistical significance was found.
Fig. 4.
Histomorphometric analysis of connective tissue content on rat cavernous tissue. a Masson’s trichrome staining of rat cavernous tissue sections from all experimental groups. Smooth muscle and connective tissues are stained in red and in blue, respectively. b Graphical presentation of quantitative changes in connective tissue content in all experimental groups, determined by computer-assisted histomorphometry. Bars represent the mean value in percentage of cavernous connective tissue content, and error bars indicate the standard error of the mean. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months. *P < 0.05, significant differences between groups; a P < 0.05 vs. 6-month C, b P < 0.05 vs. 6-month HFD
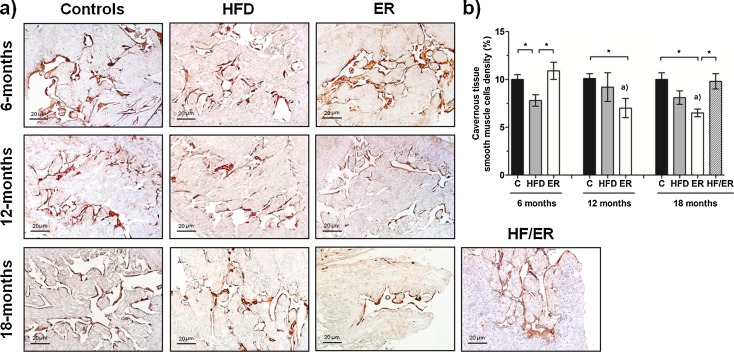
Perivascular smooth muscle cells density of cavernous tissue
In rat cavernous tissue, SMC are restricted to endothelium periphery (Neves et al. 2006, 2010). Subsequent to immunostaining of alpha-actin (Fig. 5a), differences in perivascular SML density between experimental groups were evaluated by histomorphometry (Fig. 5b). As depicted in the graph, although no significant differences were detected in SMC content in HFD and C groups along aging, a decrease in perivascular SMC density were observed in ER animals from 6 to 12 months (P = 0.045), and to 18 months (6 vs. 18 months, P = 0.019). No differences in cavernous SML density were observed between 18-month HFD rats and age-matched ER and C rats (P = 0.177 and P = 0.066, respectively). However, 6 and 12-month ER rats evidenced lower SML percentage comparatively to their paired controls (P = 0.012 and P < 0.001, respectively). HF/ER rats presented similar SMC density to either 18-month HFD (P = 0.139) or 18-month C (P = 0.850) but clearly augmented comparatively to 18-month ER rats (P = 0.006). Although aging and HF-diet regular intake are both associated with increased fat mass deposition around the vessels (Barandier et al. 2005), in this study, no adipocytes were observed in perivascular areas of cavernous tissue.
Fig. 5.
Histomorphometric analysis of smooth muscle cells density of rat cavernous tissue. a Immunohistochemical detection of alpha-actin in cavernous tissue transversal sections from all experimental groups. Note brown stain of smooth muscle cells. b Graphical presentation of quantitative changes in smooth muscle layer, determined by computer-assisted histomorphometry of all experimental groups. Bars represent the mean value in percentage of smooth muscle cells density, and error bars indicate the standard error of the mean. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months. *P < 0.05, significant differences between groups; a P < 0.05 vs. 6-month ER
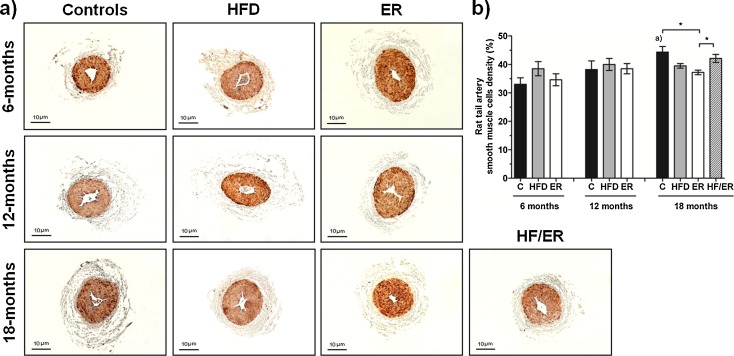
Tail artery smooth muscle cells density
SMC density of the proximal third of rat tail artery was studied after immunodetection of alpha-actin (Fig. 6a). Alpha-actin immunoreactivity was found in the tunica media that consists of concentrically arranged SMC layers. No differences in SMC density were verified along aging (Fig. 6b), except for the significant increase observed in C animals from 6 to 18 months (P = 0.009). In addition, it was found that 18-month ER animals presented thinner SMC layer than age-matched C rats (P = 0.011) but similar to 18-month HFD animals (P = 0.059).
Fig. 6.
Histomorphometric analysis of smooth muscle layer of rat’s tail artery. a Immunohistochemical detection of alpha-actin in tail artery transversal sections from all experimental groups. Note brown stain of smooth muscle cells. b Graphical presentation of quantitative changes in smooth muscle layer of rat’s tail artery, determined by computer-assisted histomorphometry of all experimental groups. Bars represent the mean value in percentage of smooth muscle cells content , and error bars indicate the standard error of the mean. C control group, HFD high-fat diet-fed group, ER energy-restricted group, HF/ER energy-restricted rats high-fat diet-fed until complete 12 months. *P < 0.05, significant differences between groups; a P < 0.05 vs. 6-month C
Discussion
MetS affects at least one quarter of the population in industrialized countries. Its prevalence is strongly associated with aging (Ford et al. 2002) and, in turn, with age-associated diseases such as ED and CVD (Jacobs et al. 2011). These clinical conditions result from complicated atheroma plaque, which stems from previous endothelial dysfunction. Therefore, age and other genetic and environmental factors that lead to endothelial dysfunction, and persistent low-grade inflammation, are key features in the pathophysiology of atherosclerosis and exhibit high incidence in MetS patients (Feldman et al. 1994; Kressel et al. 2009; Rizvi 2009).
We had hypothesized that long-term HF-diet consumption is sufficient to result in MetS. Such HF diet, lard-derived, especially rich in saturated fatty acids, is recommended for induction of obesity-related metabolic changes, as dyslipidemia and insulin intolerance (Pajvani and Scherer 2003); it contrasts with diets containing fish oils, particularly rich in polyunsaturated ω-3 fatty acids, which have beneficial effects on body composition and insulin action (Buettner et al. 2006). Beyond the ability to mimic human MetS, previous reports demonstrated that in rats, HF-fed related changes also extend to cardiovascular complications (Buettner et al. 2006).
Opposed to lard-derived HF diets, ER is the most adequate strategy to reduce BW and improve the metabolic profile (Aziz et al. 2009). In rodents, daily food intake reduction of 20–40 % significantly reduces WAT (Blüher et al. 2003), the risk to develop CVD, or other age-associated diseases (Martin et al. 2010) and extends life span by up to 40 % (Barzilai et al. 1998). Equivalent findings were observed in humans considering that 1 year of low-energy diet reduces markedly atherosclerosis risk factors such as high body mass index, blood pressure, blood levels of glucose, insulin, CRP, TG, and LDL-c, and increases the levels of HDL-c (Fontana et al. 2004).
In the current investigation, postmortem evaluation provided evidence that body composition and body fat distributions were quantitatively affected by nutritional quality of diet despite the similarity in final BW of HFD and age-matched C rats. When compared to controls and ER, HFD animals presented a significant increase in adiposity, probably accompanied by loss of lean body mass, BW, AI, and WAT. In addition, the results agree with the expected findings regarding hypertension (SBP and DBP), hyperinsulinemia, and insulin resistance (evidenced by higher HOMA-IR). Within the lipid profile of HFD animals, beyond the expected hypercholesterolemia and hypertriglyceridemia, some surprising results were obtained, e.g., the rise in HDL-c, also noticed in ER rats and controls along aging. This change is rather unexpected in view of the well-known association of atherosclerosis and lower circulating level of HDL-c in humans (Onat et al. 2010). It is possible however that HDL-c may be showing a chameleon-like lipoprotein feature: the capacity to be anti-inflammatory in the basal state (as HDL-c protects LDL-c from oxidation) and to be proinflammatory/atherogenic, injurious to the vascular system during the acute-phase responses, or chronic inflammation (G et al. 2011; Navab et al. 1996). Such inflammatory state has been associated with conditions as aging and HF diets too (Kim et al. 2008; Kressel et al. 2009; Ye and Keller 2010).
In the context of HFD and blood chemistry changes in favor of MetS, testosterone level indicates its association as well. Indeed, it declines in older animals, compared to younger ones, and also in HFD rats, compared to controls and ER. These changes appear to parallel previous human observations that recently attracted more attention, not only concerning sexual performance, but in a wider context of male health. That is so because experimental research supports the point that testosterone deficiency profoundly affects angioarchitecture of cavernous tissue and impairs the erectile capacity (Tomada et al. 2011; Traish et al. 2007) and also because hypogonadism associates with MetS and is a risk factor for CVD (Jones 2007; Frederiksen et al. 2012; Laughlin et al. 2008; Vikan et al. 2009).
The change in adiposity deserves additional comments on the location of the age-related WAT accumulation (epididymal, mesenteric, or retroperitoneal). As expected, on account of the known accretion in intra-abdominal fat upon high-energy high-fat diet intake (Sinclair 2005), pad mass and AI were higher in HFD compared to controls and ER animals. This difference was especially notable in 6-month-old animals but not as sharp in the succeeding ages.
However, whereas fat deposition at all locations exhibited a minor, age-related trend to increase in controls, considerably different results were noticed when HFD and ER were compared. In fact, while ER animals trends were similar to controls, HFD animals presented an age-related downward trend in epididymal and mesenteric fat but an upward trend in retroperitoneal fat deposition. The data suggest that aging was able to modulate epididymal and mesenteric fat deposition but not retroperitoneal, which seems more diet dependent. These different modulatory patterns of fat deposition reflect the general view that obesity is a complex disorder, dependent on genetic and environmental factors.
Besides energy storage function, WAT is an important endocrine organ that regulates many biological processes and is involved in derangements that may lead to pathological conditions. Indeed, as obesity develops, secretory activity of WAT increases and cardiometabolic risk rises (Matsuda et al. 2002). Moreover, recent studies demonstrate that adipocytes are highly active in the secretion of hormonal factors, including adiponectin, a major bioactive molecule with recognized cardiovascular beneficial properties. This peptide increases glucose uptake, reduces gluconeogenesis and lipogenesis, and enhances fat β-oxidation (Kadowaki et al. 2008). In contrast, circulating levels of adiponectin are reduced in obesity and MetS (Di Chiara et al. 2012), antedating insulin resistance (Blouet et al. 2006; Pajvani and Scherer 2003), a consequence also related to the consumption of diets rich in saturated fatty acids (Razny et al. 2011). Coherently with this point, adiponectin levels were decreased in HFD animals and substantially increased in ER animals at all ages; they inversely correlated with WAT and respective different fractions, and with AI and HOMA-IR as well.
Beyond anti-atherosclerotic properties (Matsuda et al. 2002), it is believed that adiponectin possesses anti-inflammatory effects too (Ye and Keller 2010), which would favor endothelial dysfunction prevention (Sweazea et al. 2010; Pearson et al. 2003). It is uncertain however that adiponectin may be used to assess inflammation, which is not the case for CRP, an usual marker of inflammatory conditions. Interestingly, at all time points, hypoadiponectinemia accompanied by high levels of CRP were evidenced in HFD rats compared to controls, although we did not find a noteworthy correlation between both concentrations.
These general biometric and biochemical changes observed in HFD rats indicate a trend towards the establishment of MetS and the enhancement of cardiovascular risks in a fashion similar to humans. They thus render relevance to the lard-derived HF-diet-fed rat as a model for the study of age-related metabolic derangements and consolidate the ER regimen as a contrasting companion of this experimental procedure.
Some of the observed structural features in tissues are also compatible with this trend for MetS. In fact, beyond a significant elevation in blood pressure (both SBP and DBP), HFD rats had an increased perivascular CT in the myocardium. This finding corroborates a previous report that relates hypertension to cardiac fibrosis (Berk et al. 2007) and contrasts well with the findings in ER rats, where the lowest BW, blood pressures, and myocardium CT content were verified. Still, they showed an age-related trend too.
Similarly to the heart, an enhanced CT deposition was noticed in the arterial bed of the penis in HFD rats, particularly the older ones. Although starting at a lower level of CT deposition, ER animals also evidenced an age-related trend to its increase, to reach control values at older age, further adding to age as an independent factor for CT deposition. This condition leads to vascular stiffness, compromises cavernous SM vasodilatation, and modifies the cell–cell communication. The sinusoidal spaces will become narrower, less able to retain blood, and thus the inflow of blood into cavernous spaces to elevate the pressure within the penis decreases (Jiang et al. 2005) contributing to veno-occlusive ED (Shen et al. 2001).
In contrast with that clear age-related trend for CT deposition in HFD rats’ cavernous tissue, the SMC reduction did not appear to vary when compared to controls. Also unclear is the apparent downward trend in ER animals which loose a substantial amount of SMC along aging. The meaning is uncertain, especially when it is compared with the SMC density at the tail artery, a muscular vessel widely used in the study of vascular SM (Leal et al. 2008), whose age-related features under controlled and dietary experimental conditions are here described for the first time. Loss of SMC and increment of CT deposition along aging have been repeatedly reported in corporal tissue either in animal models (Burchard et al. 2000; Carrier et al. 1997) and humans (Tomada et al. 2011). These changes provoke mechanical alterations of the penis, as reduction in its elasticity and compliance, which lead to erectile function impairment (Ferrini et al. 2009). We are convinced that the observed features in the cavernous tissue, either the CT or the vascular SMC, reflect a specific sensitivity of this reproductive tissue to reduced energy intake as well.
The group of animals that was submitted to ER after a long period of HF-diet consumption (HF/ER group) addresses a recurrent question about the value of changing dietary lifestyles after a prolonged ingestion of HF food. The rats in this experiment showed some changes that deserve a particular attention. Both BW and AI decreased to levels below HFD animals but remained above ER and controls. Diastolic blood pressure, but not systolic, presented a similar change, as well as most common blood chemistry data, including lipids, glucose, and insulin. Interestingly, circulating adiponectin, an adipocyte originated peptide, remained as if HFD had continued; in addition, while epididymal and mesenteric WAT reduced to levels similar to ER rats, retroperitoneal fat did not, indicating that biological features of such fat deposits set at younger ages are not lost later, independently of changes in its environment. Conversely, ER treatment after HF-diet feeding (HF/ER group) resulted in an important decrease of CRP, which indicates that decrease in fat depots observed in these rats reduces chronic systemic inflammation and suggests that visceral fat makes an important contribution to that. These observations suggest that different fat deposits have different, subtle regulatory mechanism and are in favor of their functional heterogeneity. For example, retroperitoneal fat appears a more diet dependent and implied in adiponectin regulation, whereas epidydimal and mesenteric are contributors to age-related inflammation and are sentinelled by CRP.
Specific unknown tissue specificities may also underlie the CT and SMC changes in the HF/ER animals. These exhibited a lower CT deposition in cavernous tissue, when compared to HFD, in contrast with CT deposition in the heart which remained similar to HF-diet-fed rats at all times. It suggests that beneficial functional effects derived from lesser energy intake may be more expected in penile vasculature, in comparison with the heart. Moreover, apparent replacement of corpora CT by SMCs favors an improved cavernous tissue performance. However, other studies are necessary to ascertain this point.
Conclusions
Taken together, the findings emphasize the importance of the diet composition and its energy value along aging; they also evidence metabolic consequences of HF-diet intake and their effect on specific target organs. HF long-term consumption increases WAT accretion, blood pressure, lipids, and CRP in plasma while reduces insulin sensibility and adiponectin secretion, and strongly favors CT increment in cavernous tissue and myocardium. These changes and the underlying endothelial dysfunction are likely to be avoided by ER implementation early in life, hoping to prevent cardiovascular events in the future. Conversely, when low-fat low-energy diet is adopted later in life, despite the improvement on insulin sensibility and lipid profile, adiponectin secretion ability is compromised, the retroperitoneal fat mobilization is limited, and the CT deposition in the myocardium is unaltered. Nevertheless, tissue structural modifications in cavernous tissue can be reversed by HF-diet withdrawal, which reinforces the role of diet in the prevention and reversion of ED.
Acknowledgments
The authors thank Dra. Emília Patrício and Dra. Luísa Rafael from Department of Clinical Pathology of Centro Hospitalar S. João for biochemical parameters and testosterone determinations, respectively. Authors are also thankful to Dr. João Pedro Ramos and Dra. Alexandra Rêgo from Department of Clinical Pathology of Centro Hospitalar S. João for insulin and adiponectin assays by RIA method. This study was supported by Fundação para a Ciência e Tecnologia (FCT), Portugal – Pluriannual funding and SFRH/BD/46009/2008.
References
- Aziz AA, Kenney LS, Goulet B, Abdel-Aal ES. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J Nutr. 2009;139:1881–1889. doi: 10.3945/jn.109.110650. [DOI] [PubMed] [Google Scholar]
- Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk BC, Fugiwara K, Lehoux S. ECM remodelling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups KL, Bank AJ, Padma-Nathan H, Katz SD, Williams RA. Erectile dysfunction as a harbinger for increased cardiometabolic risk. Int J Impot Res. 2008;20:236–242. doi: 10.1038/sj.ijir.3901634. [DOI] [PubMed] [Google Scholar]
- Blouet C, Mariotti F, Azzout-Marniche D, Bos C, Mathé V, Tomé D, Huneau JF. The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J Nutr. 2006;136:1849–1854. doi: 10.1093/jn/136.7.1849. [DOI] [PubMed] [Google Scholar]
- Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Boneti P, Lerman L, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Atheroscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36:485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- Burchard T, Burchard M, Karden J, Buttyan R, Shabsigh A, de La Taille A, Ng PY, Anastasiadis AG, Shabsigh R. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat. J Urol. 2000;164:1807–1811. doi: 10.1016/S0022-5347(05)67111-X. [DOI] [PubMed] [Google Scholar]
- Cacho J, Sevillano J, Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensibility during pregnancy in Wistar and Sprague–Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- Camici GG, Shi Y, Cosentino F, Francia P, Lüscher TF. Anti-aging medicine: molecular basis for endothelial cell-targeted strategies—a mini-review. Gerontology. 2011;57:101–108. doi: 10.1159/000314227. [DOI] [PubMed] [Google Scholar]
- Carrier S, Nagaraju P, Morgan DM, Baba K, Nunes L, Lue TF. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. J Urol. 1997;157:1088–1092. doi: 10.1016/S0022-5347(01)65147-4. [DOI] [PubMed] [Google Scholar]
- Chew KK, Finn J, Stuckey B, Gibson N, Sanfilippo F, Bremner A, Thompson P, Hobbs M, Jamrozik K. Erectile dysfunction as a predictor for subsequent atherosclerotic events: findings from a linked-data study. J Sex Med. 2010;7:192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara T, Argano C, Corrao S, Scaglione R, Licata G. Hypoadiponectinemia: a link between visceral obesity and matabolic syndrome. J Nutr Metab. 2012;2012:175245. doi: 10.1155/2012/175245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease. J Am Coll Cardiol. 2011;58:1378–1385. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychossocial correlates: results from the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, Nascimento AF, Cicogna AC, Kempinas WD. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in rat. J Sex Med. 2009;6:415–428. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Frederiksen L, Højlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr) 2012;34:145–156. doi: 10.1007/s11357-011-9213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Grundy G. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Hausman DB, Fine JB, Tagra K, Fleming SS, Martin RJ, DiGirolamo M. Regional fat pad growth and cellularity in obese Zucker rats: modulation by caloric restriction. Obes Res. 2003;11:674–682. doi: 10.1038/oby.2003.96. [DOI] [PubMed] [Google Scholar]
- Hima Bindu G, Rao VS, Kakkar VV. Friend turns foe: transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response. Cholesterol. 2011;2011:274629. doi: 10.1155/2011/274629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. Prevention of cardiovascular disease by the early identification of erectile dysfunction. Int J Impot Res. 2008;20(suppl2):S9–S14. doi: 10.1038/ijir.2008.47. [DOI] [PubMed] [Google Scholar]
- Jackson G, Rosen RC, Kloner RA, Kostis JB. The second Princeton consensus on sexual dysfunction and cardiac risk: new guidelines for sexual medicine. J Sex Med. 2006;3:28–36. doi: 10.1111/j.1743-6109.2005.00196.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M, van Greevenbroek MM, van der Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, Jansen EH, Schalkwijk CG, Stehouwer CD. The association between the metabolic syndrome and peripheral, but not coronary, artery disease is partly mediated by endothelial dysfunction: the CODAM study. Eur J Clin Investig. 2011;41:167–175. doi: 10.1111/j.1365-2362.2010.02392.x. [DOI] [PubMed] [Google Scholar]
- Jiang R, Chen JH, Jun J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005;17:417–423. doi: 10.1038/sj.ijir.3901329. [DOI] [PubMed] [Google Scholar]
- Jones TH. Testosterone associations with erectile dysfunction, diabetes, and the metabolic syndrome. Eur Urol. 2007;6:847–857. doi: 10.1016/j.eursup.2007.07.002. [DOI] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Karbowska J, Kochan Z. Role of adiponectin in the regulation of carbohydrate and lipid metabolism. J Physiol Pharmacol. 2006;57:103–113. [PubMed] [Google Scholar]
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby M, Jackson G, Betterridge J, Friedli K. Is erectile dysfunction a marker for cardiovascular disease? Int J Clin Pract. 2001;55:614–618. [PubMed] [Google Scholar]
- Kostis JB, Jackson G, Rosen R, Barrett-Connor E, Billups K, Burnett AL, Carson C, Cheitlin M, Debusk R, Fonseca V, Ganz P, Goldstein I, Guay A, Hatzichristou D, Hollander JE, Hutter A, Katz S, Kloner RA, Mittleman M, Montorsi F, Montorsi P, Nehra A, Sadovsky R, Shabsigh R. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005;96:313–321. doi: 10.1016/j.amjcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, Stichtenoth DO, Hahn A. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerotic risk. Atherosclerosis. 2009;202:263–271. doi: 10.1016/j.atherosclerosis.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of adiponectiemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.ATV.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal S, Sá C, Gonçalves J, Fresco P, Diniz C. Immunohistochemical characterization of adenosine receptors in rat aorta and tail artery. Microsc Res Tech. 2008;71:703–709. doi: 10.1002/jemt.20609. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. PNAS. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Grant PJ. Endothelial dysfunction and cardiovascular disease—the lull before the storm. Diab Vasc Dis Res. 2007;4:82–83. doi: 10.3132/dvdr.2007.024. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- Montorsi P, Montorsi F, Schullman CC. Is erectile dysfunction the “tip of iceberg” of a systemic vascular disease? Eur Urol. 2003;44:352–354. doi: 10.1016/S0302-2838(03)00307-5. [DOI] [PubMed] [Google Scholar]
- Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, Montorsi F. Association between erectile dysfunction and coronary artery disease: matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–731. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The Yin and Yang of oxidation in the development of the fatty streak: a review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.ATV.16.7.831. [DOI] [PubMed] [Google Scholar]
- Neves D, Santos J, Tomada N, Almeida H, Vendeira P. Aging and orchidectomy modulate expression of VEGF receptors (Flt-1 and Flk-1) on corpus cavernosum of the rat. Ann N Y Acad Sci. 2006;1067:164–172. doi: 10.1196/annals.1354.020. [DOI] [PubMed] [Google Scholar]
- Neves D, Tomada I, Assunção M, Marques F, Almeida H, Andrade JP. Effects of chronic red wine consumption on the expression of vascular endothelial growth factor, angiopoietin 1, angiopoietin 2, and its receptors in rat erectile tissue. J Food Sci. 2010;75(3):H79–H86. doi: 10.1111/j.1750-3841.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Conference Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. doi: 10.1001/jama.1993.03510010089036. [DOI] [PubMed] [Google Scholar]
- Onat A, Can G, Kaya H, Hergenç G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diabetes Rep. 2003;3:207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Picard F, Guarente L. Molecular links between aging and adipose tissue. Int J Obes. 2005;29:S36–S39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- Razny U, Kiec-Wilk B, Wator L, Polus A, Dyduch G, Solnica B, Malecki M, Tomaszewska R, Cooke JP, Dembinska-Kiec A. Increased nitric oxide availability attenuates high fat diet metabolic alterations and gene expression associated with insulin resistance. Cardiovasc Diabetol. 2011;10:68–81. doi: 10.1186/1475-2840-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi AA. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: emerging concepts. Am J Med Sci. 2009;338:310–318. doi: 10.1097/MAJ.0b013e3181a4158c. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Al Dashti R, Schwarz ER. Linking erectile dysfunction and coronary artery disease. Int J Impot Res. 2005;17(suppl1):S12–S18. doi: 10.1038/sj.ijir.3901424. [DOI] [PubMed] [Google Scholar]
- Shen ZJ, Jin XD, Chen ZD, Shi YH. Effect of aging on penile ultrastructure. Asian J Androl. 2001;3:281–284. [PubMed] [Google Scholar]
- Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- Shin D, Pregenzer G, Gardin JM. Erectile dysfunction. A disease marker for cardiovascular disease. Cardiol Rev. 2011;19(1):5–11. doi: 10.1097/CRD.0b013e3181fb7eb8. [DOI] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Aging Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Souza FM, Padilha AS, Stefanon I, Vassallo DV. Differences in functional and structural properties of segments of the rat tail artery. Braz J Med Biol Res. 2008;41:416–423. doi: 10.1590/S0100-879X2008005000018. [DOI] [PubMed] [Google Scholar]
- Sweazea KL, Lekic M, Walker BR. Comparison of mechanisms involved in impaired vascular reactivity between high sucrose and high fat diets in rats. Nutr Metab. 2010;7:48–57. doi: 10.1186/1743-7075-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomada I, Tomada N, Almeida H, Neves D (2011) Androgen depletion in humans leads to cavernous tissue reorganization and upregulation of Sirt1-eNOS axis. Age (Dordr). doi:10.1007/s11357-011-9328-z [DOI] [PMC free article] [PubMed]
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007;52:54–70. doi: 10.1016/j.eururo.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikan T, Schirmer H, Njolstad I, Svatberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromso Study. Eur J Endocrinol. 2009;161:435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging. 2010;2:361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]