Abstract
Age-related declines in central processing may affect corticospinal (CS) excitability that underlies the emergence of voluntary responses to external stimuli. We used single-pulse transcranial magnetic stimulation (TMS) over the primary motor cortex to explore the evolution of CS excitability in 14 young and ten elderly healthy right-handed participants. Motor-evoked potentials (MEPs) were elicited in the right or left first dorsal interosseus (FDI) during the preparatory and premotor periods of a choice reaction time (CRT) task, which required selection of left or right index finger responses. Both age groups showed significant suppression of CS excitability in the preparatory period. However, suppression was generally less pronounced in older than in young adults. Moreover, our data indicated that a reduced suppression in the right FDI during the preparatory period was associated with longer reaction times (RTs) in older adults only. In the premotor period, both age groups demonstrated comparable facilitation levels towards movement onset. Our findings indicate that increased RTs among older individuals could be directly associated with declines in preparatory processes.
Keywords: Aging, Choice reaction time, Response preparation, Transcranial magnetic stimulation, TMS
Introduction
Healthy aging is consistently associated with an overall slowing in response to visual and auditory cues (Jordan and Rabbitt 1977; Salthouse 2000). Previously, this slowing has been attributed to age-related declines in central processing (Clarkson 1978; Crossley and Hiscock 1992; Jordan and Rabbitt 1977; Walsh 1976) and working memory (Briggs et al. 1999) leading to deregulation of motor response selection and response generation. Recent EEG studies have shown that brain activity related to anticipation, preparation, and/or generation of motor responses is changed in healthy aging (Falkenstein et al. 2006; Golob et al. 2005; Roggeveen et al. 2007; Sailer et al. 2000; Sterr and Dean 2008; Yordanova et al. 2004). More specifically, Sterr and Dean (2008) showed that increased negativity of contingent negative variations (CNVs) in younger individuals during the preparatory period of a precued choice reaction time (CRT) task was associated with higher recruitment of the frontal brain network and lateralized activation over motor regions, whereas these trends were not seen in older individuals. Observations from studies using lateralized readiness potentials (LRPs) and/or event-related potentials (ERPs) have suggested that behavioral slowing is mainly due to slower response generation, rather than response selection and stimulus processing (Falkenstein et al. 2006; Roggeveen et al. 2007; Yordanova et al. 2004). Specifically, behavioral slowing in elderly individuals may be due to an enhanced and prolonged activity in the contralateral motor cortex during response generation (Falkenstein et al. 2006; Yordanova et al. 2004).
Transcranial magnetic stimulation (TMS) studies in young adults have shown a suppression of corticospinal (CS) excitability [i.e., suppression of motor-evoked potential (MEP) amplitude] towards the end of the preparation period in CRT tasks (Davranche et al. 2007; Hasbroucq et al. 1997). It is assumed that suppression of CS excitability is necessary to prevent erroneous premature responding (Davranche et al. 2007; Duque and Ivry 2009; Touge et al. 1998). During response generation, however, it has been reported that CS excitability is increased in the agonist muscle of the selected hand towards voluntary electromyographical (EMG) signal onset (Chen et al. 1998; Chen and Hallett 1999; Leocani et al. 2000).
In line with neurophysiological findings, evidence from behavioral studies indicates that aging affects the readiness of the motor system, particularly in CRT tasks (Adam et al. 1998; Bherer and Belleville 2004; Proctor et al. 2006). This may be due to a slower transition from a preparatory to an executive mode of operation (Burke and Kamen 1995) and/or an impaired ability of older individuals to benefit from precued information (Fujiyama et al. 2011).
Recently, TMS has been applied to study age-related differences in neuronal activity during response selection or event preparation for simple/go-no-go reaction tasks (Fujiyama et al. 2011, 2012b; Levin et al. 2011). However, there are virtually no studies examining age-related changes of CS excitability during preparation and motor generation in CRT tasks.
The aim of the present study was to explore differences in CS excitability patterns between young and older adults during the preparatory (i.e., from warning signal (WS) to the imperative stimulus) and premotor (i.e., from the imperative signal (IS) to the onset of voluntary EMG) periods of a precued CRT task. We hypothesized that age-related motor slowing in the CRT task is primarily attributed to a decline in the ability to modulate excitability in the motor cortex when selective tuning of the CS tracts is expected.
Methods
Subjects
A total of 24 volunteers participated in this study. They were divided in two age groups: young (n = 14, aged 21–27 years, 22.8 ± 1.7 years (mean ± SD); six male) and old (n = 10, aged 65–75 years, 69.3 ± 2.8 years (mean ± SD); two male). All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971). The average lateralization quotient (LQ) was +90.4 (±10.2 SD) for the young and +90.5 (±15.2 SD) for the older adults, with an LQ of +100 representing extreme right hand preference. All subjects had normal or corrected-to-normal vision and none of them had a history of neurological, psychiatric, cardiovascular, or neuromuscular disorders. Subjects were screened for contraindications for TMS (Wassermann 1998) and medication intake. All participants provided written informed consent prior to participation. The study was approved by the Ethics Committee for Biomedical Research at the Katholieke Universiteit Leuven, according to the Declaration of Helsinki.
Electromyographic recordings
EMG signals from both the right and left first dorsal interosseus (FDI) and the abductor digiti minimi (ADM) muscles were continuously monitored using a MESPEC 8000 EMG system (Mega Electronics Ltd., Finland). To make sure the entire hand was relaxed, the ADM muscle was included as a control muscle. Disposable, self-adhesive disc electrodes (Nutrode, Ag-AgCl sensor with Hydrogel, 35 mm diameter, GE Medical Systems Accessories Europe, Nanterre Cedex, France) were placed 2 cm apart in a belly-to-tendon montage. The raw EMG signals were amplified (gain = 1,000), filtered (bandpass 4–1,500 Hz), digitized at 5,000 Hz (CED 1401, Cambridge Electronic Design, UK), and stored on PC for offline analysis.
Transcranial magnetic stimulation
Magnetic stimulation was performed using a figure-of-eight coil (Magstim, Double 70 mm coil) connected to a Magstim 2002 (Magstim, Whitland, Dyfed, UK). Single-pulse TMS was delivered to the primary motor cortex (M1) of either the left or right hemisphere at the optimal scalp position (hotspot) to elicit motor responses in the contralateral FDI muscle. The handle of the coil was oriented towards the back of the head and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus (Mills et al. 1992). The resting motor threshold (rMT) was defined as the lowest stimulation intensity required to evoke MEPs with an amplitude larger than 100 μV peak-to-peak in at least five of ten consecutive trials (Gilio et al. 2003); the rMT was determined for each hemisphere of each individual. Stimulation intensity was set at 110 % of the rMT and was kept constant during the entire experiment.
Procedure
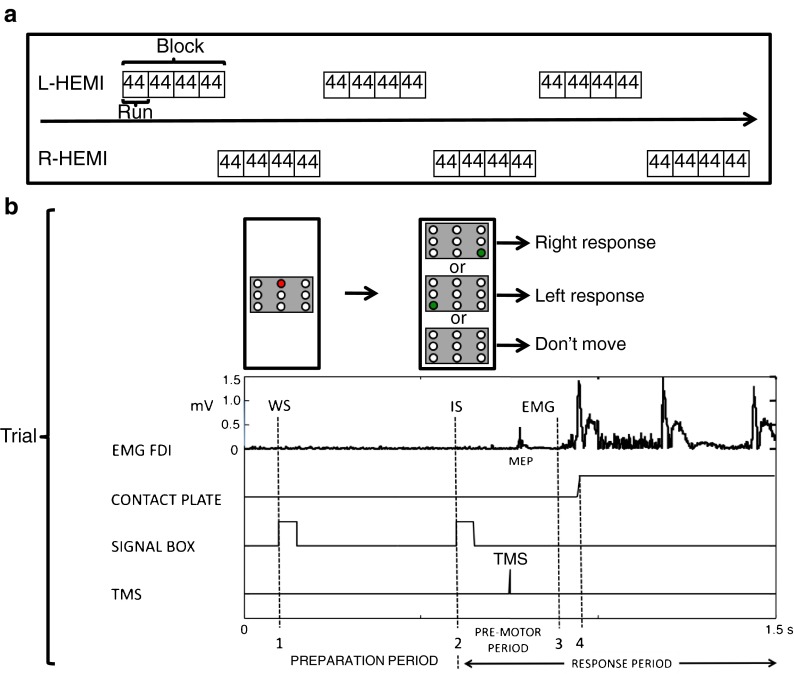
An overview of the experimental protocol is given in Fig. 1. Participants were instructed to respond with the left or right index finger in a CRT task. They were seated in a comfortable chair with both forearms pronated and the relaxed index fingers (EMG-controlled) resting on a platform, consisting of two pairs of two square (3 × 5 cm) conduction plates that were positioned 30 cm in front of the subject. A signaling box was positioned at eye level, 1 m in front of the subject. The box consisted of an upper row of three red light-emitting diodes (LEDs), a middle row of yellow LEDs, and a lower row of three green LEDs. In this experiment, only the central LED of the upper red row and the two outer LEDs of the lower green row were used. The central red LED served as the WS. The two outer green LEDs displayed the IS, either for a right (right response) or left index finger movement (left response). First, the red LED was lit (WS). After a preparation period of 500 ms, the red LED was switched off and the IS was given by switching on one of the two green LEDs. Catch trials were presented by keeping both green LEDs off until the onset of the next WS. Subjects were instructed to abduct and reposition the responding index finger as soon as possible on the conduction plate. The green LED was maintained “on” for 1,000 ms and then switched off. Intertrial intervals were randomly varied between 5 and 8 s. For each trial, data collection was initiated 100 ms prior to the onset of the WS and lasted for 1.5 s.
Fig. 1.
Experimental protocol. a The experiment consisted of six blocks. Each hemisphere was exposed to three blocks in a counterbalanced (starting left or right) and alternating sequence. Before each block, excitability at rest was administered (baseline). A block consisted of four consecutive runs, whereas a run included 44 trials. In the right index finger movement (right response) and left index finger movement (left response) conditions (18 trials each), single-pulse TMS was delivered in the preparation period (at the warning signal [WS], at −400 ms, −300 ms, −200 ms, and −100 ms before the imperative signal [IS] and at the IS) and in the response period (at 50-ms intervals, until 500 ms after the IS). In the catch trial condition, TMS was only delivered in the preparation period (six trials). Each run contained two trials (left response and right response) without TMS. b For a single trial, signals (signaling box, TMS pulse onset), muscle activation (EMG), and movement responses (contact plates) are visualized in a 1.5-s time frame. The preparation period which lasted 500 ms was defined as the time elapsing between the onset of the warning signal (WS, Point 1) and the onset of the imperative signal (IS, Point 2). In the response period (starting from Point 2), premotor time (PreMT) was defined as the time between the onset of the imperative signal and the time of onset of voluntary EMG in the FDI muscle of the moving hand (Point 3). Reaction time (RT) was defined as the time from the onset of the IS to the liftoff of the index finger (Point 4). Motor time (MT) was defined as the time elapsing between the onset of the movement-related EMG activity and the time of the index finger liftoff (Point 4–Point 3)
Prior to the beginning of the experiment, all participants performed a practice run with no TMS. The main experiment consisted of six blocks (Fig. 1a). Each hemisphere was exposed to three blocks in a counterbalanced (starting left or right) and alternating sequence. Before each block, 12 baseline MEPs were administered at rest. For each subject, MEPs administered in a block were normalized to these baseline measures to correct for effects of arousal and/or fatigue during the experiment. A block included four consecutive runs, each consisting of 44 trials, resulting in a total of 176 trials per block. Short breaks (3 min) were provided between blocks. In each trial, TMS could be delivered either in the preparation period or in the response period (Fig. 1b). During the preparation period, which was defined as the time elapsing between the onset of the WS and the onset of the IS (duration 500 ms), TMS was delivered either at the onset of the WS or 100 ms, 200 ms, 300 ms, 400 ms, or 500 ms later (=18 trials/run; six right response, six left response, and six catch trials). In the preparation period, the subject waited for the IS and no information about the forthcoming response was available. During the response period, TMS was delivered at 50 ms, 100 ms, 150 ms, 200 ms, 250 ms, 300 ms, 350 ms, 400 ms, 450 ms, 500 ms, 550 ms, or 600 ms after the onset of the IS (=24 trials/run; 12 right response and 12 left response). Here, the subject received information about the required movement and responded. Finally, to obtain pure reaction time (RT), two trials (one right response and one left response) without magnetic stimulation were included in each run (in total: 12 right responses and 12 left responses in all runs) as TMS can influence RT (Pascual-Leone et al. 1992). Together, this resulted in a total of 44 trials per run.
Data processing and statistical analysis
RT and time to onset of voluntary EMG activity were calculated from the trials without TMS. RTs were defined as the time from the onset of the IS to the liftoff of the index finger (Fig. 1b; 2–4). Trials with RTs exceeding 600 ms, erroneous, or premature responses (RT < 50 ms), or trials with no discernible onset of motor response were discarded. In total, less than 4 % of all trials were discarded. The average numbers of discarded trials per participant (mean ± SD, expressed as percent of the total number of 1,056 trials) were: 1.40 ± 1.50 % for young adults and 1.90 ± 1.51 % for older adults. The group effect was not significant (p > 0.3; Mann–Whitney U test). RT was further divided into premotor time (PreMT) and motor time (MT). The PreMT was defined as the time between the onset of the IS and the onset of voluntary EMG in the FDI muscle of the moving hand (Fig. 1b; 2–3). MT was defined as the time elapsing between the onset of the movement-related EMG activity and initiation of the index finger liftoff (Fig. 1b; 3–4).
CS excitability of the FDI muscles was evaluated for both the active and nonactive hemispheres at each condition (right response, left response, or catch trial) with respect to the TMS delivery times from the onset of the WS (preparation period) or IS (premotor period). MEPs were excluded from analysis in case of either a precontraction if they occurred during movement or if they did not appear in a 40-ms window starting 10 ms after the onset of TMS. In addition, MEPs were discarded if root mean square EMG in one of the four muscles exceeded 20 μV during the 50-ms period immediately preceding the onset of the TMS pulse. At least eight MEPs were used for the calculation of CS excitability at each time point. In total, less than 5 % of all MEPs that could be used to study excitability in the preparatory or premotor period were excluded. Because we were interested in CS excitability changes in both the preparatory and the premotor periods, MEP amplitudes were normalized to the WS and IS, respectively. More specifically, values above 100 % represent facilitation and values below 100 % represent suppression of MEPs with respect to those elicited at the WS or IS. Given the interindividual variability of the duration of the premotor period, MEPs were binned at 25 % PreMT (0.125 < t ≤ 0.375 PreMT), 50 % PreMT (0.375 < t ≤ 0.625 PreMT), and 75 % PreMT (0.625 < t ≤ 0.875 PreMT).
Advanced linear model applications (SAS 9.2, SAS Institute Inc., Cary, NC & STATISTICA 8.0, StatSoft Inc., Tulsa, OK) were used for statistical analysis. Nonparametric statistics were applied to test for significant differences: (1) between groups at individual time intervals (Mann–Whitney U test) and (2) between time intervals within each group/condition (Friedman ANOVA followed by a Wilcoxon signed-rank post hoc test). In addition, a mixed model (see Appendix) was used to estimate the rate of change (i.e., slope analysis) of CS excitability in the preparation and premotor period as function of age group, side (hemisphere), and condition.
To study the relation between performance level and CS excitability, Spearman’s Rank Correlations between RT performance (right response or left response) and TMS measures (rMT, MEP at rest and at WS; preparation period: −400 ms, −300 ms, −200 ms −100 ms, and the IS; premotor period: at the 25 % PreMT, 50 % PreMT, and the 75 % PreMT intervals) were calculated. The level of significance was set at p = 0.05.
Results
Behavioral measures
Age group means for RT, PreMT, and MT are summarized in Table 1. Significant differences between groups were noticed for RTs and PreMTs (all, p < 0.001; Mann–Whitney U test), indicating that older adults were significantly slower in responding to the IS than young adults. Within age groups, RT did not vary over the course of the experiment for both the left and the right responses (old: F = 3.80, p = 0.052; young: F = 1.10, p = 0.295; mixed model). PreMT and RT were positively correlated: older adults, for right response, Spearman’s R = 0.83 (p = 0.003) and for left response, R = 0.87 (p = 0.001); young adults, for right response R = 0.56 (p = 0.039) and for left response, R = 0.91 (p < 0.001). Therefore, we only report the results using the RT measure as regressor for the correlations with the TMS measures.
Table 1.
Mean (standard deviation) of reaction time, premotor time, and motor time in milliseconds (ms) for younger and older adults in the left and right index finger movements of the choice reaction task
| Young | Old | |||
|---|---|---|---|---|
| Left response | Right response | Left response | Right response | |
| Reaction time (ms) | 293 (24) | 284 (25) | 378 (35)* | 388 (38)* |
| Premotor time (ms) | 228 (32) | 222 (24) | 316 (45)* | 323 (35)* |
| Motor time (ms) | 65 (20) | 62 (21) | 62 (18) | 65 (17) |
*p < 0.001 (significant difference between old and young)
Resting motor threshold and baseline MEP
Age group means for rMTs and baseline (rest) MEPs are given in Table 2. rMT values for both right and left FDIs were significantly higher in older as compared to young adults (both, p < 0.001; Mann–Whitney U test). However, the amplitude of baseline MEPs elicited at 110 % of rMT was similar in both age groups for both right and left FDI muscles (both, p > 0.05; Mann–Whitney U test). For the young group, we also observed a significant difference between the right and left FDI muscles for the rMTs as well as for the amplitude of baseline MEPs (right vs. left rMT, p = 0.021; right vs. left baseline MEP, p = 0.026; Wilcoxon signed-rank test, see Table 2 for actual values). This asymmetry was not seen in the older adults (p > 0.05).
Table 2.
Mean (standard deviation) of rest motor threshold (in % of maximum stimulator output) and MEP sizes (peak-to-peak) at rest (baseline) and at onset of warning signal (WS) stimulus time in millivolt (mV) for younger and older adults in the left and right first dorsal interosseus (FDI) muscles
| Young | Old | |||
|---|---|---|---|---|
| Left FDI | Right FDI | Left FDI | Right FDI | |
| Rest motor threshold (%) | 42.7 (3.0)*** | 39.6 (4.5)*,** | 50.7 (4.9) | 51.8 (4.8) |
| MEP size at rest (mV) | 0.96 (0.49) | 1.55 (0.83)** | 1.08 (0.70) | 1.04 (0.67) |
| MEP size at WS (mV) | 1.45 (0.71) | 2.24 (1.53) | 1.69 (1.03) | 2.20 (1.54) |
*p < 0.001 (significant difference between old and young for right FDI)
**p < 0.05 (significant difference between left and right FDIs for young adults)
***p < 0.001 (significant difference between old and young for left FDI)
Correlation with RT performance
Spearman’s Rank Correlation revealed no significant trends between the RTs and the rMTs or baseline MEP amplitudes, neither for older nor for young adults (all, p > 0.05).
CS excitability
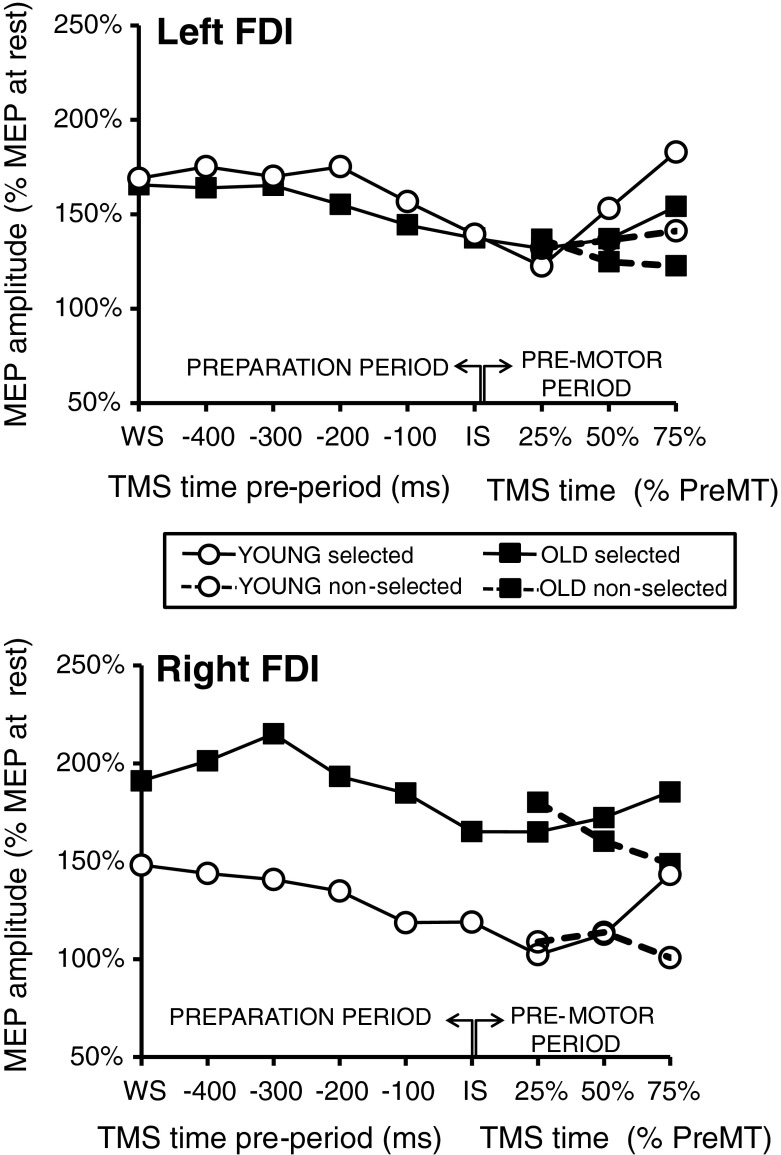
Evolution of CS excitability during the preparation period was marked by a noticeable suppression of the MEPs towards the onset of the IS and followed by a visible facilitation of MEPs in the ipsilateral active muscle (i.e., right FDI for right response or left FDI for left response) towards the onset of motor response at the end of the premotor period (Fig. 2).
Fig. 2.
Global excitability changes. Mean age group data of MEP sizes for the left and right FDI according to their latencies from the onsets of the WS (in the preparation period) or the IS (in the premotor period) for the left and right response conditions. Dots represent the young age group and squares, the old age group. In the premotor period, the black line represents the mean MEP sizes of the selected (active) hemisphere, whereas the dashed line represents MEP sizes of the nonselected (passive) hemisphere
Initiation of the preparation period
Facilitation of MEPs relative to their mean baseline amplitudes was already seen at the WS. For young adults, mean sizes of MEPs at the onset of the WS (mean ± SD, expressed as percent of mean rest MEP) were: 148 ± 59 % for right FDI and 169 ± 92 % for the left FDI (p < 0.016, Wilcoxon signed-rank test for contrasts between baseline and WS). For older adults, levels of facilitation were: 191 ± 70 % for the right FDI and 166 ± 63 % for the left FDI (p < 0.013; Wilcoxon signed-rank test). However, group differences were not significant (p > 0.05; Mann–Whitney U test).
Preparation period
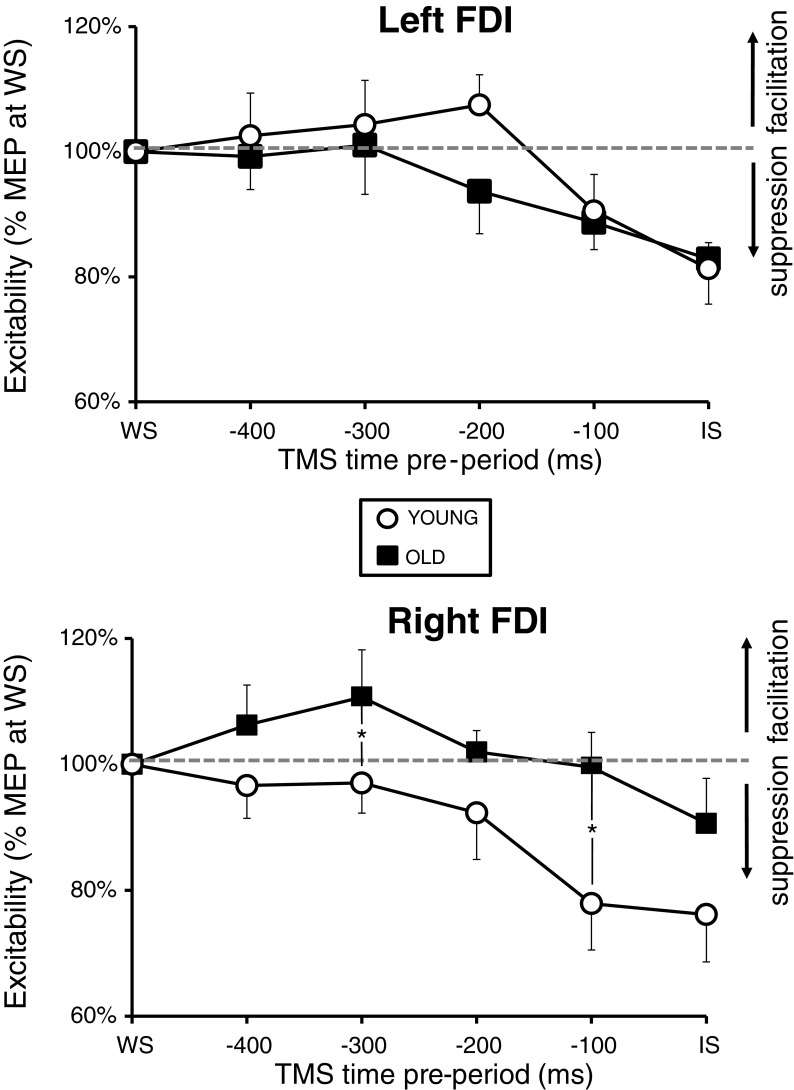
To correct for age and/or arousal-induced effects, changes in the excitability during the preparation period were expressed as percent of mean MEP at the WS (Fig. 3). Within the older adults, suppression was observed only in the left [Effect size (ES) = 17.14 %, χ2(10,4) = 10.00, p = 0.040; Friedman ANOVA] but not in the right FDI [ES = 9.38 %, χ2(10,4) = 8.64, p = 0.071; Friedman ANOVA], whereas for the young adults, a significant suppression of MEPs over time (i.e., between IS and WS) was observed in both the right FDI [ES = 23.85 %, χ2(14,4) = 15.94, p = 0.003; Friedman ANOVA] and the left FDI [ES = 18.71 %, χ2(14,4) = 19.26, p < 0.001; Friedman ANOVA]. Paired (within group) comparisons for both young and older adults (Wilcoxon signed-rank test) are given in Table 3.
Fig. 3.
Excitability changes in the preparation period. Normalized excitability changes for the left and right FDI are represented for the young and old age groups. Data correspond to mean ± SD % MEP at the warning signal. *p < 0.05
Table 3.
Paired (within-group) comparisons (Wilcoxon signed-rank test) for both young and old adults. Mean values [expressed at % of mean MEP at the warning signal (WS)] and standard deviation (SD) are given
| Young | Old | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Left FDI | Right FDI | Left FDI | Right FDI | ||||||
| Mean (SD) | p value | Mean (SD) | p value | Mean (SD) | p value | Mean (SD) | p value | ||
| WS | 100 | 100 | 100 | 100 | |||||
| −400 | 103 (29) | 0.826 | 97 (20) | 0.594 | 99 (17) | 0.721 | 106 (20) | 0.445 | |
| −300 | 104 (26) | 0.875 | 97 (18) | 0.245 | 101 (25) | 0.959 | 111 (24) | 0.114 | |
| −200 | 107 (19) | 0.300 | 92 (28) | 0.177 | 94 (21) | 0.333 | 102 (11) | 0.721 | |
| −100 | 91 (22) | 0.124 | 78 (28) | 0.019* | 89 (14) | 0.037* | 100 (18) | 0.646 | |
| IS | 81 (16) | 0.005* | 76 (28) | 0.026* | 83 (23) | 0.037* | 91 (23) | 0.285 | |
| −400 | 103 (29) | 97 (20) | 99 (17) | 106 (20) | |||||
| −300 | 104 (26) | 0.975 | 97 (18) | 0.778 | 101 (25) | 0.878 | 111 (24) | 0.798 | |
| −200 | 107 (19) | 0.975 | 92 (28) | 0.875 | 94 (21) | 0.285 | 102 (11) | 0.285 | |
| −100 | 901 (22) | 0.055 | 78 (28) | 0.048* | 89 (14) | 0.059 | 100 (18) | 0.241 | |
| IS | 81 (16) | 0.019* | 76 (28) | 0.011* | 83 (23) | 0.059 | 91 (23) | 0.139 | |
| −300 | 104 (26) | 97 (18) | 101 (25) | 111 (24) | |||||
| −200 | 107 (19) | 0.638 | 92 (28) | 0.363 | 94 (21) | 0.333 | 102 (12) | 0.169 | |
| −100 | 91 (22) | 0.064 | 78 (28) | 0.041* | 89 (14) | 0.059 | 100 (18) | 0.028* | |
| IS | 81 (16) | 0.004* | 76 (28) | 0.019* | 83 (23) | 0.047* | 91 (23) | 0.047* | |
| −200 | 107 (19) | 92 (28) | 94 (21) | 102 (11) | |||||
| −100 | 91 (22) | 0.013* | 78 (28) | 0.030* | 89 (14) | 0.169 | 100 (18) | 0.445 | |
| IS | 81 (16) | 0.001* | 76 (28) | 0.013* | 83 (23) | 0.059 | 91 (23) | 0.074 | |
| −100 | 91 (22) | 78 (28) | 89 (14) | 100 (18) | |||||
| IS | 81 (16) | 0.084 | 76 (28) | 0.683 | 83 (23) | 0.386 | 91 (23) | 0.093 | |
*p < 0.05 (significant difference between TMS intervals)
Major age group differences were noticed only for the right FDI (see Fig. 3). Significant differences between age groups were observed at the −300 ms (mean ± SD: old = 111 ± 24 % vs. young = 97 ± 18 %) and −100 ms (mean ± SD: old = 100 ± 18 % vs. young = 78 ± 28 %) intervals (both, p < 0.05; Mann–Whitney U test) but not around the onset time of the IS stimulus (mean ± SD: old = 91 ± 23 % vs. young = 76 ± 28 %, p > 0.05). For the left FDI, no significant age group differences were observed (all, p > 0.05).
The slope analysis (see Tables 4, 5, 6, and 7 in the Appendix, Type 3 test for fixed effects) revealed a significant TIME × AGE (F = 4.30, p < 0.039) and TIME × AGE × SIDE (F = 7.56, p = 0.006) interaction.
Table 4.
Mixed model solutions for rates of change of CS excitability in the preparation period. Estimated mean (standard error) values are given in s−1. The results of the Type 3 statistics are added (Table 5)
| Effect | Side | Age | Parameter | Estimate (SD) | p |
|---|---|---|---|---|---|
| TIME | β1 | 0.0329 (0.1982) | 0.8682 | ||
| TIME × SIDE | Left | β2 | 0.3332 (0.1348) | 0.0142 | |
| Right | β3 | 0 | . | ||
| TIME × AGE | Old | β4 | 0.5025 (0.1473) | 0.0008 | |
| Young | β5 | 0 | . | ||
| TIME × SIDE × AGE | Left | Old | β6 | −0.5744 (0.2089) | 0.0064 |
| Left | Young | β7 | 0 | . | |
| Right | Old | β8 | 0 | . | |
| Right | Young | β9 | 0 | . | |
| TIME × TIME | β10 | −1.5516 (0.3858) | <.0001 |
Table 5.
Type 3 tests of fixed effects
| Effect | F values | p |
|---|---|---|
| TIME | 2.86 | 0.0919 |
| TIME × SIDE | 0.19 | 0.6600 |
| TIME × AGE | 4.30 | 0.0392 |
| TIME × AGE × SIDE | 7.56 | 0.0064 |
| TIME × TIME | 16.17 | <.0001 |
Table 6.
Linear slopes for different groups
| Age | Side | Time (ms) | Estimate (SD) | p |
|---|---|---|---|---|
| Old | Left | −400 | 0.01391 (0.01760) | 0.4300 |
| Old | Right | 0.03803 (0.01760) | 0.0317 | |
| Young | Left | 0.02110 (0.01654) | 0.2034 | |
| Young | Right | −0.01222 (0.01654) | 0.4607 | |
| Old | Left | −300 | −0.00320 (0.02968) | 0.9141 |
| Old | Right | 0.04503 (0.02968) | 0.1305 | |
| Young | Left | 0.01116 (0.02713) | 0.6812 | |
| Young | Right | −0.05548 (0.02713) | 0.0420 | |
| Old | Left | −200 | −0.05135 (0.03799) | 0.1778 |
| Old | Right | 0.02100 (0.03799) | 0.5810 | |
| Young | Left | −0.02981 (0.03344) | 0.3736 | |
| Young | Right | −0.12980 (0.03344) | 0.0001a | |
| Old | Left | −100 | −0.13050 (0.04567) | 0.0046 |
| Old | Right | −0.03407 (0.04567) | 0.4564 | |
| Young | Left | −0.10180 (0.03883) | 0.0093 | |
| Young | Right | −0.23510 (0.03883) | <.0001a | |
| Old | Left | IS | −0.24080 (0.05704) | <.0001a |
| Old | Right | −0.12020 (0.05704) | 0.0362 | |
| Young | Left | −0.20480 (0.04847) | <.0001a | |
| Young | Right | −0.37140 (0.04847) | <.0001a |
aSignificant after correction for multiple comparisons (Bonferroni)
Table 7.
Differences between age groups and sides at all time points
| Difference | Side | Age | p |
|---|---|---|---|
| Old–Young | Left | 0.6262 | |
| Old–Young | Right | 0.0008 | |
| Left–Right | Old | 0.1320 | |
| Left–Right | Young | 0.0142 |
Age differences
Young adults showed faster suppression of MEPs in the right FDI as compared to older adults, (old–young: p < 0.001). No age differences in the rate of suppression were noticed for the left FDI (old–young: p > 0.05).
Side differences
Significant differences over time between left and right hemisphere excitability patterns were only reported for the young subjects (left–right: p < 0.014).
Premotor period
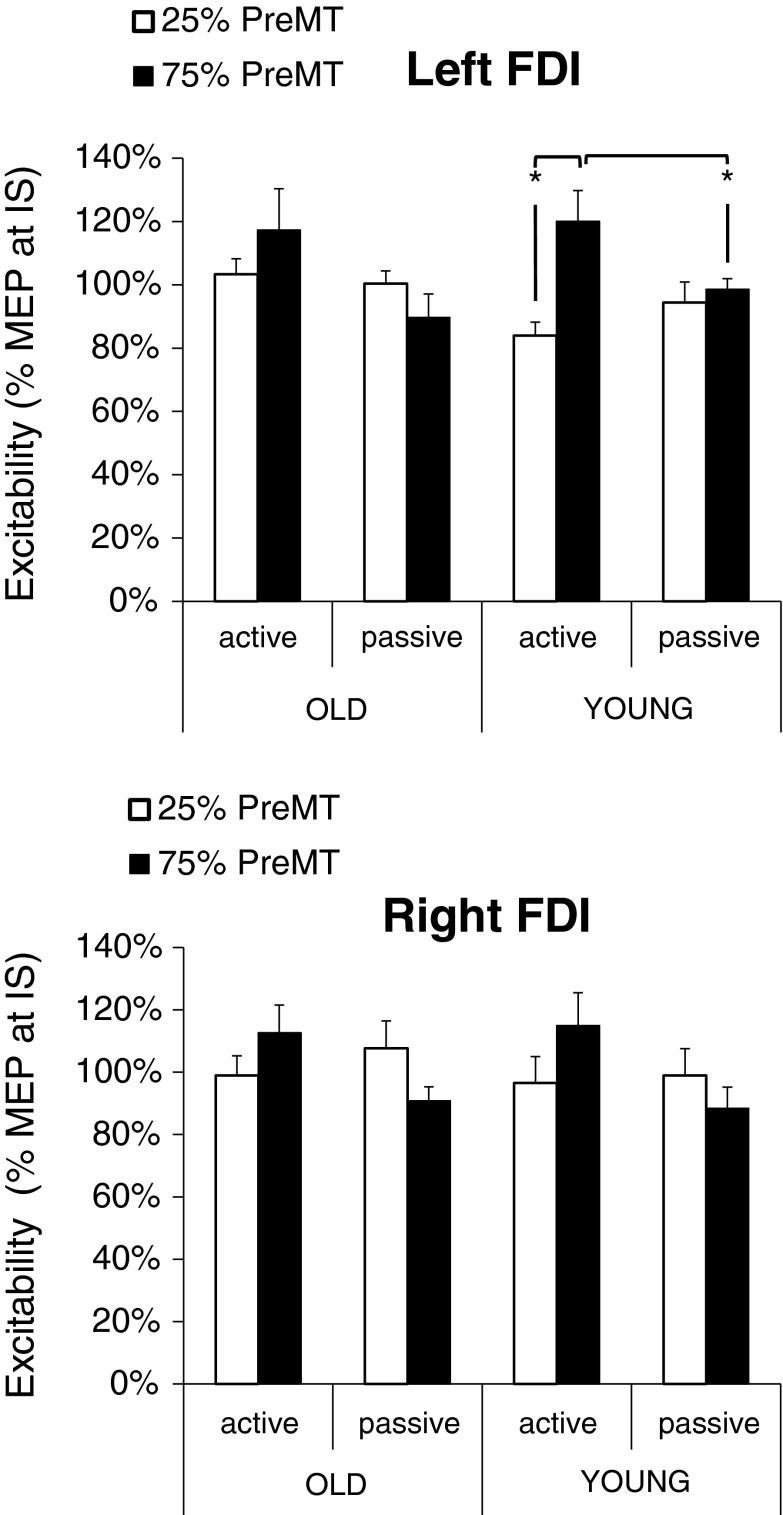
To correct for age and/or arousal-induced effects, changes in excitability during the premotor period were expressed as percent of mean MEP at the IS. Mean age group data are illustrated in Fig. 4 according to their latencies from the onsets of the IS at the 25 % PreMT and 75 % PreMT intervals. Our results showed significant changes in CS excitability as function of time only for young adults in the selected left FDI [ES = 20.06 %, χ2(12,2) = 12.67, p = 0.001; Friedman ANOVA]. Specifically, a significant facilitation of MEPs was noticed at the 75 % PreMT interval relative to the 25 % PreMT interval (p = 0.005; Wilcoxon signed-rank test). The latter effect was not seen in older adults or the right FDI (all, p > 0.05; Wilcoxon signed-rank test). No significant differences in CS excitability were found at the 50 % PreMT interval as compared to the 25 % PreMT and the 75 % PreMT interval neither for the left nor for the right FDI (all, p > 0.05).
Fig. 4.
Excitability changes in the premotor period. Gray bars representing excitability of the FDIs at 25 % of the premotor time (PreMT) and black bars, at 75 % PreMT. For both age groups, data is provided for the active and passive hemispheres. Data correspond to mean ± SD % MEP at the imperative signal. *p < 0.01
No significant group differences in CS excitability were noticed during the premotor period, in neither the selected nor nonselected FDIs (all, p > 0.05; Mann–Whitney U test). The slope analysis (see Tables 8, 9, and 10 in the Appendix, Type 3 test for fixed effects) revealed no significant interactions with AGE (F ≤ 3.80, p > 0.05).
Table 8.
Mixed model solutions for rates of change of CS excitability in the premotor period. Estimated mean (standard error) values are given in s−1. The results of the Type 3 statistics are added (Table 9)
| Effect | Age | Condition | Parameter | Estimate (SD) | p |
|---|---|---|---|---|---|
| TIME × AGE | Old | β1 | 0.01993 (0.04452) | 0.6548 | |
| Young | β2 | −0.08304 (0.03761) | 0.0281 | ||
| TIME × TIME × AGE | Old | β3 | −0.02036 (0.01549) | 0.1896 | |
| Young | β4 | 0.01866 (0.01316) | 0.1574 | ||
| TIME × TIME × CONDITION | Selected | β5 | 0.02406 (0.005200) | <.0001 | |
| Nonselected | β6 | 0 | . |
Table 9.
Type 3 tests of fixed effects
| Effect | F values | p |
|---|---|---|
| TIME × AGE | 2.54 | 0.0808 |
| TIME × TIME × AGE | 3.80 | 0.0524 |
| TIME × TIME × CONDITION | 21.40 | <0001 |
Table 10.
Differences in linear slopes for different groups
| Difference | Time | Parameter | p |
|---|---|---|---|
| Old–Young | 25 % PreMT | 0.06395 (0.03943) | 0.1059 |
| Old–Young | 50 % PreMT | 0.04985 (0.04537) | 0.2729 |
| Old–Young | 75 % PreMT | −0.04230 (0.04996) | 0.3979 |
| Passive–Active | 25 % PreMT | 0.02406 (0.00520) | <0001a |
| Passive–Active | 50 % PreMT | 0.09624 (0.02080) | <0001a |
| Passive–Active | 75 % PreMT | 0.21650 (0.04680) | <0001a |
aSignificant after correction for multiple comparisons (Bonferroni)
Correlations between CS excitability changes and RT performance
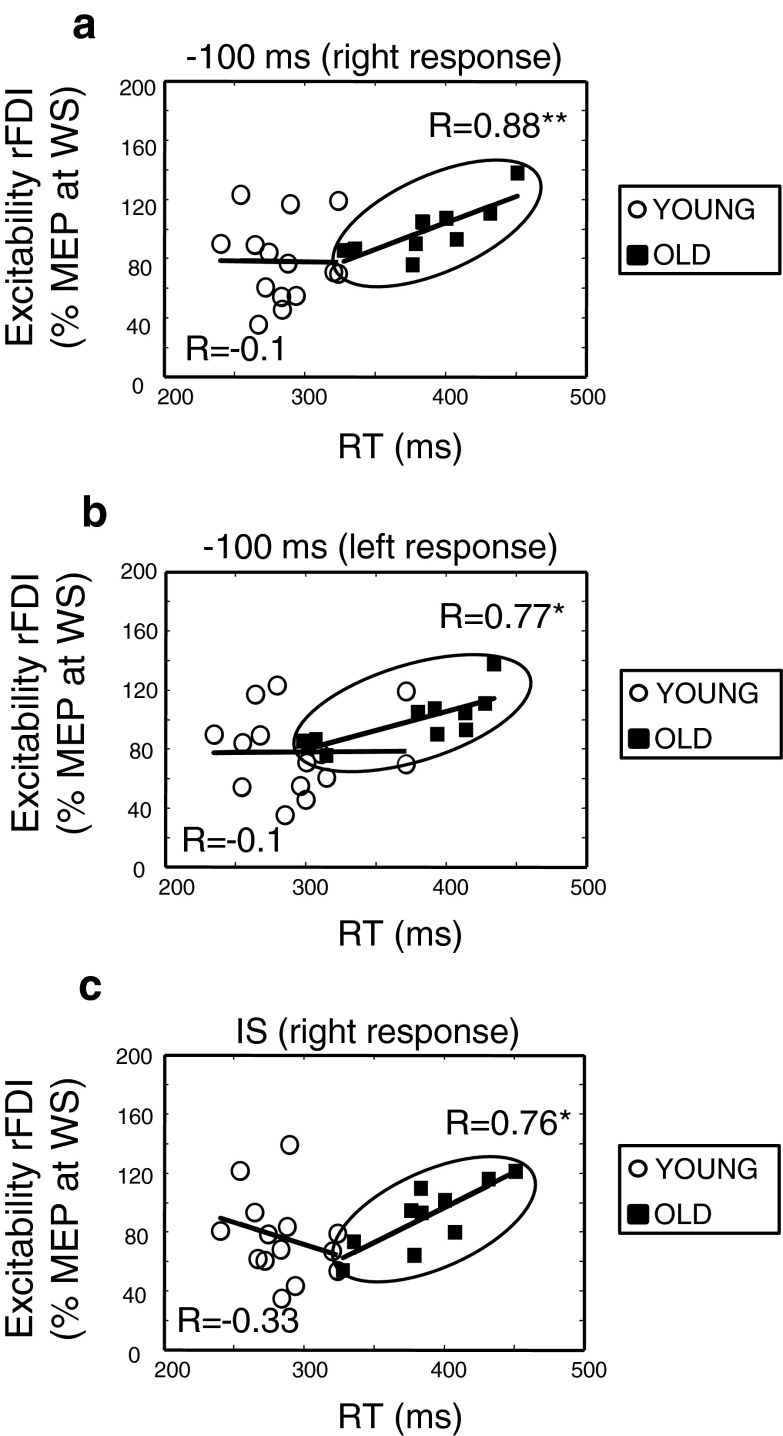
Older adults
Significant positive correlations between CS excitability and RTs were observed at the −100-ms interval of the preparation period and at the onset of the IS (Fig. 5). For the −100-ms interval, a significant positive correlation was observed between the degree of reduction in amplitude size in the right FDI and RTs for the right response (Spearman’s R = 0.88, p < 0.001) or left response (R = 0.77, p = 0.010) tasks. Similarly, at the IS, a significant positive correlation was observed only between the degree of suppression of MEPs in the right FDI and RTs for the right response (R = 0.76, p = 0.011). No significant correlations between CS excitability changes and performance on the CRT task were observed for left FDI (p > 0.05). The aforementioned observations suggest that less suppression of excitability in the dominant (left hemisphere) CS tracts in older adults was associated with slower RTs. In contrast, no significant relationships between CS excitability changes in the premotor period and performance were observed (p > 0.05).
Fig. 5.
Excitability changes and performance. Spearman’s Rank Correlations between excitability of the right FDI (rFDI) and reaction time (RT) are provided for a the right index finger movement (right response), b the left index finger movement (left response) 100 ms before the imperative signal, and c for the right response at the imperative signal (IS). Dots represent the young age group and squares, the old age group. *p < 0.05 and **p < 0.01
Young adults
Spearman’s Rank Correlation revealed no significant positive and/or negative trends between the RTs and the levels of CS excitability; neither in the preparation nor the premotor period (p > 0.05).
Discussion
The purpose of this study was to explore differences in CS excitability between young and older adults at different time intervals that span the preparation (i.e., from WS to IS) and the generation/specification (i.e., IS to the onset of voluntary EMG) of a motor response during a CRT task. Age-related slowing of RT, particularly during more complex RT conditions, is well documented (Jordan and Rabbitt 1977; Salthouse 2000) but, to date, the neural correlates of this phenomenon are not fully understood. Recent EEG studies have already indicated that information processing related to anticipation and preparation of a motor response changes during healthy aging (Golob et al. 2005; Sterr and Dean 2008). Even though evidence suggests that older adults need stronger (amplitude enhancement) and longer (prolongation of the motor-related potential) activation of the contralateral motor cortex to trigger motor responses (Falkenstein et al. 2006; Yordanova et al. 2004), it still remains unclear whether these observations reflect deficits in movement preparation or response-generation processes or both. The present study aimed to bridge this gap.
We documented the evolution of CS excitability by single-pulse TMS at different time intervals during the preparatory and premotor periods in older and young healthy individuals. Our observations indicate that: (1) suppression of MEPs in the preparatory period was stronger in young than in older adults for the dominant (right) FDI; (2) less suppression of MEPs in the preparatory period was associated with slower RTs in older adults; (3) both age groups showed comparable MEP facilitation towards the end of the premotor period; and (4) for the premotor period, no significant correlations between the amount of facilitation and RTs were noticed in both age groups.
In line with previous observations (Peinemann et al. 2001; Rossini et al. 1992), rMT values were generally higher in older adults as compared to young adults. Furthermore, young adults showed slightly lower rMT for the dominant than nondominant motor representation of the FDI muscle, whereas in older adults, the rMTs were generally higher but comparable for both FDI muscles. Both age groups showed comparable levels of facilitation at the expected onset time of the WS. Overall, no significant correlations were found between global measures of CS excitability (i.e., rMT, baseline MEP, and/or levels of CS excitability around the onset of the WS) and performance on the CRT task in older adults.
In a recent pilot study, we showed that older adults can compensate for a deficient motor activation by increasing excitability of the CS pathways to the moving effector prior to the onset of the IS in a simple RT task (Levin et al. 2011). This finding was in line with the evidence that preparatory tuning of excitability towards response generation in the simple reaction time (SRT) task can start before the onset of the IS (Pascual-Leone et al 1992; Leocani et al. 2000). Furthermore, manifestation of preparatory facilitation is expected to mask response slowing. In contrast with SRT tasks, tuning of CS excitability in the CRT task can occur only after the onset of the IS in parallel with stimulus processing. Therefore, CS excitability patterns in the present study are argued to mirror the actual dynamic properties of the aging motor network during response preparation.
In the preparatory period, suppression of CS excitability towards the onset of the IS was seen in both age groups. This result was in line with previous studies reporting decreased CS excitability towards the end of the preparation period (Davranche et al. 2007; Hasbroucq et al. 1997) that was linked to the prevention of erroneous premature responding (Davranche et al. 2007; Duque and Ivry 2009; Touge et al. 1998). In addition, our observations revealed a significant interaction between age and side. The latter effect is manifested in terms of (1) an early suppression of dominant as compared to nondominant CS activity, seen in the young adult group and (2) differences in both the depth and rate of preparatory suppression of the dominant CS activations between both age groups. Furthermore, the rate of suppression was slower in older than in young adults. More importantly, the present study showed that reduced suppression in the preparatory period is associated with increased RTs. The most reasonable interpretation of these findings is that slowing in RTs among older individuals is directly associated with a decline in preparatory processes in the dominant hemisphere. Evidence from earlier studies showed that decrease of RTs in short (500–1,000 ms) preparatory periods is accompanied by reduction in CS excitability at the expected onset of the imperative stimulus (Davranche et al. 2007; Duque and Ivry 2009; Duque et al. 2010; Fujiyama et al. 2011; Hasbroucq et al. 1997, 1999; Sinclair and Hammond 2008, 2009; Tandonnet et al. 2003, 2010). In the current study, lower suppression of MEPs yet longer RTs in the older individuals may appear seemingly contradictory. Nonetheless, it has been documented that faster RTs in CRT or Go/NoGo tasks are associated with increased activation of inhibitory interneurons (often expressed by a gradual increase of SICIs) towards the expected onset of the imperative stimulus (Fujiyama et al. 2011, 2012b; Soto et al. 2010). This observation has been argued to reflect an increased recruitment of GABAA-ergic inhibitory circuits (Kujirai et al. 1993), presumably to suppress premature responses during the preparation period. Recent work from Fujiyama et al. (2012a, b) reports that an increased capacity to regulate inhibitory function was positively associated with better performance levels in older adults. It is noteworthy that previous studies already showed that preparatory suppression might be manifested at the cortical level, including inhibitory processes in the contralateral motor area (Duque et al. 2007; Hinder et al. 2010; Talelli et al. 2008). Additionally, the involvement of higher order motor areas such as the dorsal premotor cortex cannot be ruled out (Duque et al. 2012). Nonetheless, there is no direct evidence to estimate the actual contribution of those brain areas to preparatory suppression of MEPs observed in the present study. Finally, preparatory suppression of MEPs can also be associated with subcortical and/or spinal levels of the motor system (Duque and Ivry 2009; Duque et al. 2010; Fujiyama et al. 2011; Sinclair and Hammond 2008, 2009; Tandonnet et al. 2011).
In the premotor period, facilitation of MEPs was observed in either the right or left selected FDIs at 75 % of the PreMT. Levels of facilitation were rather feeble (110 % to 120 % of MEP amplitude at the onset of the IS) and not significantly different in both age groups. This result was in line with previous studies reporting increased CS excitability in the agonist muscle of the selected hand towards voluntary EMG onset in young adults (Chen et al. 1998; Chen and Hallett 1999; Leocani et al. 2000). In addition, no correlations were found between individual levels of facilitation at the 75 % PreMT and RTs (neither for older nor for young adults), indicating that higher facilitation of MEPs in the selected FDI did not necessarily predict faster RTs. More importantly, both young and older adults showed similar trends of MEP suppression in the left FDI. These suppression levels were not associated with faster RTs in either age group. Accordingly, we hypothesize that preparatory processes in the right (nondominant) hemisphere are less affected by aging.
In summary, the present study provides new information on differences in preparatory processes between young and older adults during execution of a precued CRT task. Our data indicate that: (1) older adults show less suppression of CS excitability in the preparatory period for the dominant (right) FDI; (2) reduced suppression of CS excitability in the preparatory period is associated with slower RTs in older adults; and (3) both age groups show similar levels of MEP facilitation towards the onset of voluntary motor response. Our results provide compelling evidence that age differences in preparatory processes do predict age-related slowing and indicate that motor generation is not a source of motor slowing.
Acknowledgments
This work was supported by the Flanders Fund for Scientific Research [G0483.10, G.A114.11] and Grant P7/21 from the Interuniversity Attraction Poles program of the Belgian federal government. Koen Cuypers is supported by the Special Research Fund UHasselt.
Appendix
Mixed model
Because of the correlation between data obtained from the same subject (over time and at different hemispheres), it was necessary to use a mixed model to estimate the rate of change (slopes) of CS excitability in the preparation and premotor periods as function of age (young vs. old), side (left vs. right hemisphere), and condition (selected vs. nonselected FDI).
Methods
A mixed model including fixed effects for AGE, SIDE, CONDITION, and TIME and their interactions was used to describe the rate of change in CS excitability. Averaged MEPs per subject, side, condition, and time point were entered into the model. A random intercept for SIDE was taken into account to correct for the correlation between both sides of the same individual. Furthermore, the repetition over time was handled by estimating the correlation of the measurements obtained from the same side within a single individual as a constant [compound symmetry − PROC MIXED (REML)]. Model fit was checked based on a graphical exploration of the residuals.
Results
From the estimates of the model (Table 4), the general slopes can be calculated per time point and for all combinations of side and age group. These slope estimates are shown in Table 6. A negative estimate indicates suppression, while a positive estimate corresponds with facilitation. Although the estimates at −400 ms and −300 ms and before the imperative signal (IS) show a slight facilitation, the estimates are not significant. Towards −200 ms and −100 ms before the IS and at the IS, the estimates are negative indicating suppression, which is strongly significant.
From Table 7, furthermore, differences can be investigated between both age groups and both sides at all time points. It can be noted that there is a consistent difference between the old and the young age groups at the right-hand side for all time points (p = 0.0008), while the left- and the right-hand side are different for the young subjects at all time points (p = 0.0142).
From the estimates shown in Table 8 above, the main conclusion is that the slope for the selected condition is strongly significant and positive indicating facilitation. With respect to the nonselected condition, it can be noted that only for the young age group, it is slightly significant and negative, indicating suppression, while the older show neither facilitation nor suppression.
Table 10 shows the differences between the old and the young age groups over all time points as well as the differences between both conditions. The main conclusion here is that between age groups, there is no significant difference, while both conditions are clearly strongly significant at all time points.
References
- Adam JJ, Paas FG, Teeken JC, van Loon EM, van Boxtel MP, Houx PJ, Jolles J. Effects of age on performance in a finger-precuing task. J Exp Psychol Hum Percept Perform. 1998;24:870–883. doi: 10.1037/0096-1523.24.3.870. [DOI] [PubMed] [Google Scholar]
- Bherer L, Belleville S. Age-related differences in response preparation: the role of time uncertainty. J Gerontol B Psychol Sci Soc Sci. 2004;59:66–74. doi: 10.1093/geronb/59.2.P66. [DOI] [PubMed] [Google Scholar]
- Briggs SD, Raz N, Marks W. Age-related deficits in generation and manipulation of mental images: I. The role of sensorimotor speed and working memory. Psychol Aging. 1999;14:427–435. doi: 10.1037/0882-7974.14.3.427. [DOI] [PubMed] [Google Scholar]
- Burke JR, Kamen G. Impairments of the response preparation process in the elderly. Int J Neurosci. 1995;81:177–192. doi: 10.3109/00207459509004885. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44:317–325. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Clarkson PM. The effect of age and activity level on simple and choice fractionated response time. Eur J Appl Physiol Occup Physiol. 1978;40:17–25. doi: 10.1007/BF00420985. [DOI] [PubMed] [Google Scholar]
- Crossley M, Hiscock M. Age-related differences in concurrent-task performance of normal adults: evidence for a decline in processing resources. Psychol Aging. 1992;7:499–506. doi: 10.1037/0882-7974.7.4.499. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Yordanova J, Kolev V. Effects of aging on slowing of motor-response generation. Int J Psychophysiol. 2006;59:22–29. doi: 10.1016/j.ijpsycho.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Tandonnet C, Summers JJ. Age-related differences in corticospinal excitability during a Go/NoGo task. Psychophysiology. 2011;48:1448–1455. doi: 10.1111/j.1469-8986.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging. 2012;33:1484–14. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Tandonnet C, Garry MI, Summers JJ. Age-related differences in corticomotor excitability and inhibitory processes during a visuomotor RT task. J Cogn Neurosci. 2012;24:1253–1263. doi: 10.1162/jocn_a_00201. [DOI] [PubMed] [Google Scholar]
- Gilio F, Curra A, Inghilleri M, Lorenzano C, Suppa A, Manfredi M, Berardelli A. Abnormalities of motor cortex excitability preceding movement in patients with dystonia. Brain. 2003;126:1745–1754. doi: 10.1093/brain/awg188. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Ovasapyan V, Starr A. Event-related potentials accompanying motor preparation and stimulus expectancy in the young, young-old and oldest-old. Neurobiol Aging. 2005;26:531–542. doi: 10.1016/j.neurobiolaging.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/S0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, Depy D, Latour S, Mouret I. Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol (Amst) 1999;101:243–266. doi: 10.1016/S0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Hinder MR, Schmidt MW, Garry MI, Summers JJ. Unilateral contractions modulate interhemispheric inhibition most strongly and most adaptively in the homologous muscle of the contralateral limb. Exp Brain Res. 2010;205:423–433. doi: 10.1007/s00221-010-2379-z. [DOI] [PubMed] [Google Scholar]
- Jordan TC, Rabbitt PM. Response times to stimuli of increasing complexity as a function of ageing. Br J Psychol. 1977;68:189–201. doi: 10.1111/j.2044-8295.1977.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Levin O, Cuypers K, Netz Y, Thijs H, Nuttin B, Helsen WF, Meesen RL. Age-related differences in human corticospinal excitability during simple reaction time. Neurosci Lett. 2011;487:53–57. doi: 10.1016/j.neulet.2010.09.072. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-T. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. doi: 10.1016/S0304-3940(01)02239-X. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Vu KP, Pick DF. A deficit in older adults’ effortful selection of cued responses. J Mot Behav. 2006;38:265–284. doi: 10.3200/JMBR.38.4.265-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggeveen AB, Prime DJ, Ward LM. Lateralized readiness potentials reveal motor slowing in the aging brain. J Gerontol B Psychol Sci Soc Sci. 2007;62:78–84. doi: 10.1093/geronb/62.2.P78. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593:14–19. doi: 10.1016/0006-8993(92)91256-E. [DOI] [PubMed] [Google Scholar]
- Sailer A, Dichgans J, Gerloff C. The influence of normal aging on the cortical processing of a simple motor task. Neurology. 2000;55:979–985. doi: 10.1212/WNL.55.7.979. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/S0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res. 2008;186:385–392. doi: 10.1007/s00221-007-1241-4. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res. 2009;194:103–113. doi: 10.1007/s00221-008-1684-2. [DOI] [PubMed] [Google Scholar]
- Soto O, Valls-Sole J, Kumru H. Paired-pulse transcranial magnetic stimulation during preparation for simple and choice reaction time tasks. J Neurophysiol. 2010;104:1392–1400. doi: 10.1152/jn.00620.2009. [DOI] [PubMed] [Google Scholar]
- Sterr A, Dean P. Neural correlates of movement preparation in healthy ageing. Eur J Neurosci. 2008;27:254–260. doi: 10.1111/j.1460-9568.2007.05975.x. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandonnet C, Burle B, Vidal F, Hasbroucq T. The influence of time preparation on motor processes assessed by surface Laplacian estimation. Clin Neurophysiol. 2003;114:2376–2384. doi: 10.1016/S1388-2457(03)00253-0. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Garry MI, Summers JJ. Cortical activation during temporal preparation assessed by transcranial magnetic stimulation. Biol Psychol. 2010;85:481–486. doi: 10.1016/j.biopsycho.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Garry MI, Summers JJ. Selective suppression of the incorrect response implementation in choice behavior assessed by transcranial magnetic stimulation. Psychophysiology. 2011;48:462–469. doi: 10.1111/j.1469-8986.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/S0924-980X(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Walsh DA. Age differences in central perceptual processing: a dichoptic backward masking investigation. J Gerontol. 1976;31:178–185. doi: 10.1093/geronj/31.2.178. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/S0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Hohnsbein J, Falkenstein M. Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes: evidence from high-resolution event-related potentials. Brain. 2004;127:351–362. doi: 10.1093/brain/awh042. [DOI] [PubMed] [Google Scholar]