Abstract
Various measures incorporated in geriatric assessment have found their way into frailty indices (FIs), which have been used as indicators of survival/mortality and longevity. Our goal is to understand the genetic basis of healthy aging to enhance its evidence base and utility. We constructed a FI as a quantitative measure of healthy aging and examined its characteristics and potential for genetic analyses. Two groups were selected from two separate studies. One group (OLLP for offspring of long-lived parents) consisted of unrelated participants at least one of whose parents was age 90 or older, and the other group of unrelated participants (OSLP for offspring of short-lived parents), both of whose parents died before age 76. FI34 scores were computed from 34 common health variables and compared between the two groups. The FI34 was better correlated than chronological age with mortality. The mean FI34 value of the OSLP was 31 % higher than that of the OLLP (P = 0.0034). The FI34 increased exponentially, at an instantaneous rate that accelerated 2.0 % annually in the OLLP (P = 0.024) and 2.7 % in the OSLP (P < < 0.0001) consequently yielding a 63 % larger accumulation in the latter group (P = 0.0002). The results suggest that accumulation of health deficiencies over the life course is not the same in the two groups, likely due to inheritance related to parental longevity. Consistent with this, sib pairs were significantly correlated regarding FI34 scores, and heritability of the FI34 was estimated to be 0.39. Finally, hierarchical clustering suggests that the OLLP and OSLP differ in their aging patterns. Variation in the FI34 is, in part, due to genetic variation; thus, the FI34 can be a phenotypic measure suitable for genetic analyses of healthy aging.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9472-0) contains supplementary material, which is available to authorized users.
Keywords: Frailty, Deficits, Longevity, Aging, Heritability, Age
Introduction
Aging can be defined as the progressive decline in the ability to withstand damage and stress, which is associated with an increase in the incidence of disease and degenerative disorders (Finch 1990). This definition separates biological aging from a strict relationship with chronological age. Aging processes involve many factors, both genetic and nongenetic. The complexity of human aging is further increased by various interactions that occur among these factors in the development and progression of aging-related changes.
In an attempt to systematically approach human aging, Rowe and Kahn (1997) defined the concept of successful aging as: (1) relatively low risk of disease and disease-related disability, (2) relatively high mental and physical function, and (3) active and productive engagement with life. A quantitative approach to successful aging was developed by estimating the amount of physical and functional loss that occurs during the life course and incorporating these losses into a condition termed “frailty” (Rockwood et al. 1994, 1999). Fried et al. (2001) defined frailty as a clinical syndrome involving five features: weight loss, exhaustion, muscle weakness, slow gait speed, and low physical activity. They found that the prevalence of frailty increases with age. Mitnitski et al. (2001) developed an expanded approach to frailty by introducing a frailty index (FI), as the proportion of accumulated deficits in a set of 92 health variables surveyed for an individual at a given age. Their health variables included symptoms, signs, laboratory measurements, diseases, and disabilities. The purpose of the FI was to enumerate a broad spectrum of changes that occur in multiple domains of the human body. Since then, different FIs with different numbers of health variables have been studied (Rockwood and Mitnitski 2007; Rockwood et al. 2007), and others taking the FI approach have used the term deficit index (DI) (Kulminski et al. 2007b, 2008).
The FI appears to be a promising tool for studying human aging as an indicator of biological age and predictor of survival (Mitnitski et al. 2001, 2002a, b; Kulminski et al. 2007a, b). The FI seems to be relatively robust and consistent between studies, as long as the number of health variables is reasonably large (≥20) and sufficiently diverse to represent multiple domains of body function (Mitnitski et al. 2006; Rockwood et al. 2007; Searle et al. 2008).
Despite the potentially useful features of the FI in human aging research, studies addressing its utility in genetic analyses are extremely limited. In a twin study, Kulminski et al. (2009) found geriatric diseases can be used as cumulative indices to predict lifespan among family members. Matteini et al. (2010), in a family study, estimated the heritability of 28 health-related variables to range from 0.01 to 0.45, individually or in statistical combinations.
Here, we have constructed a FI based on 34 health variables (FI34) and studied its properties as a composite phenotype. Our 34 variables include diseases and symptoms throughout the body, deficiencies in physical and cognitive functioning, and self-rated health status. We validate the FI34 as a predictor of survival and mortality. We also describe its behavior across age groups in a family-based sample and determine its heritability. Finally, we replicate these features of the FI34 in subjects drawn from a sample of unrelated individuals, which, together with its heritability, suggests the utility of the FI34 in genetic studies of aging.
Materials and methods
Participants
Louisiana residents from the New Orleans Greater Metropolitan Area who were at least 90 years old and their offspring (N = 320) were recruited to the Healthy Aging Family Study (HAFS) (Online Resource Table 1). Ethnicity was self-declared. Eighty-nine offspring were randomly sampled each from a different family, and this group was named “offspring of long-lived parents” (OLLP).
The Louisiana Healthy Aging Study (LHAS) and demographic characteristics of its participants were described elsewhere (Jazwinski et al. 2010). Unrelated individuals (N = 869), ranging in age from 20 to over 100 years old, were recruited from eight parishes within a 40-mi radius of Baton Rouge, Louisiana, by random sampling from Voters’ Registration and CMS enrollment database files. Ethnic affiliation was determined using structure analysis (0.8 assignment probability) (Pritchard et al. 2000; Jazwinski et al. 2010), and LHAS subjects were selected to match the HAFS sample (Online Resource Table 1). Forty-eight LHAS participants were identified whose parents died at ages ≤75 and named “offspring of short-lived parents” (OSLP). The age range of these OSLP individuals approximates that of the OLLP individuals, as summarized in Table 1.
Table 1.
Age of participants sampled from the Healthy Aging Family Study (HAFS) and the Louisiana Healthy Aging Study (LHAS). Numbers are the mean age ± standard deviation (sample number)
| Study | Group | Female | Male | Both |
|---|---|---|---|---|
| HAFS | Total | 64 ± 6 (132) | 64 ± 6 (69) | 64 ± 6 (201) |
| OLLP | 64 ± 7 (57) | 63 ± 5 (32) | 64 ± 6 (89) | |
| LHAS | Total | 66 ± 24 (415) | 69 ± 22 (258) | 67 ± 23 (673) |
| OSLP | 60 ± 13 (28) | 60 ± 12 (20) | 60 ± 12 (48) |
OLLP offspring of long-lived parents, OSLP offspring of short-lived parents
Ages of participants were verified using both documentary evidence (birth certificates, passports, and driver's licenses) and demographic questionnaires. All participants provided informed consent according to protocols approved by the Institutional Review Boards.
Data management
The variables used to count health deficits in both HAFS and LHAS are listed in Online Resource Table 2. Collected data are quantitative measures, either continuous or discrete, or categorical responses from medical history questionnaires. Binary categorical responses were numerically coded: 0 for the absence of deficit and 1 for the presence of deficit. Quantitative data and multicategorical responses were recoded essentially in the same way as reported previously (Searle et al. 2008) or with modifications as shown in Online Resource Table 2. Mortality data were collected using Social Security Death Index search. For the analyses of FI34 in mortality and survival, we calculated the follow-up period (in months) for each individual as follows: for those who died, the follow-up period is the time elapsed from the date of deficit data collection to the recorded date of death (82.5 ± 20.6). For the survivors, the follow-up period is the time passed between the date of deficit data collection and the date of mortality data collection (38.3 ± 24.1).
Data analyses
All statistical analyses were performed in R (R 2008). Only Caucasian participants were included in the analyses to avoid confounding by population admixture and because of sample size considerations. The FI34, with positive skewness, was considered not normally distributed in both study samples (Online Resource Fig. 1). Therefore, in statistical tests that assume a normal distribution, we applied both parametric and nonparametric tests and compared the outcomes. In all instances, both outcomes were very similar, and we present only those from the parametric tests. Fitting of the exponential function a · e(b · age), where a and b are parameters, and weighted least squares estimation of the parameters were performed using the nls function in the R stats package. The integrate utility was used to calculate the definite integral of this function with the lower and upper limits of age set at 40 and 90 years, and permutation analysis (10,000 random samples) was used to test significance of differences between OSLP and OLLP. For hierarchical clustering of 34 variables and age, which are binary or quantitative, we used the general dissimilarity coefficient of Gower (Gower 1971), which is available in the function daisy in the cluster library with standardization (Kaufman and Rousseeuw 2005). This metric is known to be capable of handling different types of variables at the same time. Hierarchical clustering analyses based on the dissimilarity matrices were performed using the hclust function in the base package stats and plotted using the plot function in R. In addition to the “complete” method that we used to generate Fig. 2, we used different agglomeration methods, such as “ward” and “average,” all of which gave essentially the same clustering patterns. All reported P values are two-tailed.
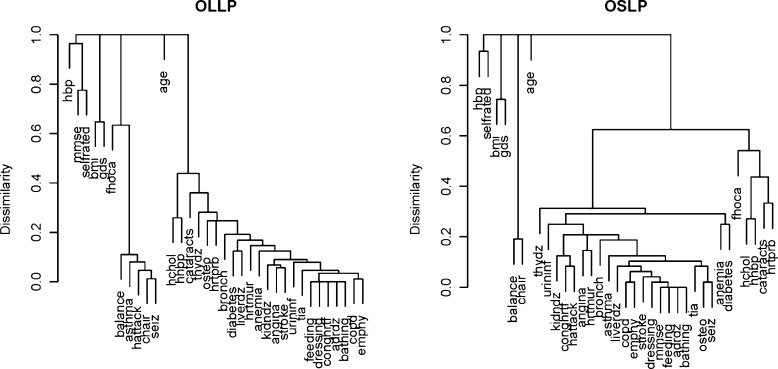
Fig. 2.
Hierarchical clustering of 34 variables and age in a the “offspring of long-lived parents” (OLLP) and b “offspring of short-lived parents” (OSLP). The variable names and descriptions are as in Online Resource Table 2. The dissimilarity matrix was constructed using the Gower metric with standardization (Gower 1971)
Heritability estimation
Heritability in the narrow sense (h2), the ratio of the additive genotypic variance to the total phenotypic variance (σa2/σ2), was estimated for 86 full sib pairs with an equal sibship size (k = 2), as described by Hartl (1980) and its standard error as described by Roff (1997) (Online Resource Table 3).
Results
FI34 as a predictor of mortality
First, we determined the extent to which the FI34 is associated with age (the chronological age at the time of test) and time to death (the time elapsed from the date of test to the date of death). We used the LHAS sample for simple correlation tests because HAFS consists of nuclear families resulting in lack of independence of certain observations. As expected, there was a strong correlation between the FI34 and age (coefficient = 0.70, P < < 0.0001), but the FI34 was better associated with time to death than age was (coefficient = −0.207, P = 0.0041 for FI34 and coefficient = −0.145, P = 0.045 for age) (Table 2).
Table 2.
Correlation between FI34, age, and time to death in the Louisiana Healthy Aging Study (LHAS)
| Variable 1 | Variable 2 | n | Coefficient | 95 % CI | P value |
|---|---|---|---|---|---|
| FI34 | Age | 673 | 0.698 | 0.657–0.735 | <<0.0001 |
| FI34 | Time to death | 191 | −0.207 | −0.339 to −0.067 | 0.0041 |
| Age | Time to death | 191 | −0.145 | −0.282 to −0.004 | 0.045 |
FI34 frailty index based on 34 items
Next, we tested the performance of the FI34 as a predictor of survival/mortality using Cox proportional hazards regression. Again, we used the LHAS sample for the survival analysis because the HAFS sample contains related individuals and the number of deaths was low. Both age and the FI34 were significantly associated with survival times, which included both censored and uncensored data (P < < 0.0001 for both). However, when the Cox regression was limited to time to death (uncensored survival times), only the FI34 had a significant effect on the hazard of death, whereas chronological age did not (P = 0.0041 for FI34 vs. P = 0.12 for age) (Table 3). These results indicate that the FI34 performs as well as previously reported FIs.
Table 3.
Cox regression for time to death as a function of FI34 or age in the Louisiana Healthy Aging Study (LHAS)
| Variable | β | Exp (β) | P value | R2 | Wald test P |
|---|---|---|---|---|---|
| FI34 | 2.358a | 10.570a | 0.0042 | 0.040 | 0.00416 |
| Age | 0.01695 | 1.017 | 0.124 | 0.014 | 0.124 |
FI34 frailty index based on 34 items
aThe coefficient (β) and its exponentiated value (Exp (β)) are for a unit increase in FI34. FI34 scores range from 0 to 1, but a FI34 score of 1 is practically impossible. Therefore, to better estimate the effect of the covariate, we should compute the values for a fractional increase, i.e., 0.1 rather than the whole unit (1). In this case, e(0.1 · β) = 1.27, which means an increase in the hazard by 27 % for a tenth of the unit increase in FI34
Differences in FI34 accumulation between OLLP and OSLP
Our goal was to test whether part of the variation in the FI34 is attributable to genetic differences and, if so, to estimate the extent of the genetic effect. To achieve this goal, we formed two study groups, OLLP from the HAFS and OSLP from the LHAS. These two groups differed in parental longevity. Differences in the FI34 between the two groups can be ascribed to differences in parental longevity, upon matching for other demographic parameters.
As shown in Table 4, the mean FI34 of the OSLP (0.163) was about 31 % higher than that of the OLLP (0.124), and this difference was statistically significant (P = 0.0034). The difference in FI34 between the OSLP and OLLP groups was more accurately assessed using a multiple linear regression to adjust for the differences in sex and age (Table 5). The group variable was significantly associated with the FI34 (P < < 0.0001), which confirms a significant difference in FI34 between the OSLP and the OLLP. Sex had no effect on the FI34 (P > > 0.05), and age was significantly associated with it as before.
Table 4.
FI34 scores of subjects in different study groups
| Study group | Sex | n | Mean ± SD | P valueb |
|---|---|---|---|---|
| OLLP | Both | 89 | 0.124 ± 0.069a | 0.0034 |
| Female | 57 | 0.128 ± 0.068 | ||
| Male | 32 | 0.116 ± 0.071 | ||
| OSLP | Both | 48 | 0.163 ± 0.077a | |
| Female | 28 | 0.159 ± 0.085 | ||
| Male | 20 | 0.168 ± 0.065 |
FI34 frailty index based on 34 items, OLLP offspring of long-lived parents, OSLP offspring of short-lived parents
aP > > 0.05 between sexes in each group
bWilcoxon rank-sum test comparing the OLLP and OSLP totals
Table 5.
Comparison of FI34 between OLLP and OSLP by multiple linear regression (FI34 = β0 + β1 · sex + β2 · age + β3 · group, where group is coded 0 for OLLP and 1 for OSLP and sex is coded 0 for female and 1 for male)
| Variable | βa | SE (β)a | P value | R2 (P value) |
|---|---|---|---|---|
| Sex | −0.00169 | 0.0111 | 0.879 | 0.285 (<<0.0001) |
| Age | 0.00389 | 0.000607 | <<0.0001 | df = 133 |
| Group | 0.0534 | 0.0115 | <<0.0001 |
FI34 frailty index based on 34 items, OLLP offspring of long-lived parents, OSLP offspring of short-lived parents
aRegression coefficient and its standard error
The association of the FI34 with parental longevity was uncovered in a cross-study comparison. To replicate this association within a single study, we used 90 additional LHAS participants with known parental longevity status. A multiple linear regression including OSLP and subjects with at least one parent aged ≥90 as a group variable showed that the FI34 was significantly associated with parental longevity (P = 0.0047) (Table 6).
Table 6.
Test for association of FI34 with parental longevity in LHAS using multiple linear regression (FI34 = β0 + β1 · sex + β2 · age + β3 · parental longevity). Parental longevity was coded 0 for those (n = 90) with either or both parents long-lived (age ≥ 90) and 1 for OSLP (n = 48) and. Sex was coded 0 for female and 1 for male
| Variable | βa | SE (β)a | P value | R2 (P for model) |
|---|---|---|---|---|
| Parental longevity | 0.0524 | 0.0182 | 0.0047 | 0.284(<<0.0001) |
| Age | 0.00369 | 0.000549 | <<0.0001 | df = 134 |
| Sex | −0.00635 | 0.00121 | 0.60 |
FI34 frailty index based on 34 items
aRegression coefficient and its standard error
Difference in FI34 between OLLP and OSLP at later ages
The two study groups were each dichotomized using age 60 as a cutoff for comparison (Online Resource Fig. 2). The mean FI34 was 34 % greater in ‘young’ (age < 60) OSLP compared to ‘young’ (age < 60) OLLP and 57 % greater in ‘old’ (age ≥ 60) OSLP compared to ‘old’ (age ≥ 60) OLLP (Online Resource Table 4). These differences between two age groups imply that the rate of deficit accumulation may be larger at later age in the OSLP than in the OLLP.
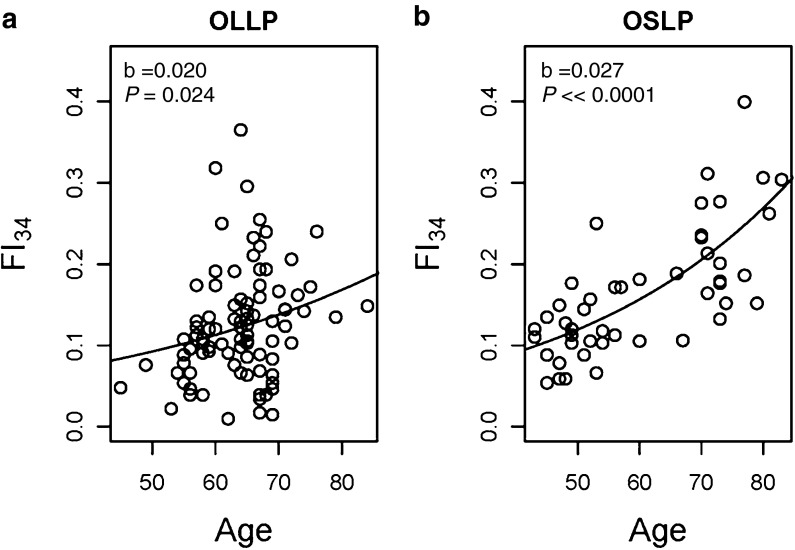
Previous studies indicated that deficits accumulate exponentially with age, and the greater difference in FI34 between OSLP and OLLP at later ages suggests this to be the case here. We estimated the rate of accumulation in each group by fitting an exponential model, a · e(b · age):
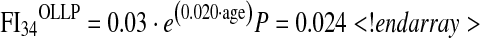 |
1 |
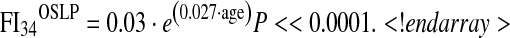 |
2 |
According to this model, the instantaneous rate of accumulation of deficits accelerates at an annual rate of 2 % in the OLLP and 2.7 % in the OSLP (Fig. 1). Integration of these equations in the interval of 40 to 90 years of age results in FI34 of 5.736 and 9.349 for OLLP and OSLP, respectively, which differs by 63 % (P = 0.0002). This confirms that the rate at which FI34 increases differs between these two groups.
Fig. 1.
Scatter plots of FI34 scores by age in the a “offspring of long-lived parents” (OLLP) and b the “offspring of short-lived parents” (OSLP), fitted with exponential curves. Using the FI34 as a dependent variable and age as an independent variable, the exponential function a · e(b · age) was fitted to estimate parameters a and b. In both cases, a = 0.03 and shown are the estimated b values with corresponding P values under the null hypothesis that slope = 0
Sib correlation and heritability of FI34
We next determined the extent of familial aggregation and heritability of the FI34. For this purpose, each OLLP individual was paired with his/her sib (or a sib was randomly selected in case of multisib (≥3) families), and using the 86 full sib pairs only, sib correlation and narrow-sense heritability were estimated. The correlation coefficient was 0.459 (df = 84, 95 % CI = 0.273–0.611, P < < 0.0001) and the estimated heritability was 0.391 (standard error = 0.209).
Group-specific profiles of healthy aging
Lastly, we checked interrelationships of the deficits by grouping them into clusters of statistically related variables, using hierarchical clustering methods. Figure 2 shows the resulting dendrograms for both OLLP and OSLP. In both cases, the 34 variables were grouped into four clusters with the variable age forming an additional cluster by itself. Most of the 34 variables were assigned to one large cluster. However, the way that these variables were grouped in this cluster was not the same between the study groups. For example, the variables ‘angina’ and ‘stroke’ were clustered together in the OLLP, but in the OSLP, they were separate and paired with different variables. The differences in clustering suggest that the pattern of deficit accumulation during aging may be associated with parental longevity.
Discussion
The main conclusion of our work is that parental longevity has a significant impact on healthy aging because FI34 scores of offspring significantly differed depending on their parents' longevity. This finding was made by comparing two different study groups and was confirmed by an analysis of a within-study sample. Using the sib pair data, we found siblings within sibships significantly correlated with each other with regard to their FI34 scores and estimated the heritability to be 0.39.
Our variables cover cardiovascular-related diseases and symptoms (10), deficiencies in physical functioning (6), respiratory functioning (4), cognitive functioning (3), and other diseases and symptoms throughout the body. Our FI34 performed as well or better than other FIs. For example, the FI34 was better correlated with time to death than age was, which replicates the previous finding by Mitnitski et al. (2001) in which their FI was based on 92 variables. The effect of our FI34 on the hazard of death, as shown by a Cox regression, is also consonant with the finding by Mitnitski et al. (2002a) based on 20 variables, though the effect we observe is stronger. They used biological age derived from their FI, but these are not independent variables. Matteini et al. (2010) performed principal component analyses on 28 variables and found no single component responsible for more than 14 % of the variance. Thus, the robustness of this type of index is likely to reflect interrelationships between biological systems at many different levels (Mitnitski et al. 2001). In this context, it is very interesting to note the differences in the clustering patterns of variables between the two groups characterized by different parental longevity. Perhaps, the difference reflects differing interactions of biological systems depending on the genetic backgrounds transmitted from previous generations.
In examining the rate of increase in FI34 with age, we rely on cross-sectional data. The trend of deficit accumulation with age may differ among different birth cohorts (Yang and Lee 2010). A few reports based on a longitudinal study and other cross-sectional data available to date suggest that accumulation of deficits increases in a nonlinear fashion (Mitnitski et al. 2001; Kulminski et al. 2007a, b; Yang and Lee 2010; Kulminski et al. 2011). Our data also fit an exponential model of deficit accumulation. The instantaneous rate of increase in deficits at a given age can be obtained by differentiating Eqs. 1 and 2. Thus, for example, the instantaneous rate of FI34 increase is 12 % for OLLP and 20 % for OSLP, respectively, at age 70.
Herskind et al. (1996) reported the heritability of human longevity ranging 0.23–0.26 with no evidence for an impact of shared (family) environment. All subsequent estimates of heritability of longevity fall between 0.15 and 0.35 (Gudmundsson et al. 2000; Kerber et al. 2001). In addition, a number of studies reported heritability during aging of physical and cognitive functions (McClearn et al. 1997; Carmelli et al. 2000; Frederiksen et al. 2002), and even a measure of health-related quality of life (Romeis et al. 2005). Our estimate of heritability of FI34, 0.39, falls within the range that Matteini et al. (2010) estimated for 28 different variables, alone and in combinations. It is also within the 95 % CI of heritability, 0.31–0.53, recently reported by Dato et al. (2012) for their “frailty phenotype,” which is based on survival, age, MMSE, Katz's index of ADL, BMI, and self-reported health rating. The inclusion of survival and age lessens the utility of this frailty phenotype as a predictive tool, however.
In genetic analyses of a complex trait such as aging, the selection of an appropriate phenotype is paramount. Ho et al. (2011) investigated association of women’s frailty with single-nucleotide polymorphisms (SNPs) in candidate genes. In that study, the FI was based on a five-point scale from measurements of muscle weakness, slow gait speed, weight loss, fatigue, and low physical activity. The candidate genes were selected based on their roles in skeletal muscle function and inflammation. However, no SNPs passed statistical significance. Edwards et al. (2011) collected data from 214 Amish subjects over age 80 for 13 variables. These variables belonged to the three domains of successful aging, as described earlier (Rowe and Kahn 1997). Linkage analysis of the binary trait of successful aging (yes/no) led to identification of three genomic regions. Although the numbers and selections of health variables were limited in these studies and the results await replication and validation, these studies suggest that a multidimensional phenotype like FI34 will be useful for genetic analyses.
In sum, we showed that (1) our FI changes with age at different rates, depending on the longevity of parents; (2) this index is heritable; and (3) it discriminates between different patterns of aging. Unraveling of these additional properties of the FI was possible due to the collected information on familial longevity.
Electronic supplementary material
(DOC 131 kb)
Acknowledgments
This study was supported by grants from the National Institute on Aging of the National Institutes of Health (K01AG027905 to SK and P01AG022064 to SMJ), by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF(2001–06)-02] (to SMJ), and by the Louisiana Board of Regents RC/EEP Fund through the Tulane-LSU CTRC at LSU Interim University Hospital. We thank the CTRC for nursing services, subject testing, and blood draw, and the core lab support for blood sample processing. We also thank the people of Louisiana for participation in our studies. The corresponding authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Sangkyu Kim, Phone: +1-504-9883091, FAX: +1-504-9888835, Email: skim5@tulane.edu.
S. Michal Jazwinski, Phone: +1-504-9888253, FAX: +1-504-9888835, Email: sjazwins@tulane.edu.
References
- Carmelli D, Kelly-Hayes M, Wolf PA, Swan GE, Jack LM, Reed T, Guralnik JM. The contribution of genetic influences to measures of lower-extremity function in older male twins. J Gerontol A Biol Sci Med Sci. 2000;55(1):B49–B53. doi: 10.1093/gerona/55.1.B49. [DOI] [PubMed] [Google Scholar]
- Dato S, Montesanto A, Lagani V, Jeune B, Christensen K, Passarino G. Frailty phenotypes in the elderly based on cluster analysis: a longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. Age (Dordr) 2012;34(3):571–582. doi: 10.1007/s11357-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Gilbert JR, Jiang L, Gallins PJ, Caywood L, Creason M, Fuzzell D, Knebusch C, Jackson CE, Pericak-Vance MA, Haines JL, Scott WK. Successful aging shows linkage to chromosomes 6, 7, and 14 in the Amish. Ann Hum Genet. 2011;75(4):516–528. doi: 10.1111/j.1469-1809.2011.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Longevity, senescence, and the genome. The John D. and Catherine T. MacArthur Foundation series on mental health and development. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23(2):110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gower JC. A general coefficient of similarity and some of its properties. Biometrics. 1971;27:857–874. doi: 10.2307/2528823. [DOI] [Google Scholar]
- Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefansson K. Inheritance of human longevity in Iceland. Eur J Hum Genet. 2000;8(10):743–749. doi: 10.1038/sj.ejhg.5200527. [DOI] [PubMed] [Google Scholar]
- Hartl DL. Principles of population genetics. Sunderland: Sinauer Associates, Inc.; 1980. [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97(3):319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Ho YY, Matteini AM, Beamer B, Fried L, Xue QL, Arking DE, Chakravarti A, Fallin MD, Walston J. Exploring biologically relevant pathways in frailty. J Gerontol A Biol Sci Med Sci. 2011;66(9):975–979. doi: 10.1093/gerona/glr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski SM, Kim S, Dai J, Li L, Bi X, Jiang JC, Arnold J, Batzer MA, Walker JA, Welsh DA, Lefante CM, Volaufova J, Myers L, Su LJ, Hausman DB, Miceli MV, Ravussin E, Poon LW, Cherry KE, Welsch MA. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell. 2010;9(5):698–708. doi: 10.1111/j.1474-9726.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ. Finding groups in data: an introduction to cluster analysis. Wiley series in probability and mathematical statistics. Hoboken: Wiley; 2005. [Google Scholar]
- Kerber RA, O'Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. J Gerontol A Biol Sci Med Sci. 2001;56(3):B130–B139. doi: 10.1093/gerona/56.3.B130. [DOI] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K, Yashin AI. Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol. 2007;42(10):963–970. doi: 10.1016/j.exger.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007;55(6):935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Ukraintseva SV, Culminskaya IV, Arbeev KG, Land KC, Akushevich L, Yashin AI. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63(10):1053–1059. doi: 10.1093/gerona/63.10.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Arbeev KG, Culminskaya IV, Ukraintseva SV, Christensen K, Yashin AI. Health-related phenotypes and longevity in Danish twins. J Gerontol A Biol Sci Med Sci. 2009;64(1):1–8. doi: 10.1093/gerona/gln051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Arbeev KG, Christensen K, Mayeux R, Newman AB, Province MA, Hadley EC, Rossi W, Perls TT, Elo IT, Yashin AI. Do gender, disability, and morbidity affect aging rate in the LLFS? Application of indices of cumulative deficits. Mech Ageing Dev. 2011;132(4):195–201. doi: 10.1016/j.mad.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteini AM, Fallin MD, Kammerer CM, Schupf N, Yashin AI, Christensen K, Arbeev KG, Barr G, Mayeux R, Newman AB, Walston JD. Heritability estimates of endophenotypes of long and health life: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1375–1379. doi: 10.1093/gerona/glq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123(11):1457–1460. doi: 10.1016/S0047-6374(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Mitnitski A, Bao L, Rockwood K. Going from bad to worse: a stochastic model of transitions in deficit accumulation, in relation to mortality. Mech Ageing Dev. 2006;127(5):490–493. doi: 10.1016/j.mad.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R (2008) Version R 2.11.1; R Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150(4):489–495. [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Roff DA. Evolutionary quantitative genetics. New York: Chapman & Hall; 1997. [Google Scholar]
- Romeis JC, Heath AC, Xian H, Eisen SA, Scherrer JF, Pedersen NL, Fu Q, Bucholz KK, Goldberg J, Lyons MJ, Waterman B, Tsuang MT, True WR. Heritability of SF-36 among middle-age, middle-class, male–male twins. Med Care. 2005;43(11):1147–1154. doi: 10.1097/01.mlr.0000183217.11811.bd. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B(2):246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 131 kb)