Abstract
Nutraceuticals are known to have numerous health and disease preventing properties. Recent studies suggest that extracts containing cranberry may have anti-aging benefits. However, little is known about whether and how cranberry by itself promotes longevity and healthspan in any organism. Here we examined the effect of a cranberry only extract on lifespan and healthspan in Caenorhabditis elegans. Supplementation of the diet with cranberry extract (CBE) increased the lifespan in C. elegans in a concentration-dependent manner. Cranberry also increased tolerance of C. elegans to heat shock, but not to oxidative stress or ultraviolet irradiation. In addition, we tested the effect of cranberry on brood size and motility and found that cranberry did not influence these behaviors. Our mechanistic studies indicated that lifespan extension induced by CBE requires the insulin/IGF signaling pathway and DAF-16. We also found that cranberry promotes longevity through osmotic stress resistant-1 (OSR-1) and one of its downstream effectors, UNC-43, but not through SEK-1, a component of the p38 MAP kinase pathway. However, SIR-2.1 and JNK signaling pathways are not required for cranberry to promote longevity. Our findings suggest that cranberry supplementation confers increased longevity and stress resistance in C. elegans through pathways modulated by daf-16 and osr-1. This study reveals the anti-aging property of widely consumed cranberry and elucidates the underpinning mechanisms.
Keywords: Cranberry, Longevity, Aging intervention, daf-16, osr-1, Caenorhabditis elegans
Introduction
Delaying aging and the onset of age-related diseases is one of the major challenges in human societies worldwide. Dietary intervention is an effective non-genetic way to combat the effect of aging and to improve health. Botanicals, such as blueberry and cranberry, contain numerous biologically active phytochemicals that are thought to be potent in preventing various diseases and promoting health (Liu 2004). The North American cranberry (Vaccinium macrocarpon) and its constituent products have been shown to provide numerous health benefits to humans, including anti-microbial, anti-virus, anti-mutagenesis, anti-angiogenesis, and antioxidation (Pappas and Schaich 2009). The anti-microbial activity of cranberry has been widely recognized due to its potency in the prevention of urinary tract infections (Walker et al. 1997) and probably stomach ulcers (Burger et al. 2002), as well as improvement of oral hygiene (Steinberg et al. 2004). The anti-microbial effect is likely attributable to the ability of cranberry to interfere with the adhesion of pathogens to the surfaces of host cells (Howell 2007; Sobota 1984). In addition, in vitro studies suggest that cranberry phenolics are capable of reducing the level of low-density lipoprotein and preventing platelet aggregation and thrombosis, which result in a reduction of blood pressure and/or inflammation (Bodet et al. 2006; Wilson et al. 1998). Cranberries have also been shown to inhibit the growth and proliferation of tumors in a broad spectrum fashion (Baur and Sinclair 2006; Liu 2004; Verma et al. 1988). The anti-cancer properties can be attributed to a variety of phytochemicals in cranberry, including proanthocyanidin oligomers, flavonols, anthocyanin glycosides, and triterpenoids. These phytochemicals can regulate cell apoptosis, DNA repair, and anti-inflammation (Bodet et al. 2006, 2007a, b; Neto 2007b; Neto et al. 2008).
A recent study showed that a mixture of cranberry and oregano extracts had a prolongevity effect in the Mexican fruit fly (Zou et al. 2010). However, it has not been determined whether the cranberry extract alone can promote longevity in any organism and the mechanisms underlying the anti-aging property of cranberry has not been defined. Here we have undertaken a study to investigate the prolongevity property of the cranberry extract (CBE) in Caenorhabditis elegans. C. elegans is a genetically tractable multi-cellular model organism that is widely used to investigate the mechanisms of aging and aging interventions (Guarente and Kenyon 2000; Kenyon 2010; Pietsch et al. 2011; Wilson et al. 2006; Zou et al. 2007). A number of genes and pathways have been identified to modulate lifespan. Many of these lifespan determinants are evolutionally conserved among diverse species. For instance, DAF-16/FOXO is a major longevity determinant and highly conserved in organisms ranging from worms, flies, rodents to humans to regulate various biological processes, including cell proliferation, apoptosis, stress resistance and carbohydrate, protein, and lipid metabolism, as well as β-cell development and function (Ai et al. 2010; van der Horst and Burgering 2007). The insulin/IGF signaling (IIS) pathway modulates lifespan in species ranging from worms and flies to mammals (Ziv and Hu 2010). In C. elegans, insulin-like ligands bind to DAF-2, the counterpart of mammalian insulin/IGF-1 receptor, to initiate the signaling cascade. Subsequently, phosphorylation of AGE-1, PDK-1, and AKT-1/2 inactivates DAF-16/FOXOs by sequestering the protein in the cytosol (Cohen and Dillin 2008; Hertweck et al. 2004; Lee et al. 2001; Morris et al. 1996). Mutations in daf-2, age-1 and akt-1/2, or increasing expression of daf-16 can lead to reduction of the IIS and extension of lifespan in C. elegans. We report here that a cranberry extract can increase both lifespan and thermotolerance in C. elegans, a species rich in genetic resources. The lifespan extension induced by CBE is not associated with changes in the worms’ resistance to oxidative stress, ultraviolet (UV) irradiation, osmotic stress, or physiological functions, such as brood size and motility. Our genetic epistasis analyses suggest that CBE promotes longevity by acting through the insulin signaling cascade and DAF-16, and requiring components of the osmotic stress response pathway, OSR-1 and UNC-43. Our findings reveal the prolongevity property of cranberry and the molecular mechanisms by which cranberry promotes longevity.
Materials and methods
Strains and growth conditions
All strains were maintained at 20 °C on nematode growth medium (NGM) seeded with Escherichia coli OP50 feeding strain. One hundred microliters of OP50 was dropped on the center of the 60-mm NGM plates, which were allowed to dry overnight before the culture assays were carried out. Strains used in this study were: N2, Bristol (wild type), daf-16 (mgDf50), sir-2.1(ok434), osr-1(rm1), sek-1(ag1), unc-43(n1186), daf-2(e1370), jnk-1(gk7), age-1(hx546), daf-16(mgDf47); xrIs87. All the strains were obtained from the Caenorhabditis Genetics Center, University of Minnesota, USA.
Cranberry extract preparation
The cranberry extract was kindly provided by R. Ghaedian (Decas Cranberry Products, Inc., Carver, MA) and was described in a previously published study (Zhu et al. 2011). To prepare cranberry supplemented food, the cranberry extract in powder was first dissolved in sterile distilled water. A 10 % fresh stock solution was prepared 1 day before the assay and then the appropriate dilutions were overlaid onto the NGM plates.
Lifespan assay
All lifespan assays were carried out at 25 °C. Synchronized populations were obtained by allowing 10–15 hermaphrodites lay eggs overnight, and the next day the parents were removed. The eggs were allowed to hatch and 30 L4/young adult worms per plate (NGM plate containing 50 μg/mL FUDR to prevent the growth of progeny) were used for each assay. All the assays were carried out in triplicates, and a minimum of two independent trials were performed for all conditions. The dead worms were counted starting the next day and exploding, protruding, bagging or contaminated worms were censored if applicable. We defined the day when we transferred the L4/young adult worms as day 0 of adult age. All statistical analyses were carried out using SPSS software (IBM SPSS Statistics). Kaplan–Meier lifespan analysis was carried out, and p values were calculated using the log-rank test. P < 0.05 was accepted as statistically significant.
Stress assays
For stress assays, worms were pretreated with 2 mg/mL CBE for two generations at 25 °C on OP50 seeded NGM plates. Worms on regular NGM plates without CBE served as controls. Each assay was carried out in three independent trials, and the data were pooled and analyzed using Student’s t test. P < 0.05 was accepted as statistically significant.
For the oxidative stress assay, we first tested the survival of L4/young adult N2 worms at 25 °C on NGM plates containing serial dilutions of paraquat (0.1–10 mM) (Sigma-Aldrich, Corp. St. Louis, MO, USA). The concentration, 5 mM, that resulted in ~20 % of worm death on the second day (LD20 = 5 mM) was selected for subsequent cranberry protection assays. CBE pretreated L4/young adult worms and their controls were transferred onto NGM plates containing 5 mM paraquat, and the survival was counted daily until all worms died (i.e., the 10th day after paraquat exposure).
The UV-induced stress assay was carried out using a UV crosslinker (CL-1000 Ultraviolet Crosslinker UVP). We first determined the LD20 by subjecting L4/young adult worms to UV irradiation in the range of 0.05 to 1.0 J/cm2 for 20, 30, and 60 s, respectively. Their survival rates were monitored on the second day. We found that the LD20 was at 0.05 J/cm2 for 20 s, which was chosen for subsequent experiments. For the cranberry protection assay, CBE-pretreated L4/young adult worms and controls were transferred to CBE (2 mg/mL) plates and NGM plates, respectively, and were irradiated at 0.05 J/cm2 for 20 s. The lifespan of the worms was followed at 25 °C.
The heat shock assay was performed as described previously (Pietsch et al. 2009; Strayer et al. 2003) with some modifications. CBE-pretreated L4/young adult stage worms and controls were transferred to CBE supplemented (2 mg/mL) plates and regular NGM plates, respectively. The plates were incubated at 37 °C for 3.5 h and then transferred back to 25 °C. The survival of the worms was monitored daily.
The osmotic stress assay was conducted as described previously (Solomon et al. 2004). For the acute osmotic stress assay, CBE-pretreated worms and their controls were exposed to high salt concentrations (300 and 500 mM NaCl) for 10 min and then transferred to regular unseeded NGM plates to monitor their motility behavior. For the chronic osmotic stress, CBE-treated and non-treated worms were subjected to a high salt concentration (500 mM) and then their mortality rate was monitored for the next 12 h.
Measurement of reactive oxygen species
Intracellular reactive oxygen species (ROS) levels were measured using 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Molecular Probes, Carlsbad, CA, USA) as described previously (Lee et al. 2009, 2010) with some modifications. CBE-pretreated and non-treated L4/young adult worms were exposed to 5 mM paraquat and collected at day 1 and day 4. Age-matched CBE-pretreated and non-treated adult worms without paraquat challenge were used as controls. For each assay, approximately 1,000 worms were collected and washed three times with M9 buffer to remove bacteria. The worms were then washed with 100 μL of PBS once and were immediately frozen by placing them in a –80 °C freezer. The worm extracts were prepared by sonication and subsequent centrifugation at 13,000 rpm at 4 °C for 30 min. The supernatant was transferred to a new tube and centrifuged again at 13,000 rpm at 4 °C for 30 min. Ten microliters of the supernatant was used for protein quantification using the Bradford method. Supernatant containing 50 μg of protein was pre-incubated with 250 μM of DCF-DA in 100 μL of PBS at 37 °C for 1 h. Fluorescence intensity was measured with a fluoremeter (molecular devices, SPECTRAmax Gemini XS, USA) at the excitation wavelength 485 nm and the emission wavelength 535 nm. The fluorescence was measured once every 30 min for 1.5 h. The time-dependent increase in the fluorescence was linear for this time frame. We therefore chose to use fluorescence intensity at 1 h for the quantification. The fluorescence intensity was normalized by subtracting the background fluorescence of 250 μM DCF-DA solution in PBS at 1 h. This assay was carried out in three independent trials. The data were pooled and analyzed using Student’s t test. P < 0.05 was accepted as statistically significant.
Brood size and body bend assays
The brood size assay was carried out by transferring a single L4/young adult worm onto an NGM plate supplemented with or without 2 mg/mL CBE (triplicates). Each worm was allowed to lay eggs at 25 °C for 24 h and was then transferred to a fresh plate until it ceased to lay eggs. The offspring were counted after they reached the L3 or L4 phase. This assay was carried out in three independent trials. The data were pooled and analyzed using Student’s t test. P < 0.05 was accepted as statistically significant.
For the motility assay, worms were pretreated with or without 2 mg/mL CBE for two generations on NGM plates at 25 °C. Ten CBE-pretreated or non-treated L4/young adult nematodes were placed onto individual NGM plates without OP50.The body bends per minute were counted. This assay was carried out in three independent trials. The data were pooled and analyzed using Student’s t test. P < 0.05 was accepted as statistically significant.
DAF-16 translocation assay
The daf-16(mgDf47); xrIs87 worms carrying a DAF-16-GFP fusion construct (Lee et al. 2001) were treated with 2 mg/mL CBE for 1 h and were then observed under a fluorescence microscope (Nikon AZ100) to monitor the nuclear translocation of DAF-16-GFP.
Gene expression analysis by quantitative PCR
The nematodes were grown on NGM plates supplemented with or without 2 mg/mL CBE at 25 °C until they reached the young adult stage. The worms were collected with M9 buffer. RNA was prepared using Absolutely RNA miniprep kit (Agilent technologies) and stored at −80 °C. Complementary DNA was prepared by using Invitrogen Superscript first strand synthesis system for real-time polymerase chain reaction (RT-PCR) (Invitrogen). Quantitative PCR (qPCR) was performed using SsoFast EvaGreen Supermix and the iCycler iQ real-time PCR detection system according to the manufacturer’s suggested protocol (Bio-Rad). The qPCR conditions were: 95 °C for 3 min followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. The act-1 was used as an internal control to normalize the expression levels of target transcripts. Each qPCR experiment was repeated three times using independent RNA preparations. The data were pooled and analyzed using Student’s t test, and p < 0.05 was accepted as statistically significant. The qPCR primers for sod-3 are: 5′-CCAACCAGCGCTG-AAATTCAATGG-3′ (forward primer (F)) and 5′-GGAACCGAAGTCGCGCTTAATAGT-3′ (reverse primer (R)). Primers for daf-16 are 5′-CCAGACGGAAGGCTTAAACT-3′ (F) and 5′-ATTCGCATGAAACGAGAATG-3′ (R). Primers for hsp-4 are 5′-TGACTCGTGCCAAGTTTGAG-3′ (F) and 5′-GCTCCTTGCCGTTGAAGTAG-3′ (R). Primers for hsp-16.1 are 5′-GCAGAGGCTCTCCATCTGAA-3′ (F), and 5′-GCTTGAACTGCGAGACATTG-3′ (R). Primers for hsp-16.49 are 5′-GCTCATGCTCCGTTCTCCATATTCTGATTCAAATGC-3′ (F) and 5′-GCAACAAAATTGATCGGAATAGAACGTGATGAG-3′ (R). Primers for hsp-70 are 5′-CGTTTCGAAGAACTGTGTGCTGATCTATTCCGG-3′ (F), and 5′-TTAATCAACTTCCTCAACAGTAGGTCCTTGTGG-3′ (R). Primers for hsp-12.6 are 5′-ATGATGAGCGTTCCAGTGATGGCTGACG-3′ (F), and 5′-TTAATGCATTTTTCTTGCTTCAATGTGAAGAATTCC-3′ (R). Primers for act-1 are: 5′- CCAGGAATTGCTGATCGTATGCAGAA-3′ (F), and 5′-TGGAGAGGGAAGCGAGGATAGA-3′ (R).
Results
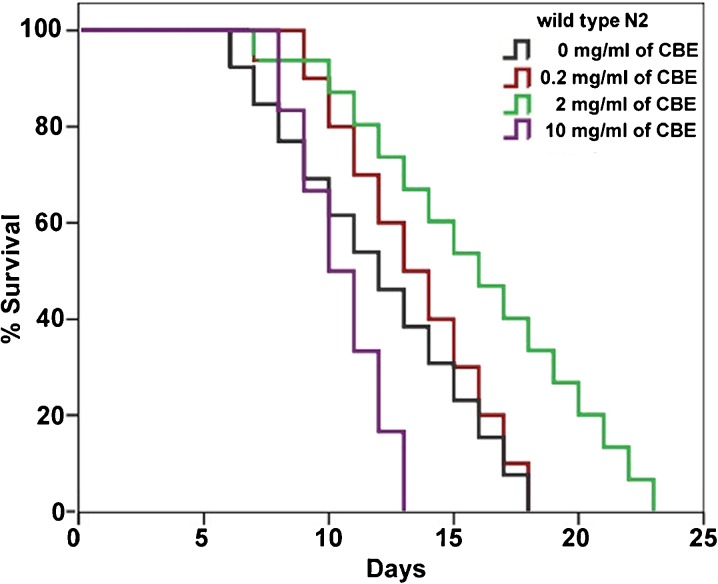
CBE supplementation extends the lifespan of C. elegans in a dose-dependent manner
To determine the prolongevity property of cranberry, we treated wild-type N2 worms with CBE at concentrations ranging from 0 to 10 mg/mL in the standard nematode growth medium (NGM) at 25 °C. CBE at 2 mg/mL increases mean lifespan from 12 to 15.9 days, while CBE at 10 mg/mL decreased the mean lifespan from 12 to 10.5 days (Fig. 1; Table 1). We found that CBE at ≥6 mg/mL reduced pH of the NGM (pH ≤ 6.0) probably due to the acidogenicity of CBE. To test whether the acidogenicity of CBE caused the shortened lifespan, we neutralized CBE with a Tris base solution (pH 9.0) before supplementing it to worms. Controls were treated with the same amount of Tris buffer but at pH 7.4. We found that the neutralized CBE affected lifespan in a similar way as the original CBE (data not shown). Taken together, these findings indicate that CBE supplementation can extend lifespan in C. elegans, and this lifespan extension is dose-dependent.
Fig. 1.
CBE extends C. elegans lifespan in a dose-dependent manner. Wild-type N2 worms were treated with CBE at 0–10 mg/mL. Each lifespan experiment was repeated at least three independent times with similar results. Quantitative data and statistical analyses for the representative experiments are included in Table 1
Table 1.
The lifespan of various worm strains at 25 °C
| Strain | Mean ± SE | Median | No. of worms | P value |
|---|---|---|---|---|
| N2a | 11.83 ± 0.946 | 12.0 | 310 | |
| N2 + 0.2 mg/mL CBEa | 13.50 ± 0.95 | 13.0 | 325 | 0.187 |
| N2 + 2 mg/mL CBEa | 15.90 ± 1.20 | 16.0 | 298 | 0.001 |
| N2 + 10 mg/mL CBEa | 10.50 ± 0.76 | 10.0 | 303 | 0.190 |
| N2b | 10.62 ± 0.96 | 11.0 | 267 | |
| N2 + 2 mg/mL CBEb | 16.73 ± 1.25 | 17.0 | 299 | 0.007 |
| daf-16(mgDf50)b | 8.66 ± 1.13 | 9.0 | 301 | |
| daf-16(mgDf50) + 2 mg/mL CBEb | 9.12 ± 1.08 | 9.0 | 287 | 0.840 |
| N2b | 11.88 ± 0.98 | 12.0 | 184 | |
| N2 + 2 mg/mL CBEb | 14.60 ± 1.29 | 15.0 | 191 | 0.047 |
| daf-2(e1370)b | 26.66 ± 1.72 | 27.0 | 167 | |
| daf-2(e1370) + 2 mg/mL CBEb | 27.94 ± 1.73 | 28.0 | 183 | 0.978 |
| N2b | 12.50 ± 0.77 | 12.0 | 193 | |
| N2 + 2 mg/mL CBEb | 15.00 ± 1.13 | 15.0 | 167 | 0.050 |
| age-1(hx546)b | 28.50 ± 1.37 | 28.0 | 189 | |
| age-1(hx546) + 2 mg/mL CBEb | 27.70 ± 1.44 | 28.0 | 174 | 0.561 |
| N2c | 12.50 ± 0.96 | 12.0 | 293 | |
| N2 + 2 mg/mL CBEc | 15.40 ± 1.11 | 15.0 | 271 | 0.040 |
| osr-1(rm1)c | 10.50 ± 0.87 | 10.0 | 300 | |
| osr-1(rm1) + 2 mg/mL CBEc | 9.90 ± 1.11 | 10.0 | 282 | 0.890 |
| unc-43(n1186)c | 14.00 ± 1.00 | 14.0 | 307 | |
| unc-43(n1186) + 2 mg/mL CBEc | 14.97 ± 1.12 | 15.0 | 282 | 0.336 |
| sek-1(ag1)c | 12.50 ± 1.04 | 12.5 | 199 | |
| sek-1(ag1) + 2 mg/mL CBEc | 14.00 ± 1.15 | 14.0 | 198 | 0.197 |
| N2d | 12.00 ± 0.82 | 12.0 | 263 | |
| N2 + 2 mg/mL CBEd | 16.60 ± 1.01 | 16.0 | 299 | 0.001 |
| jnk-1(gk7)d | 10.78 ± 0.05 | 11.0 | 275 | |
| jnk-1(gk7) + 2 mg/mL CBEd | 14.50 ± 1.56 | 15.0 | 296 | 0.027 |
| N2d | 12.57 ± 1.48 | 13.0 | 295 | |
| N2 + 2 mg/mL CBEd | 18.78 ± 1.02 | 19.0 | 284 | 0.006 |
| sir-2.1(ok434)d | 13.00 ± 1.91 | 13.0 | 293 | |
| sir-2.1(ok434) + 2 mg/mL CBEd | 16.10 ± 1.18 | 16.0 | 297 | 0.025 |
Lifespan and standard error are shown in days. The lifespan experiments were repeated at least three times with similar results, and the data for representative experiments are shown. The lifespan data were analyzed using the log-rank test and p values for each individual experiment are shown
aResults presented in Fig. 1
bResults presented in Fig. 3
cResults presented in Fig. 5
dResults presented in Fig. 4
Considering that cranberry possesses antimicrobial properties, it is possible that CBE inhibits the growth of feeding E. coli OP50 under our assay conditions, thereby extends C. elegans lifespan via dietary restriction. To test this hypothesis, we cultured OP50 in LB medium with a series of concentrations of CBE (10, 5, 2, and 0.2 mg/mL). We found that CBE at these concentrations did not inhibit the growth of E. coli OP50 at any bacterial growth phases. CBE at 2 mg/mL (the optimal concentration for lifespan extension) even promoted the growth of OP50 as compared to the control medium (data not shown). Therefore, it is unlikely that CBE prolongs C. elegans’ lifespan through its antimicrobial effect.
CBE supplementation alters worms’ response to heat shock stress and osmotic stress but not to oxidative stress or UV irradiation
Lifespan extension is generally associated with elevated stress resistance (Broughton et al. 2005; Clancy et al. 2002; Larsen 1993; Lithgow et al. 1994). To test whether CBE enhances stress resistance, we exposed C. elegans to a series of environmental stressors. We first tested oxidative stress. Adult wild-type N2 worms were pretreated with and without 2 mg/mL CBE for two generations at 25 °C and then were transferred to NGM plates containing 5 mM of paraquat. Paraquat is a chemical commonly used to generate reactive oxygen species and induce oxidative stress in the cell (Fukushima et al. 1993; Yamada and Fukushima 1993). The survival was monitored over 10 days. We found that paraquat reduced the lifespan, but CBE supplementation did not increase worms’ resistance to paraquat (Fig. 2a). Considering that paraquat is a very potent toxicant, paraquat treatment may not only induce ROS but also generate other toxic effects. It is possible that CBE supplementation is able to reduce paraquat-induced ROS, but fails to antagonize other toxic effects. To address this concern, we measured ROS levels in worms treated with or without CBE using the DCF-DA staining method. We found that CBE supplementation did not significantly reduce the ROS levels in 1-day and 4-day-old adult worms relative to age-matched non-supplemented control (Fig. 2b). Although paraquat treatment increased the ROS level relative to the non-treated groups, CBE supplementation was not effective in reducing the ROS level in worms exposed to paraquat (Fig. 2b).
Fig. 2.
CBE treatment results in the alterations of stress response to specific environmental stimuli. a The CBE-treated N2 worms showed similar survival in response to 5 mM paraquat as compared to non-treated worms. b The CBE-treated N2 worms showed similar intracellular ROS level in response to 5 mM paraquat as compared to non-treated worms. c The CBE-treated N2 worms showed similar survival after UV irradiation as compared to non-treated worms. d The CBE-treated N2 worms exhibited increased survival after 3.5 h at 37 °C when compared to non-treated worms. e The transcript levels of hsp-4, hsp-12.6, hsp-16.1, hsp-16.49, and hsp-70 in N2 worms treated with and without 2 mg/mL CBE were quantified using qRT-PCR. Controls are set to 1. f The CBE-treated N2 worms showed reduced motility after 10 min exposure in 300 mM and 500 mM NaCl relative to non-treated worms. g The CBE-treated N2 worms showed an elevated mortality rate after 12 h exposure in 500 mM NaCl compared to non-treated worms. At least triplicate samples were examined for each stress assay. “Fraction alive” indicates the average survival among the multi-replicates and error bars represent the standard deviation. P value was calculated using Student’s t test. **p < 0.05 when compared to corresponding control. Each of the stress assays was repeated at least three independent times with similar results
Next, we tested the worms’ resistance to UV irradiation. Wild-type young adult worms were exposed under 0.05 J/cm2 UV irradiation for 20 s. The survival was monitored at 25 °C over the next 10 days. CBE supplementation had no or little effect on the survival of worms after UV irradiation (Fig. 2c). Our findings indicate that CBE supplementation does not improve resistance to acute oxidative stress or UV irradiation in C. elegans.
To test the worms’ resistance to heat shock, young adult N2 worms pretreated with or without CBE for two generations at 25 °C were shifted to 37 °C for 3.5 h and then back to 25 °C. Wild-type C. elegans pretreated with CBE showed statistically significant higher resistance to heat shock compared to CBE untreated controls (Fig. 2d), indicating that CBE supplementation can elevate resistance to heat shock in C. elegans. Increasing the expression levels of heat shock proteins (HSPs) is known to have a protective effect and enhance stress resistance in C. elegans (Rea et al. 2005; Walker and Lithgow 2003; Yokoyama et al. 2002). We next measured changes in the mRNA levels of several representative HSPs, including hsp-4, hsp-12.6, hsp-16.1, hsp-16.49, and hsp-70. The mRNA level of hsp-12.6 was upregulated with CBE treatment relative to the non-supplemented controls (Fig. 2e). However, hsp-4, hsp-16.1, and hsp-16.49 were downregulated by CBE treatment, while hsp-70 did not show a significant change (Fig. 2e). These findings suggest that other mechanisms in addition to the heat shock response underlie the CBE-induced thermotolerance.
A common approach to assess the health status in C. elegans is to measure its locomotion behavior, including body bending (de Bono and Maricq 2005). Old worms generally develop motor deficits and are less active than young ones (Dillin et al. 2002; Glenn et al. 2004; Hosono et al. 1980; Huang et al. 2004; Johnson et al. 1988). Here, we use body bending frequency to examine C. elegans’ acute and chronic response to osmotic stress. To this end, young adult N2 worms were pretreated with or without 2 mg/mL CBE for two generations, and were then transferred to hyperosmolarity plates containing high concentrations of NaCl. For the acute response assay, we exposed worms to either 300 or 500 mM of NaCl for 10 min and then transferred the worms back to regular NGM plates to measure the frequency of body bending as a parameter to reflect the motility of the nematode. The motility of CBE-treated worms was dramatically reduced compared to non-treated controls (Fig. 2f). For the chronic response assay, worms were kept on the NGM plates containing 500 mM of NaCl, and dead worms were counted until all worms died. Surprisingly, the mortality rate of CBE-treated worms was higher relative to non-treated controls (Fig. 2g). Taken together, we conclude that CBE treatment increases worms’ susceptibility to osmotic stress.
Influence of CBE treatment on reproductive capacity and motility
Considering that CBE promotes lifespan and thermotolerance in C. elegans, we investigated whether CBE alters the worms’ other general physiological indexes. In this regard, we tested brood size as a reproductive parameter and motility of worms to reflect their general fitness after CBE treatment. CBE treatment did not affect total offspring quantity compared to non-supplemented controls (Table 2). For the motility assay, young adult worms pretreated with or without CBE for two generations were individually transferred to fresh NGM plates, and the body bends per minute were counted (Hart 2006). CBE did not affect the worms’ body bend frequency, namely motility, compared to non-supplemented controls (Table 2). These findings suggest that CBE-induced lifespan extension is not associated with any significant change in reproductive capacity and motility.
Table 2.
CBE supplementation does not influence the motility and reproductive capacity of C. elegans
| Temperature (°C) | Control ± SE | CBE treated ± SE | P value | Total number of worms tested | |
|---|---|---|---|---|---|
| Brood size | 16 | 213 ± 14.5 | 208 ± 15.0 | 0.64 | 9a, 9b |
| 20 | 339 ± 15.5 | 394 ± 17.5 | 0.35 | 9a, 9b | |
| 25 | 296 ± 15.0 | 254 ± 15.0 | 0.73 | 9a, 9b | |
| Motility | 25 | 8.16 ± 2.56 | 6.46 ± 2.70 | 0.45 | 30a, 30b |
These data represent the average of three independent trials for each test
aControl worms
bAssay worms
CBE supplementation modulates lifespan through the IIS pathway and DAF-16
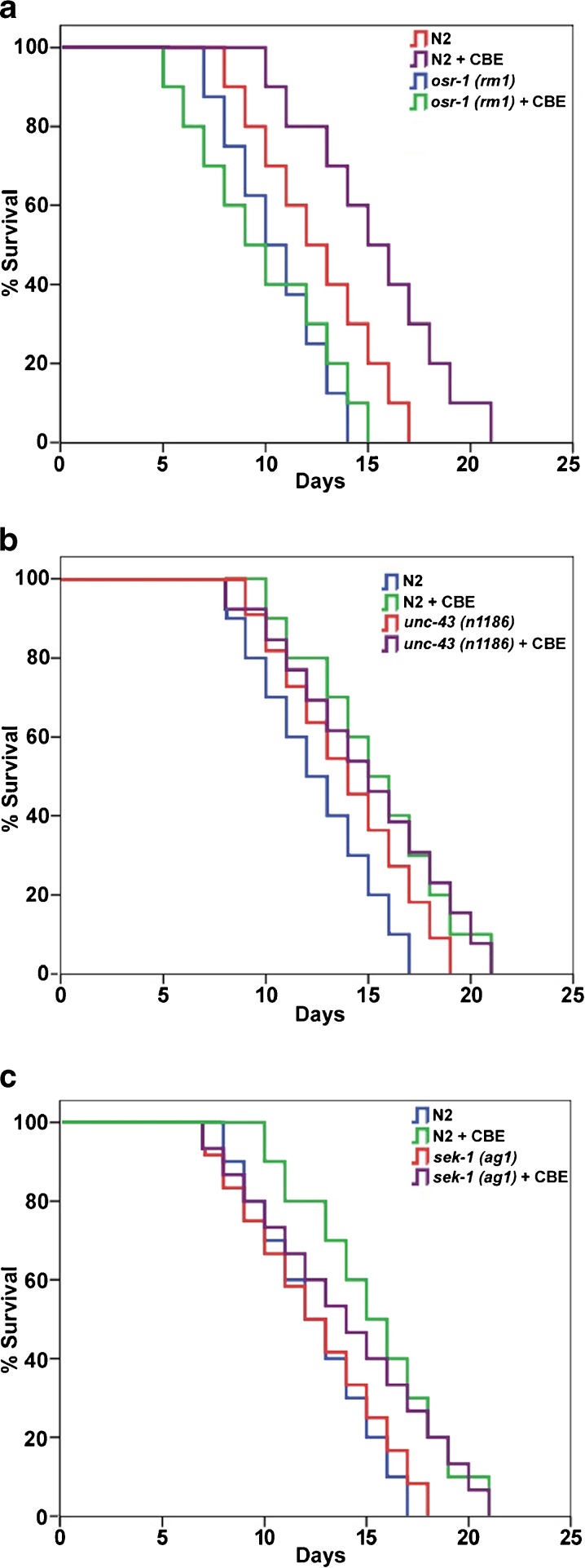
To understand the molecular mechanisms by which CBE promotes lifespan, we investigated which longevity determinants CBE genetically interacts with. We first conducted a genetic epistasis assay to test the relationship between CBE and the IIS pathway/DAF-16 (Henderson and Johnson 2001; Kenyon et al. 1993; Lee et al. 2001; Lin et al. 2001). We supplied null daf-16(mgDf50) mutant worms with 2 mg/mL CBE and found that CBE did not increase the lifespan of the daf-16(mgDf50) mutant (Fig. 3a; Table 1). This finding suggests that the longevity effect of CBE requires the activity of DAF-16. Next, we tested DAF-2 and AGE-1, two major components of the IIS pathway. Mutant worms, daf-2(e1370) and age-1(hx546), were treated with 2 mg/mL CBE, respectively. CBE at 2 mg/mL did not extend the lifespan of either daf-2(e1370) (Fig. 3b; Table 1) or age-1(hx546) (Fig. 3c; Table 1), when compared to the genotype matched non-supplemented controls. These findings suggest that CBE acts at least partly through the IIS pathway and requires DAF-16 to increase lifespan in C. elegans.
Fig. 3.
CBE modulates the lifespan of C. elegans through the IIS pathway and DAF-16. aDaf-16(mgDf50) mutant worms were treated with 2 mg/mL CBE. bDaf-2(e1370) mutant worms were treated with 2 mg/mL CBE. cAge-1(hx546) mutant worms were treated with 2 mg/mL CBE. Each lifespan experiment was repeated at least three independent times with similar results. Quantitative data and statistical analyses for the representative experiments are included in Table 1. d Transgenic worms over-expressing DAF-16::GFP (daf-16(mgDf47);xrIs87) were treated with 2 mg/mL CBE at 25 °C for 1 h. DAF-16::GFP exhibited nuclear accumulation in treated worms. Photos show the DAF-16::GFP expression pattern and DIC images of young adults. e The transcript levels of daf-16 in N2 worms treated with and without 2 mg/mL CBE were quantified using qRT-PCR. The data from three independent experiments were pooled to calculate the mean RNA level normalized to the internal control act-1. The standard errors of the mean (SEM) are shown. The normalized mean RNA level of daf-16 in non-treated N2 worms was set as 1. **p < 0.05 when compared to non-treated control. f The transcript levels of sod-3 in N2 worms treated with and without 2 mg/mL CBE were quantified using qRT-PCR. The data from three independent experiments were pooled to calculate the mean RNA level normalized to the internal control act-1. The standard errors of the mean (SEM) are shown. The normalized mean RNA level of sod-3 in non-treated N2 worms was set as 1. **p < 0.05 when compared to non-treated control
Considering that the IIS pathway regulates lifespan in C. elegans by limiting DAF-16 nuclear localization (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001), we postulated that CBE treatment may affect DAF-16 nuclear localization and transcriptional activities. To this end, we examined subcellular localization of DAF-16 using transgenic worms with a DAF-16-GFP fusion transgene (Honda and Honda 1999; Lin et al. 2001; Murphy et al. 2003). Consistent with our prediction, we observed a significant increase of DAF-16-GFP in the nucleus after a 1-h treatment of 2 mg/mL of CBE (Fig. 3d). We then measured changes of the mRNA levels of daf-16 and sod-3. Sod-3 is one of the DAF-16 direct target genes and encodes a Fe/Mn superoxide dismutase (Honda and Honda 1999; Murphy et al. 2003). The mRNA level of daf-16 was increased by three~four fold, and the expression of sod-3 was robustly elevated by eight~tenfold in CBE-treated worms compared to non-supplemented controls (Fig. 3e and f). These findings suggest that CBE treatment promotes longevity partly through increasing DAF-16 nuclear localization and transcriptional activities.
CBE-mediated lifespan extension does not require SIR-2.1 and JNK signaling
SIR-2.1, functioning as a protein deacetylase, has been implicated in lifespan modulation (Berdichevsky et al. 2006; Burnett et al. 2011; Rogina and Helfand 2004; Tissenbaum and Guarente 2001; Viswanathan et al. 2005). We examined whether SIR-2.1 is involved in lifespan extension induced by CBE. To this end, we measured the lifespan of sir-2.1(ok434) null mutant worms treated with 2 mg/mL CBE. CBE treatment significantly extended the lifespan of sir-2.1(ok434) worms (Fig. 4a; Table 1), suggesting that SIR-2.1 is dispensable for CBE to extend lifespan.
Fig. 4.
CBE-mediated lifespan extension does not require SIR-2.1 and JNK signaling. a The sir-2.1(ok434) mutant worms were treated with 2 mg/mL CBE. b The jnk-1(gk7) mutant worms were treated with 2 mg/mL CBE. Each lifespan experiment shown here was repeated at least three independent times with similar results. Quantitative data and statistical analyses for the representative experiments are included in Table 1
The JNK signaling has been shown to modulate lifespan, and phosphorylation of JNK-1 is required for JNK signaling to extend lifespan in C. elegans (Oh et al. 2005). We investigated whether lifespan extension by CBE treatment depends on JNK signaling. To test this hypothesis, we supplied a loss-of-function mutant jnk-1(gk7) with 2 mg/mL CBE. Consistent with previous work (Oh et al. 2005), jnk-1(gk7) worms showed a decreased lifespan compared to control N2 worms (Fig. 4b; Table 1). However, CBE treatment was still able to increase the lifespan of jnk-1(gk7) mutant worms, suggesting that CBE promotes longevity independent of JNK signaling.
CBE requires OSR-1 to promote longevity in an UNC-43 dependent manner
It has been reported that blueberry extracts promote longevity through the OSR-1/UNC-43/SEK-1 osmotic stress response pathway in C. elegans (Wilson et al. 2006). We tested whether CBE treatment may target the same pathway. To test this possibility, we supplied osr-1(rm1) mutant worms with 2 mg/mL CBE and found that CBE treatment was not capable of increasing the lifespan of osr-1(rm1) mutant worms (Fig. 5a; Table 1). Since osr-1(rm1) worms are short-lived (Pietsch et al. 2009; Saul et al. 2010), it is possible that osr-1(rm1) mutant worms are sick in general, which may mask any apparent longevity benefit of CBE supplementation. To address this issue, we assessed several general health parameters in osr-1(rm1) worms, including body size, motility, and touch response. osr-1(rm1) worms did not significantly alter any of these general health parameters when compared to the wild-type N2 worms (data not shown). These findings suggest that CBE requires OSR-1 to extend the lifespan in C. elegans.
Fig. 5.
CBE requires OSR-1 to extend lifespan and is partially dependent on CaMKII and p38 MAP kinase pathway. a The osr-1(rm1) mutant worms were treated with 2 mg/mL CBE. b The unc-43(n1186) mutant worms were treated with 2 mg/mL CBE. c The sek-1(ag1) mutant worms were treated with 2 mg/mL CBE. Each lifespan experiment represented here was repeated at least three independent times with similar results. Quantitative data and statistical analyses for the representative experiments are included in Table 1
We further tested whether CBE modulates lifespan in C. elegans through the CaMKII/p38 MAPK pathway (Solomon et al. 2004). OSR-1 negatively regulates the activity of CaMKII/p38 MAPK pathway. Elevated OSR-1 decreases the activities of UNC-43 and SEK-1, two major components of the CaMKII/p38 MAPK pathway. We measured the lifespan of unc-43(n1186) or sek-1(ag1) deletion mutant worms treated with CBE. Only unc-43(n1186) showed an extension in mean lifespan from 12.5 to 14 days when compared to N2 worms, while the lifespan of sek-1(ag1) mutants is similar to the N2 worms (Fig. 5b and c; Table 1). We next performed a genetic epistasis assay to further determine the role of the CaMKII/p38 MAPK pathway in CBE-induced lifespan extension. unc-43(n1186) and sek-1(ag1) mutant worms were fed 2 mg/mL CBE. CBE treatment increased the mean lifespan from 12.5 to 14 days in sek-1(ag1) worms but did not increase the mean lifespan (14 vs.14.97 days) in unc-43(n1186) worms (Fig. 5c and b; Table 1). These findings suggest that CBE modulates lifespan at least partly through OSR-1 and one of its downstream effectors, UNC-43, but not the p38 MAPK signaling.
Discussion
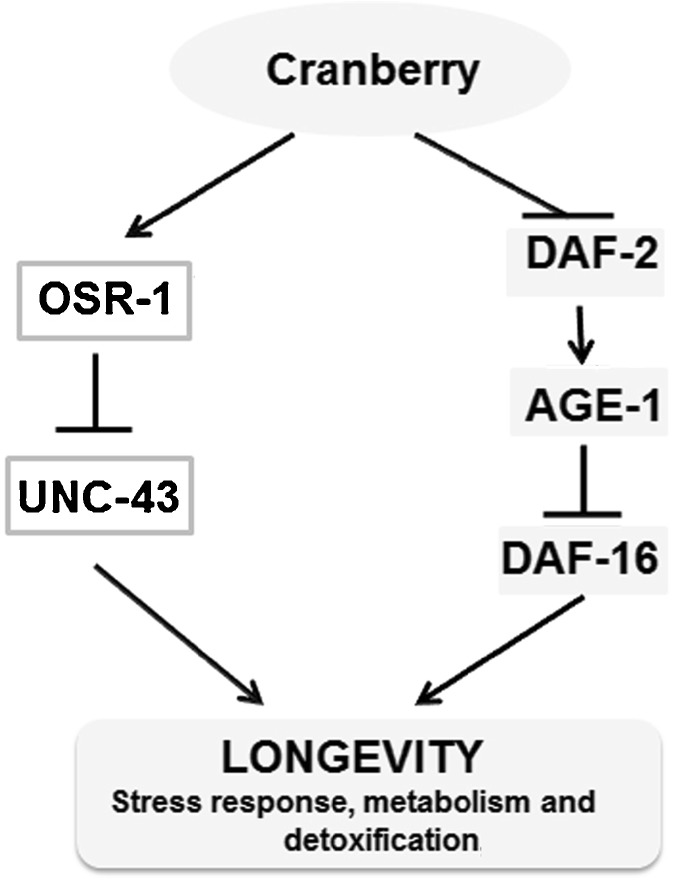
Numerous studies in humans and model organisms have shown that nutraceuticals made from various botanicals can provide a variety of health benefits, including prevention of cancer, reduction of inflammation, and delay of age-related functional decline (Lin et al. 2003; Liu 2004; Shukitt-Hale et al. 2007; Zhu et al. 2011). Our study suggests that the North American cranberry (V. macrocarpon) is a potentially effective nutraceutical for promoting healthy aging in C. elegans. CBE consumption substantially extends lifespan and elevates resistance to heat shock stress in wild-type worms. CBE supplementation requires the activity of DAF-16 through the IIS pathway to modulate lifespan and stress response. Noticeably, supplementation of CBE increases DAF-16 nuclear localization and its transcriptional activities. In addition, CBE consumption also requires OSR-1 through UNC43, but not SEK-1, to modulate lifespan extension and stress response. In contrast, SIR-2.1 and JNK signaling are not required for CBE-induced lifespan extension. Based on these findings, we propose that CBE supplementation modulates DAF-16 and OSR-1 activities to promote prolongevity and increase stress response in C. elegans through the IIS pathway and UNC-43, respectively (Fig. 6).
Fig. 6.
A putative model for cranberry’s mode of action in C. elegans. Cranberry modulates multiple longevity-related pathways to promote healthy aging and stress resistance. On the one hand, cranberry activates OSR-1 and consequently suppresses UNC-43 to promote longevity. On the other hand, cranberry suppresses DAF-2 and AGE-1 and activates DAF-16 to promote longevity
Our experiments demonstrate that CBE supplementation increases C. elegans lifespan in a dose-dependent manner, and 10 mg/mL CBE shortened C. elegans lifespan regardless of the acidic pH. It is known that cranberry contains abundant quercetin and many other unique phytochemicals (Pappas and Schaich 2009). Previous reports had shown that phytochemicals, such as quercetin, tannic acid, and rosmarinic acid, can promote longevity at relative low concentrations, but are detrimental at high concentrations in C. elegans (Pietsch et al. 2011; Saul et al. 2010). Thus, it is very likely that some phytochemicals in CBE are beneficial only at lower concentrations.
Cranberry and its constituent products have been widely consumed by humans due to their numerous health benefits (Howell 2007; Neto 2007a). Our findings ascribe the benefit of cranberry consumption to prolongevity function and show the correlation between CBE consumption and activation of DAF-16/FOXO. A critical regulation of DAF-16/FOXO activity is its phosphorylation and nuclear exclusion by the IIS pathway. Mammals have several FOXOs, the counterpart of DAF-16, which are key regulators of diabetes and obesity due to their ability to regulate energy metabolism (Michael et al. 2000; Samuel et al. 2006). In insulin-responsive tissue, FOXO1 regulates the expression of genes involved in gluconeogenesis, energy metabolism, and oxidative stress, thereby affecting the whole body energy homeostasis. When nutrient and insulin levels are low, FOXO1 promotes expression of gluconeogenic enzymes to maintain glucose level. Conversely, in the fed state, pancreatic β cells secrete insulin to inactivate FOXO1 and partially suppress gluconeogenic enzyme expression in the liver (Puigserver et al. 2003). In addition, FOXO1 also plays a vital role in the formation of skeletal muscle and adipose tissues, two major organs for the maintenance of energy homeostasis (Ahima and Flier 2000; de Lange et al. 2007). Moreover, negative signaling to FOXO1 under particular pathophysiologic conditions, such as metabolic dysfunction and insulin resistance, leads to deleterious effects, including hyperglycemia and glucose intolerance. Our study shows that DAF-16 is required for CBE-induced lifespan extension. Therefore, it is likely that CBE modulates metabolic homeostasis to promote longevity and stress resistance. This raises an intriguing possibility that CBE supplementation can be implemented as a prevention method to fight against metabolic dysfunction diseases, such as diabetes and obesity.
OSR-1 is a master regulator to prolong C. elegans survival in hyperosmotic environments (Solomon et al. 2004). OSR-1 couples with SEK-1/MAPKK through UNC-43/CAMKII to promote resistance to chronic osmotic stress. Wilson et al. found that blueberry polyphenols induced longevity requires the OSR-1/UNC-43/SEK-1 pathway in C. elegans (Wilson et al. 2006). Our genetic study indicates that OSR-1 is essential for CBE-induced lifespan extension in C. elegans, but only UNC-43, not SEK-1, is involved in this prolongevity process. This is not surprising considering that individual components in the OSR-1/UNC-43/SEK-1 pathway have been reported to differentially mediate the prolongevity effects of different phytochemicals. For instance, SEK-1, but neither OSR-1 nor UNC-43, is required for tannic acid-induced longevity (Saul et al. 2010), while SEK-1 and UNC-43, but not OSR-1, are required for quercetin-induced longevity in C. elegans (Pietsch et al. 2009). In addition, SEK-1 can regulate pathogen resistance independent of UNC-43 and OSR-1 (Kim et al. 2002). Future experiments are needed to investigate how phytochemicals in CBE differentially regulate downstream effectors of OSR-1 to modulate lifespan and stress resistance.
Our findings indicate that CBE supplementation increases C. elegans resistance to heat shock. However, CBE treatment does not improve worms’ resistance to oxidative stress or UV irradiation and even renders worms more sensitive to osmotic stress when compared to the non-supplemented controls. These results suggest that CBE treatment selectively enhances worms’ resistance to environmental stresses. A number of botanical extracts containing high levels of polyphenols have been shown to differentially promote health aging and stress resistance in diverse species. For instance, supplementation with blueberry polyphenols increases thermotolerance in C. elegans (Wilson et al. 2006). Blueberry extract promotes longevity in C. elegans and Drosophila (Peng et al. 2012; Wilson et al. 2006). Cocoa contains abundant flavonoids and promotes longevity and resistance to oxidative stress in worms (Martorell et al. 2011). Nectarine and açai extracts promote the survival in Drosophila under oxidative stress and fed high-fat diets (Boyd et al. 2011; Sun et al. 2010). Ginkgo biloba extract is widely used to improve health of the elderly due to its capacity to promote resistance to oxidative stress with its considerable volume of flavonoids (Abdel-Wahab and Abd El-Aziz 2012; DeFeudis and Drieu 2000; Wu et al. 2011). Similarly, CBE contains a high number of polyphenols, such as the proanthocyanidins, the flavonols, and the anthocyanins (Pappas and Schaich 2009). Thus, the ability of CBE to increase resistance to heat stress could be due to the similar polyphenols existing in blueberry or unique phytochemicals in cranberry. In addition, a number of experiments have shown that different phytochemicals may interact and synergize to exert their biological functions (Apostolidis et al. 2006; Liu 2004; Seeram et al. 2004). Considering that different botanical extracts have unique phytochemical profiles, CBE-induced thermotolerance may also be due to the synergistic effects generated by the interaction of unique cranberry phytochemicals. Moreover, lifespan extension induced by CBE requires DAF-16/FOXO, which is known to regulate different target genes in response to different stress stimuli (Honda and Honda 1999; Kenyon 2005; van der Horst and Burgering 2007). Therefore, it is possible that CBE fine tunes the regulation of a subset of DAF-16 target genes, and therefore improves the survival under restrictive temperature.
We have found that CBE treatment induces susceptibility to chronic osmotic stress in C. elegans. This susceptible phenomenon may be due to the depression of CaMII/p38 MAPK signaling by CBE, based on our observation that osr-1 is upregulated by CBE treatment (data not shown). This is consistent with recent studies indicating that flavonoids elevate osr-1 expression level in C. elegans (Xue et al. 2011). Further systematic studies are warranted to determine the mechanisms underlying differential effects of CBE supplementation on stress resistance in worms.
Our study highlights the prolongevity and stress resistance properties of CBE and reveals the molecular mechanisms by which CBE modulates lifespan and stress response in C. elegans. Although health benefits of CBE have been studied for many years, our study is the first to thoroughly investigate the properties of CBE on longevity and stress response, and the first to systematically analyze the genetic requirements for CBE-mediated effects. Since both IIS/DAF-16 and OSR-1 are highly conserved from C. elegans to mammals, our findings have important implications in utilizing CBE to promote healthy aging and combat age-related diseases in humans.
Acknowledgments
Caenorhabditis elegans strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). We are grateful to members of the Dong laboratory for the helpful discussions. We thank H. Knap for the assistance with qPCR, Y. Wei and A. Tietje for the help with the fluoremeter, T. Bruce for the assistance with microscopy. We especially thank S. Smith and M. Bonaccorsi for the help with worm maintenance and reagents preparation, and R. Ghaedian for providing cranberry extract. We thank E. Spangler and A. Brown for editing this paper. This work was supported by the Creative Inquiry fund at Clemson University to Y.D. and M.C and the Intramural Research Program at the National Institute on Aging, NIH to S.Z.
Contributor Information
Sige Zou, Phone: +1-410-5588461, FAX: +1-410-5588302, Email: zous@grc.nia.nih.gov.
Yuqing Dong, Phone: +1-864-6563835, FAX: +1-864-6560435, Email: ydong@clemson.edu.
References
- Abdel-Wahab BA, Abd El-Aziz SM. Ginkgo biloba protects against intermittent hypoxia-induced memory deficits and hippocampal DNA damage in rats. Phytomed Int J Phytother Phytopharmacol. 2012;19:444–450. doi: 10.1016/j.phymed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab: TEM. 2000;11:327–332. doi: 10.1016/S1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Ai J, Duan J, Lv X, Chen M, Yang Q, Sun H, Li Q, Xiao Y, Wang Y, Zhang Z, Tan R, Liu Y, Zhao D, Chen T, Yang Y, Wei Y, Zhou Q. Overexpression of FoxO1 causes proliferation of cultured pancreatic beta cells exposed to low nutrition. Biochemistry. 2010;49:218–225. doi: 10.1021/bi901414g. [DOI] [PubMed] [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr. 2006;15:433–441. [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J Dent Res. 2006;85:235–239. doi: 10.1177/154405910608500306. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Cranberry components inhibit interleukin-6, interleukin-8, and prostaglandin E production by lipopolysaccharide-activated gingival fibroblasts. Eur J Oral Sci. 2007;115:64–70. doi: 10.1111/j.1600-0722.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. J Periodontal Res. 2007;42:159–168. doi: 10.1111/j.1600-0765.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- Boyd O, Weng P, Sun X, Alberico T, Laslo M, Obenland DM, Kern B, Zou S. Nectarine promotes longevity in Drosophila melanogaster. Free Radic Biol Med. 2011;50:1669–1678. doi: 10.1016/j.freeradbiomed.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42:279–284. doi: 10.1080/10408390209351916. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nature reviews. Neuroscience. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- de Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A. Fuel economy in food-deprived skeletal muscle: signaling pathways and regulatory mechanisms. FASEB J. 2007;21:3431–3441. doi: 10.1096/fj.07-8527rev. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Yamada K, Isobe A, Shiwaku K, Yamane Y. Mechanism of cytotoxicity of paraquat. I. NADH oxidation and paraquat radical formation via complex I. Exp Toxicol Pathol Off J Ges Toxikologische Pathol. 1993;45:345–349. doi: 10.1016/S0940-2993(11)80424-0. [DOI] [PubMed] [Google Scholar]
- Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A Biol Sci Med Sci. 2004;59:1251–1260. doi: 10.1093/gerona/59.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Hart AC (2006) Behavior, wormbook, the C. elegans research community, wormbook, doi/10.1895/wormbook.1.87.1, http://www.wormbook.org
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/S0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6:577–588. doi: 10.1016/S1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hosono R, Sato Y, Aizawa SI, Mitsui Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol. 1980;15:285–289. doi: 10.1016/0531-5565(80)90032-7. [DOI] [PubMed] [Google Scholar]
- Howell AB. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51:732–737. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Conley WL, Keller ML. Long-lived lines of Caenorhabditis elegans can be used to establish predictive biomarkers of aging. Exp Gerontol. 1988;23:281–295. doi: 10.1016/0531-5565(88)90031-9. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, Ausubel FM. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/S0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lee TH, Mun JY, Han SM, Yoon G, Han SS, Koo HS. DIC-1 over-expression enhances respiratory activity in Caenorhabditis elegans by promoting mitochondrial cristae formation. Genes Cells Devoted Mol Cell Mech. 2009;14:319–327. doi: 10.1111/j.1365-2443.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Gen. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM. PGC-1beta in the regulation of hepatic glucose and energy metabolism. J Biol Chem. 2003;278:30843–30848. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49:B270–B276. doi: 10.1093/geronj/49.6.B270. [DOI] [PubMed] [Google Scholar]
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Martorell P, Forment JV, de Llanos R, Monton F, Llopis S, Gonzalez N, Genoves S, Cienfuegos E, Monzo H, Ramon D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J Agric Food Chem. 2011;59:2077–2085. doi: 10.1021/jf104217g. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- Neto CC, Amoroso JW, Liberty AM. Anticancer activities of cranberry phytochemicals: an update. Mol Nutr Food Res. 2008;52(Suppl 1):S18–S27. doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- Peng C, Zuo Y, Kwan KM, Liang Y, Ma KY, Chan HY, Huang Y, Yu H, Chen ZY. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp Gerontol. 2012;47:170–178. doi: 10.1016/j.exger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Pietsch K, Saul N, Menzel R, Sturzenbaum SR, Steinberg CE. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology. 2009;10:565–578. doi: 10.1007/s10522-008-9199-6. [DOI] [PubMed] [Google Scholar]
- Pietsch K, Saul N, Chakrabarti S, Sturzenbaum SR, Menzel R, Steinberg CE. Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology. 2011;12:329–347. doi: 10.1007/s10522-011-9334-7. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Gen. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55:2042–2050. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- Saul N, Pietsch K, Menzel R, Sturzenbaum SR, Steinberg CE. The longevity effect of tannic acid in Caenorhabditis elegans: disposable soma meets hormesis. J Gerontol A Biol Sci Med Sci. 2010;65:626–635. doi: 10.1093/gerona/glq051. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol. 1984;131:1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- Solomon A, Bandhakavi S, Jabbar S, Shah R, Beitel GJ, Morimoto RI. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics. 2004;167:161–170. doi: 10.1534/genetics.167.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D, Feldman M, Ofek I, Weiss EI. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother. 2004;54:86–89. doi: 10.1093/jac/dkh254. [DOI] [PubMed] [Google Scholar]
- Strayer A, Wu Z, Christen Y, Link CD, Luo Y. Expression of the small heat-shock protein Hsp16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB J. 2003;17:2305–2307. doi: 10.1096/fj.03-0376fje. [DOI] [PubMed] [Google Scholar]
- Sun X, Seeberger J, Alberico T, Wang C, Wheeler CT, Schauss AG, Zou S. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988;48:5754–5758. [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Walker EB, Barney DP, Mickelsen JN, Walton RJ, Mickelsen RA., Jr Cranberry concentrate: UTI prophylaxis. J Fam Pract. 1997;45:167–168. [PubMed] [Google Scholar]
- Wilson T, Porcari JP, Harbin D. Cranberry extract inhibits low density lipoprotein oxidation. Life Sci. 1998;62:PL381–PL386. doi: 10.1016/S0024-3205(98)00204-5. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TY, Chen CP, Jinn TR. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50:131–135. doi: 10.1016/j.tjog.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Xue YL, Ahiko T, Miyakawa T, Amino H, Hu F, Furihata K, Kita K, Shirasawa T, Sawano Y, Tanokura M. Isolation and Caenorhabditis elegans lifespan assay of flavonoids from onion. J Agric Food Chem. 2011;59:5927–5934. doi: 10.1021/jf104798n. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fukushima T. Mechanism of cytotoxicity of paraquat. II. Organ specificity of paraquat-stimulated lipid peroxidation in the inner membrane of mitochondria. Experimental and toxicologic pathology. Off J Ges Toxikologische Pathol. 1993;45:375–380. doi: 10.1016/S0940-2993(11)80433-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS letters. 2002;516:53–57. doi: 10.1016/S0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- Zhu M, Hu J, Perez E, Phillips D, Kim W, Ghaedian R, Napora JK, Zou S. Effects of long-term cranberry supplementation on endocrine pancreas in aging rats. J Gerontol A Biol Sci Med Sci. 2011;66:1139–1151. doi: 10.1093/gerona/glr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E, Hu D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res Rev. 2010;10:201–204. doi: 10.1016/j.arr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Zou S, Sinclair J, Wilson MA, Carey JR, Liedo P, Oropeza A, Kalra A, de Cabo R, Ingram DK, Longo DL, Wolkow CA. Comparative approaches to facilitate the discovery of prolongevity interventions: effects of tocopherols on lifespan of three invertebrate species. Mech Ageing Dev. 2007;128:222–226. doi: 10.1016/j.mad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol A Biol Sci Med Sci. 2010;65:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]