Abstract
Coenzyme Q10 (CoQ) is widely available as a dietary supplement and remains under consideration as a treatment for age-associated neurodegenerative conditions. However, no studies have determined if supplementation, initiated relatively late in life, could have beneficial effects on mild functional impairments associated with normal brain aging. Accordingly, the current study assessed the effect of CoQ intake in older mice for which cognitive and psychomotor impairments were already evident. Separate groups of young (3.5 months) and relatively old mice (17.5 months) were fed a control diet or a diet supplemented with low (0.72 mg/g) or high (2.81 mg/g) concentrations of CoQ for 15 weeks. After 6 weeks, the mice were given tests for spatial learning (Morris water maze), spontaneous locomotor activity, motor coordination, and startle reflex. Age-related impairments in cognitive and psychomotor functions were evident in the 17.5-month-old mice fed the control diet, and the low-CoQ diet failed to affect any aspect of the impaired performance. However, in the Morris water maze test, old mice on the high-CoQ diet swam to the safe platform with greater efficiency than the mice on the control diet. The old mice supplemented with the high-CoQ diet did not show improvement when spatial performance was measured using probe trials and failed to show improvement in other tests of behavioral performance. Protein oxidative damage was decreased in the mitochondria from the heart, liver, and skeletal muscle of the high-CoQ-supplemented mice and, to some extent, in the brain mitochondria. Contrasting with the deleterious effect of long-term CoQ supplementation initiated during young adulthood previously published, this study suggests that CoQ improves spatial learning and attenuates oxidative damage when administered in relatively high doses and delayed until early senescence, after age-related declines have occurred. Thus, in individuals with age-associated symptoms of cognitive decline, high-CoQ intake may be beneficial.
Keywords: Aging, Coenzyme Q10, Ubiquinone, Ubidecarenone, C57BL/6J, Oxidative damage, Mitochondria
Introduction
Coenzyme Q (CoQ) or ubiquinone (2,3-dimethoxy-5-methyl-6-polyprenyl-1,4-benzoquinone) is a lipophilic molecule found in the phospholipid bilayer of cellular membranes, that is, present in especially high concentration in the mitochondrial inner membrane (Battino et al. 1990; Lenaz et al. 1999). The tyrosine-derived quinone ring present in CoQ can exist in three different redox states: a fully oxidized form, ubiquinone (Q), a univalently reduced free radical form, ubisemiquinone (QH•), and a fully reduced form ubiquinol (QH2) (Nohl et al. 2003). CoQ plays a major role in the electron transport chain by contributing to energy production via carrying electrons from complexes I and II to complex III (Ernster and Dallner 1995; Fontaine et al. 1998; Crane 2001; Turunen et al. 2004). Because of this continuous cycle of oxidation–reduction, CoQ has the ability to act as an oxidant (autoxidation of QH• leads to the release of superoxide anion) and as an antioxidant that can directly scavenge lipid peroxyl radicals and can recycle α-tocopheroxyl radical back to α-tocopherol, a process that also results in regeneration of ascorbate (Turrens et al. 1985; Ernster and Dallner 1995; Kagan et al. 1998; Lass and Sohal 1998). Due to the inherent dual oxidative properties of CoQ, it remains uncertain whether supplementation with CoQ would reverse age-related impairments in functional capacity or whether it would exacerbate oxidative stress, thereby aiding functional losses associated with aging and/or pathological states.
Based partially on evidence that normal aging does not involve extensive neuronal degeneration, it has been suggested that many processes of brain aging are potentially reversible (Morrison and Hof 1997). In particular, the consequences of age-related increases in oxidative stress, which have been linked to cognitive and psychomotor impairments of aging rodents (Forster et al. 1996; Nicolle et al. 2001; Serrano and Klann 2004), may be amenable to late life intervention with antioxidants. This possibility is suggested by the ability of caloric restriction to attenuate oxidative damage and concurrently improve some brain functions relatively rapidly following implementation in rodents of advanced age (Forster and Lal 1999; Forster et al. 2000). Similarly, antioxidant-rich foods appear to improve age-impaired cognitive and psychomotor performance in rodents of advanced age (Bickford et al. 2000; Shukitt-Hale et al. 2006).
Several human studies have suggested that CoQ supplementation ameliorated mitochondria-related abnormalities (Bresolin et al. 1990; Morisco et al. 1993; Hofman-Bang et al. 1995) and age-related symptoms of heart failure and hypertension as well as neurodegenerative diseases including amyotrophic lateral sclerosis, Huntington’s disease, and diabetic neuropathy (Baggio et al. 1993; Beal et al. 1994; Koroshetz et al. 1997; Ferrante et al. 2002; Rosenfeldt et al. 2003, 2007; Hyson et al. 2010). Furthermore, several investigations suggest that supplementation of CoQ could have effects similar to caloric restriction or antioxidant-rich foods. When CoQ was supplemented in the food of mice for a short period (ranging from 8 to 13 weeks), there was an increase in both CoQ and α-tocopherol in the mitochondria from cerebral cortex (200 mg CoQ/kg/day; Matthews et al. 1998) and from peripheral tissues such as skeletal muscle, liver, and kidney (123 mg CoQ/kg/day; Lass et al. 1999a; 148 and 654 mg CoQ/kg/day; Kamzalov et al. 2003). Long-term supplementation with 93- or 371 mg/kg/day CoQ starting at 4 months of age have also been shown to increase CoQ concentrations in tissues (Sohal et al. 2006). In studies performed in vitro, the increase in α-tocopherol produced by CoQ was inversely proportional to the generation of superoxide (O2•−) by the mitochondria (Lass et al. 1999b). Endogenous ubiquinol in bovine submitochondrial particles inhibited both lipid peroxidation and protein oxidation (Forsmark-Andree et al. 1995) and, similarly, increasing the pool of ubiquinol in the mitochondria in the presence of succinate and antimycin resulted in a decrease of 8-hydroxy-deoxyguanosine and DNA strand breaks, both measures employed to assess DNA damage (Mukai et al. 1990; Quiles et al. 2004).
While the importance of CoQ in mitochondrial function and its status as an antioxidant have led to therapeutic applications and clinical trials in neurodegenerative diseases, the potential for CoQ to ameliorate or reverse mild cognitive and psychomotor impairments associated with normal aging has not been fully evaluated. CoQ intake (123 or 500 mg/kg/day) for 14 weeks did not yield an improvement in performance of aged mice tested under a discriminated avoidance paradigm (McDonald et al. 2005). Furthermore, CoQ supplementation (105 or 368 mg/kg/day) from 4 to 25 months of age failed to retard or attenuate age-related decline in motor and cognitive function, and the 368-mg/kg/day dose even impaired the ability of the mice to navigate to a safe platform in the Morris water maze and worsened the age-related losses in startle response to auditory and shock stimuli (Sumien et al. 2009). Under the conditions of this previous long-term study, CoQ neither facilitated nor attenuated biological indices of redox stress and oxidative damage (Sohal et al. 2006).
The current study addressed the potential risk or benefit associated with CoQ supplementation initiated during relatively advanced age as opposed to lifelong intake. Specifically, these studies addressed the important question of whether the previously reported deleterious effects of CoQ on cognition are due to the presence of CoQ during advanced age or instead reflect a long-term negative consequence of high-CoQ intake. Accordingly, the current study assessed the effect of CoQ intake initiated when mice were 17.5 months of age (when age-related impairments were already present), over a period of 16 weeks, during which the mice were given tests for spatial maze learning, discriminated avoidance, spontaneous locomotor activity, motor coordination, and startle reflex. In these studies, CoQ was added to the food in either high or low concentrations, so as to target both high doses used in clinical trials for neurodegenerative disease (trial NCT00117403) and relatively lower doses comparable to supplemental intake by healthy individuals. Protein oxidation was then assessed by measuring the concentration of carbonyls in the mitochondria from the heart, brain, liver, and skeletal muscle under the different diet conditions.
Materials and methods
Materials
Coenzyme Q10 (CoQ), obtained from Tishcon Corp. (Westbury, NY), was added to a base rodent diet, Purina diet 5001 (cat. nos. 10038 and 10039, respectively, Purina Mills Test Diet, Richmond, IN). The diet was formulated such that it yielded a low concentration of CoQ (0.72 mg/g) or a high concentration of CoQ (2.81 mg/g).
Animals
Separate groups of male C57BL/6J mice were obtained from the National Institute on Aging at 3.5 months (n = 24) and 17.5 months of age (n = 31) and subsequently maintained in the UNT Health Science Center vivarium. The mice were maintained individually in clear polycarbonate cages (28 × 17 × 12.5 cm, modified into two-mouse units with a stainless-steel divider) and had ad libitum access to food and water until they were assigned to a dietary condition. The ambient temperature was maintained at 23 ± 1 °C, under a 12-h light/dark cycle starting at 0600 hours. The animals were allowed to acclimate for a 2-week period, following which they were randomly assigned to one of the three treatment groups. The mice were fed ad libitum either a control diet (base diet) or the diet supplemented with a low (0.72 mg/g) or high (2.81 mg/g) concentration of CoQ10. The mice were weighed after 2 weeks on their respective diets and at the end of the study, and food intake was measured in a subset of old mice after 2 weeks on the diet. The daily dose of CoQ10 received was ~96 mg/kg/day for the low dose or ~457 mg/kg/day for the high dose, and this was based on the calculations of body weight and the average food intake of a mouse in 1 week.
Neurobehavioral measures
The mice were maintained on the control and CoQ-supplemented diets for a period of 6 weeks, following which they were subjected to a series of behavioral tests during which they remained on their respective diets. The behavioral tests were conducted over a period of 10 weeks in the following order: locomotor activity (spontaneous activity), coordinated running (maximum motor performance and motor learning), spatial learning and memory (visuospatial capacity), auditory and shock startle (sensory reactivity), and discriminated avoidance (cognitive flexibility). By the end of behavioral testing, the mice were 7.5 or 21.5 months of age, and brains and peripheral tissues were collected for biochemical measurements.
The behavior tests were chosen for numerous reasons: (a) performance on these tests is reliable and affected by age, (b) each test is independent as there is no correlation between performance on the different tests across individual mouse, and (c) sensitivity or lack thereof to interventions: Morris water maze performance was previously affected by CoQ supplementation whereas active avoidance was not and could serve as a negative control.
Locomotor activity
Spontaneous locomotor activity was measured using a Digiscan apparatus (Omnitech Electronics, model RXYZCM-16), as described previously (Forster and Lal 1991). Each mouse was placed in a clear acrylic test cage (40.5 × 40.5 × 30.5 cm) that was surrounded by a metal frame lined with photocells. The test cage was enclosed in a dimly lit, sound-attenuating chamber equipped with a fan that provided background noise (80 dB). During a 16-min period, movements in the horizontal plane, as well as a vertical plane 7.6 cm above the floor, were detected by the photocells and processed by a software program to yield different variables describing distance, vertical, and spatial components of spontaneous activity in the apparatus.
Coordinated running
Motor learning and maximum running performance were measured using an accelerating rotorod test described previously (Forster and Lal 1999). The apparatus was a motor-driven treadmill (Accuscan Instruments, Model # AIO411RRT525M) that consisted of a 3-cm diameter nylon cylinder mounted horizontally at a height of 35 cm above a padded surface. On a given trial, the mouse was placed on the cylinder, which then began rotating with increasing speed until the animal fell to a well-padded surface. Ability of the mice to improve running performance was assessed in a series of training sessions (two per day), each consisting of four trials at 10-min intervals. The training sessions continued until the running performance (the average latency to fall from the cylinder) failed to show improvement over three consecutive sessions. The treatment groups were compared for their average latency to fall on the first seven sessions and for the final session on which each mouse had reached its maximum stable level of performance.
Startle response
The musculoskeletal startle reflex to auditory or shock stimuli of various intensities was measured using a standard testing system (SA Lab, San Diego Instruments, San Diego, CA). For the auditory startle test, a mouse was placed inside an acrylic cylinder and presented with a series of mixed-frequency noise bursts (0, 90, 100, 110, 120, or 140 dB). Each acoustic signal (lasting 20 ms) was presented 12 times in a counterbalanced series, for a total of 72 trials. For the shock startle test, a mouse was placed inside the same acrylic cylinder, and a series of shocks (0, 0.02, 0.04, 0.08, 0.16, 0.24, 0.32, and 0.64 mA) was delivered. Each shock stimulus (100 ms in duration and scrambled across eight inputs to the grid floor of the acrylic cylinder) was given five times, for a total of 45 trials. The amplitude of the startle reflex was defined as the peak response to each auditory or shock stimulus within a 250-ms time window that began with the stimulus presentation (Sumien et al. 2006).
Morris water maze
Spatial learning and memory were measured using a Morris water maze test as described previously (de Fiebre et al. 2006; Sumien et al. 2006). On a given trial, the mouse was allowed to swim in a 120-cm diameter plastic tank filled to 34 cm from the top edge with colored water (nontoxic white paint) and maintained at 24 ± 1 °C. An escape was provided by means of a small platform (10 × 10 cm) hidden from view 1.5 cm below the surface of the water. A computerized tracking system recorded the length of the path taken by the mouse to reach the platform, as well as the swimming speed (San Diego Instruments, Model # SA-3).
During a pre-training phase, the tank was covered by a black curtain to prevent pre-exposure of the mice to visual cues present outside of the tank. In this way, mice learned the motor components of swimming and climbing onto the platform without learning its location in the tank. On each trial, the mouse was placed at one end of a 10 × 65-cm (W × L) straight alley that had a platform at the other end, and allowed to swim until it reached the platform or a maximum latency of 60 s had elapsed. The mice were given four sessions of pre-training (two per day), each consisting of five trials spaced at 5-min intervals.
After pre-training, the black curtain was removed from above the tank, and the mice were tested for their ability to learn the location of the platform using spatial cues. This testing was divided into three phases: acquisition (eight sessions with the platform in a fixed location), retention (two additional sessions after a 66-h delay interval), and reversal (four sessions with the platform at a new, fixed location). Each session consisted of five trials, at 10-min intervals, during which the mouse had to swim to the platform from one of four different starting points in the tank. Two sessions were conducted per day, separated by a period of at least 2 h, during which the mice were returned to the home cages. After the fifth trial of sessions 2, 4, 6, and 8, a probe trial was given in which the platform was submerged to a depth that prevented the mice from climbing onto it. The platform was raised after 30 s, and the trial was ended when the mouse successfully located it. On this trial, spatial bias for the platform location was evaluated in terms of the percentage of time spent within 40-cm diameter annulus surrounding the platform location.
A criterion was used to confirm that all mice used in the study used a spatial strategy for locating the platform position in the tank. According to this inclusion criterion, the mouse had to develop a spatial bias for the platform location within ten training sessions, as evidenced by at least one entry into the previous location of the platform on the first trial of reversal (session 11). The mice that did not reach this criterion were excluded from the swim maze data analysis. Six mice in the young and four mice in the old group did not reach criterion in this study.
Discriminated avoidance
A T-maze constructed of acrylic (black for the sides and clear for the top) was utilized for the discriminated avoidance task (Forster and Lal 1992; McDonald et al. 2005). The maze was divided into three compartments: a start box (10 × 6.3 × 6 cm), a stem (17.5 × 6.3 × 6 cm) and two goal arms (14.5 × 6.3 × 6 cm), each separated by clear acrylic doors. The maze rested on a grid floor wired to deliver 0.27-mA scrambled shock to the feet.
The test consisted of two sessions separated by 24 h. On each training trial, the mouse was placed in the start box, and the start door was removed to signal the beginning of the trial. On the first trial of the first session, the mouse received shock in the first arm entered and was permitted to escape shock by running to the opposite arm, which was then designated the correct arm for the remainder of the session. On subsequent trials, shock was initiated 5 s after the opening of the start door if the mouse had not entered the correct goal arm or immediately upon entry into the incorrect arm. In either case, the shock continued until the correct goal arm was entered or a maximum of 60 s had elapsed. Upon the mouse’s entry into the correct arm, the door was closed (to prevent departure), and, after 10 s, the mouse was removed (by detaching the goal arm) and allowed to enter a holding cage for 1 min. Training in this fashion continued at 1-min intervals until the mouse had met the criterion of a correct avoidance (defined as running directly to the correct arm within 5 s) on four of the last five training trials. The second session of avoidance training was a reversal such that the mice were required to run to the goal arm opposite that to which they had been trained on the previous day. Ability to learn the avoidance problem was considered inversely proportional to the number of trials required to reach criterion in each of the sessions.
Isolation of mitochondria
Upon completion of the behavioral battery, the mice were euthanized by cervical dislocation, and mitochondria were immediately prepared from fresh tissues. Mitochondria from the brain (not including the brainstem) (Sims 1993), liver (Lash and Sall 1993), heart (Arcos et al. 1968), and skeletal muscles (Trounce et al. 1989) were isolated by differential centrifugation. The mitochondrial pellets were resuspended in appropriate volume of buffer and stored at −80 °C for later analysis.
Determination of protein carbonyl concentration
Carbonyl concentrations were measured according to the method of Levine et al. (1994). The samples were incubated in the dark at room temperature for 1 h with either 2,4-dinitrophenyl hydrazine (DNPH) for the experimentals or HCl for the blanks. The difference in absorbance at 366 nm between DNPH-treated and HCL-treated samples was determined, and the results were expressed as nanomoles of carbonyls/milligram of protein using an extinction coefficient of 22.0 mM−1 cm−1.
Statistical analysis of data
The effects of age and treatment on performance on the behavioral tests were assessed using two-way analyses of variance (ANOVA) with age and diet as between-groups factors. The effect of the high-CoQ diet was considered in a balanced ANOVA that did not include data from mice on the low-CoQ diet (as this diet was not administered to young mice in this study). Planned individual comparisons between different age groups (young vs. old control) and diet groups (i.e., low or high-CoQ diet vs. age-matched control) were performed using a single degree-of-freedom F tests involving the error term from the overall ANOVA. For the weights and swim maze data, three-way ANOVAs were performed for each dependent variable, with weeks or sessions as the repeated measure. The carbonyl concentration in the mitochondria from different tissues were compared by a three-way ANOVA with age groups (young and old), diet groups (control and high CoQ), and tissues (brain, heart, liver, and skeletal muscle) as the factors. The alpha level was set at 0.05 for all analyses.
Results
Body weight
There was no change in body weight in either young or old control mice from the start to the end of the study (data not shown), an observation supported by the absence of a significant interaction between weeks and groups (P = 0.46). The initial weight difference between the young and old mice (old mice being heavier that the young ones) remained at the end of the study and was confirmed by a significant main effect of age (P = 0.029). Supplementation with CoQ did not affect the weight of young and old mice (P = 0.665).
Locomotor activity
Distance (in centimeters), rearing (counts), and center time (in seconds) were considered in the analyses of spontaneous locomotor activity (Table 1). There were declines of 26 and 15 % in rearing and distance traveled, respectively, and a 34 % reduction in center time associated with age. One- and two-way analyses of variance yielded a significant main effect of age only for rearing and center time (all P’s < 0.05), but the effects of diet and the age×diet interaction were not significant for any of the measures (all P’s > 0.3). The CoQ-supplemented groups had similar levels of locomotion, rearing, and center time when compared with their age-matched control group (all P’s > 0.4).
Table 1.
Effects of age and CoQ supplementation on functional measures
| Behavioral Measure | Young | Old | |||
|---|---|---|---|---|---|
| Control | CoQ (H) | Control | CoQ (L) | CoQ (H) | |
| Spontaneous activity | |||||
| Distance (cm) | 676 ± 47 | 627 ± 39 | 574 ± 52 | 576 ± 52 | 542 ± 71 |
| Rearing (counts) | 74.7 ± 7.0 | 81.4 ± 8.1 | 55.5 ± 6.6* | 54.2 ± 5.2 | 58.7 ± 7.0 |
| Center Time (s) | 44.7 ± 6.5 | 40.2 ± 3.5 | 29.5 ± 3.4* | 41.6 ± 5.3 | 40.2 ± 3.5 |
| Reflex/Motor | |||||
| Rotorod fall (s) | 51.5 ± 2.9 | 60.0 ± 7.5 | 32.0 ± 2.4* | 32.5 ± 1.8 | 30.8 ± 2.0 |
| Learning and Memory | |||||
| Discriminated avoidance | |||||
| Learning (trials) | 18.0 ± 1.9 | 14.4 ± 2.6 | 19.1 ± 1.7 | 18.9 ± 1.8 | 15.4 ± 2.1 |
| Reversal (trials) | 15.4 ± 2.0 | 13.4 ± 3.3 | 14.3 ± 2.4 | 13.0 ± 1.1 | 12.3 ± 1.7 |
All values are the group means±SE
*P < 0.05 (different from young control)
Coordinated running
The effects of age and supplementation on the ability of the mice to reach a criterion of stable running performance are indicated in Table 1. There was an improvement in the performance of both young and old mice over a period of seven sessions; however, the older mice performed worse overall than the young mice (data not shown). There was a trend towards improved performance in young mice supplemented with the high-CoQ diet, but this was not statistically significant (P > 0.12). The latency to fall of old control mice was reduced by 38 % when compared to treatment-matched young mice, and supplementation with CoQ failed to improve performance of old mice. A two-way ANOVA indicated a significant main effect of age (P < 0.001) but not a significant effect of Diet or a significant interaction of age and diet (all P’s > 0.204).
Morris water maze
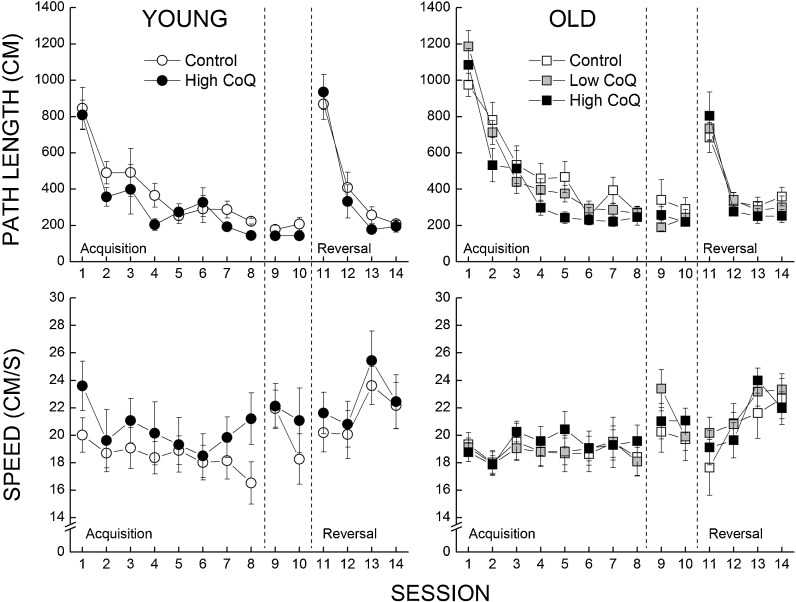
The efficiency of the mice in locating the hidden platform was assessed by the average length of the path taken over the five trials of each session. Both young and old mice had shorter path lengths as a function of sessions during acquisition phase (Fig. 1, top panels), reached a maximum level of performance that was maintained during the retention phase, and learned to locate the platform in its new position during the reversal phase (Fig. 1, top panels). Analysis of the data confirmed the effect of testing session on path length for the acquisition and reversal phases (P < 0.001) and the lack of an effect for the retention phase (P = 0.339). The older mice were less efficient in learning the location of the platform, reflected by an interaction of session and age approaching significance for the acquisition phase (P = 0.089) and significant for the reversal phase (P = 0.022).
Fig. 1.
Effects of age and CoQ supplementation on the efficiency of the mice to locate a hidden platform during acquisition, retention, and reversal sessions (top panels). Swim speed was measured during each of the sessions (bottom panels). Each value represents the mean±SEM, n = 7–11
In the acquisition phase, both young and old mice supplemented with the high-CoQ diet had shorter path length when compared to the age-matched controls, which was supported by a three-way ANOVA with sessions as a repeated measure, indicating a main effect of diet (P < 0.04), but not a significant interaction of age and diet (P = 0.586); however, individual comparisons at each session led to significant differences between control and high-CoQ groups only in the aged mice. In the retention phase, a similar statistical analysis revealed a main effect of age (P = 0.042), but neither a main effect of diet nor an interaction between age and diet (all P’s > 0.107). In the reversal phase, a comparison of the four treatment groups failed to indicate any significant effects of age, diet, or an interaction of age and diet (all P’s > 0.09).
The analysis of the swim speed data (Fig. 1, bottom panels) failed to yield significant main effects or an interaction (all P’s > 0.309).
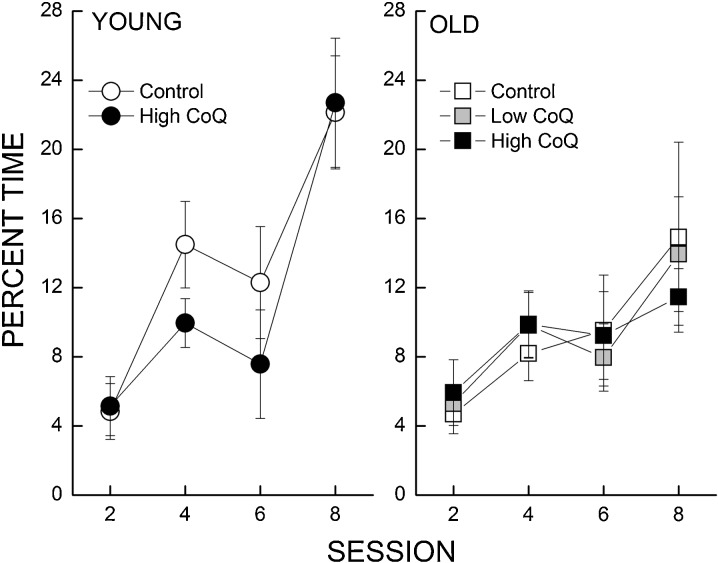
Accuracy of spatial memory was measured by conducting a probe trial as the last trial for sessions 2, 4, 6, and 8. Figure 2 illustrates the percentage of total trial time in which a mouse was within a 40-cm annulus around the target (platform) location. All the mice developed a stronger bias for the platform as a function of session and the young mice spent more time around the platform location as compared to the old mice. However, supplementation with CoQ did not affect the performance of young or old mice. These observations were supported by a three-way ANOVA which revealed a significant interaction of session and age (P = 0.045) and no interaction between session, age, and diet (P < 0.516).
Fig. 2.
Effects of age and CoQ supplementation on time spent in a 40-cm annulus during probe trials in swim maze. The percentage of time (±SE) spent in a 40-cm annulus surrounding the target area was calculated when platform was lowered in sessions 2, 4, 6, and 8. Each value represents the mean±SEM, n = 7–11
Sensory response
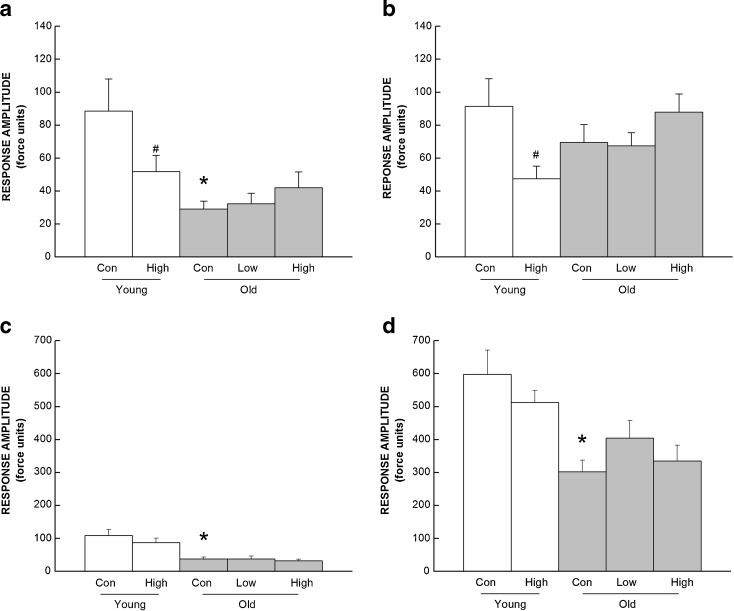
Response curves for auditory and shock startle responses were summarized and analyzed separately for low- and high-intensity sounds (Fig. 3a,b, respectively) and for low- and high-shock intensities (Fig.3c,d, respectively).
Fig. 3.
Effects of CoQ supplementation on the startle reflex to submaximal (a) and maximal (b) auditory stimuli or low (c), and high (d) shock stimuli as a function of age. Values are mean±SEM, n = 8–13. Asterisk, different from young control group, P < 0.05; number sign, different from control group of the same age, P < 0.05
Control mice had an age-related 66 % decline in responses to low sound intensities and a 24 % decrease in their responses to high sound intensities. CoQ intake affected the response at both low and high intensities differently, depending on the age of the mice. High-CoQ supplementation decreased auditory startle response to low and high intensities by 42 and 48 % in young mice, but did not affect response in the old ones. These observations were supported by a two-way ANOVA which revealed a significant interaction of age and diet for the high intensities (P = 0.023).
Control mice exhibited a lower response with age at both low (66 %) and high (49 %) intensities of shock. There was no effect of CoQ intake on shock startle response in either age group. Both observations were confirmed by two-way ANOVA, revealing a significant effect of age (P < 0.001) but no significant effect of diet or an interaction between age and diet (all P’s > 0.298).
Discriminated avoidance
The number of trials to reach the discriminated avoidance criterion on sessions 1 (learning) and 2 (reversal) is presented in Table 1. The performance of the mice was unaffected by age in both sessions. Young mice supplemented with the high-CoQ diet took fewer trials to reach criterion when compared with age-matched controls, but overall CoQ supplementation had no effect on the performance of young and old mice. A two-way ANOVA failed to indicate significant main effects or an interaction for either session (all P’s > 0.089).
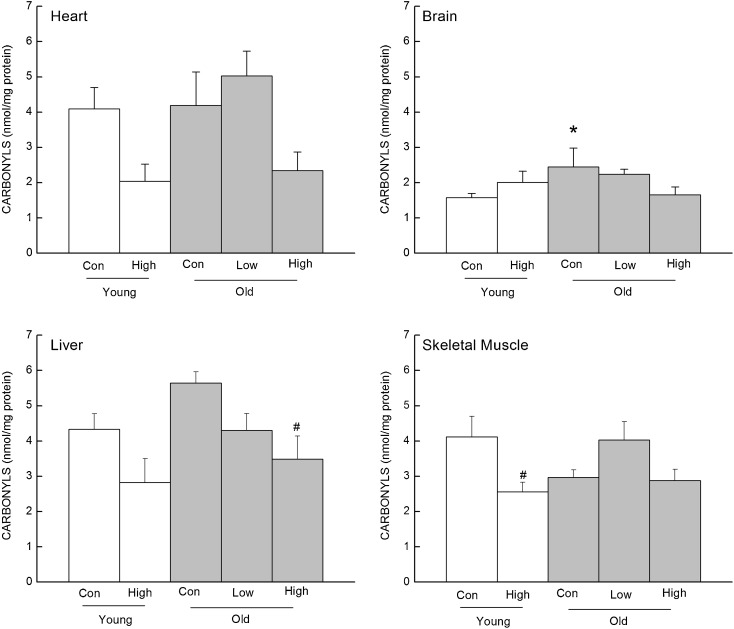
Protein oxidation in the mitochondria
Protein carbonyl content was measured in the mitochondrial fraction of the heart, brain, liver, and skeletal muscle of treated and control mice (Fig. 4). In the heart mitochondria, carbonyl concentration was 50 % lower in the young and 44 % lower in the old high-CoQ group compared to their age-matched controls, which was supported by a significant main effect of diet (P = 0.015). In the brain mitochondria, there was a 32 % decrease in carbonyl concentration associated with the high-CoQ dose in the old mice but not in the young; however, the age by diet interaction within the two-way ANOVA did not reach significance (P = 0.064). Furthermore, even though the individual comparison between old controls and old high-CoQ group did not reach significance (P = 0.055), it is noteworthy that the old high-CoQ groups was not significantly different from the young control group, whereas the old control group was (P < 0.05). In the liver mitochondria, carbonyl concentration was decreased by 35 % in the young and 38 % in old CoQ-supplemented group compared to their age-matched controls. This observation was supported by a significant main effect of diet (P = 0.004). In the skeletal muscle mitochondria, the high-CoQ diet lowered carbonyls in the young mice but not in the old. A two-way ANOVA for skeletal muscle indicated only a significant effect of diet (P = 0.044).
Fig. 4.
Effects of CoQ supplementation on carbonyl concentration in the mitochondria from the heart, brain, liver, and skeletal muscles. Values are mean±SEM, n = 3–10. Asterisk, different from young control group, P < 0.05; number sign, different from control group of the same age, P < 0.05
Discussion
The main findings of the study were (1) supplementation of CoQ (96 mg/kg/day, low dose) in aged mice for a period of 16 weeks failed to improve deficits in cognitive or psychomotor function; (2) supplementation of CoQ (457 mg/kg/day, high dose) reversed age-related declines in spatial learning; (3) supplementation of CoQ (457 mg/kg/day, high dose) decreased auditory function in young mice; and (4) supplementation of CoQ (457 mg/kg/day, high dose) decreased protein oxidation in the mitochondria of the tissues studied. These studies reveal a significant deleterious interaction of high amounts of CoQ with auditory function of healthy young mice. However, overall, the findings suggest that while long-term intake of CoQ has an apparent negative interaction with brain aging and cognitive decline, there is no detectable risk, and a modest benefit when supplementation is initiated during advanced age.
The magnitude of the age-related impairments of psychomotor function observed in mice on the control diet in the current study was similar to that reported previously (Hengemihle et al. 1999; Sumien et al. 2004, 2006). The mice receiving the low or high dose of CoQ supplementation exhibited age-related deficits in performance, similar to the control group when tested for balance (bridge walking), coordinated running (rotorod), swimming, muscle strength (wire suspension), and spontaneous activity. These results suggest that short-term intake of low or high amounts of CoQ did not lead to increased fitness or anti-aging effects for a variety of psychomotor functions, when the intake began during moderately advanced age (18 months). The high dose CoQ supplementation also did not have any effect on psychomotor function when initiated in young mice aged 4 months (Sumien et al. 2009).
Age-related impairment of cognitive function in the control diet group was also similar to that observed in previous studies (Sumien et al. 2004, 2006). It was notable that the high-CoQ diet affected various domains of cognition differently. While the high-CoQ diet had no discernible effect on the active discriminated avoidance paradigm used in this study, it led to improvement in efficiency of spatial learning in aged mice. The current results for the discriminated avoidance task are consistent with an earlier study from our laboratory in which old mice supplemented with even higher doses of CoQ (up to 500 mg/kg/day), for a period of 14 weeks, also failed to improve in their ability to learn the task (McDonald et al. 2005).
The ability of the mice to perform in the Morris water maze test is thought to require competent hippocampal, striatal, and cortical functions, which are thought to be altered with age (Winocur and Moscovitch 1990; Lee et al. 1994; Burke and Barnes 2006; Miyoshi et al. 2012). The results indicated that a high dose of CoQ reversed age-related inefficiency in locating the hidden platform during the training phase. Surprisingly, the high-CoQ diet did not influence the performance of the mice on the probe trials, which are thought to be measures of strength and accuracy of the memory (Morris 1984; McNamara and Skelton 1993). This apparent dichotomy in results suggests that these two measurements are sensitive to different functional capacities that can be affected independently by treatments. Supplementation with a high dose of CoQ improves learning but does not affect the accuracy of the learning, indicating a plausible effect of CoQ on attention or motivation rather than spatial performance. However, the lack of an effect on the active avoidance task may suggest that the latter interpretation is not the case. Furthermore, the high-CoQ diet did not affect swimming speed, suggesting that a motor improvement did not account for the spatial learning enhancement.
The current results would appear to contradict those reported previously (Sumien et al. 2009), where CoQ impaired spatial performance in mice after supplementation was initiated at 3.5 months and continued until 25 months. The results of the current study, an improvement rather than impairment, suggest that the presence of CoQ during late life is not sufficient to account for the negative effects of supplementation observed previously, but rather the timing and duration of CoQ exposure may be the major determinants. In lifelong studies, there is a possibility that the mice develop tolerance to antioxidants, leading to a diminished effect or lack thereof. Furthermore, the long-term study of CoQ also measured biomarkers of oxidative stress such as carbonyl content and glutathione ratio and the supplementation had no effect on the redox status of the brain or any other tissues studied (Sohal et al. 2006). In contrast, the current study determined that high-CoQ diet supplementation led to a decrease in oxidative damage in the brain and peripheral tissue mitochondria, suggesting an attenuation of steady-state oxidative stress, which could be associated with an amelioration in performance since previous studies have determined that behavioral test performance is strongly associated with oxidative damage in the brain (Forster et al. 1996).
In young mice, the high-CoQ diet decreased auditory sensitivity when measured as a decrease in the musculoskeletal reflex response to auditory stimuli. The decrease in response may be important as it could be associated with hearing loss, which is known to occur in C57BL/6 and other mouse strains (Willott et al. 1984; Erway et al. 1993; Prosen et al. 2003; Sumien et al. 2004). Hearing loss, detected by a decreased startle response or by attenuation of auditory-evoked brainstem response, has been widely studied in mice as a model of human auditory presbycusis (Erway et al. 1993). Furthermore, the finding that shock startle responses of the CoQ-treated mice remained unchanged implies that the deleterious effects are related to the sensory rather than the motor component of the startle reflex. Age-related hearing loss has been associated with increased oxidative damage (Someya and Prolla 2010) and down-regulation of mitochondrial respiratory chain complexes which would lead to an excess production of oxidants (Someya et al. 2007) in the cochlea. Previous studies have shown that altering the redox status of the cochlea affects hearing capacity, in particular caloric restriction has been shown to reduce presbycusis and attenuate oxidative damage (Someya et al. 2010). Other studies, in the context of noise induced and sudden sensorineural hearing loss, have determined that a low dose of CoQ, similar to the one used in the current study, has the ability to improve hearing loss in guinea pigs (Fetoni et al. 2009) and humans (Cadoni et al. 2007). Supplementation with high CoQ led to further impairments of auditory efficiency in the current study as well as in the lifelong supplementation of CoQ (Sumien et al. 2009); perhaps such high dose led to increased damage and/or mitochondrial derangement, enhancing the effect of aging on the auditory system.
It has been established that age-related decline in cognitive and psychomotor function is associated with oxidative damage in the rodent brain with aging (Forster et al. 1996; Nicolle et al. 2001; Serrano and Klann 2004). In the current study, protein oxidation was decreased by 30 % in the brain mitochondria from old CoQ-supplemented mice, but it was not a significant effect. The beneficial effects of CoQ were solely observed in a task for spatial learning and memory that relates to hippocampal, striatal, and cortical function, and previous studies clearly suggested that protein oxidation is not uniform among different regions of the brain. Therefore, the effect of CoQ on protein oxidation might have been diminished when measuring it in whole brain mitochondria. It has been previously established that oxidative damage is region-specific (Forster et al. 1996), but more importantly antioxidant distribution and more specifically CoQ distribution is region-specific (Sumien et al. 2009). A previous study demonstrated that a 3-week supplementation with CoQ led to CoQ accumulating in the neocortex more readily than in any other brain region (Sumien et al. 2009); therefore, one can hypothesize that the effect of CoQ or any antioxidants might also be region-specific.
In the mitochondria from peripheral tissues such as the heart, liver, and skeletal muscle, high-CoQ diet reduced protein oxidation. Short-term supplementation with CoQ had beneficial effects on behavior and oxidative stress status, which is in contradiction with the long-term study of the same dose (Sohal et al. 2006; Sumien et al. 2009). In this study, intake of a CoQ-enriched diet from 3.5 to 25 months of age had no effect on any of the antioxidant enzyme systems, level of oxidative stress, oxygen consumption, or ETC complex activity. Furthermore, it failed to reduce age-associated protein oxidation and had no effect on the GSH/GSSG ratio and did not affect the life-span of the mice. The decrease in oxidation in peripheral tissues could be linked to improved health, cardiovascular, or musculoskeletal function which could contribute to behavioral improvements.
The human dose equivalent for the low-CoQ diet administered to mice was ~500 mg/day CoQ, whereas the high-CoQ diet was ~2,500 mg/day CoQ (Reagan-Shaw et al. 2008). Treatment for conditions such as hypercholesterolemia, congestive heart failure, mitochondrial disorders, statin-induced CoQ depletion, and for enhancing exercise tolerance have used dosage similar to the low dose supplemented in the current study (Beal 1999; Rosenfeldt et al. 2003, 2007; Marcoff and Thompson 2007 ), whereas the high-CoQ dose can be related to doses used in clinical trials for the treatment of Parkinson’s disease (Shults and Haas 2005) or Alzheimer’s disease (trial NCT00117403), and doses up to 3,000 mg/day have been examined for short periods and determined to be void of toxic effects (Ferrante et al. 2005). The high-CoQ dose used in our study was beneficial for spatial learning and memory when used for a short period of time; when used lifelong, a similar dose led to significant deleterious effects (Sumien et al. 2009).
The mechanisms underlying the beneficial effects of CoQ intake in high amounts remain unclear. Even though previous studies have demonstrated that CoQ administration had little to no effect of oxidative stress status or mitochondrial bioenergetics markers (Sohal et al. 2006), it is conceivable that CoQ administration over short periods may lead to a shift in redox status, thereby influencing redox-dependent signaling cascades critical to memory formation and retrieval (Kamsler and Segal 2003; Watson et al. 2006; Kishida and Klann 2007).
Although it was surprising that short-term CoQ supplementation had only affected positively spatial learning, evidence suggest that combining CoQ with another antioxidant could have a more substantial impact on functionality. Concurrent administration of two antioxidants, vitamin E and CoQ, in a diet improved the ability of mice to learn the discriminated avoidance task more than did single antioxidant supplementation (McDonald et al. 2005).
In conclusion, CoQ intake for a short duration improved age-related impairments in spatial performance and attenuated protein oxidative damage. However, when initiated during adulthood and maintained throughout life, the same dose of CoQ exacerbated age-related spatial learning impairment. These differential effects provide clear evidence that starting time (age) and duration of the antioxidant intervention are critical determinants of functional outcome.
Acknowledgments
This research was supported by the grants R01 AG27353 and AG22550 from the National Institutes of Health and the National Institute on Aging. The authors also wish to thank Tishcon Corp. (Westbury, NY) for providing with CoQ for this study.
Contributor Information
Ritu A. Shetty, Phone: +1-817-7352389, FAX: +1-817-7350408, Email: ritu.shetty@unthsc.edu
Michael J. Forster, Email: michael.forster@unthsc.edu
Nathalie Sumien, Email: nathalie.sumien@unthsc.edu.
References
- Arcos JC, Sohal RS, et al. Changes in ultrastructure and respiratory control in mitochondria of rat heart hypertrophied by exercise. Exp Mol Pathol. 1968;8:49–65. doi: 10.1016/0014-4800(68)90005-1. [DOI] [PubMed] [Google Scholar]
- Baggio E, Gandini R, et al. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure (interim analysis). The CoQ10 Drug Surveillance Investigators. Clin Investig. 1993;71(8 Suppl):S145–149. doi: 10.1007/BF00226857. [DOI] [PubMed] [Google Scholar]
- Battino M, Ferri E, et al. Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem. 1990;9(3):179–190. doi: 10.3109/09687689009025839. [DOI] [PubMed] [Google Scholar]
- Beal MF. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors. 1999;9(2–4):261–266. doi: 10.1002/biof.5520090222. [DOI] [PubMed] [Google Scholar]
- Beal MF, Henshaw DR, et al. Coenzyme Q10 and nicotinamide block striatal lesions produced by the mitochondrial toxin malonate. Ann Neurol. 1994;36(6):882–888. doi: 10.1002/ana.410360613. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Gould T, et al. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/S0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- Bresolin N, Doriguzzi C, et al. Ubidecarenone in the treatment of mitochondrial myopathies: a multi-center double-blind trial. J Neurol Sci. 1990;100(1–2):70–78. doi: 10.1016/0022-510X(90)90015-F. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cadoni G, Scipione S, et al. Coenzyme Q 10 and cardiovascular risk factors in idiopathic sudden sensorineural hearing loss patients. Otol Neurotol. 2007;28(7):878–883. doi: 10.1097/MAO.0b013e3180686e4a. [DOI] [PubMed] [Google Scholar]
- Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, Sumien N, et al. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. AGE. 2006;28(3):235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271(1):195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willot JF, et al. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-H. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. 2002;22(5):1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante KL, Shefner J, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65(11):1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Piacentini R, et al. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–116. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Fontaine E, Eriksson O, et al. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex. J Biol Chem. 1998;273(20):12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- Forsmark-Andree P, Dallner G, et al. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial membranes. Free Radic Biol Med. 1995;19:749–757. doi: 10.1016/0891-5849(95)00076-A. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Neurobehavioral biomarkers of aging: influence of genotype and dietary restriction. Biomed Environ Sci. 1991;4(1–2):144–165. [PubMed] [Google Scholar]
- Forster MJ, Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav Pharmacol. 1992;3(4):337–349. doi: 10.1097/00008877-199208000-00010. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20(2):167–176. doi: 10.1016/S0197-4580(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, et al. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93(10):4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, et al. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55(11):B522–B529. doi: 10.1093/gerona/55.11.B522. [DOI] [PubMed] [Google Scholar]
- Hengemihle JM, Long JM, et al. Age-related psychomotor and spatial learning deficits in 129/SvJ mice. Neurobiology of Aging. 1999;20(1):9–18. doi: 10.1016/S0197-4580(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Hofman-Bang C, Rehnqvist N, et al. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 Study Group. J Card Fail. 1995;1(2):101–107. doi: 10.1016/1071-9164(95)90011-X. [DOI] [PubMed] [Google Scholar]
- Hyson HC, Kieburtz K, et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. Mov Disord. 2010;25(12):1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, et al. Role of coenzyme Q and superoxide in vitamin E cycling. Subcell Biochem. 1998;30:491–507. doi: 10.1007/978-1-4899-1789-8_20. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23(1):269–276. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamzalov S, Sumien N, et al. Coenzyme Q intake elevates the mitochondrial and tissue levels of Coenzyme Q and alpha-tocopherol in young mice. J Nutr. 2003;133(10):3175–3180. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9(2):233–244. doi: 10.1089/ars.2007.9.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroshetz WJ, Jenkins BG, et al. Energy metabolism defects in Huntington's disease and effects of coenzyme Q10. Ann Neurol. 1997;41(2):160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- Lash LH, Sall JM. Mitochondrial isolation from liver and kidney: strategy, techniques, and criteria for purity. In: Lash LH, Jones DP, editors. Methods in toxicology: mitochondrial dysfunction, vol. 2. San Diego: Academic; 1993. pp. 8–12. [Google Scholar]
- Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch Biochem Biophys. 1998;352(2):229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- Lass A, Forster MJ, et al. Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial alpha-tocopherol by coenzyme Q10. Free Radic Biol Med. 1999;26(11–12):1375–1382. doi: 10.1016/S0891-5849(98)00330-X. [DOI] [PubMed] [Google Scholar]
- Lass A, Kwong LK, Sohal RS. Mitochondrial coenzyme Q content and aging. Biofactors. 1999;9(IOS):199–205. doi: 10.1002/biof.5520090215. [DOI] [PubMed] [Google Scholar]
- Lee JM, Ross ER, et al. Spatial learning deficits in the aged rat: neuroanatomical and neurochemical correlates. Brain Res Bull. 1994;33(5):489–500. doi: 10.1016/0361-9230(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Fato R, et al. Localization and mobility of coenzyme Q in lipid bilayers and membranes. Biofactors. 1999;9:87–93. doi: 10.1002/biof.5520090202. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, et al. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49(23):2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L et al (1998) "Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects." Proc Natl Acad Sci USA 95(15):8892–8897. [DOI] [PMC free article] [PubMed]
- McDonald SR, Sohal RS, et al. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38(6):729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993;18(1):33–49. doi: 10.1016/0165-0173(93)90006-L. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikoski E, et al. Both the doral hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behav Brain Res. 2012;226(1):171–178. doi: 10.1016/j.bbr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Morisco C, Trimarco B, et al. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investig. 1993;71(8 Suppl):S134–S136. doi: 10.1007/BF00226854. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mukai K, Kikuchi S, et al. Stopped-flow kinetic study of the regeneration reaction of tocopheroxyl radical by reduced ubiquinone-10 in solution. Biochim Biophys Acta. 1990;1035(1):77–82. doi: 10.1016/0304-4165(90)90176-W. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, et al. Signatures of hippocampal oxidative stress in aged spatial learning-imparied rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/S0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Nohl H, Staniek K, et al. The biomolecule ubiquinone exerts a variety of biological functions. Biofactors. 2003;18:23–31. doi: 10.1002/biof.5520180204. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, et al. The functional age of hearing loss in a mouse model of presbycusis. I. Behavioral assessments. Hear Res. 2003;183(1–2):44–56. doi: 10.1016/S0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Quiles JL, Ochoa JJ, et al. Coenzyme Q supplementation protects from age-related DNA double-strand breaks and increases lifespan in rats fed on a PUFA-rich diet. Exp Gerontol. 2004;39:189–194. doi: 10.1016/j.exger.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, et al. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt F, Hilton D, et al. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors. 2003;18(1–4):91–100. doi: 10.1002/biof.5520180211. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt FL, Haas SJ, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21(4):297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3(4):431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, et al. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22(3):295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Shults CW, Haas R. Clinical trials of coenzyme Q10 in neurological disorders. Biofactors. 2005;25(1–4):117–126. doi: 10.1002/biof.5520250113. [DOI] [PubMed] [Google Scholar]
- Sims NR. Methods in toxicology: mitochondrial dysfunction. San Diego: Academic; 1993. [Google Scholar]
- Sohal RS, Kamzalov S, et al. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med. 2006;40(3):480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010;131(7–8):480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, et al. Genes encoding mitochondrial respiratory chain components are profoundly down-regulated with aging in the cochlea of DBA/2 J mice. Brain Res. 2007;1182:26–33. doi: 10.1016/j.brainres.2007.08.090. [DOI] [PubMed] [Google Scholar]
- Someya S, Tanokura M, et al. Effects of caloric restriction on age-related hearing loss in rodents and rhesus monkeys. Curr Aging Sci. 2010;3(1):20–25. doi: 10.2174/1874609811003010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, et al. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36(11):1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Sumien N, Sims MN, et al. Profiling psychomotor and cognitive aging in four-way cross mice. AGE. 2006;28:265–282. doi: 10.1007/s11357-006-9015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, et al. Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. J Nutr. 2009;139(10):1926–1932. doi: 10.3945/jn.109.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounce I, Byrne E, et al. Decline in skeletal muscle mitochrondrial respiratory chain functions: possible factor in aging. Lancet. 1989;25:637–639. doi: 10.1016/S0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, et al. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, et al. Metabolism and function of coenzyme Q. Biochem Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Watson JB, Arnold MM, et al. Age-dependent modulation of hippocampal long-term potentiation by antioxidant enzymes. J Neurosci Res. 2006;84(7):1564–1574. doi: 10.1002/jnr.21040. [DOI] [PubMed] [Google Scholar]
- Willott JF, Kulig J, et al. The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment. Hear Res. 1984;16(2):161–167. doi: 10.1016/0378-5955(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Hippocampal and prefrontal cortex contributions to learning and memory: analysis of lesion and aging effects on maze learning in rats. Behav Neurosci. 1990;97:13–27. doi: 10.1037//0735-7044.104.4.544. [DOI] [PubMed] [Google Scholar]