Abstract
Many studies have demonstrated that SIRT1, an NAD+-dependent deacetylase, reduces apoptosis in several different cells. However, the role of SIRT1 in apoptosis of disc nucleus pulposus (NP) cells remains unclear. The present study was performed to determine whether degenerative human NP would express SIRT1, and to investigate the role of SIRT1 in NP cells apoptosis. The expression of SIRT1 in disc NP of patients (>55 years) with lumbar disc degenerative disease (DDD) and the disc NP of patients (<25 years) with lumbar vertebra fracture (LVF) was assessed by immunohistochemistry, reverse transcription polymerase chain reaction, and Western blot analysis. The results showed that SIRT1 mRNA and protein levels were greater in LVF disc NP than those in DDD disc NP. Degenerative human NP cells were treated in culture with activator or inhibitor of SIRT1, resveratrol or nicotinamide, or SIRT1 small interfering RNA (siRNA), and cell apoptosis was quantified via flow cytometry. The rate of apoptosis was far fewer in resveratrol-treated NP cells than in SIRT1 siRNA-transfected or nicotinamide-treated NP cells. After SIRT1 siRNA was transfected, NP cells decreased phosphorylation of Akt, while resveratrol phosphorylated Akt. Treatment with LY294002 or Akt siRNA increased the rate of apoptosis. Our results suggested that SIRT1 plays a critical role in survival of degenerative human NP cells through the Akt anti-apoptotic signaling pathway.
Keywords: SIRT1, Apoptosis, Nucleus pulposus cells, Akt pathway, Degenerative disc disease
Introduction
Approximately 80 % aging population are suffering from low back pain, which has caused a significant socio-economic problem (Takahashi et al. 2008). It is well known that intervertebral disc (IVD) degeneration is the leading cause of low back pain and increasingly becoming a major public health issue. One of the most evident cellular and biochemical changes credited to degeneration is excessive apoptosis of nucleus pulposus (NP) cells capable of producing cartilage-specific extracellular matrix (ECM) components (Gruber and Hanley 1998, 2003; Zhao et al. 2006, 2007; Park et al. 2001a, b). The balance between ECM synthesis and degradation is disturbed in IVD degeneration, leading to a gradual loss of disc ECM, and lastly, structural failure and biomechanical change (Bibby et al. 2001; Lauerman et al. 1992). Therefore, apoptosis of NP cells plays a role in the development of IVD degeneration. With this in mind, one possible strategy to block apoptosis of NP cells may provide a means to correct ECM insufficiencies, with a goal of retarding the progression of the IVD degeneration changes.
Numerous scientific studies show that SIRT1, identified as the mammalian homolog of silent information regulation 2 (Sir2) in yeast, is a longevity gene which can inhibit apoptosis and enhance cell survival in a variety of cell systems under calorie restriction (Michan and Sinclair 2007; Cohen et al. 2004). SIRT1 is an NAD+-dependent class III histone deacetylase that is capable of mediating gene silencing (Imai et al. 2000). A number of apoptosis-associated non-histone proteins such as tumor suppressor p53 and forkhead transcription factors can also be deacetylated by SIRT1 which plays a pivotal role in many cellular functions, including cell differentiation and proliferation, apoptosis, and cell aging (Michan and Sinclair 2007; Glozak and Seto 2007; Longo and Kennedy 2006; Furukawa et al. 2007; Saunders and Verdin 2007).
Recently, it has been demonstrated that SIRT1 has vital inhibitory effects in cardiac myocyte and chondrocytes apoptosis, vascular endothelial cell and dermal fibroblast senescence, and axonal degeneration in neurons (Alcendor et al. 2007; Araki et al. 2004; Takayama et al. 2009; Gagarina et al. 2010; Ota et al. 2010; Ohguchi et al. 2010). SIRT1 has been shown to block expression of matrix metalloproteinase (MMP)-1 and MMP-3 at both mRNA and protein levels in human dermal fibroblasts (Ohguchi et al. 2010). Additionally, members of the MMP family implicate in the breakdown NP ECM in IVD degeneration. It was speculated that SIRT1 may retard IVD degeneration. It has also been shown that chondrocytes from osteoarthritis cartilage show lower levels of SIRT1 expression than normal cartilage (Takayama et al. 2009; Gagarina et al. 2010; Dvir-Ginzberg et al. 2008). In addition, SIRT1 can also enhance the expression of many cartilage-specific ECM genes, such as type II collagen (COL2A1), aggrecan (Dvir-Ginzberg et al. 2008). It has been demonstrated that disc NP cells have the same morphology and avascular supply as chondrocytes, and the incidence of degenerative disc disease increases rapidly with age. Therefore, this has led to the hypothesis that there may be an inherent relationship between SIRT1 activity and the apoptosis of NP cells.
The present study was performed to determine whether degenerative human NP would express SIRT1, and to investigate the role of SIRT1 in NP cells apoptosis, SIRT1 small interfering RNA (siRNA) was transfected into degenerative human NP cells to knock down SIRT1 expression through disc NP of patients (<25 years) with lumbar vertebra fracture (LVF), and the disc NP of patients (>55 years) with lumbar disc degenerative disease (DDD) by flow cytometry, reverse transcription polymerase chain reaction (RT-PCR), and Western blot analysis.
Materials and methods
Tissue source
The degenerative human NP samples were donated from patients (21 cases; mean age, 64; range, 55–72; 12 males and 9 females) with DDD during nucleotomy and intervertebral fusion surgery. Relatively normal NP were donated from three young male patients (aged 21, 23, and 24 years old, respectively) with LVF undergoing posterior nucleotomy, spinal fusion, decompression, and stability within 24 h of trauma, without formerly documented clinical history of low back pain, and were used as control. The NP samples were harvested under sterile conditions and delivered to the laboratory within 30 min after being harvested. Complete culture medium with serum at 4 °C was used as transport medium.
The study was approved by the ethics committee of Chongqing Medical University, and informed consent of all the patients involved in our study was obtained. Routine MRI scan of spine was performed for all degenerative patients to diagnosed DDD. The grade of disc degeneration was graded according to the Pfirrmann classification (Pfirrmann et al. 2001). There were Grades III–VI lumbar discs of all patients with DDD and Grade I lumbar discs degeneration of all three patients with LVF.
Immunohistochemical analysis
NP samples were fixed in 4 % paraformaldehyde and then embedded in paraffin. Sections of 4 μm in thickness were prepared. Afterwards, the sections were mounted onto slides, deparaffinized in xylene, and rehydrated through graded alcohol into double-distilled water, treated with 3 % H2O2 for 15 min to eliminate endogenous peroxidases activity, and blocked with special serum for 15 min. Then, they were incubated overnight at 4 °C with rabbit anti-SIRT1 (Abcam), rabbit anti-MMP-2 (Santa Cruz biotechnology)-specific antibodies, and visualized using immunohistochemical kit (Boster).
Cell isolation and culture
NP samples were separated from annulus according to their different macroscopic morphology and washed twice in phosphate-buffered saline solution (PBS). Samples were minced, and matrix was digested 20 min at 37 °C in 0.25 % trypsin solution, following 3–4 h in 0.2 % type II collagenase. Isolated cells were filtered through a 200-μm filter and then resuspended in Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F12, 1:1) containing 15 % fetal bovine serum (FBS), without any antibiotics. Monolayer cell cultures were maintained in a 5 % CO2:95 % air incubator at 37 °C. All experiments were carried out with human NP cells from passage 3 to passage 5.
Senescence-associated β-galactosidase staining
Briefly, human disc NP cells from LVF and DDD at passages 3 were cultured in 6-well plates for 24 h, fixed with fixative solution, and then senescence-associated β-galactosidase (SA-β-Gal) staining was performed according to SA-β-Gal kit (Beyotime) instructions for 12 h. The cells were then photographed under a microscope for qualitative detection of SA-β-Gal activity.
Activator and inhibitor of SIRT1 treatment
NP cells were plated in a 6-well plate 24 h prior to treatment. Cells were then treated with the indicated concentrations of activator or inhibitor of SIRT1, resveratrol (8 μM) (Sigma-Aldrich) or nicotinamide (0, 4, 8, and 12 mM; Sigma-Aldrich), in medium and incubated for 48 h. Afterwards, human NP cells were collected for flow cytometry or Western blotting. In order to induce apoptosis, TNFα (12 ng/ml; Peprotech) sometimes was added to the culture medium, or cells were cultured in the medium containing 0.5 % FBS.
siRNA transfection
Double-stranded small interfering RNA (siRNA) for human SIRT1 gene silencing was designed and chemically synthesized (Invitrogen). The expression of endogenous SIRT1 of degenerative human NP cells was suppressed by siRNA. Sequences of the SIRT1 siRNA were as follows: sense strand 5′-CCAAGCAGCUAAGAGUAAUTT-3′, antisense strand 5′-AUUACUCUUAGCUGCUUGGTT-3′. Cells were seeded in 6-well plate 24 h prior to transfection. After 24 h, cells at 60–70 % confluency were transfected with negative control or SIRT1 siRNA duplexes for 36 h at 30 nM using PepMute siRNA Transfection Reagent (SignaGen, USA) according to the manufacturer's instructions. After the following further various treatments, cells were harvested for cell apoptosis analysis, and protein extracts were subjected to Western blot experiments.
Cells apoptosis assay
Apoptotic cells of NP were assessed with a TUNEL assay kit (Roche) that enzymatically labels DNA strand breaks. Endogenous peroxidases were quenched by incubating slides with blocking solution for 10 min at 20 °C. The TUNEL reaction solution (50 μl) was then added, and the slides were incubated in a humidified atmosphere for 1 h at 37 °C. Apoptotic NP cells were quantified by counting TUNEL-labeled apoptotic cells. Results are expressed as counts of TUNEL-labeled apoptotic cells.
Apoptotic NP cells following different treatments were detected by Annexin V/PI double-staining flow cytometry. Briefly, cells were harvested, washed twice with PBS, and resuspended in binding buffer. Annexin V solution and PI were added to these cells, and the fluorescence intensities of Annexin V/PI-stained cells were analyzed by flow cytometry within 1 h. Early apoptotic cells show Annexin V+/PI−, late apoptotic shows Annexin V+/PI+, and normal cells show Annexin V−/PI−, respectively. Apoptotic cells (early stage and late stage) were counted, and the results were expressed as a percentage of the total cell count.
RNA extraction and real-time RT-PCR
Total RNA of cells was extracted using RNAiso Plus (TaKaRa Biotechnology) according to the manufacturer's manual. The isolated RNA samples were reversely transcribed to cDNA using PrimeScript® RT reagent Kit With gDNA Eraser (TaKaRa Biotechnology). Real-time quantitative RT-PCR was carried out with SYBR® Green Realtime PCR Master Mix (TOYOBO) on the iQ5 RT-PCR System (Bio-Rad, USA), and relative gene expression was analyzed according to the manufacturer's instructions. Reaction conditions used for amplification consisted of one cycle at 95 °C for 3 min, followed by 40 cycles at 95 °C for 15 s, respective annealing temperature for 20 s, and an elongation phase at 72 °C for 20 s. Primer for human genes, SIRT1 (sense, 5′-ccagaacatagacacgctggaac-3′; antisense, 5′-ctcctcgtacagcttcacagtca-3′), COL2A1 (sense, 5′-ctcaagtccctcaacaaccaga-3′; antisense, 5′-ggggtcaatccagtagtctccac-3′), aggrecan (sense, 5′-gccagcaccaccaatgtaagt-3′; antisense, 5′-agtaacaccctccacgaactcag-3′), and GAPDH (sense, 5′-ctttggtatcgtggaaggactc-3′; antisense, 5′-gtagaggcagggatgatgttct-3′) were synthesized by TaKaRa (TaKaRa Biotechnology).
Cell lysates and Western blotting
NP cells were washed in ice-cold PBS and lysed via gentle agitation in ice-cold lysis buffer added in protease and phosphatase inhibitor. Protein concentration was measured by enhanced BCA protein assay. Protein samples were mixed with 5× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample loading buffer, boiled for 10 min, and then separated on 6–10 % SDS-PAGE gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membrane (PVDF). The membranes were blocked in phosphate buffered saline (PBS) containing 0.05 % Tween-20 and 5 % bovine serum albumin (BSA) for 1 h, washed, and incubated with primary antibodies against SIRT1 (Abcam), COL2A1 (Santa Cruz biotechnology), aggrecan (Santa Cruz biotechnology), Akt (Cell Signaling Technology), phospho-Akt (Ser473; Cell Signaling Technology), active caspase-3 (Epitomics), Bcl-2 (Epitomics), acetyl-histone H3 (lys9; Cell Signaling Technology), and GAPDH (Santa Cruz biotechnology) in the same blocking buffer overnight at 4 °C. After the membranes were rinsed three times for 5 min in PBS-Tween-20 (PBS-T), they were incubated for 1 h with horseradish peroxidase-conjugated secondary antibody. After three times of washing with PBS-T, the immunocomplexes were visualized using enhanced chemiluminescence and analyzed using the software.
Statistical analysis
Data are reported as the mean ± SD of individual analysis. Statistical differences were measured by one-way analysis of variance (one-way ANOVA), and p < 0.05 was considered statistically significant.
Results
Low SIRT1 expression in NP of patients with DDD compared with that of LVF disc NP
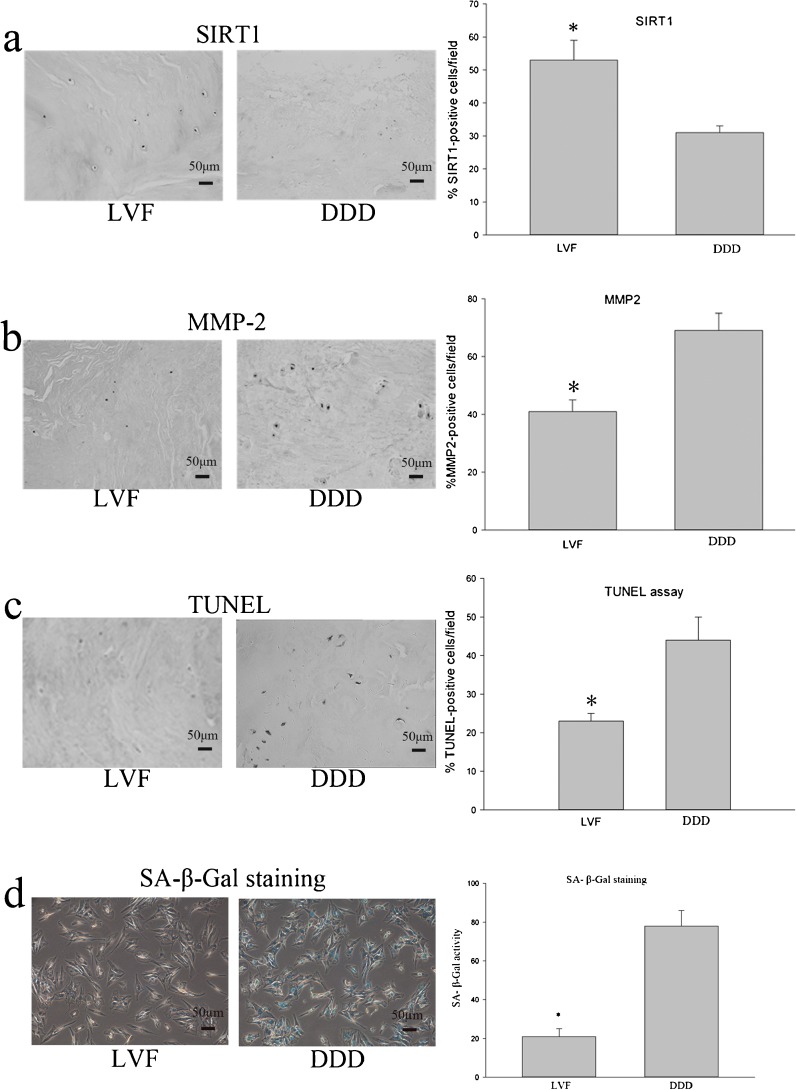
Immunohistochemical detection using specific antibodies revealed that SIRT1-postive cells in degenerative DDD NP sample were significantly decreased compared with that of the NP samples from patient with LVF (Fig. 1a). Conversely, an increase of MMP-2-positive cells was apparent in the degenerative DDD NP samples compared to that of NP samples from patient with LVF (Fig. 1b).
Fig. 1.
Examination of SIRT1 and metalloproteinase 2 (MMP-2) expression in human NP surgical sample by immunohistochemistry. a SIRT1 levels are elevated in NP surgical sample from patients with lumbar vertebra fracture (LVF). b Metalloproteinase 2 (MMP-2) levels of NP surgical sample from patients with LVF are reduced compared with those of NP surgical sample from patients with lumbar disc herniation (DDD). c The percentages of apoptotic cells in the disc NP surgical sample from LVF and DDD were detected using TUNEL assay, quantitated using image analysis. d Effect of the different disc degeneration on percentage of senescence-associated β-galactosidase-positive cells. Three independent experiments were performed, and data of image analysis represents the mean ± SD. Asterisks: significant difference with respect to DDD (p < 0.05)
TUNEL assay was performed to detect the apoptosis of surgical NP specimens from LVF and DDD. We found that the incidence of apoptotic cell of surgical NP specimens from patients with DDD was significantly greater compared with that of the LVF patients (Fig. 1c).
Both the number of SA-β-Gal-positive cells (blue) and the intensity of the staining were remarkably increased in disc NP cells from DDD according to SA-β-Gal staining (Fig. 1d).
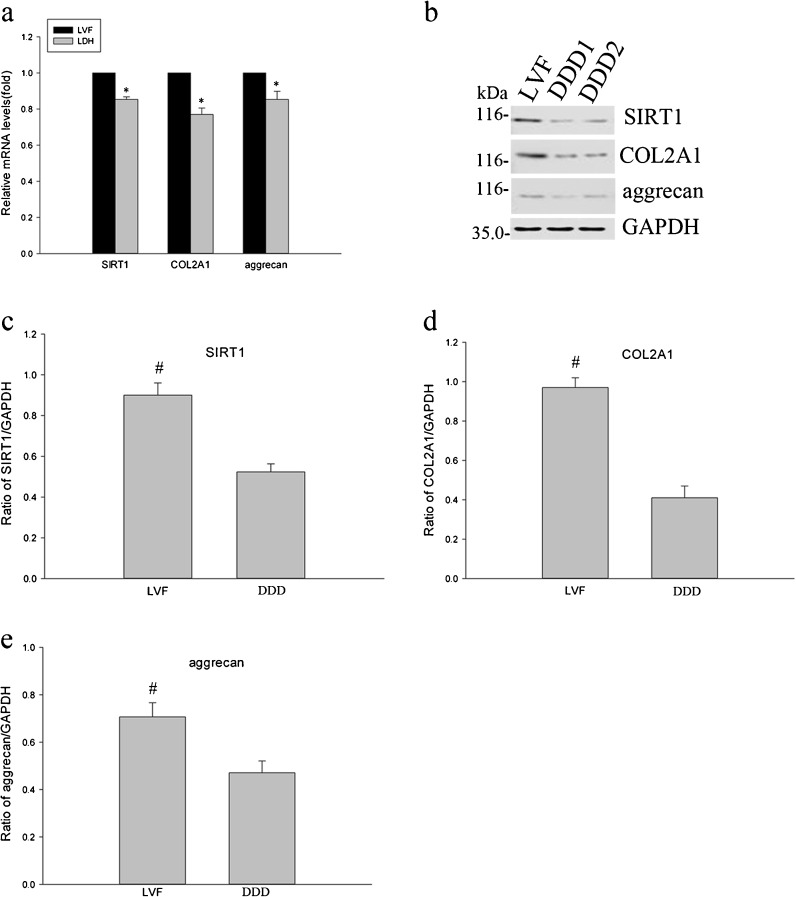
Analysis of SIRT1 mRNA and protein of NP cells by RT-PCR and Western blot demonstrated that SIRT1 levels were elevated in NP cells from patients with LVF (Fig. 2a–c). Simultaneously, the expression of COL2A1 and aggrecan was significantly decreased in the DDD NP cells compared with that of the LVF patients (Fig. 2a, b, d, e).
Fig. 2.
Analysis of SIRT1, COL2A1, and aggrecan expression in human NP cells by real-time PCR and Western blotting. a The values of real-time PCR for SIRT1, COL2A1, and aggrecan gene expression obtained from patients with LVF were normalized versus those from patients with DDD. b Western blotting analysis of SIRT1, COL2A1, and aggrecan protein expression in human NP cells from LVF and DDD. c–e Quantitative analysis of the Western blotting was performed with the software. The data are mean of three different experiments; SD. Asterisk: significant difference with respect to LVF (p < 0.05); number sign: significant difference with respect to DDD (p < 0.05)
Treatment with nicotinamide increases apoptosis of degenerative human NP cells
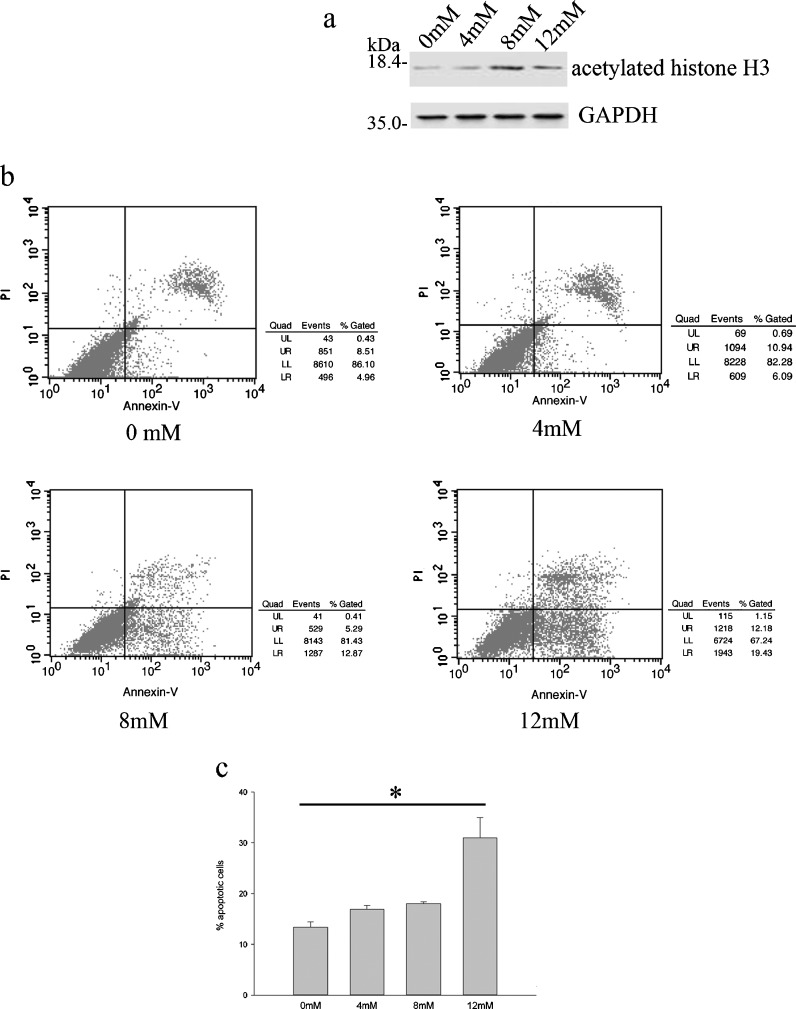
The expression level of acetylated histone H3 was increased after treatment with nicotinamide (0, 4, 8, and 12 mM; NAM, an inhibitor of SIRT1; Fig. 3a). Meanwhile, NAM markedly increased DDD NP cells apoptosis rate in a dose-dependent manner detected by Annexin V/PI double-staining flow cytometry after treatment with different concentrations of nicotinamide for 48 h (Fig. 3b, c).
Fig. 3.
Effects of SIRT1 inhibitor, nicotinamide (NAM), on apoptosis of degenerative human NP cells. Cells were treated with different concentrations of NAM (0, 4, 8, and 12 mM) for 48 h. a The levels of acetylated histone H3 in total cell lysates were analyzed by Western blot analysis. b The apoptotic percentage of degenerative NP cells measured by flow cytometry. x-axis represents annexin V staining, and y-axis represents PI staining. c The cells in early-stage (annexin V+ and PI−) and late-stage (annexin V+ and PI+) apoptosis were defined as apoptotic. Data represents mean ± SD of three independent experiments. Asterisk: p < 0.05
Knockdown of endogenous SIRT1 of human degenerative NP cells prompts apoptosis levels
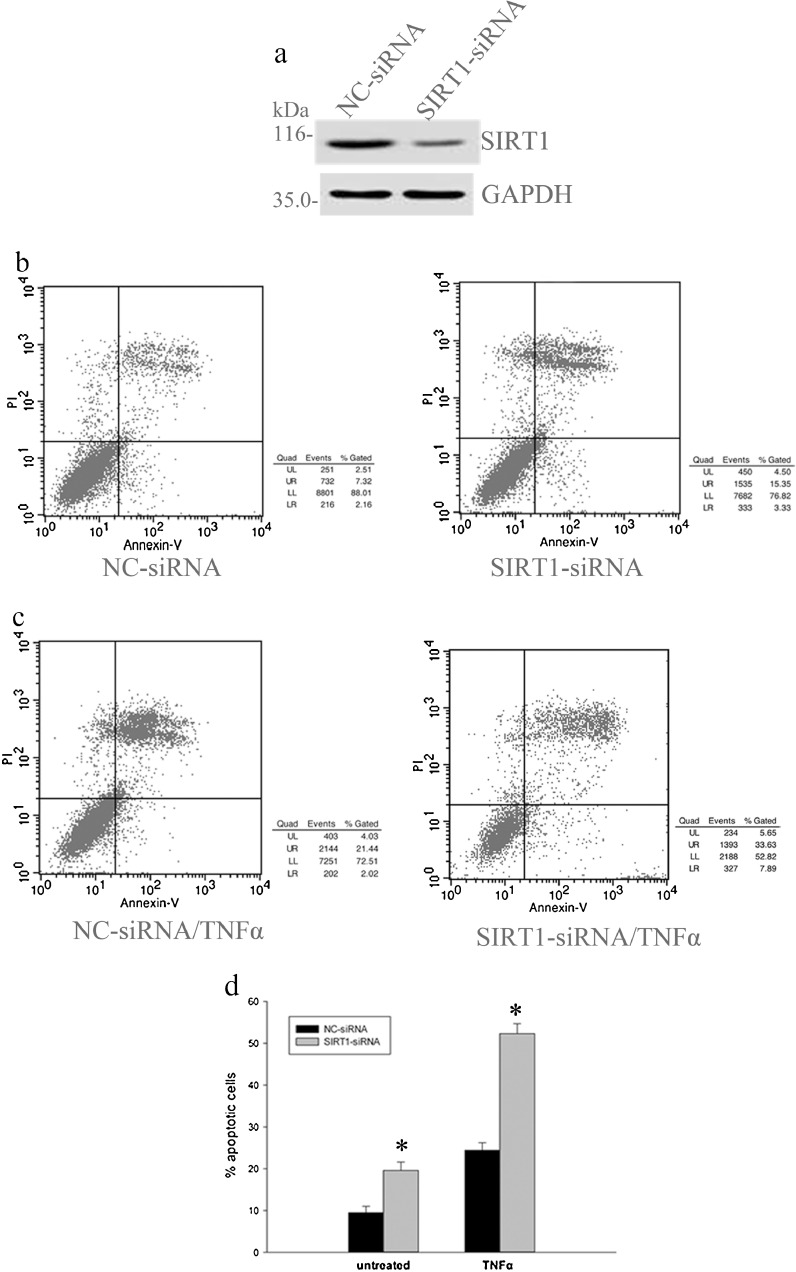
In order to confirm the SIRT1 effects on apoptosis of human degenerative DDD NP cells, we analyzed whether knockdown of endogenous SIRT1 by siRNA could affect the apoptosis rate of degenerative human NP cells. The level of SIRT1 protein expression was greatly reduced after transfection of siRNA for SIRT1 for 48 h, but the level of GAPDH was not affected (Fig. 4a). Annexin V/PI double-staining flow cytometry analysis demonstrated that apoptosis levels of DDD NP cells were significantly upregulated in transfection of siRNA for SIRT1 cells compared with that of LVF cells (Fig. 4b, d). Next, we tested whether SIRT1 siRNA transfection influences apoptotic sensitivity to tumor necrosis factor (TNFα). It was found that SIRT1 knockdown DDD NP cells increased, sensitive to apoptosis induced by TNFα (Fig. 4c, d). These results suggest that SIRT1 participates in the negative regulation of the apoptosis of degenerative NP cells.
Fig. 4.
Effects of SIRT1 siRNA transfection on apoptosis of degenerative human NP cells. Cells were transfected with negative control siRNA (NC-siRNA) or SIRT1-specific siRNA. Transfected degenerative NP cells were left untreated or treated with tumor necrosis factor-α (TNFα; 12 ng/ml) at 48 h after transfection. a The amounts of SIRT1 proteins were evaluated by Western blot analysis of total cell lysates. b–d The apoptotic percentage of degenerative NP cells determined by flow cytometry. Data represents mean ± SD of three independent experiments. Asterisk: significant difference with respect to NC-siRNA (p < 0.05)
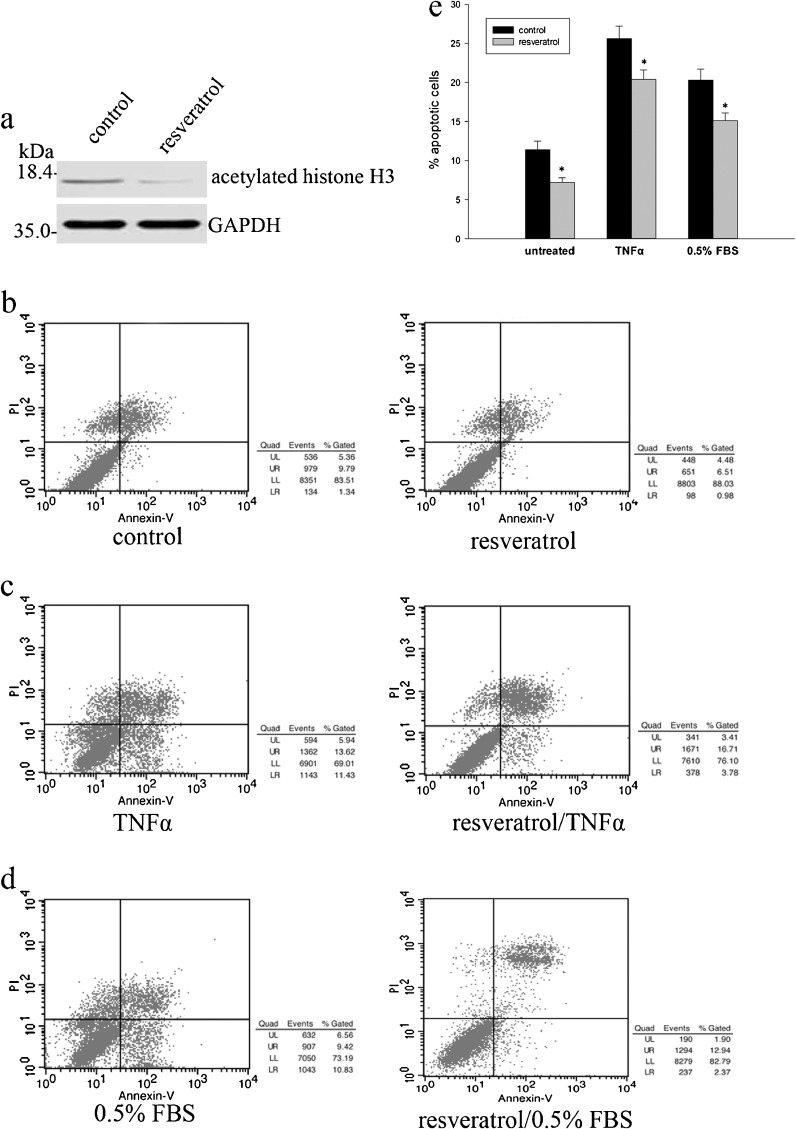
Treatment with resveratrol inhibits NP cells apoptosis levels
To further confirm the involvement of SIRT1 in the regulation of apoptosis of degenerative NP cells, we investigated the effect of SIRT1 activator, resveratrol, on NP cells apoptosis. Treatment with resveratrol reduced the levels of acetylated histone H3 (Fig. 5a). Annexin-V/PI-based apoptosis also revealed decreased apoptosis in resveratrol-treated DDD NP cells compared to that of LVF NP cells (Fig. 5b, e). To test if treatment with resveratrol is also involved in induction of apoptosis by inflammatory cytokines in DDD NP cells, we examined the effects of treatment with resveratrol on TNFα-induced cells apoptosis. Pretreatment with resveratrol significantly attenuated TNFα-stimulated cells apoptosis induction compared to that without treatment (Fig. 5c, e). Furthermore, apoptosis was also induced in DDD NP cells cultured under low serum conditions (0.5 % FBS), and the effect of addition of resveratrol on cell apoptosis was tested by flow cytometry analysis. It was found interesting that treatment with resveratrol exerted protective effect of degenerative NP cells against apoptosis induced by low nutrient condition (Fig. 5d, e). These results also suggested that SIRT1 activation inhibits apoptosis of degenerative human NP cells.
Fig. 5.
Effects of SIRT1 activator, resveratrol, on apoptosis of degenerative human NP cells. Cells were treated with resveratrol (8 μM) for 48 h. a The levels of acetylated histone H3 in total cell lysates were analyzed by Western blot analysis. b, c, e Cells were pretreated with resveratrol (8 μM) for 48 h, and then treated with or without TNFα (12 ng/ml) for 12 h, and the percentage of apoptotic cells was analyzed. d, e Degenerative NP cells treated with resveratrol were also cultured under low serum culture medium [0.5 % fetal bovine serum (FBS)], and the percentage of apoptotic cells was analyzed. Data represents mean ± SD of three independent experiments. Asterisk: significant difference with respect to control (p < 0.05)
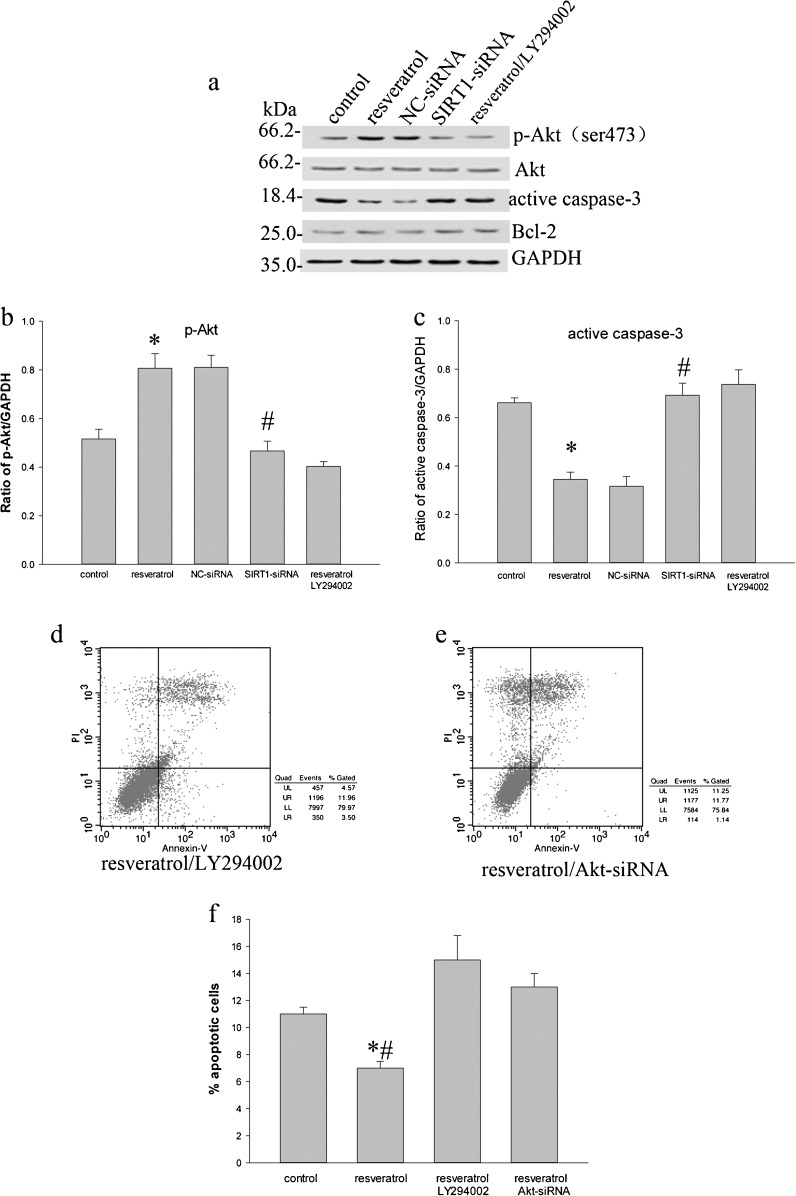
SIRT1 administration inhibits caspase-3 activation on NP cells of DDD
Since caspase-3 is known as a critical executioner of apoptosis, to observe the sensitivity of SIRT1 in cleaving caspase-3 protein levels, the resveratrol administration inhibited caspase-3 activation in DDD NP cells (Fig. 6a, c) by Western blotting. It was shown that the inhibitory effect was partly reverted by the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 administration (Fig. 6a, c). When SIRT1 siRNA were transfected, upregulation of cleaved caspase-3 level was observed in SIRT1-knockdown cells (Fig. 6a, c). In addition, it is interesting that no significant changes were observed for Bcl-2 expression in the total protein extracted from cells by treatment with resveratrol, or SIRT1 siRNA transfection (Fig. 6a).
Fig. 6.
Phosphorylated Akt (p-Akt), Akt, active caspase-3, and Bcl-2 in degenerative human NP cells detected by Western blotting. a The amounts of phosphorylation of Akt at Ser473 increased when treated with resveratrol. a Treatment with resveratrol + LY294002 (10 μmol/L) or SIRT1 siRNA inhibited phosphorylation of Akt at Ser473. Conversely, resveratrol attenuated active caspase-3, and resveratrol + LY294002 (10 μmol/L) or SIRT1 siRNA active caspase-3. a Different treatments did not cause obvious changes in the amount of Bcl-2 in total cell lysates. b, c The relative density of p-Akt and active caspase-3 is expressed as the ratio (p-Akt/GAPDH, active caspase-3/GAPDH). d–f Apoptotic rate was measured by flow cytometry after treatment with resveratrol + LY294002 or Akt-siRNA (see also Fig. 5b). Data represents mean ± SD of three independent experiments. Asterisk: significant difference with respect to control or resveratrol + LY294002 group (p < 0.05). Number sign: significant difference with respect to NC-siRNA group (p < 0.05). Asterisk and number sign: significant difference with respect to control, resveratrol + LY294002, or Akt siRNA group (p < 0.05)
SIRT1 increases survival of degenerative human NP cells through Akt pathway
PI3K/Akt pathway has demonstrated a signaling event controlling cell survival and apoptosis. To investigate the signaling pathway underlying the SIRT1-mediated degenerative human NP cells rescue from apoptosis, we examined whether SIRT1 could result in changes of Akt phosphorylation. Western blotting using an antibody to phosphorylation of Akt at Ser473 revealed that treatment with resveratrol increased the phosphorylation of Akt (Fig. 6a, b). However, the resveratrol administered with LY294002 (10 μmol/L) inhibited resveratrol-induced Akt phosphorylation (Fig. 6a, b). Afterwards, we also explored the effect of SIRT1 siRNA transfection on Akt phosphorylation. As a result, an apparent decrease was observed for Akt phosphorylation in SIRT1-knockdown cells compared to that of the negative control siRNA (NC-siRNA)-treated cells (Fig. 6a, b). Next, we further examined whether apoptosis of human degenerative NP cells might be affected by LY294002 or Akt siRNA. By addition of LY294002 for 10 h after treatment with resveratrol for 48 h, we observed that treatment with LY294002 inhibited cells survival (Figs. 5b and 6d, f). Similarly, Akt knockdown following resveratrol treatment reduced protective effect of resveratrol on human degenerative NP cells (Figs. 5b and 6e, f). These results indicate that the anti-apoptosis property of SIRT1 is attributable to stimulation of the PI3K/Akt pathway.
Discussion
SIRT1, an enzyme that removes acetyl groups from certain histones and non-histone proteins, is involved in many cellular functions, including cell differentiation and proliferation, apoptosis, and cell aging (Michan and Sinclair 2007; Glozak and Seto 2007; Longo and Kennedy 2006; Furukawa et al. 2007; Saunders and Verdin 2007; Anastasiou and Krek 2006; Qin et al. 2006; Smith and Denu 2006). However, no study has demonstrated its relationship with NP cells in degenerative human IVD.
In our study, samples of degenerative NP were obtained from patients, diagnosed as DDD by magnetic resonance imaging and clinical manifestations, undergoing nucleotomy and intervertebral fusion surgery to relieve chronic LBP. In fact, obtaining absolutely healthy human NP cells for biologic research is difficult. Therefore, relatively normal control NP obtained from young patients (<25 years old) with LVF undergoing posterior nucleotomy, spinal fusion, decompression, and stability within 24 hours of trauma, without documented clinical history of LBP, was used as control. Our study is the first to analyze expression variation of SIRT1 in the degenerative NP and normal NP. We found that the levels of SIRT1 mRNA and protein were decreased markedly both in surgical NP tissues and cultured NP cells from the degenerative NP relative to the normal control NP. We also observed changes in COL2A1, aggrecan, and MMP2 levels in the degenerative NP, and these results are consistent with the findings of previous studies (Pokharna and Phillips 1998; Buckwalter 1995; Pearce et al. 1987; Cs-Szabo et al. 2002; Antoniou et al. 1996; Roughley et al. 2006; Wallach et al. 2003; Crean et al. 1997; Kanemoto et al. 1996; Le Maitre et al. 2004). In a word, these data suggest that expression levels of SIRT1 in human NP gradually decrease with increasing age and degeneration.
It is well established that SIRT1 plays an important role in apoptosis and aging. For example, SIRT1 exerts a negative regulatory role in the production of MMP-1 and MMP-3 in human dermal fibroblasts (Ohguchi et al. 2010), in the apoptosis of human chondrocytes (Takayama et al. 2009; Gagarina et al. 2010), in cardiac myocyte apoptosis (Alcendor et al. 2007), in vascular endothelial cell senescence (Ota et al. 2010), and in axonal degeneration in neurons (Araki et al. 2004). However, to our knowledge, the effect of SIRT1 on apoptosis of degenerative human NP cells has never been reported.
Although more work is needed to further investigate the pathophysiology of degenerative disc disease, previous studies show that the number of cells in the disc declines progressively during disc aging and degeneration, a change attributable to both NP cells necrosis and apoptosis (Gruber and Hanley 1998, 2003; Zhao et al. 2006, 2007; Park et al. 2001a, b; Gruber et al. 2002; Hohaus et al. 2008). Similarly, we found that the incidence of apoptotic cell of degenerative NP specimens was significantly greater compared with the normal control. Since there is an inverse relationship between SIRT1 and cellular apoptosis in human IVD NP cells, we speculate that SIRT1 also exerts its control on cellular apoptosis processes in degenerative human NP. Indeed, treatment with resveratrol, a natural product known to activate many cellular proteins including SIRT1, leads to a reduction in levels of apoptosis and activation of caspase-3 in degenerative human NP cell, similar role of enhancing survival in chondrocyte (Takayama et al. 2009; Gagarina et al. 2010; Csaki et al. 2008; Dave et al. 2008). In contrast, apoptosis levels of degenerative human NP cells were significantly upregulated in treatment with SIRT1 siRNA transfection or NAM cells compared with control cells. Similarly, transfection of siRNA for SIRT1 cells increase activation of caspase-3 compared with the control. These observations imply that SIRT1 might protect against apoptosis of degenerative human NP cells. In a word, our result agrees with others suggesting that SIRT1 could have a pro-survival role in cells.
PI3K/Akt is known as one of the principal signaling molecules for cell proliferation and survival mediated by extracellular stimuli (Parcellier et al. 2008). Moreover, it has been recently reported that suppression of PI3K/Akt signaling pathway activity resulted in decreased proteoglycan matrix deposition and a significant reduction in aggrecan gene expression by NP cells (Cheng et al. 2009). Thus, we reasoned that PI3K/Akt pathway might involve in SIRT1-dependent protective role in human NP cells. Indeed, we discovered that treatment with resveratrol increased the phosphorylation of Akt at Ser473, while treatment with LY294002 following resveratrol partly inverted the role of Akt phosphorylation induced by resveratrol in degenerative human disc NP cells. Furthermore, SIRT1 siRNA transfection, which enhanced the caspase-3 activation and decreased anti-apoptosis property of SIRT1, inhibited phosphorylation of Akt at Ser473. Similar to other findings, SIRT1 can also activate PI3K/Akt pathway to promote survival of human osteoarthritic chondrocytes (Gagarina et al. 2010) to inhibit endothelial senescence (Ota et al. 2010).
Since Bcl-2 is known as anti-apoptosis protein related to mitochondria, we also tested regulation of Bcl-2 by SIRT1 in apoptosis of degenerative human NP cells. Surprisingly, we did not find obvious changes in the expression of Bcl-2 protein in the total protein extracted from cells with different treatment. These findings are in line with a recent study that SIRT1 exerts inapparent effect on Bcl-2 protein in the total protein extracted from chondrocytes (Gagarina et al. 2010). We did not investigate further the expression levels of Bcl-2 in the mitochondrial fraction in our paper.
Another drawback of this study is that SIRT1 plasmid transfection experiments by Lipofection 2000 were not successfully conducted because of extremely low transfection efficiency. Consequently, activator of SIRT1, resveratrol, was substituted for SIRT1 plasmid transfection in our study. To get more credible experiment results, it is no doubt that we should choose more safe and efficient SIRT1 plasmid transfection manner, for example, Nucleofector technology.
To elucidate exactly the mechanism between SIRT1 and NP cells apoptosis, we need more research to further investigate activation state of the PI3K/Akt signaling pathway upstream and downstream molecules.
Together, these results demonstrate that SIRT1 is an important mediator of apoptosis of degenerative human disc NP cells. SIRT1 is able to regulate PI3K/Akt signaling in degenerative human NP cells. Thus, SIRT1 may be a novel potential research target for further understanding of the pathophysiology of degenerative disc disease.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (NSFC; 81171751).
References
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology. 2006;21:404–410. doi: 10.1152/physiol.00031.2006. [DOI] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Bibby SR, Jones DA, Lee RB, Yu J, Urban JPG. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68:537–542. doi: 10.1016/S1297-319X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Cheng CC, Uchiyama Y, Hiyama A, Gajghate S, Shapiro IM, Risbud MV. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc. J Cell Physiol. 2009;221:668–676. doi: 10.1002/jcp.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol. 2008;75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribisyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62:1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hanley EN., Jr Biologic strategies for the therapy of intervertebral disc degeneration. Expert Opin Biol Ther. 2003;3:1209–1214. doi: 10.1517/14712598.3.8.1209. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17:492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kanemoto M, Hukuda S, Komiya Y, Katsuura A, Nishioka J. Immunohistochemical study of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 human intervertebral discs. Spine. 1996;21:1–8. doi: 10.1097/00007632-199601010-00001. [DOI] [PubMed] [Google Scholar]
- Lauerman WC, Bradford DS, Ogilvie JW, Transfeldt EE. Results of lumbar pseudarthrosis repair. J Spinal Disord. 1992;5:149–157. doi: 10.1097/00002517-199206000-00001. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguchi K, Itoh T, Akao Y, Inoue H, Nozawa Y, Ito M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br J Dermatol. 2010;163:689–694. doi: 10.1111/j.1365-2133.2010.09825.x. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–2211. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Park JB, Chang H, Kim KW. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine. 2001;26:618–621. doi: 10.1097/00007632-200103150-00011. [DOI] [PubMed] [Google Scholar]
- Park JB, Kim KW, Han CW, Chang H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine. 2001;26:142–146. doi: 10.1097/00007632-200101150-00006. [DOI] [PubMed] [Google Scholar]
- Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine. 1998;23:1645–1648. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15:S326–S332. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- Smith BC, Denu JM. Sirtuins caught in the act. Structure. 2006;14:1207–1208. doi: 10.1016/j.str.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Aoki Y, Ohtori S. Resolving discogenic pain. Eur Spine J. 2008;17:428–431. doi: 10.1007/s00586-008-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]