Abstract
Iron accumulation has been implicated in the pathogenesis of demyelinating diseases. Therefore, we hypothesized that abnormal high cerebral iron deposition may be involved in the development of white matter hyperintensities (WMHs). We used R2* relaxometry to assess whether iron levels in different brain regions correlate with the severity of WMHs. This technique has been recently validated in a postmortem study to demonstrate in vivo brain iron accumulation in a quantitative manner. Fifty-two consecutive WMH patients and 30 healthy controls with 3-T magnetic resonance imaging (MRI) were reviewed in this study. We measured WMH volume (as a marker of the severity of WMHs) on MRI, and the transverse relaxation rate R2*, as an estimate of iron content in seven brain regions. We found that R2* in globus pallidus was associated with WMH volume after adjusting for sociodemographic variables (partial correlation coefficient = 0.521, P < 0.001) and in a multivariate analysis adjusted for common vascular risk factors (partial correlation coefficient = 0.572, P = 0.033). Regional R2* in globus pallidus was also significantly higher in WMHs than in controls (P = 0.042). Iron content in globus pallidus, as assessed by R2* relaxometry, is independently linked to the severity of WMHs in our cohort of patients, suggesting that iron deposition in the brain may play a role in the pathogenesis of WMHs. This may provide prognostic information on patients with WMHs and may have implications for therapeutic interventions in WMHs.
Keywords: White matter hyperintensities, Leukoaraiosis, Susceptibility-weighted imaging, Transverse relaxation rate, Iron, Ageing
Introduction
White matter hyperintensities (WMHs), visible as hyperintense areas on T2-weighted magnetic resonance imaging (MRI) scans or fluid attenuated inversion recovery (FLAIR), are commonly seen on brain MRI of elderly subjects (Vernooij et al. 2007) and clinically important. The prevalence and the degree of cerebral WMH increased with age. The Rotterdam Scan Study demonstrated that about 13 % were completely free of subcortical white matter lesions and 32 % were free of periventricular matter lesions for subjects aged between 60 and 70 years, whereas these percentages were 0–5 % for subjects aged between 80 and 90 years (de Leeuw et al. 2001). Their presence is associated with an increased risk of stroke and stroke recurrence, dementia, and mortality (Debette and Markus 2010). We also previously found that severe WMHs are associated with larger baseline hematoma volumes and, to a lesser extent, with hematoma growth in patients with spontaneous intracerebral hemorrhage (Lou et al. 2009). However, the pathogenic mechanisms underlying WMHs are not well defined and most of current knowledge is based on postmortem MRI–pathology correlation studies. In regions of WMHs, pathological findings include myelin pallor, tissue rarefaction associated with loss of myelin and axons, and mild gliosis which is likely involved in the phagocytosis of myelin breakdown products (Erkinjuntti et al. 1996; Matsusue et al. 2006).
Iron is present in high concentrations within the oligodendrocytes and myelin (Dwork et al. 1988; Francois et al. 1981; LeVine and Macklin 1990) and is associated with the biosynthetic enzymes of myelinogenesis (Connor et al. 1995). Recently, histochemical studies have revealed that abnormal brain iron deposits are involved in the pathogenesis of demyelinating diseases (Izawa et al. 2010; LeVine and Chakrabarty 2004). Brain iron accumulation has also been reported in many neurological conditions, such as Parkinson’s disease, Alzheimer’s disease, and multiple systems atrophy. Therefore, we hypothesized that iron accumulation in the brain may be associated with the severity of WMHs.
Improved MRI techniques, such as susceptibility-weighted imaging (SWI) (Haacke et al. 2004; Ogg et al. 1999) and QSM (de Rochefort et al. 2010; Langkammer et al. 2012), made it possible to assess brain iron deposition accurately. These methods are very sensitive to iron in the forms of hemosiderin, ferritin, and deoxyhemoglobin. Recently, a quantitative MRI performed in human postmortem brains in situ showed that R2* is the preferred parameter for the assessment of iron concentration in vivo because R2* is more sensitive than R2 to variations in brain iron concentration (r2 = 0.67 for R2, r2 = 0.90 for R2*) (Langkammer et al. 2010). Thus, we used SWI R2* imaging in the present study to investigate the relationship between brain iron deposition in the iron-rich regions and the volume of WMHs.
Methods
Patients
This was an investigator-initiated, prospective, single-center study. The study protocol was reviewed and approved by our hospital ethics committee. We reviewed brain MRI for all patients admitted to our department from January 2010 to December 2011 and identified those with WMHs on MRI. We then enrolled those who met all of the following inclusion and none of the exclusion criteria into this analysis. Inclusion criteria were (1) WMH on MRI, (2) age >30, and (3) agreement to give written informed consent. Exclusion criteria were (1) patients with secondary causes of white matter lesions, such as immunological, demyelinating, metabolic, toxic, infectious, and other causes; (2) patients with abnormal brain MRI findings such as space-occupying lesions, head trauma, hemorrhage, or infarction (except lacunar infarction); (3) evidence of calcification on the CT scans or encephalomalacia in the deep gray matter structures which may influence the calculation of R2* values; and (4) patients with known iron deficiency anemia, with previous or concurrent use of iron chelators, iron supplements, or chalybeate.
We retrieved baseline clinical, demographic, laboratory, and radiological data including age, gender, years of education, the comorbid conditions such as history of hypertension, diabetes mellitus and hyperlipidemia, serum glucose level, systolic and diastolic blood pressure, total cholesterol, total homocysteine, and high-sensitivity C-reactive protein. All patients underwent a MMSE (Folstein et al. 1975).
Thirty healthy adults served as controls for the regional brain iron distribution after giving written informed consent. They were recruited for our previous study (Yan et al. 2012) or served as volunteers for our ongoing fMRI projects and had normal clinical and MRI examinations.
MRI procedures
All MRI studies were performed on a 3.0-T system (Signa Excite HD, General Electric Medical System, Milwaukee, USA) equipped with an eight-channel phased array head coil. Foam pads were inserted into the space between the subject’s head and the MRI head coil to minimize head motion. T2 FLAIR images with the following parameters were used to detect and measure the WMH volume: repetition time = 10,000 ms, echo time = 150 ms, inversion time = 2,500 ms; matrix size = 256 × 256, field of vision (FOV) = 240 × 240 mm2, slice thickness = 5.0 mm with no gap between slices, and in-plane spatial resolution of 0.4688 × 0.4688 mm/pixel. The whole brain was imaged.
The susceptibility-weighted MR images were taken parallel to the anterior–posterior commissural line and covered the nuclei of the basal ganglia, using a three-dimensional multiecho gradient-echo sequence with 11 equally spaced echoes: echo time = 4.5 ms (first echo), interecho spacing = 4.5 ms, repetition time = 58 ms, flip angle = 20°, matrix size = 256 × 256, FOV = 240 × 240 mm2, slice thickness = 2.0 mm with no gap between slices, and in-plane spatial resolution of 0.4688 × 0.4688 mm/pixel. Flow compensation was applied. R2* images were acquired and all of the data were used in further analysis.
Volumetric assessments of WMHs
We processed axial T2 FLAIR images for the volumetric quantification of WMH volume according to the published methods (Decarli et al. 1992, 1995). Briefly, a segmentation threshold for WMHs was determined a priori as three standard deviations (SDs) in pixel intensity above the mean of the fitted distribution of brain parenchyma as described previously (Decarli et al. 1995; Wen and Sachdev 2004). We measured both the AWMHV and the corrected WMH volume (CWMHV) since the intracranial volume (ICV) of each individual was not the same. The following equation was used: CWMHV = (WMHV × mean ICV) / ICV. The mean ICV is the mean value of ICV of all patients.
Measurement of R2* on MRI as an estimate of iron content
The raw SWI data were transferred to a separate workstation (ADW4.4, GE) where the reconstructed R2* map was obtained by a custom-built program. As described previously (Yan et al. 2012), we draw the regions of interests (ROIs) on the R2* map and R2* values were then measured automatically. The ROIs included the bilateral red nucleus (RN), substantia nigra (SN), globus pallidus (GP), putamen (PU), head of caudate (CA), thalamus (TH), and frontal white matter (FWM). The basal ganglia were chosen because they have high iron content and are easily visible on MR images (Bizzi et al. 1990; Hallgren and Sourander 1958). TH and FWM were selected to add regions of different iron level. The ROIs were drawn manually due to their different sizes and iron distributions in different individuals. R2* values were also measured in three areas: hyperintense white matter in WMH patients, normal white matter in WMH patients, and normal white matter in healthy controls. Two consecutive slices were used, and four measurements were done on the white matter with a total 80-mm2 square ROI and then averaged to obtain a final value.
Reliability and validity of the radiological measurements
A single trained operator (S.Y.), blinded to the participants’ age, sex, and clinical status, performed all quantitative assessments of 30 patients twice, at an interval of 3 months apart. Another operator (J.S.) independently made the measurements on the same patients. The interobserver intraclass correlation coefficients (ICCs) were 0.97 for WMH volume and 0.96 for R2*. The intraobserver ICCs were 0.95 for WMH volume and 0.93 for R2*. ICCs were described in detail elsewhere (Lee et al. 1989).
Statistical analysis
Since the CWMHV was skewed towards the left of mean, we performed natural log transformations of CWMHV before the correlation analysis. The log-transformed CWMHV appeared to be acceptably normative (a continuous probability distribution that has a bell-shaped probability density function). Pearson’s correlation analysis was used to investigate the relationship between CWMHV and continuous variables (that take on infinite number of different values), while independent samples’ two-tailed t test was used for CWMHV and dichotomous variables (that have only two values, generally 0 and 1). Bonferroni correction was used for comparison between multiple groups. Covariance (ANOVA) analysis was used to compare R2* from different tissue types. We also performed partial Pearson’s correlation analysis (that measures the degree of association between two random variables, with the effect of a set of controlling random variables removed) (Baba et al. 2004) to examine whether regional R2* is independently associated with CWMHV, by adjusting sociodemographic and vascular risk factors. All analyses were performed blinded to participant identifying information. Statistical significance was set at a probability value of ≤0.05.
Results
Patient characteristics
Fifty-two consecutive WMH patients were finally enrolled in this study. The reasons for admission of those patients were memory loss (n = 14, 26.9 %), gait disturbances (n = 8, 15.4 %), dizziness (n = 11, 21.2 %), incidental finding of WMHs on MRI (n = 7, 13.5 %), or mixed reasons above (n = 12, 23.1 %). The age of the patients ranged from 30 to 88 years, and the CWMHV ranged from 2.75 to 112.17 mL (mean = 35.95, SD = 31.85). Table 1 shows the baseline variables including the regional R2* and their associations with CWMHV.
Table 1.
Baseline sociodemographic variables, vascular risk factors, and regional R2* in relation to CWMHV
| Variable | Values (n (%) or mean ± SD) | CWMHV (mL) (mean ± SD) or correlation coefficient | P value |
|---|---|---|---|
| Gender | 0.134 | ||
| Female | 27 (51.9 %) | 42.25 ± 37.30 | |
| Male | 25 (48.1 %) | 29.14 ± 23.57 | |
| Hypertension | 0.651 | ||
| Yes | 42 (80.8 %) | 35.33 ± 31.11 | |
| No | 10 (19.2 %) | 41.19 ± 41.68 | |
| Diabetes mellitus | 0.802 | ||
| Yes | 9 (17.3 %) | 39.00 ± 36.32 | |
| No | 43 (82.7 %) | 35.58 ± 32.30 | |
| Hyperlipidemia | 0.739 | ||
| Yes | 19 (36.5 %) | 35.86 ± 29.86 | |
| No | 33 (63.5 %) | 32.58 ± 31.93 | |
| Age (years) | 66.02 ± 11.93 | r = 0.036 | 0.798 |
| Years of education (years) | 5.98 ± 3.92 | r = 0.146 | 0.303 |
| Mini-mental state examination score | 24.52 ± 4.04 | – | – |
| Systolic blood pressure (mmHg) | 143.73 ± 15.67 | r = −0.174 | 0.252 |
| Diastolic blood pressure (mmHg) | 85.04 ± 10.57 | r = −0.019 | 0.902 |
| Glucose (mmol/L) | 5.56 ± 2.19 | r = −0.027 | 0.862 |
| Total cholesterol (mmol/L) | 4.48 ± 0.98 | r = 0.000 | 0.999 |
| Total homocysteine (μmol/L) | 13.35 ± 3.88 | r = −0.208 | 0.231 |
| High-sensitivity C-reactive protein (mg/L) | 11.77 ± 13.77 | r = −0.180 | 0.254 |
| R2* measurements (s−1) | |||
| Red nucleus | 34.68 ± 5.45 | r = 0.275 | 0.048 |
| Substantia nigra | 38.04 ± 7.14 | r = 0.147 | 0.297 |
| Globus pallidus | 44.50 ± 6.40 | r = 0.505 | <0.001 |
| Putamen | 32.59 ± 6.08 | r = 0.209 | 0.136 |
| Head of caudate | 25.70 ± 4.27 | r = −0.184 | 0.191 |
| Thalamus | 18.74 ± 1.94 | r = 0.046 | 0.745 |
| Frontal white matter | 18.87 ± 1.89 | r = −0.152 | 0.283 |
Log-transformed CWMHV was used to calculate Pearson’s r value
Regional R2* correlates with brain iron level
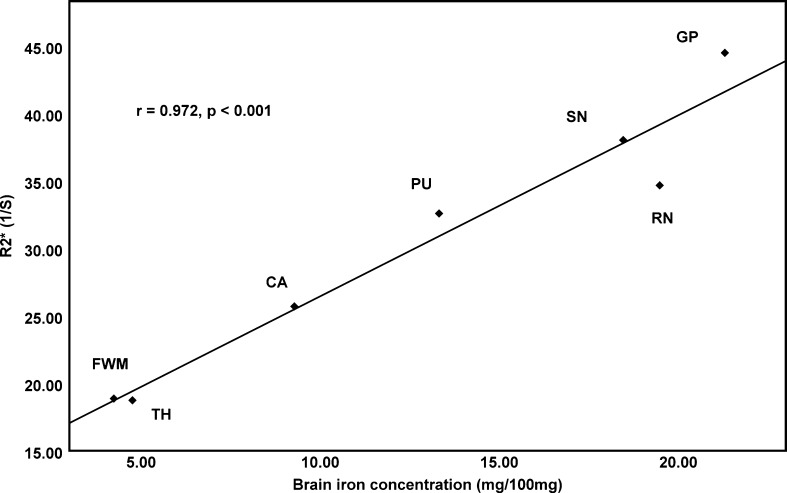
We applied Pearson’s correlation analysis to determine the correlation between our regional R2* values and iron concentration of different brain regions, as previously assessed by biochemical methods (Hallgren and Sourander 1958). We found a strong positive correlation between R2* and previously published iron concentrations in seven brain regions (r = 0.972, P < 0.001; Fig. 1), supporting the use of R2* as a viable parameter to estimate iron content in the human brain. In addition, brain regions, such as GP, PU, RN, and SN, with significantly higher iron content had a higher R2*, as compared with FWM and TH (Table 1).
Fig. 1.
The scatter plot between R2* and brain iron concentration as published by Hallgren and Sourander (1958). CA caudate, PU putamen, GP globus pallidus, TH thalamus, RN red nucleus, SN substantia nigra, FWM frontal white matter
Relationship between R2* and WMHs
As seen in Table 1, the correlation was significant between CWMHV and R2* in GP (r = 0.505, P < 0.001) and in RN (r = 0.275, P = 0.048). No significant correlation was observed between CWMHV and other variables. Table 2 presents the results of different partial Pearson’s correlation analysis models. In the analysis adjusted for age, sex, and years of education, log-transformed CWMHV was positively associated with R2* in GP and RN. The partial correlation coefficients were 0.521 (P < 0.001) and 0.321 (P = 0.025), respectively. Further adjustment for baseline vascular risk factors yielded similar results for R2* in GP (partial correlation coefficients = 0.572, P = 0.033), but the association between log-transformed CWMHV and R2* in RN became weaker (partial correlation coefficients = 0.302, P = 0.183). Examples were given in Fig. 2.
Table 2.
Associations between regional R2* and CWMHV using partial Pearson correlation analysis models
| Brain regions | Log-transformed corrected WMH volume | ||
|---|---|---|---|
| Partial Pearson correlation (model 1, r (P)) | Partial Pearson correlation (model 2, r (P)) | Partial Pearson correlation (model 3, r (P)) | |
| Red nucleus | 0.281 (0.046) | 0.321 (0.025) | 0.302 (0.183) |
| Substantia nigra | 0.148 (0.299) | 0.178 (0.221) | 0.274 (0.229) |
| Globus pallidus | 0.510 (<0.001) | 0.521 (<0.001) | 0.572 (0.033) |
| Putamen | 0.206 (0.146) | 0.209 (0.150) | 0.291 (0.200) |
| Head of caudate | −0.194 (0.172) | −0.229 (0.114) | −0.139 (0.548) |
| Thalamus | 0.065 (0.652) | 0.078 (0.595) | −0.028 (0.903) |
| Frontal white matter | −0.148 (0.301) | −0.146 (0.317) | −0.107 (0.644) |
Model 1 adjusted for age, Model 2 adjusted for age and other sociodemographic variables (sex and years of education), Model 3 adjusted for age, sex, years of education, and vascular risk factors (hypertension, diabetes, hyperlipidemia, serum glucose level, systolic and diastolic blood pressure, total cholesterol, total homocysteine, and high-sensitivity C-reactive protein)
Fig. 2.
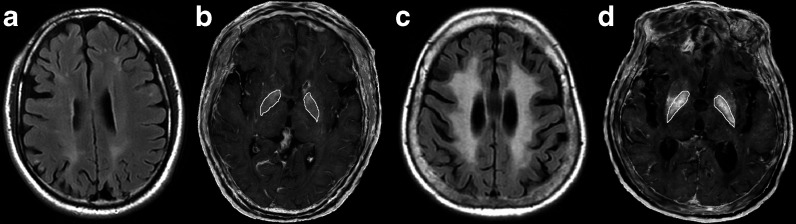
FLAIR image (a, c) and R2* maps (b, d) of two WMH patients. The CWMHV was 88.51 mL (c) and 2.79 mL (a). Mean R2* in the globus pallidus was increased (52.88 vs 37.81) in the severe WMH patient (d), compared with mild WMH patient (b), which exemplarily reflected increased iron deposition in WMH. All maps are scaled identically. The ROIs of the globus pallidus were illustrated
Comparison of R2* between WMHs and healthy control
As presented in Table 3, regional R2* in globus pallidus was significantly higher in WMHs than in controls. Based on this finding, we used regional R2* in globus pallidus for further analyses in relation to sociodemographic variables and vascular risk factors in WMH patients, as shown in Table 4. No significant correlations were observed. Regional R2* values were also compared in hyperintense white matter in WMH patients (mean = 18.47, SD = 1.93), normal white matter in WMH patients (mean = 18.76, SD = 1.97), and normal white matter in healthy controls (mean = 19.16, SD = 1.66). However, no significant difference was found among them (P = 0.383).
Table 3.
Comparison of regional R2* between WMHs and control group
| WMHs group (n = 52) | Control group (n = 30) | P value | |
|---|---|---|---|
| Female | 27 (51.9 %) | 16 (53.3 %) | 0.902 |
| Ages (years) | 66.02 ± 11.93 | 66.47 ± 11.01 | 0.561 |
| R2* measurements (s−1) | |||
| Red nucleus | 34.68 ± 5.45 | 33.52 ± 5.24 | 0.159 |
| Substantia nigra | 38.04 ± 7.14 | 36.63 ± 4.82 | 0.293 |
| Globus pallidus | 44.50 ± 6.40 | 41.75 ± 4.53 | 0.042 |
| Putamen | 32.59 ± 6.08 | 30.59 ± 4.45 | 0.129 |
| Head of caudate | 25.70 ± 4.27 | 23.68 ± 3.45 | 0.046 |
| Thalamus | 18.74 ± 1.94 | 16.91 ± 2.32 | <0.001 |
| Frontal white matter | 18.87 ± 1.89 | 18.53 ± 1.67 | 0.417 |
Table 4.
The relationship between baseline sociodemographic variables, vascular risk factors, and regional R2* in globus pallidus
| Variable | Values (n (%) or mean ± SD) | R2* in GP (s−1) (mean ± SD) or correlation coefficient | P value |
|---|---|---|---|
| Gender | 0.792 | ||
| Female | 27 (51.9 %) | 44.28 ± 6.33 | |
| Male | 25 (48.1 %) | 44.75 ± 6.61 | |
| Hypertension | 0.272 | ||
| Yes | 42 (80.8 %) | 44.98 ± 6.71 | |
| No | 10 (19.2 %) | 42.49 ± 4.66 | |
| Diabetes mellitus | 0.299 | ||
| Yes | 9 (17.3 %) | 46.54 ± 5.82 | |
| No | 43 (82.7 %) | 44.08 ± 6.50 | |
| Hyperlipidemia | 0.184 | ||
| Yes | 19 (36.5 %) | 46.07 ± 5.94 | |
| No | 33 (63.5 %) | 43.60 ± 6.57 | |
| Age (years) | 66.02 ± 11.93 | r = −0.076 | 0.591 |
| Years of education (years) | 5.98 ± 3.92 | r = 0.070 | 0.622 |
| Systolic blood pressure (mmHg) | 143.73 ± 15.67 | r = −0.012 | 0.940 |
| Diastolic blood pressure (mmHg) | 85.04 ± 10.57 | r = 0.142 | 0.352 |
| Glucose (mmol/L) | 5.56 ± 2.19 | r = 0.104 | 0.507 |
| Total cholesterol (mmol/L) | 4.48 ± 0.98 | r = −0.011 | 0.943 |
| Total homocysteine (μmol/L) | 13.35 ± 3.88 | r = −0.245 | 0.155 |
| High-sensitivity C-reactive protein (mg/L) | 11.77 ± 13.77 | r = −0.003 | 0.988 |
Discussion
In the current study, we found an independent association between iron content in globus pallidus, measured by R2* map and CWMH volume, suggesting that excessive iron deposits in the brain deep gray matter might be important in the pathogenesis of WMHs.
In several previous studies, SWI phase shifts were used to estimate iron concentration (Haacke et al. 2007; Pfefferbaum et al. 2009). However, the phase value also changes with the difference of the magnetic susceptibility of the surrounding tissue of interest, i.e., is related to the nonlocal distribution of iron (Haacke et al. 2009). In two reports, phase-sensitive methods did not correlate with iron content, compared with R2* (Yao et al. 2009) or FDRI technique (Pfefferbaum et al. 2009), and our previous study also revealed that phase value of SWI may not be used for assessing the iron content in some brain regions especially globus pallidus (Yan et al. 2012). We found a strong and statistically significant positive correlation between R2* and brain iron concentration (Hallgren and Sourander 1958) in all analyzed regions of the brain (r = 0.972, P < 0.001). Our results are consistent with previous studies (Langkammer et al. 2010) and highlight the potential utility of R2* technique as a viable and practical tool to assess in vivo regional brain iron content in patients.
Postmortem studies confirm that the basal ganglia, in particular GP, contain the highest levels of iron in the brain (Bizzi et al. 1990; Chen et al. 1993; Hallgren and Sourander 1958). Iron in the GP increases rapidly and almost reaches constant value after the age of 30 years in previous studies (Aquino et al. 2009). These observations also suggest that the iron content of GP may be an indicator of the total brain iron load. We also found that iron content in RN was slightly higher than that in SN, while R2* in RN was less than that in SN. This finding is consistent with another study with 3-T MRI (Gelman et al. 1999). We speculate that the different distribution of iron concentration in separated parts of RN (lower iron in the medullary lamellae) may cause the decrease of measured R2* in RN (Pu et al. 2000).
The mechanistic link(s) between iron deposition in the brain and WMHs are unclear. It is not known if it is the result or the cause of WMHs. The mechanism of damage to the brain by iron might be related, at least in part, to oxidative stress induced by the generation of reactive oxygen species (LeVine and Macklin 1990). However, iron overload can also damage the myelin sheath across the cerebrospinal region by triggering the immune cascade (Singh and Zamboni 2009). The iron deposits represent a powerful chemotactic stimulus that attracts macrophages laden with stored iron, thus, accelerating the damage of myelin (Adams 1988). A recent postmortem microarray RNA expression analysis found that approximately 10.6 % of WMH-related gene was involved in immune regulation (Xu et al. 2010). Biomarkers of inflammation were also observed to be increased in WMH patients (Fornage et al. 2008). It is, therefore, reasonable to speculate that iron overload might predispose to WMHs by activating the immune regulation and damaging the myelin in the periventricular (WMHs) regions. On the other hand, the source of increased iron deposition in WMHs may be myelin/oligodendrocyte debris and concentrated iron in the macrophages. The ischemic condition of white matter in WMHs may lead to accumulation of free radicals and acidosis, which are favorable for the deposition of iron. Because of the injury of white matter, it might be possible that iron is still taken up by deep gray matter structures, but its centrifugal transportation is impaired leading to local accumulation. Interestingly, there is evidence to suggest that iron deposition may be a consequence of altered cerebral venous return (Singh and Zamboni 2009) and that periventricular intraparenchymal venular disease correlates with the severity of WMHs (Black et al. 2009). Therefore, it is plausible that periventricular venular disease may be a possible cause of iron deposits in WMH. Further studies are clearly needed to elucidate the pathophysiologic link(s) and causal relationship between iron and WMHs.
Previous pathology study with ferric iron staining has shown the increased diffuse iron staining among WMH tissues, compared by normal WM in controls (Gebril et al. 2011). We thus compared the R2* values between hyperintense and healthy WM in both patients and controls, in order to directly investigate the relation between iron content and WMH volume. However, we did not find significant differences. There are some possible explanations for this finding. According to MR relaxation theory, R2* includes the effects due to both local magnetic field inhomogeneities and intrinsic tissue properties. R2* in the brain tissue with hyperintensities itself would thus be decreased in the WMH region. In addition, the estimation of iron concentrations in white matter regions was shown less accurate and more complex because the counteracting contribution from diamagnetic myelinated neuronal fibers confounds the interpretation (Langkammer et al. 2012). Our finding also suggests that direct measurement of local WM iron might not be a reliable indicator to reflect the severity of WMHs.
Our study has limitations. Firstly, we did not perform age-paired comparison of R2* between WMHs and normal control. The prevalence of WMH ranges from 68 to 87 % in adults aged between 60 and 70 years, whereas these percentages were more than 95 % for subjects aged between 80 and 90 years (de Leeuw et al. 2001). It is thus difficult to find normal old subjects for age-matched comparison. Secondly, the investigated cohort only included Chinese patients at a single institution who had symptoms or distinct imaging changes. Therefore, our cohort does not represent the full spectrum of WMHs and limits the generalizability of our results. Thirdly, we have not investigated the natural course over time of WMH based on the observation of R2*. Future longitudinal studies are needed to clarify the prediction of R2* on WMH progression.
Regardless, our finding of an independent association between iron deposits and WMH volume is novel and could have important prognostic and therapeutic implications. The amount of iron deposition could be used as a biomarker of the progression of WMHs. Moreover, our results open new avenues to explore the utility of iron-modifying agents as therapeutic interventions in WMHs (Selim et al. 2011).
Acknowledgments
We are grateful for the support from our patients and healthy volunteers. This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LR12H09001), the National Natural Science Foundation of China (grant numbers 81070915 and 81171095), the Science and Technology Department of Zhejiang Province (grant number 2008C14078), and the Health Bureau of Zhejiang Province (grant number WKJ2010-2-010).
Abbreviations
- WMHs
White matter hyperintensities
- SWI
Susceptibility-weighted imaging
- QSM
Quantitative susceptibility mapping
- GP
Globus pallidus
- MMSE
Mini-mental state examination
- AWMHV
Absolute white matter hyperintensity volume
- CWMHV
Corrected white matter hyperintensity volume
- ICV
Intracranial volume
References
- Adams CW. Perivascular iron deposition and other vascular damage in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1988;51(2):260–265. doi: 10.1136/jnnp.51.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252(1):165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Baba K, Shibata R, Sibuya M. Partial correlation and conditional correlation as measures of conditional independence. Aust New Z J Stat. 2004;46(4):657–664. doi: 10.1111/j.1467-842X.2004.00360.x. [DOI] [Google Scholar]
- Bizzi A, Brooks RA, Brunetti A, Hill JM, Alger JR, Miletich RS, Francavilla TL, Di Chiro G. Role of iron and ferritin in MR imaging of the brain: a study in primates at different field strengths. Radiology. 1990;177(1):59–65. doi: 10.1148/radiology.177.1.2399339. [DOI] [PubMed] [Google Scholar]
- Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- Chen JC, Hardy PA, Kucharczyk W, Clauberg M, Joshi JG, Vourlas A, Dhar M, Henkelman RM. MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. AJNR Am J Neuroradiol. 1993;14(2):275–281. [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer’s diseased brains. J Neurochem. 1995;65(2):717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu J, Wang Y. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging. Magn Reson Med. 2010;63(1):194–206. doi: 10.1002/mrm.22187. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/WNL.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Schon EA, Herbert J. Nonidentical distribution of transferrin and ferric iron in human brain. Neuroscience. 1988;27(1):333–345. doi: 10.1016/0306-4522(88)90242-4. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Benavente O, Eliasziw M, Munoz DG, Sulkava R, Haltia M, Hachinski V. Diffuse vacuolization (spongiosis) and arteriolosclerosis in the frontal white matter occurs in vascular dementia. Arch Neurol. 1996;53(4):325–332. doi: 10.1001/archneur.1996.00550040053014. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, Tracy RP, Longstreth WT., Jr Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke. 2008;39(7):1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Nguyen-Legros J, Percheron G. Topographical and cytological localization of iron in rat and monkey brains. Brain Res. 1981;215(1–2):317–322. doi: 10.1016/0006-8993(81)90510-2. [DOI] [PubMed] [Google Scholar]
- Gebril OH, Simpson JE, Kirby J, Brayne C, Ince PG. Brain iron dysregulation and the risk of ageing white matter lesions. Neuromol Med. 2011;13(4):289–299. doi: 10.1007/s12017-011-8161-y. [DOI] [PubMed] [Google Scholar]
- Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP, Knight RA. MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology. 1999;210(3):759–767. doi: 10.1148/radiology.210.3.r99fe41759. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52(3):612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Ayaz M, Khan A, Manova ES, Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F, Kirsch W. Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging. 2007;26(2):256–264. doi: 10.1002/jmri.22987. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30(1):19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3(1):41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Izawa T, Yamate J, Franklin RJ, Kuwamura M. Abnormal iron accumulation is involved in the pathogenesis of the demyelinating dmy rat but not in the hypomyelinating mv rat. Brain Res. 2010;1349:105–114. doi: 10.1016/j.brainres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257(2):455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62(3):1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Koh D, Ong CN. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med. 1989;19(1):61–70. doi: 10.1016/0010-4825(89)90036-X. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Macklin WB. Iron-enriched oligodendrocytes: a reexamination of their spatial distribution. J Neurosci Res. 1990;26(4):508–512. doi: 10.1002/jnr.490260415. [DOI] [PubMed] [Google Scholar]
- Lou M, Al-Hazzani A, Goddeau RP, Novak V, Selim M. Relationship between white-matter hyperintensities and hematoma volume and growth in patients with intracerebral hemorrhage. Stroke. 2009;41(1):34–40. doi: 10.1161/STROKEAHA.109.564955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, Ogawa T. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci. 2006;5(2):99–104. doi: 10.2463/mrms.5.99. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Langston JW, Haacke EM, Steen RG, Taylor JS. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging. 1999;17(8):1141–1148. doi: 10.1016/S0730-725X(99)00017-X. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. NeuroImage. 2009;47(2):493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Liu Y, Hou J, Fox PT, Gao JH. Demonstration of the medullary lamellae of the human red nucleus with high-resolution gradient-echo MR imaging. AJNR Am J Neuroradiol. 2000;21(7):1243–1247. [PMC free article] [PubMed] [Google Scholar]
- Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, Schlaug G, Torbey M, Waldman B, Xi G, Palesch Y. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42(11):3067–3074. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. J Cereb Blood Flow Metab. 2009;29(12):1867–1878. doi: 10.1038/jcbfm.2009.180. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. NeuroImage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Xu H, Stamova B, Jickling G, Tian Y, Zhan X, Ander BP, Liu D, Turner R, Rosand J, Goldstein LB, Furie KL, Verro P, Johnston SC, Sharp FR, Decarli CS. Distinctive RNA expression profiles in blood associated with white matter hyperintensities in brain. Stroke. 2010;41(12):2744–2749. doi: 10.1161/STROKEAHA.110.591875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SQ, Sun JZ, Yan YQ, Wang H, Lou M. Evaluation of brain iron content based on magnetic resonance imaging (MRI): comparison among phase value, R2* and magnitude signal intensity. PLoS One. 2012;7(2):e31748. doi: 10.1371/journal.pone.0031748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Li TQ, Gelderen P, Shmueli K, de Zwart JA, Duyn JH. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. NeuroImage. 2009;44(4):1259–1266. doi: 10.1016/j.neuroimage.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]