Abstract
In rats, as in humans, normal aging is characterized by a decline in hippocampal-dependent learning and memory, as well as in glutamatergic function. Both growth hormone (GH) and insulin-like growth factor-I (IGF-I) levels have been reported to decrease with age, and treatment with either GH or IGF-I can ameliorate age-related cognitive decline. Interestingly, acute GH and IGF-I treatments enhance glutamatergic synaptic transmission in the rat hippocampus of juvenile animals. However, whether this enhancement also occurs in old rats, when cognitive impairment is ameliorated by GH and IGF-I (des-IGF-I), remains to be determined. To address this issue, we used an in vitro CA1 hippocampal slice preparation and extracellular recording techniques to study the effects of acute application of GH and IGF-I on compound field excitatory postsynaptic potentials (fEPSPs), as well as AMPA- and NMDA-dependent fEPSPs, in young adult (10 months) and old (28 months) rats. The results indicated that both GH and IGF-I increased compound-, AMPA-, and NMDA-dependent fEPSPs to a similar extent in slices from both age groups and that this augmentation was likely mediated via a postsynaptic mechanism. Initial characterization of the signaling cascades underlying these effects revealed that the GH-induced enhancement was not mediated by the JAK2 signaling element in either young adult or old rats but that the IGF-I-induced enhancement involved a PI3K-mediated mechanism in old, but not young adults. The present findings are consistent with a role for a GH- or IGF-I-induced enhancement of glutamatergic transmission in mitigating age-related cognitive impairment in old rats.
Keywords: Plasticity, Postsynaptic, Phosphorylation, Glutamate receptors, Cell signaling
Introduction
It is well-established that both growth hormone (GH) and insulin-like growth factor-I (IGF-I) have important roles in mammalian growth and development. These polypeptide hormones play major roles in the regulation of cellular processes in tissues throughout the body (Gahete et al. 2009) including, but not limited to, protein synthesis (Lo and Ney 1996), regulation of bone metabolism (Ohlsson et al. 1998), immune and cardiovascular function (Cittadini et al. 1999; Conti et al. 2008; Hattori 2009). However, in addition to the peripheral effects of GH and IGF-I, these factors also impact the central nervous system, in particular, the hippocampus of adult animals (Lobie et al. 2000).
Cognitive function, including hippocampal-dependent learning and memory, declines with advancing age (Frick et al. 1995; Rosenzweig et al. 1997; Ramsey et al. 2004). Parallel to this age-related decline in hippocampal-dependent cognition, the levels of GH and IGF-I also decrease across lifespan (Sonntag et al. 1980; Richman et al. 1981; Carter et al. 2002; Sonntag et al. 2005; Ramsey et al. 2004). Importantly, GH and IGF-I supplementation in rodents have been reported to ameliorate hippocampal-dependent cognitive deficits associated with normal aging (Markowska et al. 1998; Thornton et al. 2000; Ramsey et al. 2004), and there has been debate on whether there is a direct role of GH in mediating these effects or whether GH acts through its anabolic mediator, IGF-I (Molina et al. 2011). One potential mechanism for the improvement of age-related cognitive impairment by GH and IGF-I is an enhancement of excitatory synaptic transmission. Excitatory synaptic transmission in the hippocampus is mediated primarily by glutamatergic receptors, principally the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartic acid (NMDA) types of the glutamate receptor. Subunits that comprise the AMPA and NMDA receptors in the hippocampus have been reported to decline with age (Foster 2002; Shi et al. 2007; Adams et al. 2008; Newton et al. 2008) and AMPA and NMDA receptor-mediated synaptic transmission is reduced as well (Barnes 1990; Barnes et al. 1992, 1997, 2000a, b; Bauman et al. 1992; Deupree et al. 1993; Papatheodoropoulos and Kostopoulos 1996; Jovenceau et al. 1998). Moreover, administration of either GH or IGF-I has been reported to increase mRNA and protein levels of those subunits (Sonntag et al. 2000; Le Grevès et al. 2005). Notably, GH and IGF-I can also enhance glutamatergic synaptic transmission in rat hippocampal slices of juvenile animals (Ramsey et al. 2005; Mahmoud and Grover 2006), an effect that may contribute to improved cognitive performance (Porrino et al. 2005; Goff et al. 2008; Hamlyn et al. 2009; Hampson et al. 2009).
The cognitive benefits of GH and IGF-I supplementation have been demonstrated in aged animals; however, the enhancement of hippocampal synaptic transmission by GH and IGF-I has been shown only in juvenile rats, and little is known about the effects of GH or IGF-I on glutamatergic synaptic transmission in adulthood or old age. The question then arises as to whether GH and IGF-I can augment synaptic transmission in old animals when chronic supplementation with these hormones ameliorates impaired hippocampal-dependent cognitive function. Thus, in the present study, we used extracellular recording methods to evaluate the effect of acute GH and IGF-I application on hippocampal glutamatergic synaptic transmission in brain slices from young adult and old rats. The results demonstrate that both GH and IGF-I enhance AMPA and NMDA function to a similar extent in young adult and old rats, supporting the notion that acute effects of either hormone may contribute to the reported GH and IGF-I mediated amelioration of age-related cognitive impairment. Our findings also provide initial evidence of possible age-dependent differences in the signaling cascades that mediate the effects of these hormones on rat glutamatergic synapses.
Methods
Experimental subjects and slice preparation
Juvenile (1–2 months), young (10 months) and old (28 months) male Fisher 344 × Brown Norway rats (F344 × BN) rats from the National Institute of Aging Colony at Harlan Sprague–Dawley were anesthetized with halothane and decapitated. The brain was removed and submerged in chilled, oxygenated artificial cerebrospinal fluid (aCSF) containing 124 mM NaCl, 3.3 mM KCl, 2.4 mM MgCl2, 2.5 mM Ca Cl2, 1.2 mM KH2PO4, 10-d-glucose, and 25.9 mM NaHCO3 and saturated with 95 % O2–5 % CO2. The right and left hemispheres were separated and cut coronally into 400-μm thick slices using a vibratome (Leica VT1000S; Vashaw Scientific, Atlanta, GA). The hippocampus in each slice was dissected from the surrounding tissue and was equilibrated in an incubation chamber with oxygenated aCSF at 20–25 °C for 90 min. During experiments, slices were placed in a recording chamber and perfused with oxygenated aCSF at a flow rate of 2 mL/min at 32 °C. All experiments were conducted in accordance with the Guidelines for the Care and Use of Experimental Animals and approved by the Institutional Animal Care and Use Committee.
Field excitatory postsynaptic potential recordings
Electrodes were prepared from filamented borosilicate glass capillary tubes (0.86 mm ID) using a horizontal micropipette puller (Sutter P-97; Sutter, Novato, CA) and filled with aCSF. Slices were transferred to a recording chamber, a twisted bipolar stimulating electrode (FHC, Bowdoinham, ME) was placed in the Schaffer collaterals of CA3, and Field excitatory postsynaptic potential (fEPSPs) were recorded from the stratum radiatum of CA1. Stimulation was adjusted to elicit ~30 % of the peak amplitude (pAmp), and responses were recorded every 20 s, prior to growth hormone (GH) or des-insulin-like growth factor-I (des-IGF-I) application. Compound (CMPD) responses were defined as fEPSPs elicited in the absence of either AMPA or NMDA inhibitors. For experiments evaluating AMPA- and NMDA-mediated synaptic transmission, appropriate inhibitors were added for 20 min (see “Drug preparation”) before a baseline was recorded. To ensure that NMDA and AMPA were effectively isolated AMPA- and NMDA-dependent fEPSPs were blocked using AMPA and NMDA receptor antagonists, respectively. In addition, CMPD responses were completely abolished by bath application of AMPA and NMDA receptor antagonists supporting that the CMPD response consisted of AMPA and NMDA fESPSPs, consistent with previous studies (Foy et al. 1999; Frazier et al. 1998; Alkondon et al. 2003; McQuiston 2010; data not shown). Baseline, under all conditions was defined as 10 min of stable recordings where the pAmp remained within 10 % of the mean. In the case of AMPA- and NMDA-dependent recordings, the baseline was recorded after 20 min of inhibitor application. GH or des-IGF-I was applied for 30 min following baseline recordings. The enhancement of CMPD, AMPA- and NMDA-dependent fEPSPs after GH or des-IGF-I application in young adult and old hippocampal slices was determined by comparing the last 5 min of GH or IGF-I application to the last 5 min of baseline. The same procedure for determining the GH and IGF-I enhancement was followed for all conditions. Paired-pulse ratio studies were conducted by delivering two consecutive stimuli with an interpulse interval of 50 ms. The ratio between the second stimulus pulse (P2) and the first stimulus (P1) was calculated in the presence and absence of GH or des-IGF-I.
Drug preparation
The selective NMDA receptor blocker d-(-)2-amino-5-phosphonovaleric acid (APV, Sigma, St. Louis, MO; 20 μM) was used for the pharmacological isolation of AMPA-dependent fEPSPs. To isolate NMDA-dependent fEPSPs, 6,7-dinitroquinoxaline-2,3-dione (DNQX, Sigma, St. Louis, MO; 50 μM), a selective AMPA/KA receptor blocker, combined with bicuculline methylbromide (BIC, Sigma, St. Louis, MO; 30 μM) a gamma-aminobutyric acid type A (GABAA) channel blocker, and glycine (Sigma, St. Louis, MO; 1 μM), an allosteric modulator of NMDA receptors were used. The drugs were prepared as stock solutions in dimethyl sulfoxide (DMSO; final DMSO concentration <0.05 %, Sigma, St. Louis, MO) for DNQX or in deionized water for APV and BIC.
Porcine GH (Ozbiopharm, Knoxfield, Australia) was prepared as a stock solution in deionized water. Human des(1-3)-IGF-I (Gropep, Thebarton, Australia) was prepared as a stock solution in 0.1 N glacial acetic acid (final concentration 0.1 acetic acid <0.005 %). Des-IGF-I is a truncated form of IGF-I that does not interact with the IGF-I binding proteins that normally sequester circulating IGF-I (Ballard et al. 1996). Furthermore, while des-IGF-I has been shown to more potent than IGF-I in vitro, both des-IGF-I and IGF-I have similar effects (Russo and Werther 1994; Guan et al. 1996). Drugs used to block signaling components involved in GH- and IGF-I-mediated effects were: tyrphostin AG 490 (Sigma, St. Louis, MO; 10 μM), a Janus kinase 2 (JAK2) inhibitor and wortmannin (Sigma, St. Louis, MO; 1 μM), a phosphoinositide 3-kinase (PI3K) inhibitor. Both inhibitors were made up as stock solutions in DMSO (final DMSO concentration <0.05 %). During recordings, all drugs were applied with aCSF in known concentrations via calibrated syringe pumps (Razel, Stamford, CT).
Statistics
Post-treatment effects of GH and des-IGF-I on pAmp of fEPSPs were defined as a percent of pre-treatment baseline values. The mean of the last 5 min post-treatment was compared to the mean of the last 5 min pre-treatment baseline. Paired Student’s t tests were used to compare post-treatment to pre-treatment values for compound, AMPA- and NMDA-dependent fEPSPs. Signaling cascade inhibitor effects on GH- and des-IGF-I-mediated changes in fEPSPs pAmp were analyzed by one-way ANOVA followed by Newman–Keuls test for pairwise comparison, when appropriate. All statistical analyses were performed using Sigmastat version 3.5 (SYSTAT Software, Point Richmond, CA). Statistical significance was defined as p < 0.05. All error bars in the figures represent standard error. Values for dose response studies are represented as mean percent pAmp above baseline ± standard error. Values for percent potentiation for GH and IGF-I enhancement and percent potentiation for signaling studies are represented as mean percent potentiation ± standard error.
Results
Concentration–response curves
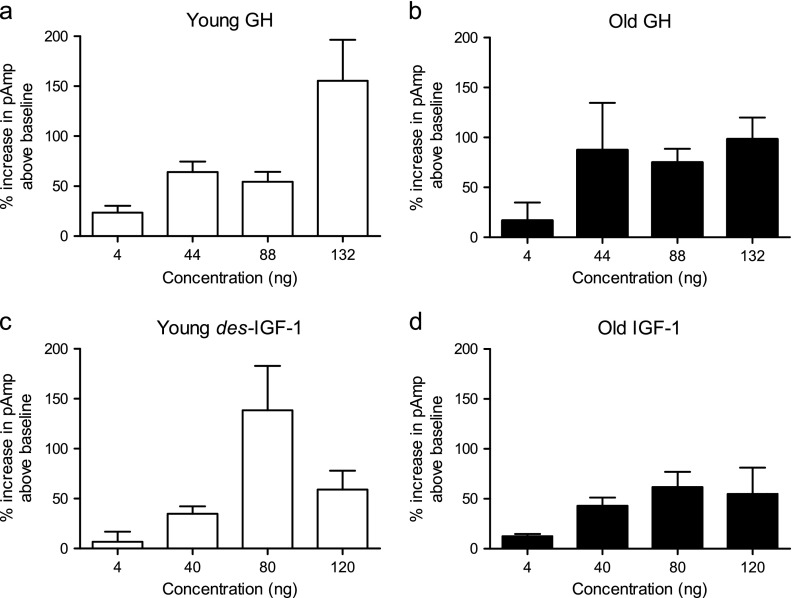
In the first set of experiments, concentration–response curves were generated to determine the optimal GH and des-IGF-I concentrations for the present studies of the effects of these hormones on excitatory synaptic transmission in the CA1 field of hippocampal slices from young adult and old rats (Fig. 1a–d). Statistical assessment of age versus drug effects for both GH and IGF-I demonstrated that there was an overall concentration effect for both GH (p < 0.005) and des-IGF-I (p < 0.0001); however, there was no significant age effect at any of the concentrations (p = 0.7737). Concentrations of 88 ng/mL of GH and 80 ng/mL of des-IGF-I were selected because both were intermediate among the concentrations tested and both exerted significant enhancement above baseline in young adult and old rats (Fig. 1a–d).
Fig. 1.
Concentration–response curves. Concentration-dependence of GH (a, b) and des-IGF-I (c, d) potentiation of compound fEPSPs in young adult (a, c) and old (b, d) rats
GH and des-IGF-I enhance fEPSPs in young adult and old rats
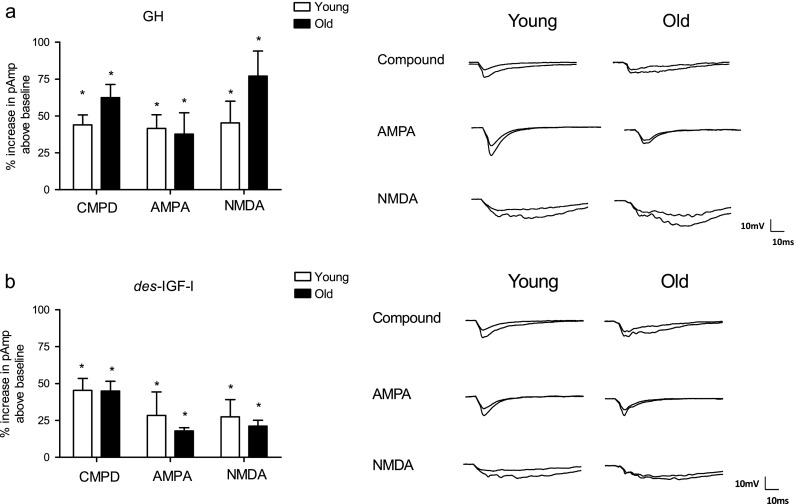
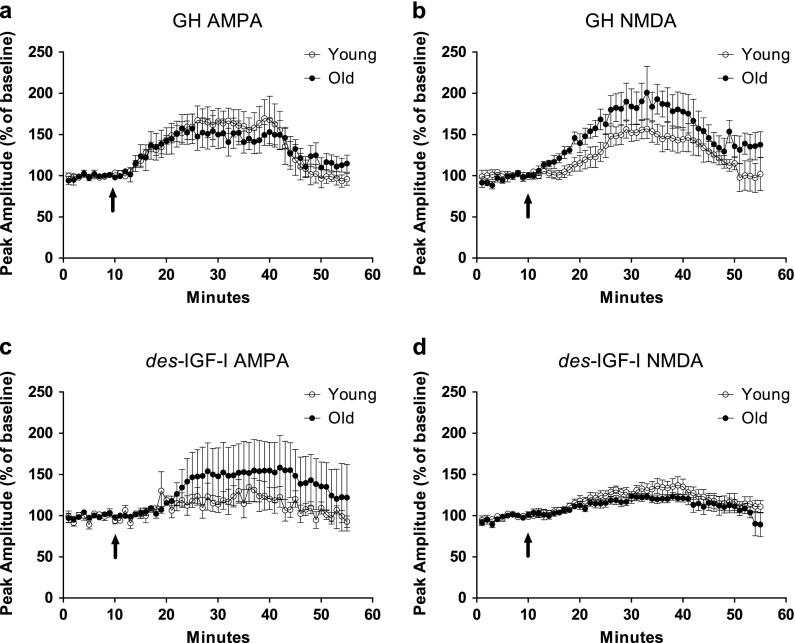
Age-related differences in the effect of GH or des-IGF-I on glutamatergic synaptic transmission were assessed in hippocampal slices (Figs. 2 and 3). Baseline recordings were performed for each slice during which pAmp remained within 10 % of the mean value for 10 min. CMPD and AMPA-dependent fEPSPs were characterized by short latency negative-deflecting potentials (Karnup and Stelzer 1999), whereas NMDA-dependent fEPSPs were characterized by prolonged negative-deflecting potentials preceeded by an initial, rapid negative deflection. These NMDA-dependent fEPSPs were similar in appearance to those described previously (Eckles-Smith et al. 2000). Our results reveal that GH induced a statistically significant enhancement above baseline in CMPD (144 ± 7 %, n = 18), AMPA- (142 ± 15 %, n = 8), and NMDA-dependent (145 ± 15 %, n = 7) fEPSPs in slices from young adult rats (Fig. 2a, b). A similar, significant GH-induced enhancement of CMPD (161 ± 7 %, n = 14), AMPA- (138 ± 15 %, n = 8), and NMDA-dependent (178 ± 17 %, n = 7) fEPSPs was also observed in slices from old rats (Fig. 2a, b). Des-IGF-I also produced a statistically significant enhancement above baseline in young adult, CMPD (140 ± 8 %, n = 20), AMPA- (123 ± 13 %, n = 12), and NMDA- (133 ± 8 %, n = 12,) dependent synaptic transmission, as well as in old CMPD (159 ± 11 %, n = 17), AMPA (118 ± 2 %, n = 11) and NMDA (122 ± 3 %, n = 12) fEPSPs (Fig. 2c, d). Furthermore, time–course waveforms of average fEPSPs across time for GH (Fig. 3a, b) and des-IGF-I (Fig. 3c, d) under, AMPA- and NMDA-dependent conditions were similar for young and old slices. In addition, in Fig. 3, the arrow indicates the onset of the drug application and since these time courses are not different directly following the onset of the drug this likely indicates the enhancement is not due to a change in the speed of the release of the neurotransmitter. Thus, our results demonstrate that CMPD, AMPA-, and NMDA-dependent synaptic transmission was increased above baseline by either GH or des-IGF-I and that the enhancement was comparable in young adult and old rats (p values: GH CMPD, p = 0.08; AMPA, p = 0.83; NMDA, p = 0.18; p values: des-IGF-I CMPD, p = 0.18; AMPA, p = 0.72; NMDA, p = 0.25 (Fig. 2).
Fig. 2.
GH and des-IGF-I enhancement of excitatory transmission in young and old rats. Effect of GH (a) and des-IGF-I (b) on compound (CMPD) fEPSPs and pharmacologically isolated AMPA- and NMDA-mediated fEPSPs in young adult and old hippocampal slices. Field EPSP enhancement above baseline (100 %) was significant in both young adult and old F344 × BN rats (all p values <0.05) and there were no significant effects of age under any of the recording conditions (all p values >0.05)
Fig. 3.
GH and des-IGF-I effects on excitatory transmission in young and old rats. Time course for GH (a, b) and des-IGF-I (c, d) application on pharmacologically isolated AMPA- and NMDA-mediated fEPSPs in young adult and old hippocampal slices. Application onset of GH and IGF-I is depicted by the arrow. Comparison of time courses for young adult and old hippocampal slices demonstrated no significant age-related differences in the response to GH or des-IGF-I
Paired-pulse facilitation is unaltered by GH and IGF-I
Since GH and des-IGF-1 significantly enhanced glutamatergic synaptic transmission in slices from young adult and old animals, we next sought to characterize the synaptic locus underlying these effects. Alterations in the paired-pulse ratio (PPR) of fEPSPs can be used as an indicator of changes in neurotransmitter release and thus may reveal changes that are of a presynaptic origin (Foster and McNaughton 1991; Schultz et al. 1994). Therefore, we measured the PPR of CMPD fEPSPs following direct application of these hormones (Fig. 4). Although bath application of GH significantly potentiated the fEPSPs, GH had no effect on the PPR in recordings from either young adult (pre-treatment GH = 1.311; post-treatment GH 1.217; n = 4; Fig. 4a) or old rats (pre-treatment GH = 1.635; post-treatment GH = 1.299, n = 7; Fig. 4a). Similarly, des-IGF-I application also enhanced CMPD fEPSPs with no effect on PPR in either young adult (pre-treatment des-IGF-I = 1.394; post-treatment des-IGF-I = 1.435; n = 5; Fig. 4b) or old hippocampal slices (pre-treatment des-IGF-I = 1.524, n = 7; post-treatment des-IGF-I = 1.034, n = 7; Fig. 4b). The observation that neither hormone altered the PPR is consistent with the notion that GH and des-IGF-I enhancement of synaptic transmission in young adult and old hippocampal slices is not mediated by an increase in glutamate release and instead likely involves postsynaptic mechanisms.
Fig. 4.
GH and des-IGF-1 effects on presynaptic facilitation in young and old rats. Effect of GH (a) and des-IGF-I (b) on paired-pulse ratios of CMPD fEPSPs. A paired-pulse stimulus train was generated in the hippocampal slices at an interpulse interval of 50 ms. The ratio of the second (P2) pulse over the first pulse (P1) was determined under baseline conditions (pre-treatment) and after GH and des-IGF-I application (post-treatment). Neither GH nor IGF-I application altered paired-pulse ratios significantly in young adult or old rats
GH and IGF-I signaling cascade elements are altered with age
In light of the comparable enhancement of glutamatergic transmission observed in young adult and old hippocampal slices treated with GH or des-IGF-I, we also conducted initial studies to investigate whether there were any age-dependent changes in the dominant elements of the GH and des-IGF-I signaling cascades that could underlie these effects. Studies indicate that the non-intrinsic tyrosine kinase GH receptor dimerizes and associates with a non-receptor cytoplasmic tyrosine kinase JAK2 for its activation (Carter-Su et al. 1996). Furthermore, JAK2 phosphorylation has been proposed to be critical for GH signal transduction (Jin et al. 2008). To compare the role of this element of the GH signaling cascade in young adult and old rats, the JAK2 inhibitor tyrphostin AG 490 was added to slices from both groups 20 min prior to and throughout GH application. The results demonstrated that tyrphostin AG 490 did not block GH enhancement in either young adult (GH = 144 ± 6 %, n = 19, GH + Tyrphostin AG 490 = 143 ± 5 %; n = 10; Fig. 5a) or old slices (GH = 161 ± 7 %, n = 14, GH + Tyrphostin AG 490 = 167 ± 7 %, n = 4; Fig. 5a). These findings are in contrast to reports that GH enhancement is diminished by tyrphostin AG 490 in 2–3-month-old juvenile rats (Mahmoud and Grover 2006). Therefore, in order to ensure the effectiveness of our inhibitor, we assessed inhibition of the JAK2 signaling cascade by tyrphostin AG 490 in slices from rats in that age range. We found a significant inhibition of GH-induced enhancement similar to that described by Mahmoud and Grover (2006), (GH = 188 ± 18 %, n = 7, GH + tyrphostin AG 490 = 136 ± 9, n = 9, p = 0.0171; data not shown). These data indicate that tyrphostin AG 490 can inhibit JAK2 in juvenile but not in young or old rats. This suggests that the signaling cascade mediating GH enhancement in young adult and old rats is not dependent on JAK2, and based on current evidence from the literature, a second tyrosine kinase, a Src family kinase, that activates the Ras/MAP kinase pathway may be involved (Zhu et al. 2002; Barclay et al. 2010).
Fig. 5.
Effect of age on the signaling cascades responsible for GH and des-IGF-1 potentiation of excitatory transmission. For blocking the GH-induced JAK-STAT pathway (a), tyrphostin AG 490 was used in slices from young adult and old rats. Tyrphostin AG 490 did not block the GH enhancement of fEPSPs. For blocking the des-IGF-1-induced PI3K pathway wortmannin was used (b). Wortmannin partially blocked des-IGF-1 enhancement in slices from young adult but not old rats (*p values <0.05)
One of the major signaling cascades initiated by IGF-I receptor activation is the phosphatidylinositol 3-kinase (PI3K) signaling cascade. It has been proposed that the PI3K-dependent pathway contributes to the maintenance of cognitive function (Sun et al. 2005) and this pathway is involved in the IGF-I potentiation of hippocampal AMPA EPSCs in juvenile rats (Ramsey et al. 2005). To determine if there were age-dependent changes in this signaling cascade, we tested the effect of the PI3K inhibitor wortmannin on des-IGF-I potentiation of fEPSPs in young adult and old rats. The results demonstrated that wortmannin reduced des-IGF-I enhancement in slices prepared from old (IGF-I = 151 ± 8 %, n = 16, IGF-I + wortmannin 124 ± 7, n = 5, p = 0.0262; Fig. 5b) but not young adult rats (IGF-I = 140 ± 9 %, n = 15, IGF-I = 141 ± 11 %, n = 5; Fig. 5b). Thus, our data suggest that the IGF-I-dependent enhancement may be partially mediated by PI3K in old rats, but not in young. In young animals, the IGF-I enhancement may be mediated by a parallel pathway, the Ras/MAP kinase pathway, through the recruitment of GRB2/SOS and its interaction with a phosphorylated IRS-1 or SHC that is activated by the phosphorylation of the IGF-I receptor (Neuman-Haefelin et al. 2008; Perrini et al. 2010).
Discussion
The results of this study provide the first evidence that both acute GH and des-IGF-I application significantly augment excitatory synaptic transmission in the hippocampus of young adult and old rats. Isolation of AMPA- and NMDA-dependent fEPSPs demonstrated that both hormones significantly increased AMPA- and NMDA-mediated synaptic transmission in young adult and old rats. PPR experiments suggested that the GH- and des-IGF-I-induced enhancement is not mediated by a presynaptic facilitation of glutamate release. Finally, our data provided initial evidence of significant age-dependent changes in the signaling cascades responsible for these effects. Although the JAK2 pathway is required for GH potentiation of fEPSPs in juvenile rats, blockade of this pathway had no effect on GH modulation of glutamatergic transmission in slices from young adult and old rats. Moreover, inhibition of PI3K revealed that des-IGF-I enhancement involves the PI3K signaling cascade in old rats, but not in young adults, suggesting a shift such that the PI3K pathway is utilized in juvenile and old rats, but not young animals.
The present results indicate that application of GH or des-IGF-I potentiates excitatory synaptic transmission in the hippocampus of young adult and old rats. A previous study reported that the GH augmentation involved both AMPA and NMDA receptors in hippocampal slices from juvenile rats (Mahmoud and Grover 2006). The present study extends those findings by demonstrating that (1) GH also increased AMPA- and NMDA-dependent synaptic transmission in young adult and old rats and (2) the level of enhancement was comparable in young adult and old rats. A similar change in excitatory glutamatergic transmission was also evident following application of des-IGF-I in slices from adult and old rats. Importantly, we found that des-IGF-I enhances both AMPA- and NMDA-dependent fEPSPs. This finding is in contrast to a previous report that des-IGF-I potentiated AMPA-dependent, but not NMDA-dependent synaptic transmission in juvenile rats (Ramsey et al. 2005). These data, taken together with the present results, suggest that there may be age-related changes in the glutamate receptors responsible for the des-IGF-I augmentation of hippocampal excitatory synaptic transmission. Specifically, the des-IGF-I enhancement appears to only involve AMPA receptors early in life (1–2 months; Ramsey et al. 2005) but utilizes both AMPA and NMDA receptors by young adulthood (10 months of age, present findings). This developmental change could be due to the addition or maturation of NMDA receptor subunit complexes. NMDA receptors are comprised of heteromeric complexes consisting of an NR1 subunit and a complement of NR2 subunits (A-D) which exhibit distinct physiological and pharmacological properties (Meguro et al. 1992; Kutsuwada et al. 1992; Cull-Candy et al. 2001), and the relative proportions of these subunits have been documented to demonstrate functionally significant changes during development (Monyer et al. 1994; Portera-Cailliau et al. 1996; Bellone and Nicoll 2007).
The enhancement of hippocampal glutamatergic synaptic transmission by GH and des-IGF-I demonstrated in the present study provides a mechanism for the reported amelioration of age-related cognitive impairment by GH and IGF-I supplementation. Specifically, we show here that GH- and des-IGF-I each can directly modulate hippocampal synaptic transmission not only in young adults, but also at old age when cognitive performance can be improved by either GH or IGF-I administration (Markowska et al. 1998; Ramsey et al. 2004). Compromised AMPA- and NMDA-dependent synaptic transmission has been reported in rats with cognitive deficits (Porrino et al. 2005; Liu et al. 2008; Goff et al. 2008; Kessels and Malinow 2009; Lin and Tsien 2009; Hampson et al. 2009; Hamlyn et al. 2009), and increased activation or expression of glutamatergic receptors maintains cognitive function (Tang et al. 1999; Porrino et al. 2005; Cao et al. 2007; Hampson et al. 2009; Hamlyn et al. 2009). While GH and IGF-I levels were not measured in the animals in the present study, it has been well-documented in the literature that they decline with age (Sonntag et al. 1980; Richman et al. 1981; Carter et al. 2002; Sonntag et al. 2005; Ramsey et al. 2004), and these declines may contribute to age-related cognitive deficits. Future studies will be directed at correlating the amount of the enhancement in excitatory synaptic transmission with the hormone levels within individual subjects to determine whether animals with and without cognitive deficits are still responsive to GH and/or IGF-I. Finally, it has been recently shown by our group that in two well-characterized models of decreased circulating GH and IGF-I levels, the local levels of these hormones remain constant (Adams et al. 2009; Molina et al. 2011). Thus, it will be important in future studies to document whether the local levels of these hormones also change and whether these levels correlate with alterations in synaptic plasticity.
The results of our PPR experiments, taken together with the data indicating no change in the speed of release of the neurotransmitter, suggest that the effects of GH and des-IGF-I observed here may not involve increased glutamate release and may instead reflect changes in the glutamate receptors in the postsynaptic membrane. For example, increased trafficking of NMDA or AMPA subunits to the postsynaptic membrane could occur following GH or IGF-I administration. In the cerebellum, IGF-I mediates NMDA receptor trafficking by subunit phosphorylation (Chen and Roche 2007) and other growth factors also have been shown to enhance NMDA subunit phosphorylation (Suen et al. 1998; Lin et al. 1998, 1999). Moreover, the expression of both NR2A and NR2B subunit protein levels in old rats is enhanced by IGF-I (Sonntag et al. 2000) and GH increases the level of NMDA receptor expression in the hippocampus (Le Grevès et al. 2005). Finally, the literature suggests that AMPA subunit phosphorylation and insertion at the synapse increases the receptor fEPSP amplitude (Malinow and Malenka 2002) and increases in NMDA receptor levels occur simultaneously with an increase in the NMDA-mediated receptor fEPSP (Eckles-Smith et al. 2000). Thus, the effects of GH and/or IGF-I on subunit phosphorylation, as well as expression, may be essential in the enhanced synaptic transmission reported here.
Although the ability of GH and des-IGF-I to enhance synaptic transmission does not change appreciably between 10 and 28 months of age, the signaling pathway implicated in the IGF-I-mediated enhancement appears to be modified during that time. Specifically, blocking the PI3K, a signaling pathway involved in the maintenance of cognitive function (Sun et al. 2005), reduces the des-IGF-I-mediated enhancement in old, but not in young adult rats. In addition to IGF-I downstream signaling events being mediated through the phosphorylation of PI3K, activation of the Ras/MAP kinase pathway through SHC adaptor protein phosphorylation has been observed to occur in parallel to PI3K (Neuman-Haefelin et al. 2008; Perrini et al. 2010). It may be that as animals age there is a switch from a dependence on an SHC-MAPK signaling pathway, which is more utilized in young adult rats (Kim et al. 1997; Sullivan et al. 2008) to a greater reliance on the PI3K pathway in old rats. This type of age-related switch has been reported to occur in the expression patterns of neurotrophins TrkA and p75NTR and is thought to involve IGF-I receptor activation (Costantini et al. 2006). In contrast, blocking the JAK2 pathway, reported to be a major signaling pathway for GH induced effects, was equally ineffective in reducing GH-mediated synaptic enhancement in young adult and old rats. While most of GH-dependent effects have been thought to occur through the phosphorylation of JAK2 that occurs after the GH receptor activation, several studies have demonstrated that some of GH’s functions are independent of JAK2 phosphorylation (Zhu et al. 2002; Barclay et al. 2010). It is possible that in young adult and old rats, GH mediates its effects primarily by recruiting other tyrosine kinases such as c-Src, which activates the Ras/MAP kinase pathway, and that the effects mediated through the recruitment of c-Src are independent of JAK2 (Zhu et al. 2002). Furthermore, it has been demonstrated that some GH events GH can induce an increase in calcium entry independent of JAK2 (Billestrub et al. 1995). Thus, a GH-induced increase in intracellular Ca2+ might be responsible for the enhancement of synaptic transmission during JAK2 inhibition (Zhang et al. 2004). While JAK2 has a much greater response to GH receptor activation, JAK1 has also been observed to be phosphorylated by JAK1 activation (Smit et al. 1996), and therefore, the AMPA- and NMDA-dependent results that we observe could be mediated by JAK1. In addition, 17β-estradiol has been observed to exert rapid action on glutamatergic, currents in the hippocampus (Gu et al. 1999; Kim et al. 2006). It is possible that the GH-mediated enhancement that we observed on AMPA- and NMDA-dependent fEPSPs might be as a result of rapid action of GH on AMPA and NMDA receptors without requiring post-translational modification. This would occur by GH directly modulating AMPA and NMDA receptor-dependent functions.
IGF-I is known to be the anabolic mediator of the biological effects of GH. Interestingly, we previously showed that chronic GH treatment enhances excitatory synaptic transmission in the absence of an increase in IGF-I levels in aged rats and suggested that GH may be having a direct effect on the brain. Thus, the current data demonstrating that acute GH application enhances compound and pharmacologically isolated NMDA- and AMPA-receptor fEPSPs in young adult and old animals, together with evidence of the presence of GH receptor mRNA in the brain (Fraser et al. 1990; Walsh et al. 1990; Burton et al. 1992; Lobie et al. 1993, 2000; Zhai et al. 1994; Hull and Harvey 1998) and a demonstration that GH crosses the blood–brain barrier (Pan et al. 2005), support the conclusion that GH may directly influence glutamate receptor function. This also provides a potential mechanism for the GH-induced amelioration of cognitive function in aged rats.
Summary
Taken together, the results presented here demonstrate that either GH or des-IGF-I application to hippocampal slices enhanced synaptic transmission in both young adult and old rats. These findings reveal for the first time that both GH and IGF-I have direct effects on AMPA and NMDA-dependent synaptic transmission at a time in the lifespan that administration of either GH or IGF-I is able to ameliorate age-related cognitive decline. Understanding the mechanisms by which GH and IGF-I mediate synaptic enhancement is essential for understanding how the chronic administration of these factors ameliorates the age-related decline cognitive function. The involvement of AMPA and NMDA receptors in the GH and des-IGF-I-induced enhancement and the alteration to the IGF-I signaling cascade between young adulthood and old age provide opportunities for understanding the targets of the GH and/or IGF-I-induced amelioration of cognitive impairment in the elderly, and thus contribute to the development of alternative therapeutic strategies that could benefit this population.
Acknowledgments
This work was supported by NIH grants: NIA PO1 AG11370 and KO1 AG027828. M.M. Adams is currently supported by an Installation Grant from the European Molecular Biology Organization.
References
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Forbes ME, Linville MC, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors. 2009;27(3):181–188. doi: 10.1080/08977190902863639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Alburquerque EX. NMDA and AMPA receptors contribute to nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J Neurophysiol. 2003;90(3):1613–1625. doi: 10.1152/jn.00214.2003. [DOI] [PubMed] [Google Scholar]
- Ballard FJ, Wallace JC, Francis GL, Read LC, Thomas FM (1996) Des(1-3)IGF-I: a truncated form of insulin like growth factor-I. Int J Biochem Cell Biol 28(10):1085–1087 [DOI] [PubMed]
- Barclay JL, Kerr LM, Arthur L, Rowland JE, Nelson CN, Ishikawa M, d’Aniello EM, White M, Noakes PG, Waters MJ. In vivo targeting of the growth hormone receptor (GHR) box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol Endocrinol. 2010;24(1):204–217. doi: 10.1210/me.2009-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Effects of aging on the dynamics of information processing and synaptic weight changes in the mammalian hippocampus. Prog Brain Res. 1990;86:89–104. doi: 10.1016/S0079-6123(08)63169-6. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Foster TC, McNaughton BL. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus. 1992;2:457–468. doi: 10.1002/hipo.450020413. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-d-aspartate R-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–452. doi: 10.1016/S0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path–granule cell synapse. Neurobiol Aging. 2000;21:613–620. doi: 10.1016/S0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Orr G. Age-related decrease in the Schaffer collateral-evoked EPSP in awake, freely behaving rats. Neural Plast. 2000;7:167–178. doi: 10.1155/NP.2000.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman LA, Mahle CD, Boissard CG, Gribkoff VK. Age-dependence of effects of A1 adenosine receptor antagonism in rat hippocampal slices. J Neurophysiol. 1992;68:629–638. doi: 10.1152/jn.1992.68.2.629. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Billestrub N, Bouchelouche P, Allevato G, Ilondo M, Nielsen JH. Growth hormone receptor C-terminal domains required for growth hormone-induced intracellular free Ca2+ oscillations and gene transcriptions. Proc Natl Acad Sci. 1995;92(7):2725–2729. doi: 10.1073/pnas.92.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology. 1992;131:958–963. doi: 10.1210/en.131.2.958. [DOI] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Carter-Su C, King AP, Argetsinger LS, Smit LS, Vanderkuur J, Campbell GS. Endocr J. 1996;43:S65–S70. doi: 10.1507/endocrj.43.Suppl_S65. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittadini A, Longobardi S, Fazio S, Sacca L. Growth hormone and the heart. Miner Electrolyte Metab. 1999;25:51–55. doi: 10.1159/000057420. [DOI] [PubMed] [Google Scholar]
- Conti E, Musumeci MB, Assenza GE, Quarta G, Autore C, Volpe M. Recombinant human insulin-like growth factor-1: a new cardiovascular disease treatment option? Cardiovasc Hematol Agents Med Chem. 2008;6:258–271. doi: 10.2174/187152508785909456. [DOI] [PubMed] [Google Scholar]
- Costantini C, Scrable H, Puglielli L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J. 2006;25:1997–2006. doi: 10.1038/sj.emboj.7601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-Z. [DOI] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain research. Mol Brain Res. 2000;78:154–162. doi: 10.1016/S0169-328X(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Foster TC. Regulation of synaptic plasticity in memory and memory decline with aging. Prog Brain Res. 2002;138:283–303. doi: 10.1016/S0079-6123(02)38083-X. [DOI] [PubMed] [Google Scholar]
- Foster TC, McNaughton BL. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Fraser RA, Attardo D, Harvey S. Growth hormone receptors in hypothalamic and extra-hypothalamic tissues. J Mol Endocrinol. 1990;5:231–238. doi: 10.1677/jme.0.0050231. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998;18(20):8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Duran-Prado M, Luque RM, Martinez-Fuentes AJ, Quintero A, Gutierrez-Pascual E, Cordoba-Chacon J, Malagon MM, Gracia-Navarro F, Castano JP. Understanding the multifactorial control of growth hormone release by somatotropes: lessons from comparative endocrinology. Ann N Y Acad Sci. 2009;1163:137–153. doi: 10.1111/j.1749-6632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, Manschreck T, Freudenreich O, Johnson SA. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2008;33:465–472. doi: 10.1038/sj.npp.1301444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainite-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140(2):660–666. doi: 10.1210/en.140.2.660. [DOI] [PubMed] [Google Scholar]
- Guan J, Williams CE, Skinner SJ, Mallard EC, Gluckman PD. The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: evidence for a role for IGF binding proteins. Endocrinology. 1996;137:893–898. doi: 10.1210/en.137.3.893. [DOI] [PubMed] [Google Scholar]
- Hamlyn E, Brand L, Shahid M, Harvey BH. The ampakine, Org 26576, bolsters early spatial reference learning and retrieval in the Morris water maze: a subchronic, dose-ranging study in rats. Behav Pharmacol. 2009;20:662–667. doi: 10.1097/FBP.0b013e328331ba1b. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Espana RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology (Berl) 2009;202:355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19:187–197. doi: 10.1016/j.ghir.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Hull KL, Harvey S. Autoregulation of growth hormone receptor and growth hormone binding protein transcripts in brain and peripheral tissues of the rat. Growth Horm IGF Res. 1998;8:167–173. doi: 10.1016/S1096-6374(98)80107-X. [DOI] [PubMed] [Google Scholar]
- Jin H, Lanning NJ, Carter-Su C. JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol. 2008;22:1825–1841. doi: 10.1210/me.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovenceau A, Dutar P, Billard JM. Alteration of NMDA receptor-mediated synaptic responses in CA1 area of the aged rat hippocampus: contribution of GABAergic and cholinergic deficits. Hippocampus. 1998;8:627–637. doi: 10.1002/(SICI)1098-1063(1998)8:6<627::AID-HIPO5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Karnup S, Stelzer A. Temporal overlap of excitatory and inhibitory afferent input in guinea-pig CA1 pyramidal cells. J Physiol. 1999;516:485–504. doi: 10.1111/j.1469-7793.1999.0485v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Leventhal PS, Saltiel AR, Feldman EL. Insulin-like growth factor-I-mediated neurite outgrowth in vitro requires mitogen-activated protein kinase activation. J Biol Chem. 1997;272:21268–21273. doi: 10.1074/jbc.272.34.21268. [DOI] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Taguay A, Berger TW, Brinton RD. 17beta-estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141(1):391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Le Grevès M, Le Grevès P, Nyberg F. Age-related effects of IGF-I on the NMDA-, GH- and IGF-I receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374. doi: 10.1016/j.brainresbull.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Lin F, Tsien JZ. Memory and NMDA receptors. N Engl J Med. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postysynaptic densities. Brain Res Mol Brain. 1998;55:20–27. doi: 10.1016/S0169-328X(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lin SY, Wu K, Len GW, Xu JL, Levine ES, Suen PC, Mount HT, Black IB. Brain-derived neurotrophic factor enhances association of protein tyrosine phosphatase PTP1D with the NMDA receptor subunit NR2B in the cortical postsynaptic density. Brain Res Mol Brain Res. 1999;70:18–25. doi: 10.1016/S0169-328X(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–841. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- Lo HC, Ney DM. GH and IGF-I differentially increase protein synthesis in skeletal muscle and jejunum of parenterally fed rats. Am J Physiol. 1996;271:E872–E878. doi: 10.1152/ajpendo.1996.271.5.E872. [DOI] [PubMed] [Google Scholar]
- Lobie PE, Garcia-Aragon J, Lincoln DT, Barnard R, Wilcox JN, Waters MJ. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Res Dev Brain Res. 1993;74:225–233. doi: 10.1016/0165-3806(93)90008-X. [DOI] [PubMed] [Google Scholar]
- Lobie PE, Zhu T, Graichen R, Goh EL. Growth hormone, insulin-like growth factor I and the CNS: localization, function and mechanism of action. Growth Horm IGF Res. 2000;10(Suppl B):S51–S56. doi: 10.1016/S1096-6374(00)80010-6. [DOI] [PubMed] [Google Scholar]
- Mahmoud GS, Grover LM. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J Neurophysiol. 2006;95:2962–2974. doi: 10.1152/jn.00947.2005. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/S0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. Cholinergic modulation of excitatory synaptic input integration in hippocampal CA1. J Physiol. 2010;588(Pt19):3727–3742. doi: 10.1113/jphysiol.2010.188581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Molina DP, Ariwodola OJ, Linville C, Sonntag WE, Weiner JL, Brunso-Bechtold JK, Adams MM (2011) Growth hormone modulates hippocampal excitatory synaptic transmission and plasticity in old rats. http://dx.doi.org/10.1016/j.neurobiolaging.2011.09.014; PMID:220151312 [DOI] [PMC free article] [PubMed]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Neuman-Haefelin E, Qi W, Finkbeiner E, Walz G, Baumesister R, Hertweck M. SHC-1/p52Shc targets the insulin/IGF-1 and JNK signaling pathways to modulate life span and stress response in C. elegans. Genes Dev. 2008;22(19):2721–2735. doi: 10.1101/gad.478408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging. 2008;29:1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/er.19.1.55. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ (2005) Permeation of growth hormone across blood-brain barrier. Endocrinology 146(12):5533–5539 [DOI] [PubMed]
- Papatheodoropoulos C, Kostopoulos G. Age-related changes in excitability and recurrent inhibition in the rat CA1 hippocampal region. Eur J Neurosci. 1996;8:510–520. doi: 10.1111/j.1460-9568.1996.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF-I axis and signaling pathways in muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. N-methyl-d-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. J Neurochem. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- Richman RA, Weiss JP, Hochberg Z, Florini JR. Regulation of growth hormone release: evidence against negative feedback in rat pituitary cells. Endocrinology. 1981;108:2287–2292. doi: 10.1210/endo-108-6-2287. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Russo VC, Werther GA. Des (1-3) IGF-I potently enhances differentiated cell growth in olfactory bulb organ culture. Growth Factors. 1994;11:301–311. doi: 10.3109/08977199409011003. [DOI] [PubMed] [Google Scholar]
- Schultz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of growth hormone receptor and JAK1 and JAK2 kinases in the activation of Stats 1,3, and 5 by GH. Mol Endocrinol. 1996;10(5):519–533. doi: 10.1210/me.10.5.519. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-d-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/S0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Xu JL, Lin SY, Levine ES, Black IB. NMDA receptor subunits in the postsynaptic density of rat brain: expression and phosphorylation by endogenous protein kinases. Brain Res Mol Brain Res. 1998;59:215–228. doi: 10.1016/S0169-328X(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Kim B, Feldman EL. Insulin-like growth factors in the peripheral nervous system. Endocrinology. 2008;149:5963–5971. doi: 10.1210/en.2008-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55:B106–B112. doi: 10.1093/gerona/55.2.B106. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Mangurian LP, Posner BI. The distribution of lactogen receptors in the mammalian hypothalamus: an in vitro autoradiographic analysis of the rabbit and rat. Brain Res. 1990;530:1–11. doi: 10.1016/0006-8993(90)90651-Q. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Lai Z, Roos P, Nyberg F. Characteristics of growth hormone binding sites in rat brain. Acta Paediatr Suppl. 1994;406:92–95. doi: 10.1111/j.1651-2227.1994.tb13433.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kohler M, Yang SN, Zhang F, Larsson O, Berggren PO. Growth hormone promotes Ca(2+)-induced Ca2+ release in insulin-secreting cells by ryanodine receptor tyrosine phosphorylation. Mol Endocrinol. 2004;18:1658–1669. doi: 10.1210/me.2004-0044. [DOI] [PubMed] [Google Scholar]
- Zhu T, Ling L, Lobie PE. Identification of a JAK2-independent pathway regulating growth hormone (GH)-stimulated p44/42 mitogen-activated protein kinase activity. GH activation of Ral and phospholipase D is Src-dependent. J Biol Chem. 2002;277:45592–45603. doi: 10.1074/jbc.M201385200. [DOI] [PubMed] [Google Scholar]