Abstract
Increased susceptibility to respiratory infections such as influenza is the hallmark of advancing age. The mechanisms underlying the impaired immune response to influenza are not well understood. In the present study, we have investigated the effect of advancing age on dendritic cell (DC) function because they are critical in generating robust antiviral responses. Our results indicate that monocyte derived DCs from the aged are impaired in their capacity to secrete interferon (IFN)-I in response to influenza virus. Additionally, we observed a severe reduction in the production of IFN-III, which plays an important role in defense against viral infections at respiratory mucosal surfaces. This reduction in IFN-I and IFN-III were a result of age-associated modifications in the chromatin structure. Investigations using chromatin immunoprecipitation with H3K4me3 and H3K9me3 antibodies revealed that there is increased association of IFN-I and IFN-III promoters with the repressor histone, H3K9me3 in non-stimulated aged DCs compared to young DCs. This was accompanied by decreased association of these promoters with activator histone, H3K4me3 in aged DCs after activation with influenza. In contrast to interferons, the association of TNF-alpha promoter with both these histones was comparable between aged and young subjects. Investigations at 48 h suggested that these changes are not stable and change with time. In summary, our study demonstrates that myeloid DCs from aged subjects are impaired in their capacity to produce IFNs in response to influenza virus and that age-associated altered histone expression patterns are responsible for the decrease in IFN production.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9477-8) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Aging, IFN-I, IL-29, Histones, Chromatin Immunoprecipitation
Introduction
Respiratory infections such as influenza are the major cause of age-related morbidity and mortality and account for nearly 80 % of deaths in persons aged 65 years or older (Falsey and Walsh 2005; Thompson et al. 2003). The infection is often more severe in the elderly requiring hospitalization. Furthermore, there is a significant increase in the tendency to develop secondary complications such as pneumonia and asthma (Griffin et al. 2002; Yap et al. 2004). Reduced efficacy of vaccination against influenza in the elderly further compounds the problem (Goodwin et al. 2006). Recent studies suggest that increase in influenza in the last 20 years could be related in part due to an increase in the aging population. Thus it is important to identify the age-related alterations in the immune responses that are responsible for the enhanced susceptibility of the elderly to influenza infections.
Although most of the studies point to adaptive immunity as the primary culprit for the defective control of influenza viral infection during aging, innate immune responses are the early responders against viral activity. The rapid production of type I interferon (IFN-α/β) (Garcia-Sastre and Biron 2006) and recently discovered IFN-III (IL-28/29) by innate immune cells such as DCs is required to control the infection (Ank and Paludan 2009). These IFNs induce antiviral responses which serve as primary host defense mechanisms against infection with influenza. In addition to anti-viral activity, IFNs modulate both innate and adaptive immune response. Type I interferons promote the differentiation of CD4+ naïve T cells to Th1 helper cells (Farrar et al. 2000; Rogge et al. 1998) and contribute to prolonging the survival of activated T cells (Marrack et al. 1999; Tough et al. 1996). They enhance the cytotoxic activity of CD8+T and NK cells. The critical role of IFN-α in enhancing antibody production and inducing isotype switching in B cells is very well documented (Braun et al. 2002; Jego et al. 2003; Le Bon et al. 2006). Furthermore, IFN-I also enhances the cross-presentation and DC maturation by up-regulating co-stimulatory molecules, Toll like receptors and pro-inflammatory cytokines (Beignon et al. 2003; Tough 2004). Recently discovered type III IFNs — IFN-III (IL28/29) — display type I IFN-like antiviral activity and induction of typical IFN-inducible genes except that they provide better protection against viral infections at respiratory mucosal surfaces (Ank and Paludan 2009). It has been reported that IFN-III contributes greatly in defense against pathogens that infect respiratory tract such as Influenza A virus, influenza B virus, RSV which interestingly affect predominantly the elderly population (Jewell et al. 2010; Mordstein et al. 2008, 2010). Unlike IFN-alpha receptors which are broadly expressed on most cell types, including leukocytes, IFN-lambda receptors are largely restricted to cells of epithelial origin which may explain their effectiveness against respiratory pathogens.
Plasmacytoid dendritic cells (pDCs) are considered the primary producers of type I IFNs (Liu 2005). They produce copious amounts of IFN-I very early in the infection. We (Sridharan et al. 2010) and others (Canaday et al. 2010; Jing et al. 2009; Shodell and Siegal 2002; Stout-Delgado et al. 2008; Qian et al. 2011) have reported decreased production of IFNs by pDC population in the aged subjects. In addition to pDC, the myeloid dendritic cells (mDCs) subset is also capable of producing IFNs in response to viral infection. Though the magnitude of IFN secreted is significantly lower than pDC nevertheless, unlike pDCs, mDCs retain the ability to express interferons for a long period of time after infection (Garcia-Sastre and Biron 2006). mDCs are also the primary producers of IFN-III, which is critical for protection against infection at respiratory surfaces (Iversen et al. 2010). Since mDC are comparatively more abundant than pDCs (Shortman and Liu 2002) IFN production by them may compensate for the reduced IFN secretion by pDCs from aged. In this study, we investigated the response of aged and young monocyte derived DCs (which are similar to mDCs) to influenza virus with primary focus on IFN-I and IFN-III secretion. Our data suggested that mDCs from aged were impaired in their capacity to secrete IFN-I and IFN-III in response to influenza.
Accumulating evidence has demonstrated that chromatin structure plays a critical role in gene activation and induction of inflammatory genes. Chromatin in cellular DNA is composed of the core histones H2A, H2B, H3 and H4 (Strahl and Allis 2000). Local changes in histone modification play a major role in epigenetic regulation of gene expression by affecting chromatin compaction and thereby DNA accessibility. Euchromatin or open chromatin states enhance gene expression by facilitating transcription factor recruitment and Pol II binding to promoter and transcribed regions (Shilatifard 2006). Heterochromatin or closed chromatin states on the other hand cause gene silencing. The gene expression is differentially modulated by the nature and the position of modification on histone tail residue. Acetylation of H3 (H3Ac) (Roh et al. 2006; Wang et al. 2008), and methylation on H3 on lysine 4, 36 and 79 (H3K4, H3K36, H3K79) are associated with transcriptional activation (Barski et al. 2007; Schubeler et al. 2004) whereas methylation of H3 on lysine 9,27 (H3K9, and H3K27) are associated with transcriptional repression or gene silencing (Jacobs et al. 2001; Nielsen et al. 2001). Different types of methylation mono-, di-, and tri-methyl groups on histone tails may also affect the chromatin structure. For example di- and tri-methylation of H3K4 are generally found at genes that are competent for activation, with H3K4 trimethylation often linked to active transcription (Shilatifard 2006).
Profound changes in chromatin structure have been reported with advancing age. There is decreased genomic stability and altered transcription (Busuttil et al. 2007; Maslov and Vijg 2009). The pattern of histone expression is also altered with age. For example, a decrease in yeast lifespan was associated with dramatically reduced histone levels. A similar decrease in histone expression was also reported in human fibroblasts in culture (Dang et al. 2009). A more recent study reports a significant age-associated decrease in the expression of H3K9me3 in drosophila (Wood et al. 2010). In contrast, decreased heterochromatin-specific H3K9me3 due to reduced expression of the recruiting protein HP1 has been reported in aged humans (Scaffidi and Misteli 2006). A recent study (Kreiling et al. 2011) reported an age-associated increase in heterochromatin marks in several tissues of mice and primates. There is a scarcity of information regarding age-associated changes in chromatin organization changes particularly in human immune cells. Such information would prove to be extremely beneficial in identification of key mediators of the aging immune process.
In the present study, we investigated whether aging leads to alterations in the response of DCs from aged subjects to influenza. Furthermore, the role of chromatin modifications in the reduced secretion of IFNs by DCs from aged in response to influenza was also examined.
Materials and methods
Donors
Blood was collected from healthy aged (65–90 years) and young (20–35 years) donors. Aged subjects were of middle-class socioeconomic status, and were living independently. Cohort description is provided in Table 1. The Institutional Review Board of the University of California, Irvine, approved this study.
Table 1.
Description of the aged and young cohorts
| Young (n = 22) | Aged (n = 22) | |
|---|---|---|
| Age (range) | 27 (20–35) | 77 (65–88) |
| Gender, female | 15 (68 %) | 11 (50 %) |
| Comorbidities | ||
| Osteoarthritis | 0 | 9 (41 %) |
| Hypertension | 0 | 8 (36 %) |
| Dyslipidemia | 0 | 7 (31 %) |
| Diabetes | 0 | 0 |
| Medications | ||
| Vitamins | 0 | 14 (64 %) |
| Antioxidants | 0 | 9 (41 %) |
Culture and treatment of human monocyte-derived DCs
DCs were prepared essentially as described (Agrawal et al. 2007, 2009). Briefly, peripheral blood mononuclear cells (PBMCs) were separated by Ficoll–Hypaque density gradient centrifugation. Monocytes were purified from the PBMCs by positive selection with anti-CD14 microbeads (Stemcell Sep, Vancouver, BC). The purity of the isolated CD14+ monocytes was >90 % as determined by flow cytometry. For the induction of DC differentiation, purified CD14+ monocytes were cultured in a humidified atmosphere of 5 % CO2 at 37 °C in RPMI 1640 supplemented with10% FBS, 1 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 ng/ml human rGM-CSF (PeproTech, Rocky Hill, NJ), and 10 ng/ml human rIL-4 (Peprotech). Half of the medium was replaced every 2 days and DCs (CD14−HLA-DR+CD11c+ cells) were collected after 6 days. The purity of the DC was >95 % as determined by the expression of CD14, CD11c and HLA-DR. DCs were stimulated with 10 μg/ml formalin-inactivated influenza virus A/PR/8/34-INF (Charles River, North Franklin, CT) for 48 h. Cytokines, IFN-α, IL-6, tumor necrosis factor (TNF)-α, IL-10, IL-1β, MCP-1, IL-8, IP-10, IL-12p40 in the supernatants were measured by Flow Cytomix (BD Pharmingen, San Diego, CA) as per the manufacturer’s protocol. IL-29 was measured using specific ELISA kit (PBL Biomedicals, Piscataway, NJ).
For detection of IFN-A2, IL-29, and TNF-α gene expression by real-time polymerase chain reaction (PCR) DCs (5×105) from aged and young subjects were stimulated with influenza for 3 h. RNA was extracted using Trireagent. After cDNA synthesis real-time PCR was performed using TNF-α, IFNA2 and IL-29 validated specific primers (real-time primers, Elkins Park, PA) using SYBR green as described for chip. Data was normalized to the GAPDH and is represented as fold increase over unstimulated DC.
DC phenotype
Influenza stimulated/unstimulated DCs were stained for surface markers CD40, CD83, CD80, CD86, HLA-ABC using directly conjugated antibodies and isotype controls (BD Pharmingen, San Jose, CA). Gated CD14-CD11c + HLA-DR+DCs were acquired using FACSCalibur (BD PharMingen, San Diego, CA). Data were analyzed using Flow Jo (Treestar Inc).
Chromatin immunoprecipitation (ChIP)
For the ChIP assay DCs were stimulated with influenza for 3 h. For the experiments in Fig. 4, DCs were stimulated with influenza for 48 h. ChIP assays were performed using ChIP express kit from Active Motif (Carlsbad, CA) according to the manufacturer’s manual with minor modifications. For each experiment DCs were pooled from three aged and three young subjects. Influenza stimulated and unstimulated aged and young DCs (~4×106) were collected in 15 ml conical tube and centrifuged at 1,000 rpm for 5 min. Cells were then cross-linked using formaldehyde (1 %) for 10 min at Room temperature on a rocking platform. Glycine at a final concentration of 1× were added to stop the fixation and then the cells were washed with ice cold PBS. Cell pellet was lysed with lysis buffer for 30 min at 4°C and then passed through twenty dounce cycles. To ensure that cell lysis was complete and nuclei were released from the cells, an aliquot of the lysate was examined under the phase contrast microscope. The chromatin was sheared by enzymatic shearing cocktail (200 U/ml) for 10 min at 37°C, generating DNA fragments in the range of 200–400 bp. Next, 10 μl was reserved as input DNA sample and the remaining chromatin was subjected to immunoprecipitation for overnight at 4°C in rotation. Then, 5 μl of anti tri-methyl-H3K4me3 (Actif motif, Carlsbad, CA), anti tri-methyl-H3K9me3 (Epigenetk, Farmingdale, NY), and anti-IgG (Actif motif) ChIP-validated antibodies were used in this study. Immunoprecipitates were washed and then eluted from the magnetic beads. Protein-DNA cross links were reverted, along with the input DNA sample at 95°C for 15 min, and then treated with proteinase K for 1 h at 37°C. The resulting DNA was purified using CHIP clean up (Zymospin) and stored at −20°C until subsequent PCR analysis.
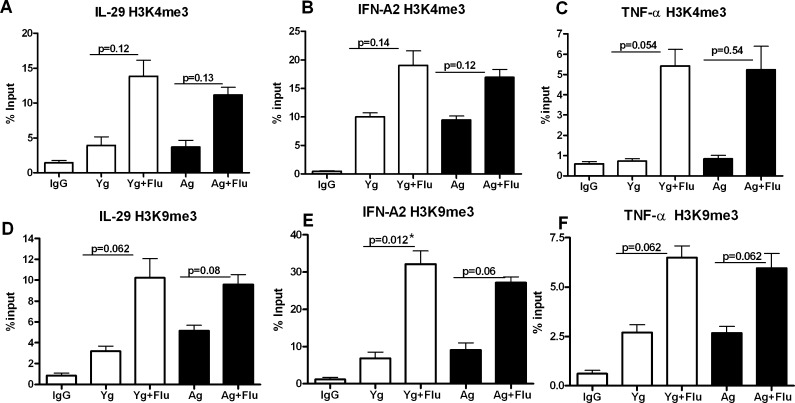
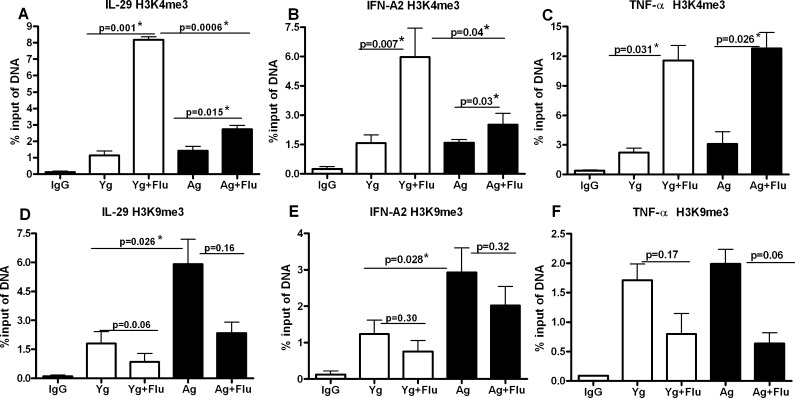
Fig. 4.
Association of IFN-A2, IL-29 and TNF-α promoter to H3K4me3 and H3K9me3 is not stable and alters with time. Chromatin from influenza stimulated (48 h) aged and young DCs was precipitated using H3K4me3 and H3K9me3 antibodies. Association of IFN-A2, IL-29 and TNF-α promoters with these histones was determined using real-time PCR. Data were normalized to the input DNA and is represented as % of input. a Real-time PCR analysis of binding of IL-29 promoter to H3K4me3. b Real-time PCR analysis of binding of IFN-A2 promoter to H3K4me3. c Real-time PCR analysis of binding of TNF-α promoter to H3K4me3. d Real-time PCR analysis of binding of IL-29 promoter to H3K9me3. e Real-time PCR analysis of binding of IFN-A2 promoter to H3K9me3. f Real-time PCR analysis of binding of TNF-α promoter to H3K9me3. Data represent the mean ± SEM of three experiments. Pooled DCs from three aged and three young subjects were used for each of the experiments
Real-time PCR
PCR analysis was performed in a 10-μl volume containing 3 μl ChIP DNA, 1 μM of each primer, H2O and 5 ml of SYBR green PCR master mix (Roche Applied Science). The PCR conditions were as follows: reactions were pre-incubated at 95°C for 10 min, amplified at 95°C for 10 s, 59°C for 10 s, 72°C for 15 s using LightCycler® 480 Multiwell Plate 384 system (Roche Applied Science). Data were normalized to the input DNA and is represented as % of input. Primers used are described in Table 2. Primers for IL-29 and IFN-A2 promoters were designed by identifying the probable promoter region from the sequence to which most transcription factors were binding (www.cbrc.jp/research/db/TFsearch.html). TNF-α promoter primers were from El Gazzar et al. (2009).Non-specific primers for IL-29 and IFN-A2 were designed from an area upstream from the promoter.
Table 2.
Human primer sequences
| Purpose | Gene | Primer sequence 5′-3′ | Genomic Segment | PCR product |
|---|---|---|---|---|
| Promoter | IFNA2F +68 | AATGGCCTTGACCTTTGCTTTACT | Promoter/Exon 1 | 152 |
| IFNA2R +220 | ATGGAGGACAGGGATGGTTTC | |||
| Negative control | IFNA2F −7137 | GCGAATGAGCATCATATGACTG | Promoter/Upstream | 302 |
| IFNA2R −6835 | TCTTCCTGCCTGCCAATGC | |||
| Promoter | IL29F −167 | CTGCCCACACCTGTTCCCTCATCA | Promoter/Upstream | 232 |
| IL29R +65 | GCCAAGCCTAGCACCAAAGTCACC | |||
| Negative control | IL29F −5249 | GAGCTCTGTTCACACATTTCTGG | Promoter/Upstream | 186 |
| IL29R −5063 | ACTCTACTGCTGTCCTGGGGC | |||
| Promoter | TNF-αFa (−122) | TACCGCTTCCTCCAGATGAG | Promoter/Upstream | 190 |
| TNF-αR (+68) | TGCTGGCTGGGTGTGCCAA |
aEl Gazzar et al. (2009)
Statistical analysis
Data was analyzed using GraphPad Prism™ 4.00 software (Graph Pad Software, San Diego, CA). Significant differences between groups were determined by Mann–Whitney test. A p value <0.05 was considered significant.
Results
The phenotype of aged and young DCs was comparable after activation with influenza virus
DCs are the major antigen presenting cells of the body that initiate and regulate the immune response to infection. Aged subjects are impaired in their ability to clear influenza infection. Here, we investigated the ability of DCs from aged subjects to respond to influenza infection.
Subjects are described in Table 1. The control population included 22 individuals in the age range of 20–35 years with an average age of 27 years. The geriatric population consisted of 22 individuals in the age range of 65–88 years with an average age of 77 years. The younger individuals were healthy and not on medications.
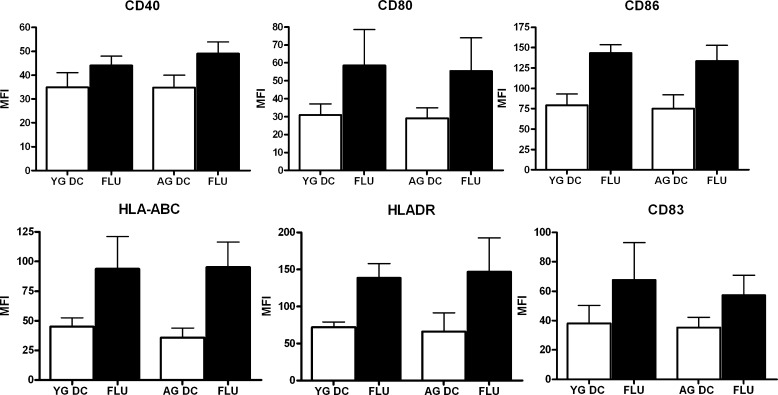
Briefly, DCs from aged and young subjects were stimulated with heat killed influenza A virus for 48 h. As shown in Fig. 1, stimulation with the virus resulted in substantial activation of both aged and young DCs. Comparable levels of up-regulation of CD40, CD80, CD86, CD83, HLADR, HLA-ABC were observed in aged and young DCs. These data suggest that aged DCs are not impaired in their capacity to respond to influenza virus.
Fig. 1.
The phenotype of aged and young DCs was comparable after activation with influenza virus. Bar graphs depict the mean fluorescence intensity (MFI) of the expression of CD40, CD80, CD86, HLADR, HLA-ABC, CD83 on aged and young DCs before and 48 h after stimulation with influenza. Data represent the mean ± SE of six different aged and young subjects
DCs from aged individuals are impaired in their ability to produce IFN-I and IFN- III in response to influenza virus
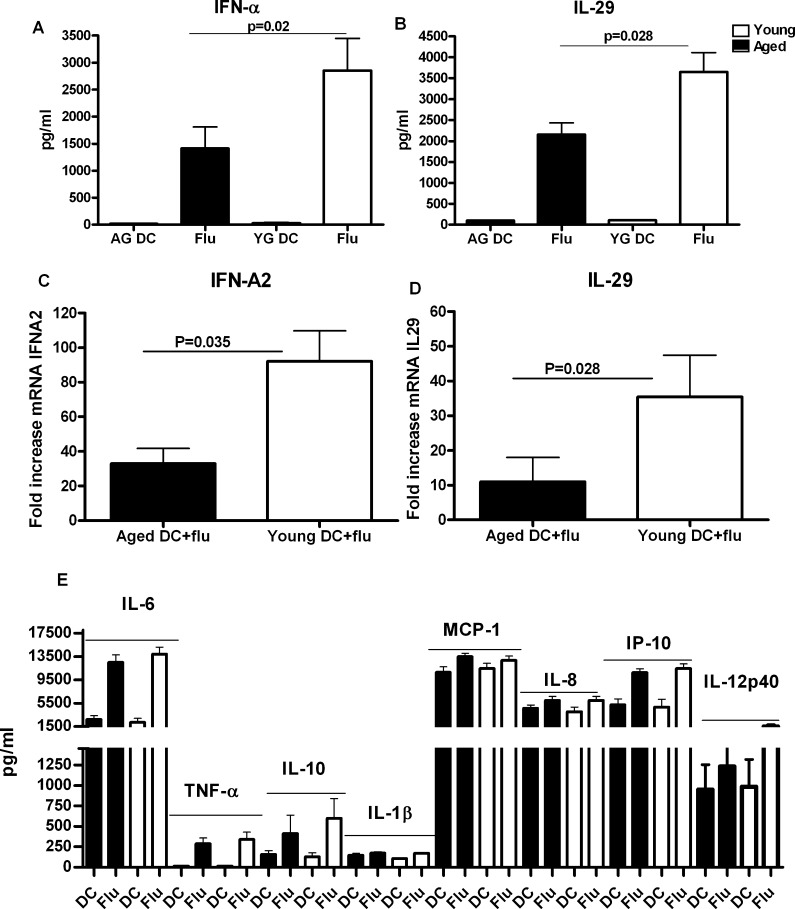
In addition to activation markers, cytokines secreted by DCs are also critical for host defense. As shown in Fig. 2a and b, both aged and young DCs produced IFN-I and IFN-III when activated with Influenza. However, aged DCs produced significantly (p = 0.02) reduced quantity of IFN-I when compared to young DCs (Fig. 2a). Interestingly, IFN-III secretion was higher than IFN-I but was also impaired (p = 0.028) in aged DCs (Fig. 2b). These results were further confirmed with real-time PCR. As is evident in Fig. 2c and d, induction of IFN-A2 and IL-29 in response to influenza stimulation was significantly impaired (p < 0.05) in aged DCs as compared to young DCs.
Fig. 2.
DCs from aged individuals are impaired in their ability to produce IFN-I and IFN-III in response to influenza virus. Monocyte derived DCs from aged and young subjects were stimulated with heat killed influenza (Flu). Cytokine secretion was determined after 48 h. a Level of type I IFN secretion by aged and young DCs. b Level of type III IFN secretion by aged and young DCs. Data represent the mean ± SE of 22 different aged and young subjects. c Gene expression levels of IFN-A2 in influenza stimulated aged and young DCs over unstimulated aged and young DCs 3 h after stimulation. d Gene expression levels of IL-29 in influenza stimulated aged and young DCs over unstimulated aged and young DCs 3 h after stimulation. Data represent the mean ± SE of eight different aged and young subjects. e Levels of MCP-1, IL-8, IL-1β, TNF-α, IL-6, IL-10, IL-12p40 and IP-10 secretion by aged and young DCs. Data represent the mean ± SE of 22 different aged and young subjects. Black aged, white young
The secretion of several other cytokines and chemokines was also assayed (Fig. 2e). IL-6, MCP-1, IL-8, IP-10 and IL-12p40 were secreted at very high levels by both aged and young DCs. Other cytokines such as TNF-α, IL-10 and IL-1β were also produced albeit at a lower level compared to the aforementioned mediators. Real-time PCR for induction of TNF-α in response to influenza was also comparable between aged and young DCs (Fig. S1). The secretion of these cytokines by DCs was comparable in aged and young individuals suggesting that expression of influenza sensing PRRs is not altered with age; instead, the defect lies only in the induction of interferons, IFN-I and IFN-III.
In aged subjects, there were several subgroups based on comorbidities (Table 1). For some of the subgroups we had enough subjects to do a subgroup analysis. Osteoarthritis was the most common comorbid condition in the aged population studied. IFN-I and IFN-III levels were comparable between arthritis positive and negative subjects (p = 0.33, IFN-I; p = 0.14, IFN-III). Aged subjects with hypertension and dyslipidemia were also not different in their induction of IFN-I and IFN-III (p > 0.5). Many of the aged subjects were also taking vitamins and antioxidants however, we did not observe any difference in IFN levels between the two groups (p > 0.8, p > 0.2). Based on these subgroup analyses, we feel fairly confident that the comparisons between the young control and aged subject populations are yielding valid results across a general geriatric group.
Association of IFN-A2 and IL-29 promoter to H3K4me3 and H3K9me3 is altered in aged DCs as compared to young DCs
In order to investigate whether epigenetic changes in aged DCs are responsible for the reduced production of interferons, ChIP experiments were performed. Briefly, aged and young DCs were activated with heat killed influenza virus for 3 h. DNA was precipitated with anti-H3K4me3 and anti-H3K9me3 antibodies, followed by real-time PCR amplification with IFN-A2 and IL-29 promoter specific genes as described in Materials and methods section.
As shown in Fig. 3a, the association of IL-29 promoter with H3K4me3 after influenza activation increase significantly (p = 0.015) in aged DCs. However, a much more robust increase in the association of IL-29 promoter with H3K4me3 is observed in young DCs (p = 0.0006) compared to aged DC. IFN-A2 promoter also displayed a similar pattern of association (Fig. 3b) with significant increase (p = 0.03) in association with H3K4me3 in aged DCs and a much more dramatic increase in young DCs (p = 0.007). These changes were specific since amplification with non-specific primers did not exhibit this pattern (Table 2, Fig. S2). As with interferons, TNF-α promoter also showed significantly increased association with H3K4me3 (Fig. 3c) in both aged (p = 0.026) and young DCs (p = 0.031) after stimulation with influenza. However, in contrast to IFN-A2 and IL-29, the association of TNF-α promoter with H3K4me3 (Fig. 3c) was comparable between aged and young DCs. This was in keeping with the ELISA (Fig. 1e) and PCR (Fig. S1) results for TNF-α which did not show a difference between the two age groups. The striking decrease in association of IL-29 and IFN-A2 promoters with H3K4me3 (a histone signature associated with transcription activation) in aged DCs after activation demonstrates that chromatin modifications in aged DCs are also responsible for the impaired secretion of interferons in the elderly.
Fig. 3.
Association of IFN-A2 and IL-29 promoter to H3K4me3 and H3K9me3 is altered in aged DCs as compared to young DCs. Chromatin from influenza stimulated (3 h) aged and young DCs was precipitated using H3K4me3 and H3K9me3 antibodies. Association of IFN-A2, IL-29 and TNF-α promoters with these histones was determined using real-time PCR. Data were normalized to the input DNA and is represented as % of input. a Real-time PCR analysis of binding of IL-29 promoter to H3K4me3. b Real-time PCR analysis of binding of IFN-A2 promoter to H3K4me3. c Real-time PCR analysis of binding of TNF-α promoter to H3K4me3. d Real-time PCR analysis of binding of IL-29 promoter to H3K9me3. e Real-time PCR analysis of binding of IFN-A2 promoter to H3K9me3. f Real-time PCR analysis of binding of TNF-α promoter to H3K9me3. Data represent the mean ± SEM of three experiments. Pooled DCs from three aged and three young subjects were used for each of the experiments
Further confirmation of the involvement of chromatin modifications was obtained with the results from ChIP of the DNA with the repressor histone, H3K9me3 antibodies. The association of IL-29 promoter to H3K9me3 was significantly increased (p = 0.026) in aged DC when compared to young DCs at the basal level, without stimulation (Fig. 3d). After stimulation with influenza, there was a decrease in association of IL-29 promoter with H3K9me3 in aged (p = 0.16) and young DCs (p = 0.06), which was not significant. Association of IFN-A2 promoter to H3K9me3 was also increased significantly (p = 0.028) in aged DCs at the basal level as compared to young DC. Stimulation with the virus resulted in decrease in the association of IFN-A2 promoter with H3K9me3 in both aged and young DCs (Fig. 3e) which was not significant (p > 0.05). Here, again, the increased association of interferon promoters was not observed with non-specific primers (Fig. S2). In contrast to interferon promoters, the association of TNF-α promoter to H3K9me3 was comparable at the basal level between aged and young subjects (Fig. 3f). Stimulation with influenza resulted in decreased association of TNF-α promoter with H3K9me3 in both aged and young DCs (Fig. 3f) with a concomitant increase in association with H3K4me3 (Fig. 3e) suggesting activation. The decrease in TNF-α promoter with H3K9me3 was comparable between aged and young subjects.
These data demonstrate that the association of IL-29 and IFN-A2 promoters to repressor histone, H3K9me3 is increased in aged DCs at the basal level which would prevent transcription of IFN genes. Decreased association of IFN promoters with the activator histone, H3K4me3 in aged DCs after activation with influenza will also impair the production of IFNs. The activator and repressor histones thus work in tandem to silence the transcription of IFNs in aged DCs. These age-related changes in the associations of the interferon promoters to histones may therefore be responsible for the decreased secretion of interferons observed in the aged subjects. The data also suggests that these changes are specific to interferons as these changes were not evident in TNF-α.
Association of IFN-A2, IL-29 and TNF-α promoter to H3K4me3 and H3K9me3 is not stable and alters over time
Next, we determined whether the histone associated promoter changes are stable over time. To investigate this, ChIP was performed as described for Fig. 3 except that DCs were stimulated with influenza for 48 h instead of 3 h. As shown in Fig. 4a, b and c, the association of H3K4me3 with IL-29, IFN-A2 and TNF-α promoters is increased after stimulation with influenza though the increase is not significant (p > 0.05). However, compared to 3 h where there was decreased association of IL-29 and IFN-A2 promoter with H3K4me3 in aged DC as compared to young DC, at 48 h, there is no significant difference between the two aged groups. TNF-α association with H3K4me3 after influenza association is also no longer significantly increased over unstimulated DCs (p > 0.05).
Association of all three promoters with H3K9me3 exhibits an even more dramatic alteration at 48 h compared to 3 h (Fig. 4d–f). There is increased association of all three promoters with H3K9me3 after stimulation with influenza at 48 h in contrast to 3 h where the association was decreased. This increased association is present in both aged and young DCs. These results suggest that activation of all three genes is silenced by 48 h due to their increased association with H3K9me3. Furthermore, the data also suggest that association of promoters to histones is a dynamic process which changes with time of stimulation.
Discussion
Increased susceptibility to viral infection in the elderly is often ascribed to alteration in T cell functions (Effros 2004; Gupta et al. 2004; Haynes and Maue 2009). Contribution of the innate immune response that impact anti-viral defense mechanisms in aging, remain largely unexplored. The first line of defense after influenza virus infections consists of robust production of interferons by innate immune cells (Billiau 2006; Isaacs and Lindenmann 1957). These interferons have potent anti-viral properties and subsequently regulate innate and adaptive immunity (Randall and Goodbourn 2008).
Numerous studies have reported a decrease secretion of IFN-α by aged pDCs, which are the most potent IFN producing cells (Sridharan et al. 2010; Stout-Delgado et al. 2008; Diebold et al. 2003; Jing et al. 2009). Besides pDCs, the mDCs subset of DCs is also capable of producing robust amount of IFNs in response to viral infection (Katashiba et al. 2011; Diebold et al. 2003). In this study, we show that similar to aged pDCs, monocyte derived DCs (which are similar to mDCs) from aged subjects are also impaired in their capacity to secrete IFNs in response to influenza virus (Fig. 2a–d). The reduced secretion of interferons by mDCs adds further to the deficit in interferon secretion by pDCs.
In addition to IFN-I, aged mDCs were also deficient in the production of IL-29 (IFN-III). IL-29 is a recently discovered cytokine that has similar mode of anti-viral activity as IFN-I (Dumoutier et al. 2004; Meager et al. 2005; Ank et al. 2008; Uze and Monneron 2007). However, IFN-III seems to have a more specialized role in epithelial and mucosal antiviral defense (Brand et al. 2005; Okabayashi et al. 2011) since the expression of IFN-III receptor is restricted to renal and mucosal epithelial surfaces (Iversen et al. 2010; Li et al. 2009; Sommereyns et al. 2008; Mordstein et al. 2010). Except for one recent study from our laboratory reporting decreased IL-29 secretion by pDCs from aged, there is a scarcity of information regarding the status of IL-29 production during aging. It is essential to gain this information as recent reports suggest that IFN-III, but not IFN-I acts as the first line of host response against upper respiratory epithelium viral infections (Okabayashi et al. 2011). It has been shown that IFN-III has certain unique functions which are different from that of IFN-Is. For example, IL-29-treated macrophages were more responsive to IFN-γ, because IL-29 enhanced IFN-γ induced IL-12p40 and TNF production by macrophages on TLR stimulation while, IFN-I suppressed the production of these cytokines (Liu et al. 2011). More recently, a very important role for IFN-III was reported in protection from allergic airway disease. IFN-III levels were found to be profoundly down-regulated in allergic asthma which led to exacerbation of allergic airway inflammation by augmenting Th2 and Th17 responses, and IgE levels (Koltsida et al. 2011). Therefore, profound decline in the production of IFN-III would not only increase the risk of elderly population to influenza and other respiratory infections but may also be responsible for development of asthma in the elderly as poorly resolve respiratory infections are often the forerunners of asthma attacks in the population.
Decline in IFN secretion by pDCs in aged subjects has been demonstrated to be due to defect in signaling mechanisms that result in impaired phosphorylation of Interferon Regulatory Factor-7 (IRF-7) (Sridharan et al. 2010; Stout-Delgado et al. 2008). More recently, Qian et al. (2011) have reported decreased IFN-I secretion in monocyte derived DCs from older donors in response to West Nile virus due to decreased induction of STAT-1, IRF-7 and IRF-1. A growing body of evidence demonstrates that chromatin modification is another important mechanism that regulates cytokine expression (Barth and Imhof 2010; Bartova et al. 2008; Kouzarides 2007; Strahl and Allis 2000). Many immune related genes such as MCP-1, TNF-α, IL-12, and IL-4 are regulated by epigenetic changes characterized by histone metylation and acetylation (Weinmann et al. 1999; Fields et al. 2002; Barthel and Goldfeld 2003; Boekhoudt et al. 2003; Goriely et al. 2003; Wen et al. 2008). Furthermore, TLR- induced chromatin modification of H3K4me3 and histone acetylation are shown to control the inflammation by silencing the pro-inflammatory mediators (Foster et al. 2007). Dynamic epigenetic changes are also shown to occur also during differentiation of monocytes to macrophages and DCs (Tserel et al. 2010). Interestingly, genes related to phagocytic activity have more active chromatin marks (H3K4me3 and H3Ac) in monocytes and macrophages where the phagocytosis is more efficient. Whereas genes related to antigen processing and presentation have more active chromatin marks in macrophages and DCs which are the primary antigen presenting cells. Thus epigenetic mechanisms provide a new framework for understanding the complexity of immune regulation.
Studies reporting age related alteration in histone modifications in immune cells are rare. El Mezayen et al. (2009) found that up-regulation of IL-23 gene in aged DCs is associated with increased binding of IL-23p19 promoter to H3K4me3. Our findings with ChIP experiments demonstrate that the binding of IFN-A2 and IL-29 promoters to gene-repressor histone, H3K9me3 is increased in non-activated aged DCs (Fig. 3d and e). This means that for these promoters to be activated, H3K9me3 must relax to allow the dissociation of the promoters and subsequent binding to activator histones to start transcription of IFN genes. In the young DCs, on the other hand since there is no association of the promoters with H3K9me3, they are free to bind to the activator histones when required. Our results with H3K4me3 ChIP support this scenario since there was decreased association of these promoters with activator histone, H3K4me3 in aged DCs after activation with influenza which correlates with decreased secretion of these cytokines by aged DCs. Young DCs, on the other hand, were fully competent to produce the IFNs.
The association of the promoters with the histones was dynamic and changed by 48 h when we observed increased binding of all three promoters to H3K9me3 indicating a silencing of these genes. This is not surprising since DCs are innate immune cells that, on activation with influenza, secrete the interferons and TNF-α. This secretion is fast and occurs early on in the immune response and by 48 h the DCs have already migrated to the lymph nodes to prime T cells in vivo.
In summary, we have shown that aged monocyte derived DCs are impaired in the secretion of IFN-I and IFN-III in response to influenza virus. This is due to age-associated changes in chromatin structure since we find increased binding of IFN-I and IFN-III promoters to H3K9me3 in aged DCs. This results in decreased association of IFN-I and IFN-III promoters with H3K4me3 after activation with influenza resulting in reduced production of these cytokines by aged DCs. A better knowledge of the molecular mechanisms that control cytokine secretion by DCs will provide deeper understanding of the initiation and development of the inflammatory processes occurring in response to infection.
Electronic supplementary material
(JPEG 2 kb)
(JPEG 75 kb)
High resolution image file (TIFF 1027 kb)
Acknowledgements
This study was supported by New Scholar grant from the Ellison Medical Foundation. We thank the Institute of Clinical and Translational Science (ICTS), UCI, for providing blood from young donors.
References
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178(11):6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182(2):1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180(4):2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors. 2009;35(1):82–87. doi: 10.1002/biof.19. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci. 2010;35(11):618–626. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Barthel R, Goldfeld AE. T cell-specific expression of the human TNF-alpha gene involves a functional and highly conserved chromatin signature in intron 3. J Immunol. 2003;171(7):3612–3619. doi: 10.4049/jimmunol.171.7.3612. [DOI] [PubMed] [Google Scholar]
- Bartova E, Krejci J, Harnicarova A, Galiova G, Kozubek S. Histone modifications and nuclear architecture: a review. J Histochem Cytochem. 2008;56(8):711–721. doi: 10.1369/jhc.2008.951251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beignon AS, Skoberne M, Bhardwaj N. Type I interferons promote cross-priming: more functions for old cytokines. Nat Immunol. 2003;4(10):939–941. doi: 10.1038/ni1003-939. [DOI] [PubMed] [Google Scholar]
- Billiau A. Interferon: the pathways of discovery: I. Molecular and cellular aspects. Cytokine Growth Factor Rev. 2006;17(5):381–409. doi: 10.1016/j.cytogfr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Boekhoudt GH, Guo Z, Beresford GW, Boss JM. Communication between NF-kappa B and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J Immunol. 2003;170(8):4139–4147. doi: 10.4049/jimmunol.170.8.4139. [DOI] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diebold J, Diepolder H, Adler B, Auernhammer CJ, Goke B, Dambacher J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G960–968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol. 2002;14(4):411–419. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- Busuttil R, Bahar R, Vijg J. Genome dynamics and transcriptional deregulation in aging. Neuroscience. 2007;145(4):1341–1347. doi: 10.1016/j.neuroscience.2006.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday DH, Amponsah NA, Jones L, Tisch DJ, Hornick TR, Ramachandra L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J Immunol. 2010;30(3):373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459(7248):802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424(6946):324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279(31):32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- Effros RB. Replicative senescence of CD8 T cells: effect on human ageing. Exp Gerontol. 2004;39(4):517–524. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, Yoza BK, Chen X, Garcia BA, Young NL, McCall CE. Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol. 2009;29(7):1959–1971. doi: 10.1128/MCB.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8(5):553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005;22(7):577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JD, Smith JD, Murphy TL, Murphy KM. Recruitment of Stat4 to the human interferon-alpha/beta receptor requires activated Stat2. J Biol Chem. 2000;275(4):2693–2697. doi: 10.1074/jbc.275.4.2693. [DOI] [PubMed] [Google Scholar]
- Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169(2):647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312(5775):879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Goriely S, Demonte D, Nizet S, De Wit D, Willems F, Goldman M, Van Lint C. Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites. Blood. 2003;101(12):4894–4902. doi: 10.1182/blood-2002-09-2851. [DOI] [PubMed] [Google Scholar]
- Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus-related morbidity in chronic lung disease. Arch Intern Med. 2002;162(11):1229–1236. doi: 10.1001/archinte.162.11.1229. [DOI] [PubMed] [Google Scholar]
- Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Characterization of naive, memory and effector CD8+ T cells: effect of age. Exp Gerontol. 2004;39(4):545–550. doi: 10.1016/j.exger.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21(4):414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference: I. The interferon. Proc R Soc Lond B. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 2010;84(9):4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SA, Taverna SD, Zhang Y, Briggs SD, Li J, Eissenberg JC, Allis CD, Khorasanizadeh S. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 2001;20(18):5232–5241. doi: 10.1093/emboj/20.18.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol. 2010;84(21):11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70(10):777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katashiba Y, Miyamoto R, Hyo A, Shimamoto K, Murakami N, Ogata M, Amakawa R, Inaba M, Nomura S, Fukuhara S, Ito T. Interferon-alpha and interleukin-12 are induced, respectively, by double-stranded DNA and single-stranded RNA in human myeloid dendritic cells. Immunology. 2011;132(2):165–173. doi: 10.1111/j.1365-2567.2010.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, Kotenko SV, Sideras P, Lehr HA, Tepe M, Klucher KM, Doyle SE, Neurath MF, Finotto S, Andreakos E. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3(6):348–361. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kreiling JA, Tamamori-Adachi M, Sexton AN, Jeyapalan JC, Munoz-Najar U, Peterson AL, Manivannan J, Rogers ES, Pchelintsev NA, Adams PD, Sedivy JM. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10(2):292–304. doi: 10.1111/j.1474-9726.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176(4):2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86(1):23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- Liu B-S, Janssen HLA, Boonstra A. IL-29 and IFNα differ in their ability to modulate IL-12 production by TLR-activated human macrophages and exhibit differential regulation of the IFNγ receptor expression. Blood. 2011;117(8):2385–2395. doi: 10.1182/blood-2010-07-298976. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189(3):521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Vijg J. Genome instability, cancer and aging. Biochim Biophys Acta. 2009;1790(10):963–969. doi: 10.1016/j.bbagen.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and −29: comparison with type I interferons. Cytokine. 2005;31(2):109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4(9):e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84(11):5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, O'Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera RE, Kouzarides T. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412(6846):561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, Himi T, Fujii N, Sawada N. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus. 2011;160(1–2):360–366. doi: 10.1016/j.virusres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011;203(10):1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rogge L, D'Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, Adorini L, Sinigaglia F. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161(12):6567–6574. [PubMed] [Google Scholar]
- Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci U S A. 2006;103(43):15782–15787. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubeler D, MacAlpine DM, Scalzo D, Wirbelauer C, Kooperberg C, van Leeuwen F, Gottschling DE, O'Neill LP, Turner BM, Delrow J, Bell SP, Groudine M. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 2004;18(11):1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56(5):518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan A, Esposo M, Kaushal K, Tay J, Osann K, Agrawal S, Gupta S, Agrawal A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age. 2010;33(3):363–376. doi: 10.1007/s11357-010-9191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181(10):6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Tough DF. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma. 2004;45(2):257–264. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Tserel L, Kolde R, Rebane A, Kisand K, Org T, Peterson H, Vilo J, Peterson P. Genome-wide promoter analysis of histone modifications in human monocyte-derived antigen presenting cells. BMC Genomics. 2010;11:642. doi: 10.1186/1471-2164-11-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uze G, Monneron D. IL-28 and IL-29: newcomers to the interferon family. Biochimie. 2007;89(6–7):729–734. doi: 10.1016/j.biochi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann AS, Plevy SE, Smale ST. Rapid and selective remodeling of a positioned nucleosome during the induction of IL-12 p40 transcription. Immunity. 1999;11(6):665–675. doi: 10.1016/S1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood. 2008;111(4):1797–1804. doi: 10.1182/blood-2007-08-106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Hillenmeyer S, Lawrence C, Chang C, Hosier S, Lightfoot W, Mukherjee E, Jiang N, Schorl C, Brodsky AS, Neretti N, Helfand SL. Chromatin remodeling in the aging genome of Drosophila. Aging Cell. 2010;9(6):971–978. doi: 10.1111/j.1474-9726.2010.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap FH, Ho PL, Lam KF, Chan PK, Cheng YH, Peiris JS. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73(4):617–623. doi: 10.1002/jmv.20135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPEG 2 kb)
(JPEG 75 kb)
High resolution image file (TIFF 1027 kb)