Abstract
The role of caffeine consumption on insulin action is still under debate. The hypothesis that chronic caffeine intake reverses aging-induced insulin resistance in the rat was tested in this work. The mechanism by which caffeine restores insulin sensitivity was also investigated. Six groups of rats were used: 3 months old (3 M), 3 months old caffeine-treated (3MCaf), 12 months old (12 M), 12 months old caffeine-treated (12MCaf), 24 months old (24 M), and 24 months old caffeine-treated (24MCaf). Caffeine was administered in drinking water (1 g/l) during 15 days. Insulin sensitivity was assessed by means of the insulin tolerance test. Blood pressure, body weight, visceral and total fat, fasting glycemia and insulinemia, plasma nonesterified fatty acids (NEFA), total antioxidant capacity (TAC), cortisol, nitric oxide, and catecholamines were monitored. Skeletal muscle Glut4 and 5′-AMP activated protein kinase (AMPK) protein expression and activity were also assessed. Aged rats exhibited diminished insulin sensitivity accompanied by hyperinsulinemia and normoglycemia, increased visceral and total fat, decreased TAC and plasma catecholamines, and also decreased skeletal muscle Glut4 and AMPK protein expression. Chronic caffeine intake restored insulin sensitivity and regularized circulating insulin and NEFA in both aging models. Caffeine neither modified skeletal muscle AMPK expression nor activity in aged rats; however, it decreased visceral and total fat in 12 M rats and it restored skeletal muscle Glut4 expression to control values in 24 M rats. We concluded that chronic caffeine intake reverses aging-induced insulin resistance in rats by decreasing NEFA production and also by increasing Glut4 expression in skeletal muscle.
Keywords: Caffeine, Insulin resistance, Aging, Glut4 transporters, AMPK
Introduction
Aging is characterized by a gradual decline in the functional reserve of body organs and systems, with the net result of a reduction in the ability to maintain homeostasis. It is well established that aging is associated with an increase in insulin resistance accompanied by a decline in glucose tolerance (De Fronzo 1981; Rowe et al. 1983; Fink et al. 1986). Aging alters body composition, leading to an increase in central fat mass, which contributes to the development of insulin resistance (for a review, see Seals and Bell 2004).
In fact, several studies have attempted to assign insulin resistance to aging-associated obesity, instead of to aging itself (Bartke 2008; Carrascosa et al. 2011; Seals and Bell 2004). This hypothesis has been challenged by work showing that nonobese elderly humans also present decreased insulin sensitivity (Fink et al. 1983).
In the past few years, several epidemiological studies point toward a protective role for coffee in the development of type 2 diabetes (Van Dam and Feskens 2002; Van Dam et al. 2006), contrasting with reports that caffeine can lower insulin sensitivity when administered acutely (Keijzers et al. 2002; Moisey et al. 2008). The primary mechanism proposed for the protective role of caffeine in the development of type 2 diabetes was weight loss attributed to increased thermogenesis, lipolysis, and fat oxidation induced by this xanthine (Lopez-Garcia et al. 2006; Van Dam et al. 2006; Zheng et al. 2004; Jeukendrup and Randell 2011) via sympathetic nervous system (SNS) activation (Dulloo et al. 1992; Graham et al. 2000). Nevertheless, this hypothesis remains controversial since several authors have shown that chronic coffee consumption does not cause significant weight reduction (Astrup et al. 1992; Conde et al. 2012).
An increase in SNS activity is associated with enhanced circulating catecholamines which ultimately lead to insulin resistance (see Seals and Bell 2004). Increased circulating catecholamines contribute to insulin resistance mainly by stimulating lipolysis in adipose tissue, leading to an increase in circulating nonesterified fatty acids (NEFA) (Lafontan and Langin 2009), and by stimulating both gluconeogenesis and glycogenolysis, contributing to increased hepatic glucose production (Exton and Park 1968; Exton et al. 1972). This increase in endogenous glucose production associated with decreased skeletal muscle glucose uptake and decreased fatty acid oxidation (Young et al. 1985; Acheson et al. 2004; Mulder et al. 2005) leads to hyperglycemia and tissue triglyceride deposition, major pathophysiological hallmarks of insulin resistance. We have recently shown that chronic caffeine intake prevents the development of insulin resistance and decreases circulating catecholamines in diet-induced insulin-resistant rats (Conde et al. 2012). We have also shown that the pharmacological inhibition of the SNS with carvedilol, an antagonist of α1, β1, and β2 adrenoceptors, mimics the protective effect of caffeine, which suggests that chronic caffeine intake prevents diet-induced insulin resistance through a decrease in SNS activity. These contradictory results related to the role of caffeine on SNS modulation strongly support the hypothesis that acute and chronic caffeine administrations have opposite effects and different pharmacological effects/targets. Recently, caffeine has also been implicated in skeletal muscle regulation of glucose and lipid metabolism. Caffeine stimulates insulin-independent muscle glucose transport (Wright et al. 2004; Jensen et al. 2007) and increases Glut4 mRNA and/or protein expression and fatty acid metabolism (Mukwevho et al. 2008), having metabolic effects similar to the ones of 5′-AMP activated protein kinase (AMPK).
AMPK is a metabolic sensor that plays a key role in regulating lipid metabolism, as well as glucose homeostasis. By simultaneously inhibiting lipogenesis and lipolysis in adipose tissue, AMPK activation decreases circulating lipids and ectopic fat deposition(Long and Zierath 2006); it also increases lipid oxidation both in the liver and muscle (Luo et al. 2005; Ruderman and Saha 2006), contributing to increased whole-body insulin sensitivity. AMPK also decreases hepatic glucose production and increases glucose uptake in skeletal muscle, playing an important role in glucose homeostasis, and AMPK activation in the pancreatic beta cell decreases insulin secretion (Long and Zierath 2006). Recently, it has been shown that caffeine can increase Glut4 mRNA in skeletal muscle cells and insulin-independent glucose transport through an AMPK-mediated process (Egawa et al. 2009).
Herein, we tested the effects of long-term caffeine administration on aging-induced insulin resistance. In addition, we investigated the mechanism by which chronic caffeine improves insulin action in aging models.
Methods
Animals and experimental procedures
The experiments were performed in Wistar rats of both sexes. All along their life span, the animals were kept in the vivarium of Faculdade de Ciências Médicas with a seasonal day/light rhythm, with temperature controlled between 22 ± 2.0 °C and with water and food ad libitum. Six groups of rats were used: 3 months old (3 M) (n = 12), 3 months old caffeine-treated (3MCaf) (n = 12), 12 months old (12 M) (n = 12), 12 months old caffeine-treated (12Mcaf) (n = 12), 24 months old (24 M) (n = 8), and 24 months old caffeine-treated (24MCaf) (n = 8). The number of females and males in each group was equal. Rats’ weight was 292.43 ± 16.54 g at 3 months old, reaching 447.50 ± 49.27 g at 12 months and 627.78 ± 41.89 g at 24 months of age. Rats were fed with standard chow (7.4 % fat + 75 % carbohydrate (4 % sugar) + 17 % protein; SDS diets RM1; Probiológica, Sintra, Portugal). Caffeine was administered in drinking water (1 g/l) during 15 days before the experiments and liquid intake was monitored (Gasior et al. 2002), attaining a concentration of 25–30 mg/day. Body weight and animal behavioral changes were assessed twice per week during caffeine administration. Animal behavioral changes were monitored using the following parameters: response to handling, orientation in cage, and open field reflexes (Whishaw et al. 1983). On the last day before the experimental protocol, rats were fasted overnight and allowed free access to caffeine solutions. Afterwards, the animals were anesthetized with intraperitoneal sodium pentobarbital (60 mg/kg) and transferred to a heating pad to maintain body temperature at 37.5 ± 0.5 °C throughout the experiment. At the end of the experiments, the rats were euthanized by an intracardiac overdose of sodium pentobarbital. A low rate of mortality was observed; in fact, only one rat from the 24 M group was euthanized prior to experiments due to existence of tumor mass in the abdominal area. Principles of laboratory care were followed in accordance with the European Union Directive for the Protection of Vertebrates Used for Experimental and Other Scientific Ends (86/609/CEE). Experimental protocols were previously approved by the Ethics Committee for Animal Care and Use at the Faculty of Medical Sciences, New University of Lisbon (FCM-UNL; Faculdade de Ciências Médicas, Universidade Nova de Lisboa).
Measurement of insulin sensitivity
The insulin tolerance test (ITT) was used to measure insulin sensitivity. The ITT consists in the administration of an intravenous insulin bolus of 0.1 U/kg body weight after an overnight fast, followed by measuring the decline in plasma glucose concentration over 15 min. The constant rate for glucose disappearance (KITT) was calculated using the formula 0.693/t1/2. Glucose half-time (t1/2) was calculated from the slope of the least square analysis of plasma glucose concentrations during the linear decay phase. Blood samples were collected by tail tipping and glucose levels were measured with a glucometer (Abbott Diabetes Care, Portugal) and test strips (Abbott Diabetes Care, Portugal).
Blood pressure measurements
To measure mean arterial pressure (MAP), the femoral artery was cannulated under a dissection microscope and the catheter connected to a pressure transducer (model 603, HSE-HA GmgH, Harvard Apparatus, Madrid, Spain) and a pressure amplifier (Plugsys Housings, model 603, HSE-HA GmgH, Harvard Apparatus, Madrid, Spain). MAP data were acquired with an HSE-Harvard Pulmodyn W software (Harvard Apparatus, Madrid, Spain).
Measurement of insulin, nonesterified fatty acids, nitric oxide, total antioxidant capacity, cortisol, and catecholamines
Plasma and serum were collected after heart puncture to ethylenediaminetetraacetic acid (EDTA) precoated tubes and to Eppendorfs, respectively, and kept on ice. Plasma samples were centrifuged (Sigma, Madrid, Spain) at 3,000 × g for 10 min (4 °C) and serum samples were centrifuged in a microfuge (Eppendorf, Madrid, Spain) at 12,000 × g for 10 min. Plasma and serum were stored at −80 °C in an ultralow freezer (Heraeus, Madrid, Spain). Insulin, nitric oxide (NO), and total antioxidant capacity (TAC) were quantified in plasma, and NEFA, cortisol, and catecholamines were quantified in serum.
Insulinemia was quantified with an enzyme-linked immunosorbent assay (ELISA) kit (Mercodia Ultrasensitive Rat Insulin ELISA Kit, Mercodia AB, Uppsala, Sweden), free fatty acids with a colorimetric assay (Zenbio, North Carolina, USA), TAC by a 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) Antioxidant Assay Kit (Zenbio, North Carolina, USA), and cortisol by an immunoassay (Assay Designs, Hines Drive, USA).
For NO/NO−3 determination, plasma samples obtained were deproteinized by adding two volumes of ethanol (0 °C). After 30 min on ice, samples were centrifuged in a microfuge (Eppendorf, Madrid, Spain) at 12,000 × g for 10 min. NO/NO−3 concentration was determined by using a specific and sensitive NO/ozone chemiluminescence technique (NO-Analyzer 280, Sievers Research Inc., Boulder, CO, USA).
To quantify serum catecholamines by serum quantification, 500 μl of serum samples were purified and catecholamines were extracted using 30 mg OASIS Hlb Wat cartridges (Waters, Milford, MA, USA) and eluted in 500 μl of mobile phase. One hundred microliters of the samples were directly injected into a high-performance liquid chromatography system composed of a Waters 600 controller pump, a Waters C18 (particle size, 4 μm) column, a Waters 717 plus autosampler, a Bioanalytical Systems LC-4A electrochemical detector (set at a holding potential of 0.65 mV and a sensitivity of 1 nA). An isocratic elution was used: the mobile phase consisted of a solution of Na2HPO4 25 mM with 6 % of methanol, pH 3.55, running at a flux of 1.0 ml/min. The signal coming out of the detector was fed to an analog to digital converter controlled by the Peak Sample Chromatography System Software (Buck Scientific, East Norwalk, CT, USA). Identification and quantification of catecholamines were done against external standards.
Collection and weight of abdominal fat
Renal, genital, and visceral fat were collected after an abdominal laparotomy and weighted (Mettler Toledo, Lisbon, Portugal).
Western blot analysis of Glut4 expression, AMPKα1 expression, and AMPK activity assessed as AMPKα1 Thr172 phosphorylation in skeletal muscle
Muscles were homogenized in Zurich medium (Tris–HCl, 10 mM; EDTA, 1 mM; NaCl, 150 mM; Triton X-100, 1 %; sodium cholate, 1 %; sodium dodecyl sulfate, 0.1 %) containing a cocktail of protease inhibitors. Protein concentrations of the homogenates were measured by a Micro-BCA Protein Assay (Pierce, Madrid, Spain). Samples of the homogenates (25 μg) and the prestained molecular weight markers (Precision, BioRad, Madrid, Spain) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10 % with a 5 % concentrating gel) under reducing conditions and electrotransferred to polyvinylidene difluoride membranes (0.45 μM, Millipore, Spain). After blocking for 1 h at room temperature with 5 % nonfat milk in Tris-buffered saline, pH 7.4 containing 0.1 % Tween 20 (TTBS) (BioRad, Spain), the membranes were incubated overnight at 4 °C with goat polyclonal anti-Glut4 (sc-1608, Sta Cruz Biotechnology, USA) diluted 1:200, goat polyclonal anti-AMPKα1 (sc-19128, Sta Cruz Biotechnology, USA) diluted 1:200, and rabbit polyclonal antiphosphorylated Thr172 of AMPKα1 (sc-101630, Sta Cruz Biotechnology, USA). After three washing periods of 15 min with TTBS, the membranes were incubated with donkey antigoat (1:2,000, Sta Cruz Biotechnology, USA) and goat antirabbit (1:2,000, Sta Cruz Biotechnology, USA) in TTBS for 1 h at room temperature and developed with enhanced chemiluminescence reagents according to the manufacturer’s instructions (Immobilon Western, Millipore, Spain). The intensity of the signals was detected in a Chemidoc Molecular Imager (Chemidoc BioRad, Spain) and quantified using the Quantity One software (BioRad, USA). The membranes were then reprobed and tested for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoreactivity (bands in the 37-kDa region) in order to compare and normalize the expression of proteins with the amount of protein loaded. Briefly, the membranes were washed several times with TTBS and then incubated for 2 h at room temperature with monoclonal antimouse GAPDH (1:200, Sta Cruz Biotechnology, USA). After three washing periods of 15 min with TTBS, the membranes were incubated with goat antimouse horseradish peroxidase (1:2,000, Sta Cruz Biotechnology, USA) as previously described. The mean intensity of control samples in each membrane was used as reference for controlling gel-to-gel variation.
Drugs and chemicals
Aprotinin, bovine serum albumin, EDTA, epinephrine, KH2PO4, KOH, leupeptin, metaphosphoric acid, NaCl, Na2HPO4, norepinephrine (NE), perchloric acid, pepstatin, phenylmethylsulfonyl fluoride, sodium cholate, sodium orthovanadate, Tris, trypsin inhibitor, and Triton X-100 were obtained from Sigma-Aldrich (Madrid, Spain). Tween 20 was obtained from BioRad (Madrid, Spain). All antibodies were purchased from Sta Cruz Biotechnology (USA). Insulin was commercially available as Humulin® Regular (Lilly, Portugal) in a concentration of 100 UI/ml.
Data analysis
Data were evaluated using GraphPad Prism Software, version 4 (GraphPad Software Inc., San Diego, CA, USA) and was presented as the mean ± SEM. The significance of the differences between the means was calculated by one-way and two-way analysis of variance (ANOVA) with Dunnett’s and Bonferroni multiple comparison tests, respectively. Differences were considered significant at p ≤ 0.05.
Results
Liquid intake (in milliliters per day) was similar in all animal models tested and chronic ingestion of caffeine did not modify liquid intake or animal behavior within groups (data not shown). Caffeine intake in all groups was between 25 and 30 mg/day. Fasting glycemia was not significantly different in control, 12 M, and 24 M rats. Chronic caffeine intake did not modify fasting glycemia in any of the groups tested (Table 1).
Table 1.
Effect of chronic caffeine intake (1 g/l) on glycemia and insulinemia in 3-, 12-, and 24-month-old rats (mean values with their standard errors for all the individual samples were obtained from 8 to 12 rats)
| Months | Plasma glucose (mM) | Plasma insulin (nM) | |
|---|---|---|---|
| 3 | Without caffeine | 5.84 ± 0.23 | 0.35 ± 0.07 |
| With caffeine | 5.75 ± 0.17 | 0.19 ± 0.04 | |
| 12 | Without caffeine | 5.37 ± 0.18 | 0.95 ± 0.02* |
| With caffeine | 5.75 ± 0.32 | 0.71 ± 0.07** | |
| 24 | Without caffeine | 5.36 ± 0.17 | 0.93 ± 0.05* |
| With caffeine | 5.66 ± 0.35 | 0.64 ± 0.08** | |
*p < 0.001, mean value was significantly different from those obtained in 3-month-old rats (two-way ANOVA with Bonferroni multicomparison test); **p < 0.5, mean value was significantly different from 12- and 24-month-old rats that do not drink caffeine (two-way ANOVA with Bonferroni multicomparison test)
Fasting insulinemia was 0.35 ± 0.07 nM in control rats. Both 12 M and 24 M rats showed a significant increase in insulinemia (p < 0.001) when compared with the control group. Caffeine significantly decreased insulinemia, although without reaching control 3 M rat values (Table 1).
Effect of chronic caffeine consumption on insulin sensitivity
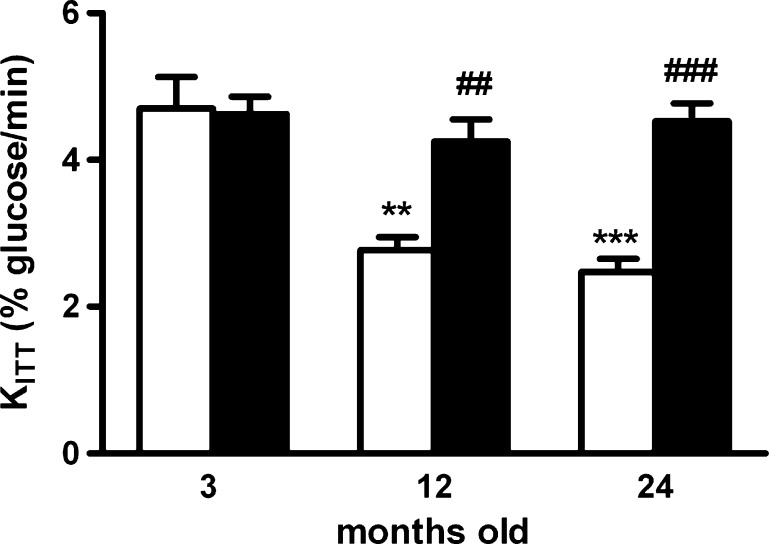
Figure 1 presents the effect of chronic caffeine intake (1 g/l) on insulin sensitivity in 3 M, 12 M, and 24 M rats. Aging caused a decrease in insulin sensitivity, as KITT significantly decrease in 12 M and 24 M rats from a control value of 4.67 ± 0.42 to 2.77 ± 0.17 % glucose/min (p < 0.01) and to 2.47 ± 0.18 % glucose/min (p < 0.001), respectively. Chronic caffeine intake did not modify insulin sensitivity in control animals (KITT Caf = 4.62 ± 0.23 % glucose/min); however, it completely reversed the aging-induced insulin resistance both in 12 M and 24 M animals (KITT 12MCaf = 4.25 ± 0.30 % glucose/min; KITT 24MCaf = 4.52 ± 0.25 % glucose/min).
Fig. 1.
Effect of chronic caffeine intake (1 g/l) on insulin sensitivity in 3-, 12-, and 24-month-old rats, determined by ITT, expressed as constant for glucose disappearance (KITT). Values represent the mean ± SEM; values without caffeine represent the mean of 8–12 experiments; values with caffeine represents the mean of 8–11 experiments. **p < 0.01, ***p < 0.001, compared with control values without caffeine; ##p < 0.01, ###p < 0.001, compared with values without caffeine within 3-, 12-, and 24-month-old animals (two-way ANOVA with Bonferroni multicomparison test)
Effect of chronic caffeine consumption on mean arterial pressure
Table 2 displays the effect of chronic caffeine consumption on MAP in control and aged rats. Neither aging nor caffeine modified MAP.
Table 2.
Effect of chronic caffeine (1 g/l) on MAP, NO, and plasma TAC in 3-, 12-, and 24-month-old rats (mean values with their standard errors for all the individual samples were obtained from 8 to 12 rats)
| Months | 3 | 12 | 24 | |||
|---|---|---|---|---|---|---|
| Without caffeine | With caffeine | Without caffeine | With caffeine | Without caffeine | With caffeine | |
| MAP (mmHg) | 91.78 ± 3.96 | 87.46 ± 5.48 | 90.15 ± 9.74 | 93.86 ± 2.81 | 88.90 ± 4.96 | 94.29 ± 4.37 |
| NO (μM) | 15.43 ± 0.99 | 17.33 ± 0.67 | 16.08 ± 2.62 | 21.49 ± 4.13 | 15.21 ± 2.64 | 18.45 ± 2.19 |
| TAC (μM Trolox equivalent) | – | – | 166.81 ± 35.08 | 172.53 ± 41.41 | 270.53 ± 39.04 | 259.03 ± 19.4 |
Two-way ANOVA with Bonferroni multicomparison test
Effect of chronic caffeine intake on lipid metabolism: body weight, visceral fat, and nonesterified fatty acids
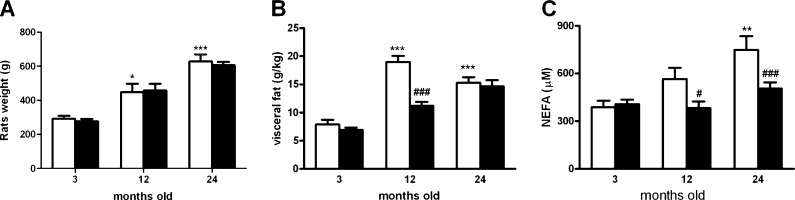
Figure 2a shows the effect of chronic caffeine administration on body weight. At 3 months of age, rats presented a mean body weight of 292.43 ± 16.54 g. At 12 months of age, mean body weight reached 447.50 ± 49.27 g, and at 24 months old, it reached 627.78 ± 41.89 g.
Fig. 2.
Effect of chronic caffeine intake (1 g/l) on body weight (a), visceral fat corrected to body weight (b), and NEFA (c) in 3-, 12-, and 24-month-old rats. Values are presented as the mean ± SEM. Values for body weight represent a mean of 8–12 experiments and a mean of 9–11 in the absence and the presence of caffeine, respectively. Values for visceral fat represent a mean of 8–21 experiments and a mean of 8–19 in the absence and the presence of caffeine, respectively. Values for NEFA represent a mean of 7–23 experiments and a mean of 9–23 in the absence and the presence of caffeine, respectively.*p < 0.05, **p < 0.01, ***p < 0.01, compared with control values without caffeine; #p < 0.05, ###p < 0.001 compared with values without caffeine within 3-, 12-, and 24-month-old animals (two-way ANOVA with Bonferroni multicomparison test)
Chronic caffeine intake did not significantly modify body weight in any of the groups tested (Fig. 2a). Visceral fat significantly increased with age (3 M rats, 7.92 ± 0.81 g/kg; 12 M rats, 18.97 ± 1.08vg/kg; 24 M rats, 15.31 ± 0.98 g/kg) (Fig 2b). Chronic caffeine intake did not modify visceral fat both in 3 M and 24 M rats, but it significantly reduced visceral fat mass in 12 M rats (11.20 ± 0.68 g/kg).
Figure 2c depicts the effect of chronic caffeine consumption on NEFA in 3-, 12-, and 24-month-old rats. NEFA show a tendency to increase with age that became statistically significant at 24 months old. Chronic caffeine intake did not modify NEFA in 3 M rats (NEFA 3 M = 389.18 ± 40.45 μM; NEFA 3MCaf = 406.57 ± 29.51 μM), but it decreased NEFA to control values both in 12MCaf (382.99 ± 41.05 μM) and 24MCaf (503.55 ± 40.70 μM) rats.
Effect of chronic caffeine intake on oxidative status: plasma NO/NO−3 concentration and on plasma total antioxidant capacity
In Table 2, the effect of chronic caffeine intake on plasma NO/NO−3 is depicted. Aging did not alter NO levels compared to 3-month-old rats (3 M = 15.43 ± 0.99 μM). Chronic consumption of caffeine did not significantly modify NO levels either in control rats or in 12- and 24-month-old rats. Chronic caffeine intake did not modify TAC in 12- and 24-month-old rats (Table 2).
Effect of chronic caffeine intake on stress biomarkers: cortisol and serum catecholamines
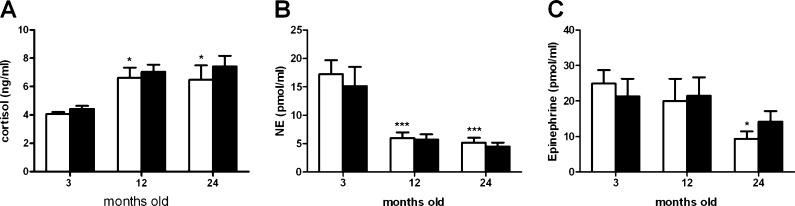
The effect of chronic caffeine intake on cortisol levels in 3-, 12-, and 24-month-old rats is depicted in Fig. 3a. Aging increases cortisol levels in 12 M and 24 M rats in comparison with 3 M rats (cortisol 3 M = 4.077 ± 0.14 ng/ml; 12 M = 6.62 ± 0.71 ng/ml; 24 M = 6.48 ± 1.01vng/ml), but chronic caffeine intake did not significantly alter cortisol levels (Fig. 3a).
Fig. 3.
Effect of chronic caffeine intake (1 g/l) on plasma cortisol (a), NE (b), and epinephrine (c) concentrations in 3-, 12-, and 24-month-old rats. Values are presented as the mean ± SEM. Cortisol (without caffeine n = 7–12; with caffeine n = 6–9); NE (without caffeine n = 7–12; with caffeine n = 7–12); epinephrine (without caffeine n = 7–12; with caffeine n = 7–12). *p < 0.05, ***p < 0.001, compared with values obtained in 3-month-old rats without caffeine intake (two-way ANOVA with Bonferroni multicomparison test)
Figure 3b, c presents the effect of chronic caffeine intake on circulating NE and epinephrine levels, respectively. NE levels were significantly decreased by 66 and 72 % in 12 M and 24 M rats in comparison with 3 M rats (NE = 18.37 ± 2.60 μM) (Fig. 3b). Although epinephrine levels were significantly diminished in 24 M rats (epinephrine 3 M = 24.88 ± 3.82 μM; epinephrine 24 M = 9.3 ± 2.06 μM) (Fig. 3c), chronic caffeine intake did not significantly modify NE levels or epinephrine levels at 3 M, 12 M, or 24 M rats.
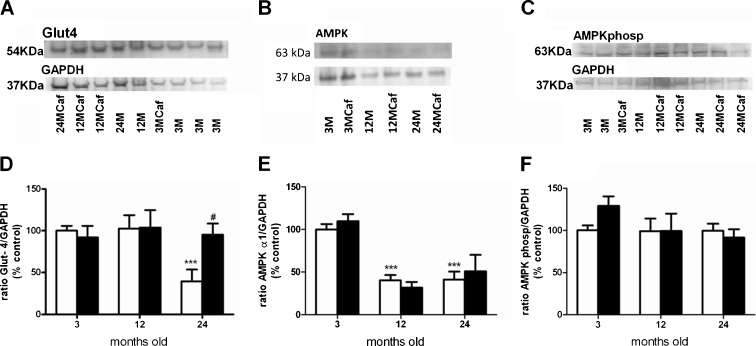
Effect of chronic caffeine intake on Glut4, AMPKα1, and AMPKα1 Thr172 phosphorylation expression in skeletal muscle
Figure 4a–c presents the Western blots comparing Glut4, AMPKα1, and AMPKα1 Thr172 phosphorylation immunoreactivity in the skeletal muscle, corresponding to 54, 63, and 63 kDa bands, respectively, when comparing 3-, 12-, and 24-month-old rats with and without caffeine intake. As it can be shown in Fig. 4a, d, the expression of Glut4 transporters in skeletal muscle decreased significantly by 60.5 % at 24 M and chronic caffeine intake restore Glut4 expression to control levels. The expression of AMPKα1 in skeletal muscle decreased by 59.8 and 58.8 % in 12 M and 24 M, respectively; however, the long-term caffeine intake did not modify these values (Fig. 4b, e). Additionally, the activity of AMPK, assessed as AMPKα1 Thr172 phosphorylation expression, was not altered in aged rats and chronic caffeine intake did not modify this expression (Fig. 4c, f).
Fig. 4.
Effect of chronic caffeine intake on Glut4, AMPKα1, and AMPKα1 Thr172 phosphorylation expression in skeletal muscle in 3-, 12-, and 24-month-old rats. a–c Western blot comparing Glut4, AMPKα1, and AMPKα1 Thr172 phosphorylation immunoreactivity in skeletal muscle, corresponding to 54, 63, and 63 kDa bands, respectively, when comparing 3-, 12-, and 24-month-old rats with and without caffeine intake; d–f the average relative Glut4, AMPKα1, and AMPKα1 Thr172 phosphorylation immunoreactivity in the different paradigms expressed in relation to GAPDH immunoreactivity. *p < 0.05, **p < 0.01, ***p < 0.001, compared with 3-month-old rats without caffeine intake; #p < 0.05, compared with values without caffeine within groups (two-way ANOVA with Bonferroni multicomparison test, comparing all the groups with the control group). Data are presented as the means ± SEM; Glut4 expression (without caffeine n = 6–10; with caffeine n = 7–10); AMPK and AMPKα1 Thr172 phosphorylation expression (without caffeine n = 4–18; with caffeine n = 4–8)
Discussion
We demonstrate herein that chronic caffeine consumption reverses insulin resistance induced by aging in rats. The increase in insulin sensitivity induced by caffeine was not attributable to weight loss, increased NO production, caffeine-mediated antioxidant effects, decreased cortisol levels, or decreased SNS activity. Caffeine restored circulating NEFA in aged animals to values observed in young 3 M control rats. Moreover, in 24 M rats, caffeine increased skeletal muscle Glut4 expression. Our results suggest that the metabolic effects of caffeine in aged animals are mediated by inhibition of lipolysis and/or increased lipid oxidation and also by stimulation of skeletal muscle Glut4 expression.
The concentration of caffeine tested was chosen based on the literature, since findings by Holtzman’s group (e.g., Holtzman 1990) and replicated by Goldberg’s group (Gasior et al. 2002) showed that rats exposed to 1 g/l of caffeine in drinking water displayed plasma caffeine levels comparable to those in moderate to low consumers of caffeinated beverages. We also have previously described (Conde et al. 2012) that plasma concentrations of caffeine obtained with the dose 1 g/l are in the same range as those previously described in rats by Gasior et al. (2002) and Cognato et al. (2010) and correspond to a consumption of three to four cups of coffee per day in humans (Fredholm et al. 1999).
Chronic caffeine treatment did not significantly change fasting glycemia in any of the groups tested but it significantly decreased aging-induced hyperinsulinemia. Caffeine can acutely increase insulin secretion (Greenberg et al. 2006), but the effects of sustained administration of the xanthine on insulin production by the pancreas appear to be, as many others, completely opposite from the ones observed after acute administration. We have previously shown that plasma insulin levels decrease after chronic caffeine treatment in hyperinsulinemic animal models of diet-induced insulin resistance (Conde et al. 2012), although we could not ascertain the mechanism responsible for the lowering of plasma insulin induced by caffeine. It still remains to be clarified whether the hypoinsulinemic effect induced by chronic caffeine intake in insulin-resistant animal models is a direct effect of caffeine on the pancreas, possibly by the activation of AMPK (Long and Zierath 2006) or simply a negative feedback at the β cell ensuing improved peripheral insulin sensitivity.
In the present work, we also demonstrated that chronic caffeine intake did not modify blood pressure, endogenous NO production, or antioxidant capacity in aged animals. These results suggest that acknowledged mechanisms of the action of caffeine, like increased NO production (Ofluoglu et al. 2009; Corsetti et al. 2008) or its antioxidant activity (Scharf et al. 2001)) are not involved in the reversal of age-related insulin resistance induced by the drug.
Reversal of insulin resistance through the administration of caffeine involves a decrease in SNS activity (Conde et al. 2012). It is indubitable that overfeeding hypercaloric diets is associated with an increase in sympathetic activity that contributes to insulin resistance (Landsberg and Young 1978); however, in aging-induced insulin resistance, the involvement of this mechanism is not clear. Although several molecular and cellular mechanisms contribute to aging-induced insulin resistance, particularly oxidative stress (Pitocco et al. 2010), skeletal muscle mitochondrial dysfunction (Chanséaume and Morio 2009; Kim et al. 2008), inflammation (Zeyda and Stuling 2009), and SNS overactivity (Di Nardo et al. 2009), the pathogenesis of aging-induced insulin resistance is still controversial. In contrast with the data of Di Nardo’s group, our results show that sympathetic activity, measured as circulating catecholamines, is decreased in aged animals despite the fact that insulin sensitivity is markedly diminished. This led us to the conclusion that sympathetic overactivity is not in the genesis of aging-induced insulin resistance. These results are in the same line of evidence than those obtained by Lee et al. (2001), where they observed decreased plasma monoamines in 12- and 24-month-old rats, as well as those reported by Cizza et al. (1995), in which aged rats showed reduced ability to activate their central and peripheral catecholaminergic systems when compared to young rats in a stressed state.
In contrast to the absence of effect of chronic caffeine on SNS activity, a very significant effect was observed in adipose tissue homeostasis. Visceral fat mass was increased both in 12 M and 24 M animals, with a more pronounced effect in the 12 M group. Supporting our data, it has been observed that the decrease of insulin sensitivity until 8 months of age is paralleled by a significant increase in visceral and total fat mass, but between 8 and 24 months of age, insulin sensitivity decreases further without significant changes in percent visceral adiposity (Escrivá et al. 2007). In our experimental setting, caffeine significantly decreased visceral fat mass in the 12 M, but not in the 24 M, group. Escrivá and coworkers also showed that in 24-month-old rats, the accumulation of retroperitoneal and total body fat is more significant than visceral fat buildup (Escrivá et al. 2007). We may have missed a significant effect of caffeine on adipose tissue in 24 M rats, considering that we did not assess whole body fat.
The decrease in visceral fat mass induced by long-term caffeine administration observed in the 12 M group was not due exclusively to increased lipolysis, a known effect of this xanthine (Acheson et al. 2004; Jeukendrup and Randell 2011), since it was accompanied by a significant decrease in circulating NEFA. Additionally, it has been suggested by Benowitz that the increase in lipolysis mediated by caffeine occurs through a rise in circulating epinephrine (Benowitz 1990), but in our experimental conditions, circulating catecholamines were decreased in aged animals and caffeine did not modify neither NE nor epinephrine plasma concentrations. In agreement with our results, others have also described that plasma and cerebral catecholamines are decreased in aged rats (Lee et al. 2001).
The effects of caffeine on adipose tissue metabolism are probably contributing significantly to the insulin-sensitizing effect of the drug in the 12 M aging animal model. The increase in KITT induced by caffeine is not related to changes in Glut4 skeletal muscle expression or AMPK expression or activity, but coexists with decreased visceral fat mass, decreased NEFA, and decreased insulinemia. Since increased circulating NEFA is a pathophysiological hallmark of insulin resistance, the beneficial effect of caffeine in this aging model seems to be directly related to the modulation of adipose tissue homeostasis (Kovacs and Stumvoll 2005).
In contrast, the increase in insulin sensitivity observed in 24 M rats is accompanied by a decrease in NEFA and plasma insulin without changes in visceral fat mass and also by increased Glut4 expression in skeletal muscle. In older animals, the metabolic effect of caffeine seems to be less dependent from the adipose tissue, and this fact may be explained by a decrease in fat mass observed in control 24 M when compared to control 12 M animals. At 24 months of age, the rats have less fat mass, which renders caffeine inhibition of lipogenesis less effective than in 12 M animals.
The 24 M animals had lower skeletal muscle Glut4 expression compared to the 3 M control group, which was not observable in 12 M animals. Our results suggest that caffeine administration restored Glut4 expression in the elderly group, but it was not able to increase Glut4 expression above a maximal level in the 12 M group. The mechanism by which caffeine increases Glut4 expression is still unclear, but the effect appears to be extant to several cell types: rat skeletal muscle primary cultures (Egawa et al. 2009) and myoblasts (Mukwevho et al. 2008), although contrasting results have been obtained in adipose tissue cells where caffeine suppressed insulin-induced Glut4 translocation (Akiba et al. 2004).
In conclusion, we have shown that chronic caffeine administration reverses insulin resistance associated with aging in animal models, an effect that was independent of weight loss, increased NO production, antioxidant status, cortisol levels, and SNS activity. The major target tissue for the beneficial effect of caffeine on metabolic homeostasis in aging animals seems to be the adipose tissue due to a significant decrease in circulating NEFA induced by chronic administration of the drug. In older animals, caffeine was even able to restore glucose uptake, increasing skeletal muscle Glut4 expression. We believe this work strengthens the conclusions of recent epidemiological studies concerning the protective role of coffee consumption in type 2 diabetes and the metabolic syndrome (Van Dam and Feskens 2002; Van Dam et al. 2006; Natella and Scaccini 2012). Furthermore, it provides new insights into the mechanism of action of caffeine on insulin resistance and supports the need for a paradigm shift on advised lifestyle changes, like coffee withdrawal, in insulin-resistant and hypertensive patients.
Acknowledgments
We are very grateful to Elena Gonzalez Muñoz and Constancio Gonzalez from the Faculty of Medicine of the University of Valladolid for the serum catecholamines quantification. We also thank to Inês Faustino for her technical support and to Dr. Michael Bright for reviewing the English. The work was financially supported by L’Oreal-Unesco-FCT Awards for Women in Science 2009-Portugal and by Portuguese Foundation for Science and Technology grant PTDC/SAU-ORG/111417/2009. There are no conflicts of interest.
References
- Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, Fay LB, Gay LJ, Schneiter P, Schindler C, Tappy L. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr. 2004;79:40–46. doi: 10.1093/ajcn/79.1.40. [DOI] [PubMed] [Google Scholar]
- Akiba T, Yaguchi K, Tsutsumi K, Nishioka T, Koyama I, Nomura M, Yokogawa K, Moritani S, Miyamoto K. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochem Pharmacol. 2004;68:1929–1937. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Astrup A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial. Int J Obes Relat Metab Disord. 1992;16:269–277. [PubMed] [Google Scholar]
- Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med. 1990;41:277–288. doi: 10.1146/annurev.me.41.020190.001425. [DOI] [PubMed] [Google Scholar]
- Carrascosa JM, Andrés A, Ros M, Bogónez E, Arribas C, Fernández-Agulló T, De Solís AJ, Gallardo N, Martínez C. Development of insulin resistance during ageing: involvement of central processes and role of adipokines. Curr Protein Pept Sci. 2011;12:305–315. doi: 10.2174/138920311795906655. [DOI] [PubMed] [Google Scholar]
- Chanséaume E, Morio B. Potential mechanisms of muscle mitochondrial dysfunction in aging and obesity and cellular consequences. Int J Mol Sci. 2009;10:306–324. doi: 10.3390/ijms10010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza G, Pacak K, Kvetnansky R, Palkovits M, Goldstein DS, Brady LS, Fukuhara K, Bergamini E, Kopin IJ, Blackman MR. Decreased stress responsivity of central and peripheral catecholaminergic systems in aged 344/N Fischer rats. J Clin Invest. 1995;95:1217–1224. doi: 10.1172/JCI117771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognato GP, Agostinho PM, Hockemeyer J, Müller CE, Souza DO, Cunha RA. Caffeine and an adenosine A(2A) receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J Neurochem. 2010;112:453–462. doi: 10.1111/j.1471-4159.2009.06465.x. [DOI] [PubMed] [Google Scholar]
- Conde SV, Nunes da Silva T, Gonzalez C, Mota Carmo M, Monteiro EC, Guarino MP. Chronic caffeine intake decreases circulating catecholamines and prevents diet-induced insulin resistance and hypertension in rats. Br J Nutr. 2012;107:86–95. doi: 10.1017/S0007114511002406. [DOI] [PubMed] [Google Scholar]
- Corsetti G, Pasini E, Assanelli D, Bianchi R. Effects of acute caffeine administration on NOS and Bax/Bcl2 expression in the myocardium of rat. Pharmacol Res. 2008;57:19–25. doi: 10.1016/j.phrs.2007.07.007. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and ageing. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- Di Nardo F, Burattini R, Cogo CE, Faelli E, Ruggeri P. Age-related analysis of insulin resistance, body weight and arterial pressure in the Zucker fatty rat. Exp Physiol. 2009;94:162–168. doi: 10.1113/expphysiol.2008.044529. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Seydoux J, Girardier L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: adenosine antagonism or phosphodiesterase inhibition? Metabolism. 1992;41:1233–1241. doi: 10.1016/0026-0495(92)90015-3. [DOI] [PubMed] [Google Scholar]
- Egawa T, Hamada T, Kameda N, Karaike K, Ma X, Masuda S, Iwanaka N, Hayashi T. Caffeine acutely activates 5′adenosine monophosphate-activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metabolism. 2009;58:1609–1617. doi: 10.1016/j.metabol.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Escrivá F, Gavete ML, Fermín Y, Pérez C, Gallardo N, Alvarez C, Andrés A, Ros M, Carrascosa JM. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194:131–141. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- Exton JH, Park CR. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3′,5′-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968;243:4189–4196. [PubMed] [Google Scholar]
- Exton JH, Friedmann N, Wong EH, Brineaux JP, Corbin JD, Park CR. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J Biol Chem. 1972;247:3579–3588. [PubMed] [Google Scholar]
- Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in ageing. J Clin Invest. 1983;71:1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink RI, Wallace P, Olefsky JM. Effects of ageing on glucose-mediated glucose disposal and glucose transport. J Clin Invest. 1986;77:2034–2041. doi: 10.1172/JCI112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology (Berl) 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- Graham TE, Helge JW, MacLean DA, Kiens B, Richter EA. Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. J Physiol. 2000;529:837–847. doi: 10.1111/j.1469-7793.2000.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr. 2006;84:682–693. doi: 10.1093/ajcn/84.4.682. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Caffeine as a model drug of abuse. Trends Pharmacol Sci. 1990;11:355–356. doi: 10.1016/0165-6147(90)90175-8. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007;293:E286–E292. doi: 10.1152/ajpendo.00693.2006. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Randell R (2011) Fat burners: nutrition supplements that increase fat metabolism. Obes Rev 12:841–851 [DOI] [PubMed]
- Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- Kim JA, Wei Y, Sowers JR (2008) Role of mitochondrial dysfunction in insulin resistance. Circ Res 102:401–414 [DOI] [PMC free article] [PubMed]
- Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Pract Res Clin Endocrinol Metab. 2005;19:625–635. doi: 10.1016/j.beem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Landsberg L, Young JB (1978) Fasting, feeding and regulation of the sympathetic nervous system.N Engl J Med 298:1295–1301 [DOI] [PubMed]
- Lee JJ, Chang CK, Liu IM, Chi TC, Yu HJ, Cheng JT. Changes in endogenous monoamines in aged rats. Clin Exp Pharmacol Physiol. 2001;28:285–289. doi: 10.1046/j.1440-1681.2001.03439.x. [DOI] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia E, van Dam RM, Rajpathak S, Willett WC, Manson JE, Hu FB. Changes in caffeine intake and long-term weight change in men and women. Am J Clin Nutr. 2006;83:674–680. doi: 10.1093/ajcn.83.3.674. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Moisey LL, Kacker S, Bickerton AC, Robinson LE, Graham TE. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr. 2008;87:1254–1261. doi: 10.1093/ajcn/87.5.1254. [DOI] [PubMed] [Google Scholar]
- Mukwevho E, Kohn TA, Lang D, Nyatia E, Smith J, Ojuka EO. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am J Physiol Endocrinol Metab. 2008;294:E582–E588. doi: 10.1152/ajpendo.00312.2007. [DOI] [PubMed] [Google Scholar]
- Mulder AH, Tack CJ, Olthaar AJ, Smits P, Sweep FC, Bosch RR. Adrenergic receptor stimulation attenuates insulin-stimulated glucose uptake in 3T3-L1 adipocytes by inhibiting Glut4 translocation. Am J Physiol Endocrinol Metab. 2005;289:E627–E633. doi: 10.1152/ajpendo.00079.2004. [DOI] [PubMed] [Google Scholar]
- Natella F, Scaccini C. Role of coffee in modulation of diabetes risk. Nutr Rev. 2012;70:207–217. doi: 10.1111/j.1753-4887.2012.00470.x. [DOI] [PubMed] [Google Scholar]
- Ofluoglu E, Pasaoglu H, Pasaoglu A. The effects of caffeine on l-arginine metabolism in the brain of rats. Neurochem Res. 2009;34:395–399. doi: 10.1007/s11064-008-9790-x. [DOI] [PubMed] [Google Scholar]
- Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of ageing. J Clin Invest. 1983;71:1581–1587. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK. Metabolic syndrome: adenosine monophosphate-activated protein kinase and malonyl coenzyme A. Obesity (Silver Spring) 2006;14:25S–33S. doi: 10.1038/oby.2006.279. [DOI] [PubMed] [Google Scholar]
- Scharf G, Prustomersky S, Huber WW. Elevation of glutathione levels by coffee components and its potential mechanisms. Adv Exp Med Biol. 2001;500:535–539. doi: 10.1007/978-1-4615-0667-6_82. [DOI] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Feskens EJ (2002) Coffee consumption and risk of type 2 diabetes mellitus. Lancet 360:1477–1478 [DOI] [PubMed]
- van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29:398–403. doi: 10.2337/diacare.29.02.06.dc05-1512. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B, Sutherland RJ. The analysis of behaviour in the laboratory rat. In: Robinson TE, editor. Behavioral approaches to brain research. New York: Oxford University Press; 1983. pp. 141–211. [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- Young DA, Wallberg-Henriksson H, Cranshaw J, Chen M, Holloszy JO. Effect of catecholamines on glucose uptake and glycogenolysis in rat skeletal muscle. Am J Physiol. 1985;248:C406–C409. doi: 10.1152/ajpcell.1985.248.5.C406. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance—a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004;18:55–62. [PubMed] [Google Scholar]