Abstract
Patients with Alzheimer's disease have an impairment of inhibitory control for reasons that are currently unclear. Using an eye-tracking task (the gap-overlap paradigm), we examined whether the uncorrected errors relate to the task of attentional disengagement in preparation for action. Alternatively, the difficulty in correcting for errors may be caused by the working memory representation of the task. A major aim of this study was to distinguish between the effects of healthy aging and neurodegenerative disease on the voluntary control of saccadic eye movements. Using the antisaccade task (AST) and pro-saccade task (PST) with the ‘gap’ and ‘overlap’ procedures, we obtained detailed eye-tracking measures in patients, with 18 patients with probable Alzheimer's disease, 25 patients with Parkinson's disease and 17 healthy young and 18 old participants. Uncorrected errors in the AST were selectively increased in Alzheimer's disease, but not in Parkinson's disease compared to the control groups. These uncorrected errors were strongly correlated with spatial working memory. There was an increase in the saccade reaction times to targets that were presented simultaneously with the fixation stimulus, compared to the removal of fixation. This ‘gap’ effect (i.e. overlap–gap) saccade reaction time was elevated in the older groups compared to young group, which yielded a strong effect of aging and no specific effect of neurodegenerative disease. Healthy aging, rather than neurodegenerative disease, accounted for the increase in the saccade reaction times to the target that are presented simultaneously with a fixation stimulus. These results suggest that the impairment of inhibitory control in the AST may provide a convenient and putative mark of working memory dysfunction in Alzheimer's disease.
Keywords: Alzheimer's disease, Parkinson's disease, Attention, Antisaccade, Working memory, Eye tracking
Introduction
Patients with early Alzheimer's disease (AD) usually present with a symptom of a deterioration in episodic memory. However, there is increasing evidence of early deficits in executive function and attention, including the inhibitory control of cognition and behaviour in AD (Greenwood et al. 1997; Amieva et al. 2004; Collette et al. 2009; Tse et al. 2010). The control of attention affects information encoding and memory functions and can have a direct impact on memory recall in AD. For example, it has been shown that patients with AD are deficient in the strategic selection of the important items in a memory recall task (Castel et al. 2009). Saccadic eye movement paradigms have been widely used as a tool to examine impairments in attention and the cognitive control of behaviour (Broerse et al. 2001; Crawford et al. 2002; Leigh and Kennard 2004; Hutton and Ettinger 2006). The pro-saccade task (PST) requires an eye movement to be directed towards the visual target. In contrast, the antisaccade task (AST) is cognitively more complex and requires the observer to trigger an eye movement in the opposite direction to the stimulus. The paradigm affords the ability to distinguish between bottom-up and top-down control of behaviour under identical stimulus conditions by a simple change in the instructions of the task. Using the AST, Crawford et al. (2005) revealed a self-monitoring deficit in the inhibitory control of saccadic eye movements in patients with mild to moderate AD. There was a marked reduction in the ability to generate a corrective eye movement, following a saccade that was incorrectly directed towards the target. This abnormality of uncorrected errors in AD has now been replicated (Boxer et al. 2006; Garbutt et al. 2008; Kaufman et al. 2012). Crawford et al. (2005) reported that the magnitude of the errors exceeded that in healthy aging, and there was a positive correlation with the dementia severity on the mini-mental state examination (MMSE).
However, the cognitive source of these uncorrected errors during the AST is unclear. One of the aims of the current work was to examine the attentional disengagement and working memory hypotheses in relation to this abnormality in AD and to distinguish between the effects of neurodegenerative disease and aging. Several cognitive factors contribute to the control of antisaccades (Crawford et al. 2011). These include the disengagement of attentional from the target, the processing speed of the reflexive (bottom-up) saccadic pathways and working memory (top-down). The AST requires participants to disengage attention from the peripheral target and then to programme a saccadic eye movement into the opposite hemifield (Crawford et al. 2006). Parasuraman and colleagues (Greenwood et al. 1997) (1) used the Posner cueing task (Posner and Cohen 1984) to investigate the characteristics of spatial attention in patients with AD. Greenwood et al. (1997) reported that ADs were much slower to respond in the condition where attention should be disengaged rapidly from the currently attended location of the invalid cue and then transferred to the opposite hemifield. This raises the possibility that the impairment of antisaccades in AD might be caused by a similar mechanism. We refer to this as the ‘attention disengagement’ hypothesis.
There is a well-established procedure for the evaluation of oculomotor disengagement that employs a temporal ‘gap’ and ‘overlap’ between the offset of the fixation point and presentation of the peripheral target. In the ‘gap’ procedure, the fixation point is removed before the onset of the new peripheral target. In the ‘overlap’ procedure, the fixation point remains on for a period that ‘overlaps’ with the presentation of the peripheral target. The reaction time of a saccadic eye movement is facilitated by the removal of the fixation point (Saslow 1967; Fischer and Weber 1993). The ability to disengage attention from the fixation point is estimated by the difference in the saccade reaction times between the two conditions. Therefore, we can establish whether oculomotor disengagement is impaired in AD and distinguish between healthy aging and any specific or general candidate markers of neurodegenerative disease with detailed measures of saccadic eye movements.
Other factors that have been considered in the control of antisaccades are the processing speeds of the reflexive saccadic pathways and working memory (Hallett 1978; Crawford et al. 2011). The role of the processing speed in the control of antisaccades is consistent with a competitive race model, in which the processing of the voluntary antisaccade competes with the saccade that is automatically triggered towards the target. Various studies also support working memory as an important factor in the control of antisaccades (Roberts et al. 1994; Kimberg and Farah 2000; Mitchell et al. 2002; Eenshuistra et al. 2004, 2007; Unsworth et al. 2004). Indeed in AD, working memory may play a key role in the difficulty that they experience in correcting for the spatial errors in the AST, given that the deterioration in short-term memory is usually the earliest noticeable cognitive complaint. However, as yet, there is no clear evidence to substantiate this.
Roberts et al. (1994) first proposed that working memory has a key function in the AST, by maintaining the task set when faced with competition from the propotent stimulus. However, the specific working memory operations that are responsible for the failure of uncorrected AST errors in AD are unknown. In this current work, we distinguished between verbal and spatial memory span measures (Baddeley and Hitch 1974; Baddeley 2007) and between the forwards and reverse recall operations. When verbal or spatial items are recalled in the same order as they are presented (i.e. ‘forwards version’), there is no complex manipulation of the memory items, as the items can be stored in a temporary buffer and then read off, verbatim. In contrast, a more complex working memory procedure is required when the items are recalled in the reverse sequence to the order in which they were presented. The ‘forwards’ format provides a simple measure of memory span. The ‘reverse’ format requires the working memory processes of storage, inhibition and resequencing. We used the ‘reverse’ formats to estimate spatial and verbal working memory in common with previous clinical research (Boxer et al. 2006; Garbutt et al. 2008). On the basis of previous studies, we predicted that the uncorrected errors would be strongly related to spatial working memory. Given that the ‘reverse’ format provides a better estimate of working memory, we predicted that the ‘reverse’ format should correlate more reliably with AST errors than the ‘forwards’ version. Parkinson's disease (PD) is a relatively common neurodegenerative disorder that is also characterised by deficits of inhibitory control (Crawford et al. 2002). We therefore examine whether these uncorrected AST errors are specific to AD or are a general feature of a neurodegenerative disorder. Using the same eye-tracker and visual stimulus, we contrasted the frequency of uncorrected errors in a cohort of AD (Crawford et al. 2005), a sample of patients with PD and a healthy group of younger participants.
The goal of identifying specific cognitive operations in patients with a dementia and ruling out non-specific factors presents a formidable challenge for neuropsychiatric research. Crawford et al. (2005) stated, ‘The psychological complications of the disease make it difficult to distinguish any generic cognitive impairment from the secondary effects of the disorder. Experimental studies must address the inevitable uncertainties concerning the source of the poor performance in AD. Does poor performance reflect an inability to perform the task, a failure to comprehend the task or simply a lack of motivation?’ Given a major aim of the current work, to distinguish the specific cognitive effects of disease from healthy aging, it is important to identify task-related impairments that might be attributed to non-specific factors. Ideally, we would distinguish the neurocognitive impairments in AD from any non-specific effects due to poor comprehension, motivation or abnormalities in sensorimotor functions. With this in mind, detailed behavioural measures of the task performance were processed to identify potential sources of impairment. The frequency of AST inhibitory errors and the proportion and timing of uncorrected errors were the principal index of inhibitory control and error monitoring. The spatial precision of saccadic eye movements (i.e. saccade amplitudes) was measured, towards and away from the target, to identify any general deficits of sensorimotor function. Older participants are generally slower on traditional cognitive tests, and this slowing can account for some of the cognitive difficulties in old age (Salthouse 1992; Faust et al. 1999). Therefore, a measure of saccadic reaction times (SRTs) was obtained to determine whether the processing speed of the saccadic eye movement was a potential contributory factor. Anticipation errors are generated when a participant pre-empts the onset signal of the task, with an anticipatory eye movement in advance of the target. The frequency of these errors would provide insight into the specificity of inhibition failures and general understanding of the tasks. A saccade omission occurs when a participant fails to generate a saccade on a given trial; they provide an index of sustained attention and task compliance. Prone to saccade omissions will be those participants who may have difficulties in sustained attention or working memory. If the final landing position of the eye (FEP), after any intervening eye movements in the AST, is relatively close to the target and is similar across the groups, we can have greater confidence that factors such as poor task comprehension, motivation and goal neglect (Duncan et al. 1996) have been undermined. Therefore, FEP provided a further index of task comprehension, motivation and compliance.
Methods and materials
Participants
Twenty-five participants with mild to moderate idiopathic Parkinson's disease (PD), diagnosed by consultant neurologists were recruited from the Department of Neurology, Royal Preston Hospital (RPH), Lancashire Teaching Hospitals NHS Trust, Lancashire, U.K. (16 males, 9 females), Hoehn and Yahr (1967) mean score = 2.12; SD = 0.845. PD patients were free of dementia as assessed with the MMSE (Folstein et al. 1975) and European Alzheimer's Disease Assessment Scale Cognitive Test (ADAS). Twenty-three of the PDs were on levodopa medication, two patients were on no PD medication; none of the PD patients were on anticholinergic medication. AD participants were recruited from the AD Research Project from Lytham Hospital Memory Clinic, United Kingdom. The patient group consisted of 18 patients with early dementia (13 males, 5 females) who fulfilled the criteria for the American Psychiatric Association's DSM IV (APA 2000) and the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) for probable AD. All AD patients underwent a detailed clinical history, physical/neurological examination and routine investigations: hemoglobin, full blood count, erythrocyte sedimentation rate, urea and electrolytes, liver function tests, blood glucose, thyroid function tests, serum vitamin B12 and folate, serology for syphilis and urinalysis. Computerized tomography (CT) scans were obtained for eight patients. Ten patients were taking cholinesterase inhibitors: galantamine (3), rivastigmine (4), donepezil (3). An old control group (OC) consisted of 18 healthy participants (8 males, 10 females) were volunteers from the local Lytham community. All OC participants underwent a detailed neuropsychological assessment. Any OC who scored less than 27 on the MMSE or more than 12 on the ADAS was excluded. All participants were screened for visual acuity using the Snellen's chart and for visual neglect with the line bisection task (Schenkenberg et al. 1980). In order to distinguish the effects of normal healthy aging from neurodegenerative disease, we recruited a sample of 17 young controls (YC) undergraduates from Lancaster University, U.K (mean age = 23.8; SD = 1.7; 9 females, 8 males), who did not undergo the full workup of cognitive and clinical assessments. Written informed consent was obtained from all participants after a detailed description of the study, which was approved by Blackpool, Wyre, and Fylde Local Research Ethics Committee and the Local Research Ethics Committee for the NHS Trust, UK (2001).
Materials
All PD, AD and OC groups completed a detailed battery of neuropsychological assessments within 2 weeks from the completion of the eye-tracking tests. In this report, we investigate the relationships of working memory and saccadic eye movement data. Verbal working memory scores were based on the forwards and reverse scores digit span from the Wechsler Adult Intelligence Scale III (Wechsler 1997a). Spatial working memory was based on the forwards and backwards spatial span using the Corsi block spatial memory task (Milner 1971; Wechsler 1997b).
Procedure
An infra-red scleral reflection device ‘ExpressEye’ (Optom, Freiburg, Germany) recorded saccadic eye movements monocularly with a temporal resolution of 1 ms and spatial resolution of 0.1°. The system is linear across 15° field of view. The stimulus display presented a central fixation point within an unfilled 0.75° × 0.75° empty square marker; the target itself was a red 0.4° spot projected in the horizontal plane. The device projected these stimuli from a head-mounted laser onto a white tangential screen at 57 cm. The laser output was 0.2 mW, with a wavelength of 635 nm with a luminance of 66.37 cd/m2. The three head-mounted lasers therefore helped to compensate for any effects of lateral head motion.
Target paradigms
Gap pro-saccade task
Each trial started with a central fixation point (see Fig. 1) that was projected within a central square marker for 1,000 ms. This central square remained visible throughout all of the trials and provided a useful reference point for the stabilisation of the head. The fixation point was then removed 200 ms (i.e. ‘GAP’) before the onset of the pro target which was displayed at ±4° (randomized) in the horizontal plane for 2,000 ms. The target offset was followed by an inter-trial interval of 1,200 ms when only the central square was visible. The central fixation point was then presented to signal the start of the next trial. Participants were instructed to direct their gaze quickly, and as accurately as possible, to the target and then to return back to the central square.
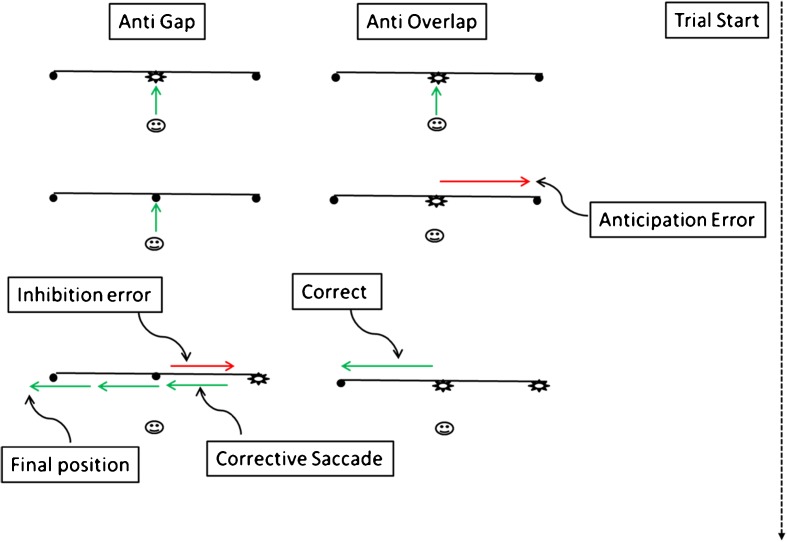
Fig. 1.
In the gap tasks, the fixation point is removed before the onset of the target, while in the overlap task the fixation point is visible when the target is presented. In the antisaccade task, the observer is required to saccade away from the target. The diagram illustrates the different types of errors that are generated in the gap and overlap paradigms. The green arrows indicate correctly directed saccade and fixations; red arrows show incorrect saccades and anticipations
Pro-saccade task overlap
The overlap display was similar to the gap display except with respect to the timing of the removal of the central fixation point. Here, the fixation point ‘overlapped’ for a period of 200 ms with the onset of the pro-saccade target in contrast to the gap task when the fixation point was removed before the target was presented.
Anti-saccade task gap
The stimulus conditions (Fig. 1) were identical in the corresponding pro-saccade task (PST) and antisaccade task (AST), only the instructions to the participants were different. In the ASTs, the participants were instructed to ‘look’ away from the target in the opposite direction towards a location that was approximately equally distant from the fixation point, and this should be done as quickly and accurately as possible.
Anti-saccade task overlap
The target display for this paradigm was identical to the PST overlap condition. Participants were instructed to ‘look’ towards the location, equal distance but in the opposite direction, from the anti-target, as quickly and as accurately as possible. The experiments were conducted in a darkened room in the oculomotor laboratory at Lytham Hospital. Each paradigm consisted of 24 trials and was completed in separate blocks. The tasks were preceded by a set of five practice trials to ensure that participants had a clear understanding of their tasks. All sessions began with the PST to avoid any carry over effect from the AST (Roberts et al. 1994) and to avoid confusion.
Measurement of saccadic parameters
The start and end of a saccade was initially detected at the point at which the eye velocity crossed 30°/s threshold. An experienced eye-tracking researcher (SH) confirmed the detection of all saccades interactively. Our work with elderly patients yielded various types of error in response to the task instructions. Therefore, in addition to the standard measures of saccadic eye movement, we conducted a detailed assessment of the major types of errors. This would enable us to evaluate the category of the errors, task motivation and comprehension measures in addition to the inhibition frequency data. These measurements include: the amplitudes and reaction times of the primary saccade that was generated towards or away from the target, anticipation errors, the reaction times and amplitudes of any corrective saccades, final eye position after any corrections and the proportion of trials where there was no response to the target (i.e. omission errors). Anticipatory errors were defined as saccadic eye movements with a reaction time less than 80 ms after the target was presented. The analyses also included inhibition errors and the number of uncorrected errors. Tests for homogeneity of variance were conducted using IBM SPSS Statistics 19. Post hoc comparisons applied the Bonferroni alpha adjustment. Variables with unequal variance were submitted to nonparametric analyses using the Kruskal–Wallis test (χ2) for a between groups analyses.
Results
Theses analyses address the following questions:
What are the effects of aging and AD on saccadic eye movements?
Would the high frequency of uncorrected AST errors be relatively specific to AD, or is this a general feature of neurodegenerative disorders or healthy aging?
Would the uncorrected AST errors in AD be related to the degree of spatial working memory impairment or to a difficulty in disengaging attention?
Would the ‘reverse’ format of the spatial memory task be a better predictor of AST errors in AD than the ‘forwards’ version?
Cognitive Scores: Table 1 shows the demographic and cognitive scores for the group OC, AD and PD groups. AD and OC were matched on mean age however, the PD group was younger than the AD and OC (F(2,60) = 42.17, p = 0.001). These groups did not differ significantly in the number of years of education (F(2,60) = 1.78, p = 0.177). As expected, the AD showed greater impairment on the cognitive scores compared to the PD and OC on the MMSE (F(2,60) = 65.6, p = 0.001), the ADAS (F(2,60) = 60.66, p = 0.001), number of items recalled correctly in the reversed digit span (F(2,60) = 4.8, p = 0.018), spatial span, both forwards (F(2,60) = 18.72, p = 0.001) and reverse forms (F(2,60) = 20.85, p = 0.001). The OC group read more words correctly on the NART in comparison to the PD and AD groups (F(2,60) = 4.315, p = 0.018), who did not differ significantly from each other. The groups did not differ significantly on the number of items recalled correctly in the forwards digit span test (F(2,60) = 2.79, p = 0.07).
Table 1.
Mean and standard deviations of demographic data and cognitive scores
| Groups (N) | PD (25) | AD (18) | OC(18) | Sig. |
|---|---|---|---|---|
| Age (years) | 63 (7.4) | 78 (4.8) | 75 (3.6) | PD vs. (AD and OC)a |
| Education (years) | 11.76 (2.1) | 12.6 (1.8) | 11.3 (1.9) | NS |
| MMSE | 28.8 (1.2) | 20.9 (4.3) | 29.2 (1.1) | AD vs. (PD and OC)a |
| ADASCog | 6.7 (2.5) | 23.5 (8.9) | 8.2 (2.5) | AD vs. (PD and OC)a |
| NART | 107 (9.3) | 106 (10.9) | 115 (9.7) | CON vs. (AD and PD)a |
| Digit span (F) | 9.8 (1.7) | 8.7 (2.2) | 10.2 (2) | NS |
| Digit span (R) | 6.7 (2.3) | 4.1 (1.9) | 7.1 (2.2) | AD vs. (PD and OC)a |
| Spatial span (F) | 8 (1.5) | 5.1 (1.7) | 7 (1.6) | AD vs. (PD and OC)a |
| Spatial span (R) | 7.1 (1.4) | 4.1 (1.9) | 6.5 (1.4) | AD vs. (PD and OC)a |
AD Alzheimer's disease, OC old controls, PD Parkinson's disease, MMSE mini-mental state examination, ADASCog Alzheimer's disease cognitive assessment scale, NART National adult reading test
aSignificant effect p < 0.01, groups with parentheses did not differ from each other
Age effects
Table 2 reveals that there were some clear effects of healthy aging (see OC vs YC contrasts), primarily in the PST gap. YC generated significantly larger saccade amplitudes than the other groups (F(3,77) = 5.41, p = 0.002; OC vs. YC p = 0.02), and fewer target omissions (χ2(3, N = 78) = 12.26, p = 0.007; OC vs. YC p = 0.03). No age-related effects emerged in the PST overlap. The YC group generated significantly fewer target omissions in the AST overlap (χ2(3, N = 76) = 18.63, p = 0.001; OC vs. YC p = 0.027). YC were also significantly faster to generate a corrective saccade (correction SRT) in the AST overlap (χ2(3, N = 68) = 24.89, p = 0.001; OC vs. YC p = 0.01).
Table 2.
Means, standard deviations (SD), group comparisons
| Nonparametric analysesa | ANOVA | Main effect | Post hoc contrasts (adjust p) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccadic paradigm | Task format | Saccadic parameter | AD [SD] | PD [SD] | OC [SD] | YC [SD] | DF | χ2 | F | Sig | Age (OC v YC) | OC v PDb | OC v ADb | AD v PDb |
| PST | Gap | SRT (ms) | 225 (49) | 183 (50) | 209 (32) | 202 (29) | 3,77 | 3.62 | 0.017c | 1.0 | 0.325 | 1.0 | 0.011c | |
| Amplitude (deg) | 3.0 (0.6) | 3.22 (0.9) | 3.2 (0.8) | 3.9 (0.4) | 3,77 | 5.41 | 0.002c | 0.02c | 1.0 | 1.0 | 1.0 | |||
| Omissions (%) | 4.6 (9.7) | 0.7 (2.2) | 6.5 (11.5) | 0 (0) | 3 (n = 78) | 12.26 | 0.007c | 0.03c | 0.179 | 1.0 | 0.223 | |||
| Anticipations (%) | 2.1 (5.1) | 8.9 (8.4) | 4.4 (6.0) | 1.0 (2.4) | 3 (n = 78) | 23.59 | 0.001c | 0.33 | 0.178 | 0.6 | 0.001c | |||
| PST | Overlap | SRT (ms) | 294 (48) | 242 (75) | 257 (29) | 211 (44) | 3,70 | 5.84 | 0.001c | 0.131 | 1.0 | 0.51 | 0.04c | |
| Amplitude (deg) | 3.1 (0.7) | 3.3 (1.3) | 3.2 (0.6) | 3.6 (0.4) | 3,70 | 0.909 | 0.44 | – | – | – | – | |||
| Omissions (%) | 11.9 (21.3) | 4.1 7.5) | 1.9 (3.5) | 0 (0) | 3 (n = 71) | 13.39 | 0.004c | 0.71 | 1.0 | 0.42 | 1.0 | |||
| Anticipations (%) | 2.4 (4.5) | 1.5 (2.7) | 0.8 (3.2) | 2.1 (5.2) | 3,70 | 0.48 | 0.697 | – | – | – | – | |||
| AST | Gap | SRT (ms) | 331 (121) | 298 (60) | 293 (45) | 244 (38) | 3 (n = 77) | 12.49 | 0.006c | 0.073 | 1.0 | 1.0 | 1.0 | |
| Amplitude (deg) | 3.5 (2.5) | 4.3 (1.8) | 4.2 (1.6) | 3.9 (1.4) | 3,76 | 0.874 | 0.459 | – | – | – | – | |||
| Omission (%) | 10.4 (12.0) | 4.13 (14.3) | 5.8 (12.6) | 1.2 (2.4) | 3 (n = 78) | 16.22 | 0.001c | 1.0 | 0.68 | 0.19 | 0.001c | |||
| Anticipations (%) | 5.3 (6.0) | 2.4 (3.7) | 2.6 (5.0) | 3.6 (7.5) | 3,77 | 1.124 | 0.345 | – | – | – | – | |||
| Inhibition Errors (%) | 53.4 (23.6) | 25.5 (16.5) | 18.4 (13.4) | 10.8 (9.2) | 3 (n = 77) | 30.88 | 0.001c | 0.98 | 1.0 | 0.001c | 0.008c | |||
| UnC Errors (%) | 25.4 (22.4) | 2.02 (4.73) | 2.5 (7.0) | 1.0 (1.8) | 3 (n = 78) | 27.67 | 0.001c | 1.0 | 1.0 | 0.001c | 0.001c | |||
| Correction SRT (ms) | 500 (112) | 387 (130) | 365 (84) | 327 (74) | 3,70 | 7.5 | 0.001c | 1.0 | 1.0 | 0.004c | 0.009c | |||
| Correction Amp (deg) | 6.2 (1.7) | 5.7 (1.9) | 6.5 (2.8) | 7.6 (3.0) | 3,70 | 1.97 | 0.126 | – | – | – | – | |||
| Correction FEP (deg) | 3.6 (1.6) | 3.4 (1.7) | 4.1 (2.1) | 4.6 (2.9) | 3,70 | 1.2 | 0.315 | – | – | – | – | |||
| AST | Overlap | SRT (ms) | 349 (142) | 340 (104) | 325 (55) | 251 (60) | 3,75 | 3.4 | 0.02c | 0.216 | 1.0 | 1.0 | 1.0 | |
| Amplitude (deg) | 3.6 (2.2) | 3.8 (1.3) | 3.9 (1.6) | 3.9 (1.0) | 3,75 | 0.178 | 0.911 | – | – | – | – | |||
| Omissions (%) | 13.2 (18.5) | 3.8 (11.5) | 9.1 (14.2) | 0.0 (0.0) | 3 (n = 76) | 18.63 | 0.001c | 0.027c | 0.231 | 1.0 | 0.012c | |||
| Anticipations (%) | 4.4 (4.4) | 1.9 (4.4) | 2.9 (6.2) | 4.4 (11.7) | 3,75 | 0.655 | 0.582 | – | – | – | – | |||
| Inhibition Errors (%) | 48.4 (22.9) | 26.1 (18.4) | 12.3 (9.6) | 6.3 (6.5) | 3 (n = 76) | 37.8 | 0.001c | 1.0 | 0.13 | 0.13 | 0.07 | |||
| UnC Errors (%) | 18.3 (14.9) | 2.3 (5.3) | 1.4 (2.9) | 0.5 (2.1) | 3 (n = 76) | 35.36 | 0.001c | 1.0 | 1.0 | 0.001c | 0.001c | |||
| Correction SRT (ms) | 640 (240) | 656 (140) | 552 (151) | 346 (83) | 3 (n = 68) | 24.89 | 0.001c | 0.01c | 1.0 | 1.0 | 1.0 | |||
| Correction Amp (deg) | 5.4 (2.4) | 5.5 (2.5) | 5.7 (2.2) | 6.7 (0.9) | 3,67 | 0.997 | 0.4 | – | – | – | – | |||
| Correction FEP (deg) | 2.8 (1.9) | 2.6 (1.3) | 3.0 (2.1) | 3.7 (1.4) | 3,67 | 1.156 | 0.334 | – | – | – | – |
AD Alzheimer's, PD Parkinson's, OC old controls, YC young controls, PST prosaccade task, AST antisaccade, FEP final eye position, SRT saccade reaction time, Amps amplitude
aKruskal–Wallis test for nonparametric data
bPost hoc comparison used Bonferonni alpha adjustment
cSignificant group effects
The gap effect: attention disengagement
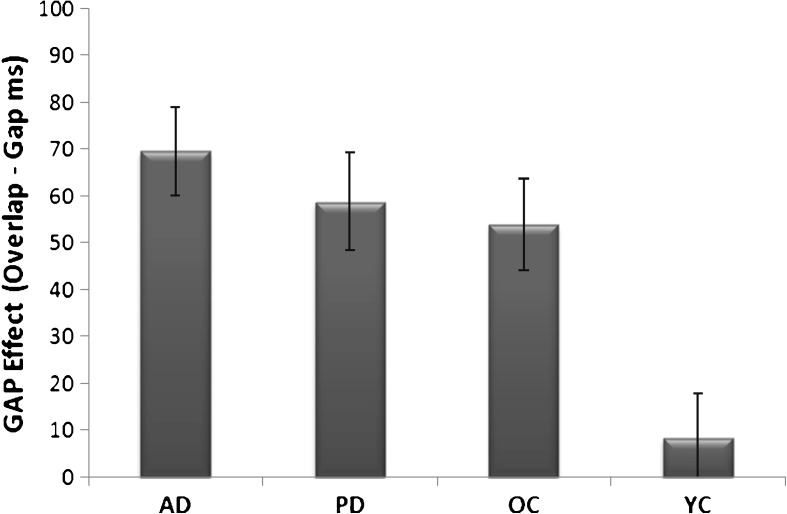
The gap effect refers to the increase in saccade reaction time in the overlap task in comparison to a gap task. All groups showed an increase in reaction times in the overlap compared to the gap PST. Figure 2 shows that the mean gap effect was significantly reduced in the YC in comparison to the other groups (F(3,70) = 6.468, p = 0.001). The size of the gap effect did not differ among the OC, AD and PD groups.
Fig. 2.
The mean gap effect (overlap–gap) and standard error bars for saccade reaction times in Alzheimer's disease (AD), Parkinson's disease (PD), old controls (OC) and young control (YC) groups
Effects of neurodegenerative disease
Table 2 reveals that there were no disease specific (AD vs. OC or PD vs. OC) impairment of saccades in the PST gap task, although the AD group had slower mean reaction times (SRT) (mean = 225 ms) than the PDs (mean = 209 ms) (F(3,77) = 3.62, p = 0.017; PD vs. AD p = 0.011). The proportion of anticipations were also elevated in PD (p = 0.001) in the PST gap compared to the other groups. There were no AD- or PD-specific impairments in relation to the OC group for saccades in the PST task. As in the gap task, the AD group were slower to initiate a saccadic eye movement (SRT) in the PST overlap task compared to the PD group (F(3,70) = 5.84, p = 0.001; PD vs. AD p = 0.04) but not in comparison to the OC group. Table 2 shows in the AST gap task that there was a significant increase in AD inhibition errors (χ2(3, N = 77) = 30.88, p = 0.001) and uncorrected errors (χ2 (3, N = 78) = 27.67, p = 0.001) in comparison to all other groups. The AD were also slower to generate a corrective saccade after their errors (F(3,70) = 7.5, p = 0.001) and had a higher proportion of target omissions (p = 0.001). The AD pattern of inhibition errors in the AST overlap reflected the results for the AST gap task. The AD group produced a higher frequency of inhibition errors (χ2(3, N = 76) = 37.8, p = 0.001) and uncorrected errors (χ2 (3, N = 76) = 35.36, p = 0.001).
The following questions were addressed in these analyses to identify any non-specific effects, in relation to the AD results. Do the AD group show a general sensorimotor impairment? The AD group showed no reliable differences in saccadic amplitudes, revealing that there was no fundamental sensorimotor deficit in spatial programming (see Table 2). Does the AD group show a general slowing in saccadic reaction times? The AD group showed no reliable differences in SRTs, which helps to rule out processing speed as a causal factor. Does the AD group show a general impairment in task completion? Measures of non-specific effects of general task motivation, understanding and compliance, as indexed by final eye position (i.e. goal maintenance (Duncan et al. 1996)), were also preserved in AD (see Table 2). The following saccadic parameters of sensorimotor function, processing speed and motivation revealed no reliable differences between the OC and AD groups. PST gap: SRT, amplitude, omissions, anticipations. PST overlap: SRT, amplitude, omissions, anticipations. AST gap: SRT, omissions, anticipations, correction amp, correction FEP. AST overlap: SRT, amplitude, anticipations, correction amp and correction FEP (see Table 2). Although the AD group displayed no increase in PST Gap omissions, there was an increase in target omissions in the PST overlap and in both of the AST tasks suggesting a possible deficit in sustained attention and/or working memory.
AST errors and working memory in Alzheimer's disease
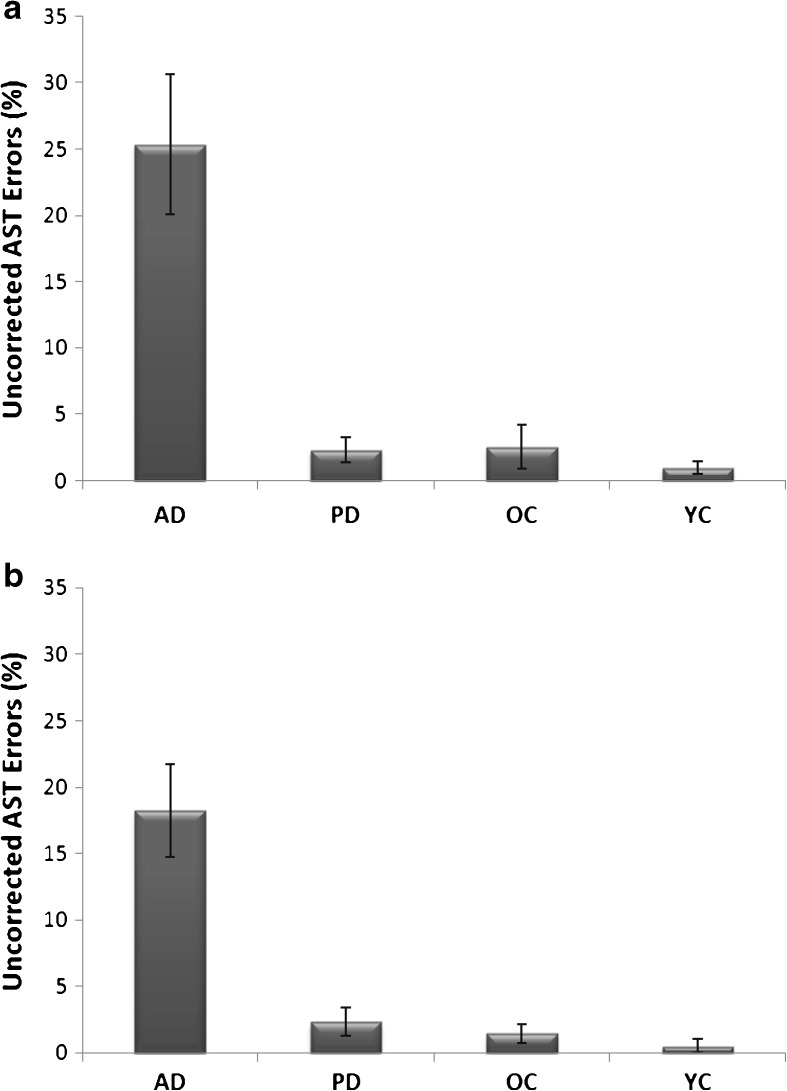
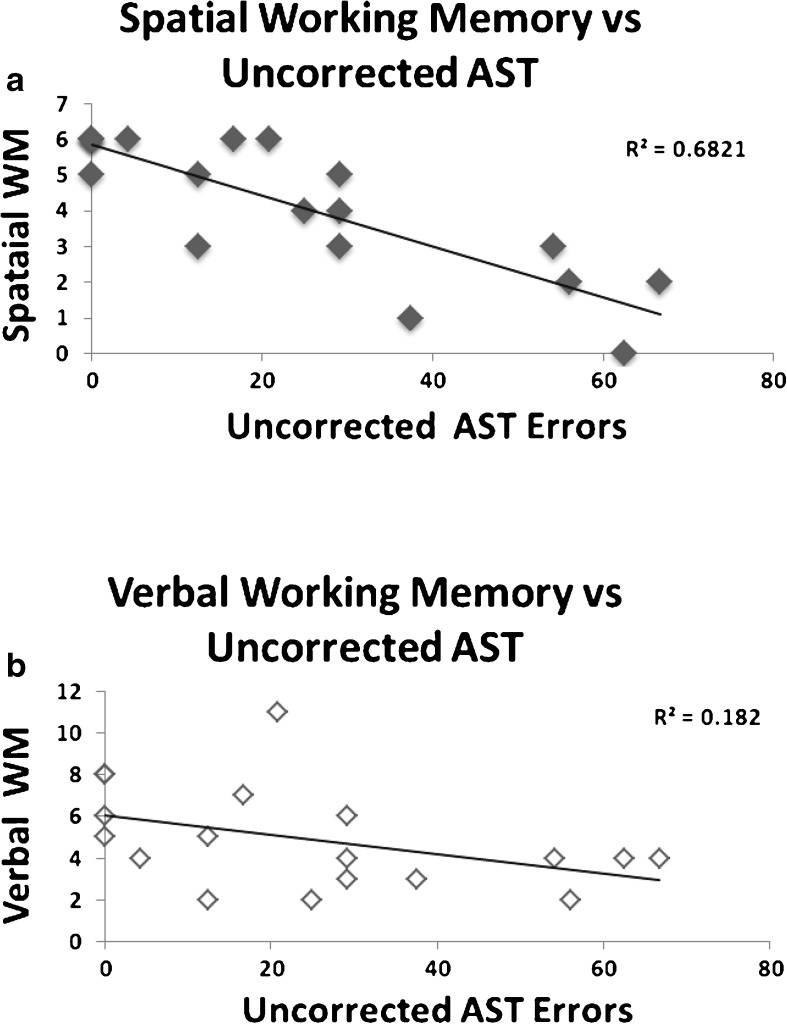
Figure 3 shows the proportion of uncorrected saccades in the AST gap and overlap tasks across all the groups. Uncorrected errors were confined predominantly to the AD group in the AST gap (F(3,77) = 18.99, p < 0.001) and AST overlap (F(3,75) = 19.869, p < 0.001). Given the low frequency of uncorrected errors in the other groups the following analyses were restricted to the AD group. Pearson's correlations and multiple regression analyses were conducted to determine the relationship between uncorrected AST errors and verbal memory and spatial working memory. Table 3 reveals that there was a significant correlation between the frequency of uncorrected errors and the spatial working memory (i.e. reverse format) (r(18) = −0.826, p = 0.001) and verbal working memory (r(18) = −0.427 (p = 0.039) in AD. Figure 4a, b reveals a tighter relationship of uncorrected errors with spatial than verbal working memory. In contrast, forwards memory span scores were not correlated with the uncorrected AST errors. Thus, the strongest correlations with the uncorrected errors in the AD emerged for the working memory, rather than the simple span scores. A multiple regression analyses was conducted to determine the best predictor of the uncorrected errors and to establish whether there was unique variance in association with each of the working memory components. Multiple regression confirmed that the spatial working memory score, was the best predictor of the AST uncorrected errors (estimated coefficient = −10.469, SE = 2.094, t = −4.99, p < 0.001). Spatial working memory scores accounted for 68.2 % proportion of variance. No other measure explained any unique proportion of the variance. These results were also supported by a step-wise regression model.
Fig. 3.
a, b Mean uncorrected errors and standard error bars in gap (upper graph) and overlap (lower graph antisaccade tasks (AST). AD Alzheimer's disease; PD Parkinson's disease; OC old controls; YC young controls
Table 3.
Alzheimer's disease Pearson correlation (r) with antisaccade task uncorrected errors (N = 18)
| R | P | |
|---|---|---|
| Spatial Span (R) | −0.826 | 0.001 |
| Spatial Span (F) | −0.252 | ns |
| Digit Span (R) | −0.427 | 0.039 |
| Digit Span (F) | 0.340 | ns |
Fig. 4.
a, b Scatter plot of uncorrected antisaccade task errors (AST) against spatial working memory (spatial span reversed) and verbal working memory (digit span reversed scores). The r2 values reveal a more robust relationship between spatial working memory and uncorrected AST errors (upper graph)
A logistic regression analysis was conducted to determine whether the uncorrected AST errors were able to differentiate patients from controls, over and above the contribution of spatial working memory. Group status (OC vs. AD) was entered as the dependent logistic variable; spatial working memory and then AST uncorrected errors, respectively, were entered as predictors in the regression model (see Table 4). With spatial working as the first predictor the classification accuracy was 72.2 % and the model was highly significant (χ2(1) = 18.137, p = 0.000). The classification accuracy improved significantly to 80.6 % (χ2(1) = 5.032, p = 0.025) when the uncorrected AST errors was entered into the regression model (χ2(2) = 23.169, p = 0.000). Uncorrected AST errors can therefore improve the classification of AD from the OC group, even after taking into account spatial working memory.
Table 4.
Logistic regression of group status (old control vs Alzheimer's disease) using predictor variables working memory and uncorrected AST errors
| Predictor | SE | Wald | df | P | |
|---|---|---|---|---|---|
| Constant | 5.996 | 3.49 | 2.923 | 1 | 0.087 |
| Spatial WM | −1.212 | 0.608 | 3.973 | 1 | 0.046 |
| Uncor AST | 0.116 | 0.058 | 3.932 | 1 | 0.047 |
| Test | χ2 | df | p | ||
| Omnibus test | 23.169 | 2 | 0.000 | ||
| −2 Likelihood ratio | 26.737 | ||||
| Cox and Snell r2 | 0.475 | ||||
| Nagelkerkr r2 | 0.633 |
UNCor AST uncorrested antisaccade task errors, WM working memory
Discussion
Alzheimer's disease and uncorrected errors
People with AD generated an excessive proportion of uncorrected errors in the AST which is consistent with the dysregulation of a self-monitoring and error correction neural network. A number of studies have argued that working memory is critically important for inhibitory control in the AST. Using dual-task methodology, several studies have shown that tasks which impose a load on working memory will disrupt eye movements in the AST (Roberts et al. 1994; Mitchell et al. 2002; Eenshuistra et al. 2004). Although there are some unresolved issues with the dual-task methodology (Crawford et al. 2011), there is converging evidence that a top-down cognitive control process is involved in the AST. Here, we examined the relationship of these errors in relation to both spatial and verbal working memory. The frequency of the uncorrected errors was unaffected in patients with PD or the OC control group. These results clearly revealed that these errors are not a general characteristic of a neurodegenerative disorder or healthy aging. Spatial working memory was highly correlated with frequency of uncorrected AST errors and accounted for the majority (68 %) of the variance in the errors. Note that it is not the failure of inhibitory control in the AST per se, that is the distinctive feature of the AD. The problem of inhibitory control is common to several other neuropsychiatric disorders including schizophrenia, obsessive compulsive disorder, attention-deficit and hyperactivity disorder and Huntington's disease. It appears to be the difficulty that AD patients experience in the monitoring and correcting of their errors that distinguishes AD from several other disorders. It is unlikely that people with AD simply forget the context of the task. The majority of error trials were in fact corrected (approx. 70 %) even in the AD group. It appears to be the unreliable access to this error monitoring and correction function that is a major problem for the AD group, and this accessibility appears to require an intact spatial representation. It is also unlikely that people with AD suffer from an inability to attend to or track the location of the stimulus (saccade amplitude in the PST was relatively preserved in AD). We suggest that the particular difficulty for people with AD emerges in the AST because the attention for tracking the location of the target competes with the requirement to also monitor and correctly recall the intended location of their eye movements. Thus, the deterioration in spatial working memory is an important contributory factor in the account of uncorrected errors in AD. Importantly, the contrast of OC vs. AD revealed no differences in the oculomotor anticipations, amplitude, FEPs or reaction times. Thus, the effects on inhibitory control cannot be attributed to non-specific effects due to task motivation, compliance or general sensorimotor impairments.
Previous reports have supported the uncorrected AST errors as a promising early marker of dementia in AD (Boxer et al. 2006; Garbutt et al. 2008; Kaufman et al. 2010, 2012). The current findings revealed that uncorrected AST errors are not a general characteristic of neurodegenerative disorders. In contrast to AD patients, where uncorrected AST errors were common, these errors were not characteristic of PD. Uncorrected AST errors discriminated well among AD patients, OCs and PDs. These results provide further support for the uncorrected AST errors as a useful diagnostic marker in AD. However, this impairment is not confined to Alzheimer's disease. Uncorrected AST errors alone did not distinguish between AD, corticobasal syndrome and progressive supranuclear palsy (Garbutt et al. 2008). (Progressive nuclear palsy was easily distinguished by the broad range of ocular abnormality including hypometric and slow vertical gaze). AST uncorrected errors could serve a useful diagnostic aid in distinguishing between some disorders with very similar profiles on traditional cognitive tests (e.g. AD vs. semantic dementia) (Boxer et al. 2006; Garbutt et al. 2008).
Saccadic eye movements in Parkinson's disease
Studies typically report that pro-saccades are relatively spared in patients with mild or moderate PD (Lueck et al. 1990; Fukushima et al. 1994; Vidailhet et al. 1994b; Kingstone et al. 2002; Cameron et al. 2010). A recent meta-analysis suggested that the eccentricity of the visual target has a critical effect on PST reaction times in PD. PD patients trigger saccades more rapidly when the target is relatively close to fixation point and more slowly for targets that are more distant (Chambers and Prescott 2010). However, there is a lack of consensus on antisaccades (Lueck et al. 1990; Kitagawa et al. 1994; Vidailhet et al. 1994a; Crevits and DeRidder 1997; Briand et al. 2001; Cameron et al. 2012). Inhibition errors may depend on the extent of cognitive impairment (Crawford et al. 2002) as PD is not homogenous in the extent of prefrontal impairments. Our findings in relation to the gap effect in PD are consistent with that of Chan et al. (2005) and van Koningsbruggen et al. (2009) who found an equivalent effect in older controls. Crevits et al. (2004) also reported a reliable gap effect in PD, although in their study, there was an apparent significant interaction between the magnitude of the effect and group.
General effects of aging on saccadic eye movements
There have been several studies of the effects of aging on saccadic eye movements (Spooner et al. 1980; Abel et al. 1983; Sharpe and Zackon 1987; Moschner and Baloh 1994; Fischer et al. 1997; Pratt et al. 1997), but few have attempted to distinguish the effects of neurodegenerative disease from normal aging within a single study design. A number of distinct age-related saccadic characteristics emerged from these data. Table 2 revealed that younger healthy adults generally produced more accurate saccades and fewer errors compared to the older groups, although there were a number of parameters that withstood the effects of old age. For example, saccade reaction times in the PST gap task differed between the YC and OC groups by a margin of only 7 ms on average. Even in the more complex AST, mean saccade amplitudes were remarkably similar for the YC and OC groups. Also, Fig. 2 revealed that the gap effect was clearly larger in the older groups in comparison to the YC group. The mean reaction time results shown in Table 2 revealed that there was a greater increase in saccadic reaction time in the PST overlap in the older groups. Clearly, it was more difficult for older groups to disengage their attention from a visual target, and this slows down the initiation of a subsequent saccade. This implies a larger benefit from the offset of the fixation point for the older groups. This change in the gap effect with increasing age indicates that there is an age-related change in the ability to disengage attention from a current stimulus implying a reduction in the inhibition of fixations cells in the superior colliculus. However, this hypothesis should be regarded with caution in the light of the findings of Munoz et al. (1998) of an increase in the gap effect in young children, but no increase in the gap effect across the age range in older adults. The increase in the gap effect in older groups that we detected in this study is consistent with some findings by Pratt et al. (1997), although they attributed this to a statistical effect due to the general slowing of reaction times in their older participants.
Conclusions
Patients with AD generate an excessive proportion of uncorrected errors in the AST as they have great difficulty in generating a corrective eye movement, after the eyes have automatically moved in the wrong direction. These uncorrected errors are strongly correlated with the degree of impairment of their spatial working memory. To our knowledge, this is the first study to have identified a cognitive source of the abnormality. An increasing number of studies support the idea that the oculomotor system can provide a potential biological marker of dementia in AD. The current work supports the uncorrected errors in AST as a strong candidate marker (possibly in combination with other saccadic indicators) that: (1) discriminates well between AD and other groups, (2) are predictive of MMSE scores and (3) is reflective of the working memory impairment in AD. These findings have potentially important implications in terms of expanding the future options for the early detection and monitoring of people in the early stages of AD. It would be interesting to determine in future work, whether this simple laboratory task reflects the inability to recall the location of the door keys, or the credit card, that is so often the experience of people with early AD. However, these findings have emerged from a limited sample; therefore, future research will be required with larger and more diverse populations.
Acknowledgments
This work was funded by the Lytham League of Friends, the Bial Foundation and The Sir John Fisher Foundation. We are grateful to T. Renvoize, J. Patel, A. Suriya, S. Tetley and the late Professor D. Mitchell for help with original data collection and to Ms. Lilith Kostandian for her help with processing of the PD data. We are grateful to two anonymous referees for their helpful comments on an earlier draft.
References
- Abel L, Troost B, Dell'Osso L. The effects of age on normal saccadic characteristics and their variability. Vis Res. 1983;23:33–37. doi: 10.1016/0042-6989(83)90038-X. [DOI] [PubMed] [Google Scholar]
- Amieva H, Phillips LH, Della Sala S, Henry JD. Inhibitory functioning in Alzheimer's disease. Brain. 2004;127:949–964. doi: 10.1093/brain/awh045. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders. Washington: American Psychiatric Association; 2000. [Google Scholar]
- Baddeley A. Working memory, thought and action. Oxford: Oxford University Press; 2007. [Google Scholar]
- Baddeley A, Hitch G. Working memory. In: Bower G, editor. The psychology of learning and motivation: advances in research and theory. New York: Academic; 1974. pp. 47–89. [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26:6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand KA, Hening W, Poizner H, Sereno AB. Automatic orienting of visuospatial attention in Parkinson's disease. Neuropsychologia. 2001;39:1240–1249. doi: 10.1016/S0028-3932(01)00045-8. [DOI] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/S0028-3932(00)00155-X. [DOI] [PubMed] [Google Scholar]
- Cameron IG, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson's disease: response automaticity and task switching. Neuropsychologia. 2010;48:1948–1957. doi: 10.1016/j.neuropsychologia.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Cameron IG, Pari G, Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. Impaired executive function signals in motor brain regions in Parkinson's disease. NeuroImage. 2012;60:1156–1170. doi: 10.1016/j.neuroimage.2012.01.057. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DB. Memory efficiency and the strategic control of attention at encoding: impairments of value-directed remembering in Alzheimer's disease. Neuropsychology. 2009;23:297–306. doi: 10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Prescott T. Response times for visually guided saccades in persons with Parkinson's disease: a meta-analytic review. Neuropsychologia. 2010;48:887–899. doi: 10.1016/j.neuropsychologia.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong I, Pari G, Riopelle R, Munoz D. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia. 2005;43:784–796. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Collette F, Schmidt C, Scherrer C, Adam S, Salmon E. Specificity of inhibitory deficits in normal aging and Alzheimer's disease. Neurobiol Aging. 2009;30:875–889. doi: 10.1016/j.neurobiolaging.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Bennett D, Lekwuwa G, Shaunak S, Deakin JF. Cognition and the inhibitory control of saccades in schizophrenia and Parkinson's disease. Prog Brain Res. 2002;140:449–466. doi: 10.1016/S0079-6123(02)40068-4. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Higham S, Renvoize T, Patel J, Dale M, Suriya A, Tetley S. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer's disease. Biol Psychiatry. 2005;57:1052–1060. doi: 10.1016/j.biopsych.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Kean M, Klein RM, Hamm JP. The effects of illusory line motion on incongruent saccades: implications for saccadic eye movements and visual attention. Exp Brain Res. 2006;173:498–506. doi: 10.1007/s00221-006-0392-z. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Parker E, Solis-Trapala I, Mayes J. Is the relationship of prosaccade reaction times and antisaccade errors mediated by working memory? Exp Brain Res. 2011;208:385–397. doi: 10.1007/s00221-010-2488-8. [DOI] [PubMed] [Google Scholar]
- Crevits L, DeRidder K. Disturbed striatoprefrontal mediated visual behaviour in moderate to severe parkinsonian patients. J Neurol Neurosurg Psychiatry. 1997;63:296–299. doi: 10.1136/jnnp.63.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevits L, Vandierendonck A, Stuyven E, Verschaete S, Wildenbeest J. Effect of intention and visual fixation disengagement on prosaccades in Parkinson's disease patients. Neuropsychologia. 2004;42:624–632. doi: 10.1016/j.neuropsychologia.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williamms P, Johnson R, Freer C. Intelligence and the frontal lobe: the organzation of goal-directed behaviour. Cogn Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Eenshuistra RM, Ridderinkhof KR, van der Molen MW. Age-related changes in antisaccade task performance: inhibitory control or working-memory engagement? Brain Cogn. 2004;56:177–188. doi: 10.1016/j.bandc.2004.02.077. [DOI] [PubMed] [Google Scholar]
- Eenshuistra R, Ridderinkhof K, Weidema M, van der Molen M. Developmental changes in oculomotor control and working-memory efficiency. Acta Psychol. 2007;124:139–158. doi: 10.1016/j.actpsy.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychol Bull. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci. 1993;16:553–610. doi: 10.1017/S0140525X00031575. [DOI] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Res. 1997;754:285–297. doi: 10.1016/S0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-Mental State Examination. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Miyasaka K, Yamashita I. Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biol Psychiatry. 1994;36:21–30. doi: 10.1016/0006-3223(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, Dean D, Kramer J, Neuhaus J, Miller BL, Lisberger SG, Boxer AL. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131:1268–1281. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Controlling the focus of spatial attention during visual search: effects of advanced aging and Alzheimer's disease. Neuropsychology. 1997;11:3–12. doi: 10.1037/0894-4105.11.1.3. [DOI] [PubMed] [Google Scholar]
- Hallett P. Primary and secondary saccades to goals defined by instructions. Vis Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442 [DOI] [PubMed]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiol. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Kaufman LD, Pratt J, Levine B, Black SE. Antisaccades: a probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. J Alzheimers Dis JAD. 2010;19:781–793. doi: 10.3233/JAD-2010-1275. [DOI] [PubMed] [Google Scholar]
- Kaufman LD, Pratt J, Levine B, Black SE. Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain Behav. 2012;2:15–21. doi: 10.1002/brb3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D, Farah M. Is there an inhibitory module in the prefrontal cortex? Working memory and the mechanisms underlying cognitive control. In: Monsell S, Driver J, editors. Attention and performance, vol. XVIII. Cambridge: MIT Press; 2000. [Google Scholar]
- Kingstone A, Klein R, Morein-Zamir S, Hunt A, Fisk J, Maxner C. Orienting attention in aging and Parkinson's disease: distinguishing modes of control. J Clin Exp Neuropsychol. 2002;24:951–967. doi: 10.1076/jcen.24.7.951.8387. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Fukushima J, Tashiro K. Relationship between antisaccades and the clinical symptoms in Parkinson's disease. Neurology. 1994;44:2285–2289. doi: 10.1212/WNL.44.12.2285. [DOI] [PubMed] [Google Scholar]
- Leigh R, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain. 2004;127:460–477. doi: 10.1093/brain/awh035. [DOI] [PubMed] [Google Scholar]
- Lueck CJ, Tanyeri S, Crawford TJ, Henderson L, Kennard C. Antisaccades and remembered saccades in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1990;53:284–288. doi: 10.1136/jnnp.53.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin 27. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae C, Gilchrist ID. Working memory and the suppression of reflexive saccades. J Cog Neurosci. 2002;14:95–103. doi: 10.1162/089892902317205357. [DOI] [PubMed] [Google Scholar]
- Moschner C, Baloh RW. Age-related-changes in visual tracking. J Gerontol. 1994;49:M235–M238. doi: 10.1093/geronj/49.5.M235. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Posner M, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Components of visual orienting, vol. 10. Hillsdale: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Pratt J, Abrams R, Chasteen A. Initiation and inhibition of saccadic eye movements in younger and older adults: an analysis of the gap effect. J Gerontololgy. 1997;52:P103–P107. doi: 10.1093/geronb/52B.2.P103. [DOI] [PubMed] [Google Scholar]
- Roberts J, Ralph J, Hager LD, Heron C. Prefrontal cognitive processes: working memory and inhibition in the antisaccade task. J Exp Psychol Human. 1994;123:374–393. doi: 10.1037/0096-3445.123.4.374. [DOI] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychol. 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-H. [DOI] [PubMed] [Google Scholar]
- Saslow M. Latency for saccadic eye movement. J Opt Soc Am. 1967;57(8):1030–1033. doi: 10.1364/JOSA.57.001030. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurological impairment. Neurology. 1980;30:509–517. doi: 10.1212/WNL.30.5.509. [DOI] [PubMed] [Google Scholar]
- Sharpe J, Zackon D. Senescent saccades. Effects of aging on their accuracy, reaction time and velocity. Acta Otolaryngol. 1987;104:422–428. doi: 10.3109/00016488709128270. [DOI] [PubMed] [Google Scholar]
- Spooner J, Sakala S, Baloh R. Effect of aging on eye tracking. Arch Neurol. 1980;37:575–576. doi: 10.1001/archneur.1980.00500580071012. [DOI] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Yap MJ, Duchek JM, McCabe DB. Effects of healthy aging and early stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. J Exp Psychol Learn Mem Cogn. 2004;30:1302–1321. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- van Koningsbruggen MG, Pender T, Machado L, Rafal RD. Impaired control of the oculomotor reflexes in Parkinson's disease. Neuropsychologia. 2009;47:2909–2915. doi: 10.1016/j.neuropsychologia.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouider-Khouja N, Pillon B, et al. Eye movements in Parkinson's syndromes. Ann Neurol. 1994;35:420–426. doi: 10.1002/ana.410350408. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouiderkhouja N, Pillon B, Bonnet AM, Gaymard B, Agid Y, Pierrotdeseilligny C. Eye movements in Parkinsonian syndromes. Ann Neurol. 1994;35:487–490. doi: 10.1002/ana.410350408. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. San Antonio: The Psychological Corporation; 1997. [Google Scholar]