Abstract
Studies linking insulin-like growth factor-1 (IGF-1) to age-related bone loss in humans have been reported but remain only correlative. In this investigation, we characterized the bone phenotype of aged WT C57BL/6J male mice in comparison to that of C57BL/6J mice with reduced serum IGF-1 levels arising from an igfals gene deletion (ALS knockout (ALSKO)). During the aging process, WT mice showed an increase in fat mass and decrease lean mass while ALSKO mice had stable lean and fat mass values. Skeletal analyses of femora from WT mice revealed an expansion of the marrow area and a significant accumulation of intracortical porosity associated with increased intracortical remodeling. In contrast, ALSKO mice showed only small age-related declines in the amount of cortical bone tissue and minimal intracortical porosity, at 2 years of age. Accordingly, mechanical tests of femora from 2-year-old WT mice revealed reduced stiffness and maximal load when compared to bones from ALSKO mice. We show here that lifelong reductions in serum IGF-1 compromise skeletal size in development leading to slender bones; they are also associated with decreased intracortical bone remodeling and preservation of bone strength during aging.
Keywords: IGF-1, Bone, ALSKO mice, Micro-computed tomography, Endocrine, Aging
Introduction
Age-related bone loss is a major determinant of osteoporosis and fracture risk in the elderly (Melton et al. 2007; Rivadeneira et al. 2007; Sornay-Rendu et al. 2009). In humans, the typical pattern of cancellous bone loss is indicated by a decrease in bone mineral density (BMD), which arises due to trabecular thinning and deterioration of trabecular architecture (Djuric et al. 2010; Lochmuller et al. 2008). In cortical bone, there are also noticeable reductions in BMD as well as an increase in medullar area and intracortical bone loss (increased cortical bone porosity) (Boyce and Bloebaum 1993; McCalden et al. 1993; Ruff and Hayes 1984; Simmons et al. 1991). During growth, insulin-like growth factor-1 (IGF-1) plays a significant role in establishing bone size and tissue amount; however, during aging, the role of IGF-1 remains unclear. Prior studies have shown that during aging in humans, serum IGF-1 levels decrease and these decreases have been correlated with decreases in BMD (Langlois et al. 1998; Patel et al. 2005; Mezquita-Raya et al. 2004; Rhee et al. 2004; Gillberg et al. 2002) and also increases in total number of hip fractures (Boonen et al. 1999). Thus, it is possible that serum IGF-1 levels may be linked to decreased bone mass with age. However, interpretation of these associations should be considered carefully, as developmental patterns that may contribute to an increased risk of bone loss during aging have not been addressed by these correlative studies. Indeed, in a recently published study, we demonstrated that inducible reductions in serum IGF-1 after establishment of peak bone mass did not affect cortical BMD or morphology in the adult mouse (Courtland et al. 2011).
Studies of the natural aging process in inbred mice have shown increased femoral cortical porosity and increased medullar area by 121 weeks of age (~70 human years) (Silbermann et al. 1987), as well as decreases in femoral cortical and trabecular bone volume as early as 78 weeks (~50 human years) (Weiss et al. 1991). Thus, mice appear to be a good model for human age-related bone loss. ALS knockout (ALSKO) is a unique mouse model, where the igfals gene encoding the acid-labile subunit is ablated. As a result, serum IGF-1 levels are reduced by 50 % (Yakar et al. 2006). We have previously shown that ALSKO mice have significant decreases in cortical bone size and tissue amount during puberty and through adulthood in both genders (Courtland et al. 2010). In the present study, we sought to examine the aging bone phenotype of C57BL/6J mice and determine whether age-related bone loss is exacerbated by significant reductions in serum IGF-1 levels that begin during early growth and development.
Methods
Animals
ALSKO mice were generated as described previously (Yakar et al. 1999) and backcrossed to C57BL/6J (B6) for ten generations. B6 (WT) and ALSKO male littermates were housed two to five animals per cage in a 12-h light/dark cycle facility where food and water were freely available. Male WT B6 and ALSKO mice were killed at 21 (for body weight and composition only), 52, 80, and 104 weeks of age. For histomorphometric analysis, the animals were injected with calcein (10 mg/kg) 2 days prior to killing as well as 2 weeks (for 52 weeks animals) and 30 days (for 80 and 104 weeks animals) prior to killing. Relative fat and lean mass (as a percentage of body weight) were assessed in live (non-anesthetized) animals using an EchoMRI™ 3-in-1 system (Echo Medical Systems, LLC). All procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine.
Serum and plasma parameters
Serum and plasma were collected through orbital bleeding immediately after sacrifice. Plasma IGF-1 and serum insulin levels were measured using commercial radioimmunoassays as previously described (Yakar et al. 2006; Yakar et al. 2002). Plasma osteocalcin levels were measured using a commercial radioimmunoassay (American Laboratory Products Company, Inc., Salem, NH, USA). Serum GH and tartrate-resistant acid phosphatase (TRAP) levels were measured using commercial ELISA kits (Millipore Corporation, Billerica, MA, USA and Immunodiagnostic Systems Limited, Boldon, Tyne & Wear, UK, respectively).
Bone morphology and tissue mineral density
WT and ALSKO left femora were scanned using a high-resolution SkyScan micro-CT system (SKYSCAN, Kontich, Belgium). Images were acquired using a 10-MP digital detector, 10-W power energy (100 kV and 100 mA), and a 0.5-mm aluminum filter with 6.7 μm image voxel size. A fixed global threshold method was used based on the manufacturer’s recommendations and preliminary studies, which showed that mineral variation between groups was not high enough to warrant adaptive thresholds. The cortical region of interest was selected as the 2.0-mm mid-diaphyseal region directly below the third trochanter, which includes both the mid-diaphysis and more proximal cortical regions. Cortical bone measurements consisted of total cross-sectional area (Tt.Ar), cortical area (Ct.Ar), marrow area (Ma.Ar), cortical thickness (Ct.Th), polar moment of inertia (Jo), tissue mineral density (TMD), and relative intracortical porosity (Pc, closed vascular porosity not continuous with the marrow cavity within the sample regions was counted; expressed as percent of Ct.Ar). Trabecular measurements included bone volume relative to total volume (BV/TV), bone mineral density (BMD), trabecular number (Tb.N), trabecular spacing (Tb.Sp), and trabecular thickness (Tb.Th). Additional whole bone measurements included femoral length (Le), robustness (Tt.Ar/Le), and relative cortical area (RCA = Ct.Ar/Tt.Ar).
Mechanical testing
In order to assess mechanical integrity, left femora were loaded to failure in four-point bending using a servo-hydraulic materials testing machine (MTS, Eden Prairie, MN USA). Tests were carried out in the posterior-to-anterior direction at a loading rate of 0.05 mm/s with the anterior aspect of the bone in tension. From the resulting load vs. displacement curves, structural properties were calculated to describe the specific aspects of the tissues’ mechanical behavior under load. Those properties included stiffness (in newtons per millimeter) and maximum force at failure (in newton). Stiffness was defined as the slope of the elastic portion of the load/displacement curve, while max force was defined as the largest load value registered during the test. In addition, post-yield displacement and work-to-fracture were determined to assess brittleness and fracture toughness.
Histology
Right femora from WT and ALSKO mice were fixed in 10 % zinc formalin and cut in half at the midshaft. Proximal halves of cut femora were decalcified using EDTA and processed for paraffin sectioning and stained with either hematoxylin and eosin (for osteocyte number) or TRAP (for osteoclast number). For osteocyte measurements, 4',6-diamidino-2-phenylindole (DAPI)-stained sections were used to identify lacunae with osteocytes; their number density (Ot.N, in number per millimeter squared bone area) was sampled across four femoral midshaft quadrants (lateral-posterior, medial-posterior, lateral-anterior, and medial-anterior), and these values were then averaged to give a mean osteocyte lacunar density. TRAP staining was performed as described previously (Elis et al. 2010). Distal halves were processed and embedded in polymethyl methacrylate for thick sectioning on a low-speed saw. Thick sections were mounted on glass slides and polished to ~50 μm for study. Bone histomorphometry studies were performed using OsteoMeasure (OsteoMetrics, Atlanta, GA).
Primary osteoclast cultures
Bone marrow cells were flushed from femurs of 104-week male mice using a 26-gauge needle, collected in α-modified minimum essential medium (αMEM) and drawn through an 18-gauge needle to achieve single cell suspension. Cells were then washed in αMEM and cultured in αMEM + 10 % fetal bovine serum (FBS) overnight. The following day, nonadherent cells were cultured in osteoclast differentiation media (αMEM, 10 % FBS, 60 ng/ml RANKL, and 40 ng/ml M-CSF) for 7 days. Primary osteoclast cultures were then washed two times with 1× phosphate-buffered saline (PBS) and fixed with 10 % glutaraldehyde solution for 15 min at 37 °C. Following two washes with 1× PBS, a 37 °C warmed TRAP staining solution (0.05 M sodium acetate, 0.025 M sodium tartrate, 0.125 mg/ml Fast Red Violet LB Salt, and 0.125 mg/ml Naphthol AS-MX phosphate) was added for 10–15 min and then washed with water. Multinucleated, stained cells were observed and counted using light microscopy.
Statistical analysis
Data are presented as mean values + standard deviation. Significant differences among groups and ages were tested using ANOVA (p < 0.05) with Bonferroni post-hoc tests. In cases of unequal variance among two or more groups, data were log transformed before performing ANOVA. For statistical analyses of osteocyte numbers and histomorphometric parameters on each bone surface of 104-week animals, t tests were performed.
Results
Decreases in serum IGF-1 levels are associated with reduced body weight and fat mass during aging
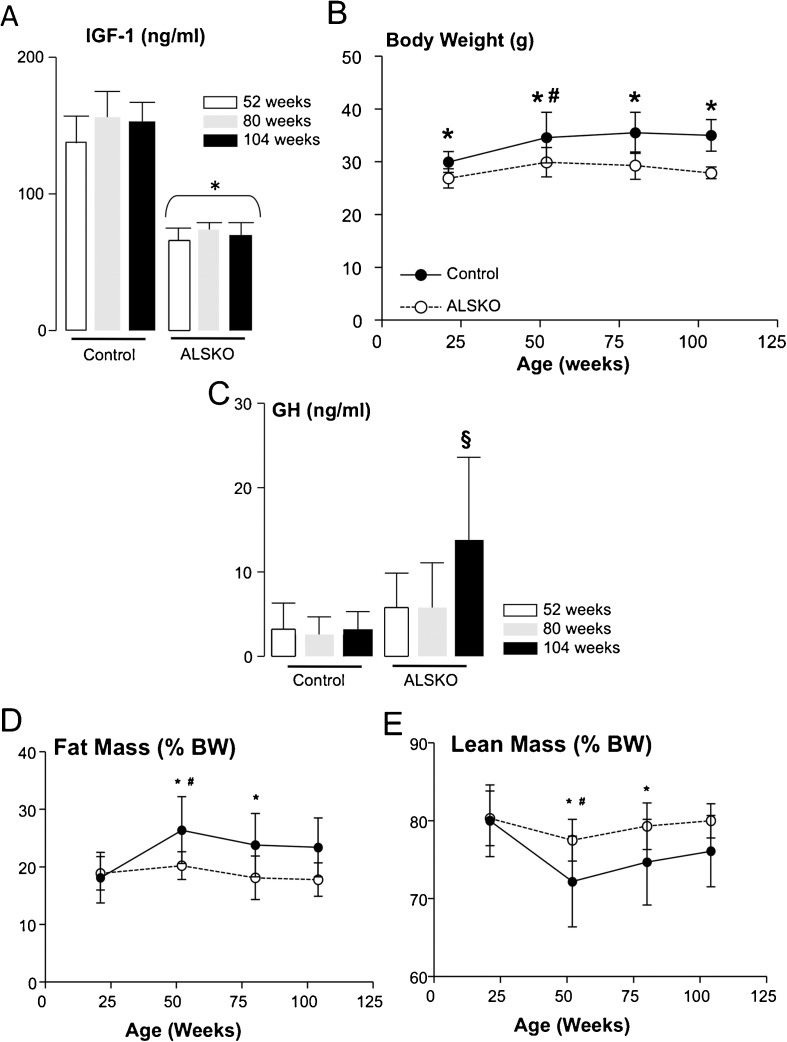
Our previous studies of ALSKO mice during growth showed reductions in serum IGF-1 levels that were associated with decreases in body size. Likewise, during aging, ALSKO mice exhibited decreases in plasma IGF-1 levels (Fig. 1a) and reductions in body weight at 52, 80, and 104 weeks of age (Fig. 1b, Table 1). For both WT and ALSKO mice, body weight increased significantly from 21 to 52 weeks and then remained constant through 104 weeks (Fig. 1b).
Fig. 1.
Igfals gene inactivation leads to decreased serum IGF-1 levels and affects body size and body composition through aging. a Plasma IGF-1 levels at 52, 80, and 104 weeks of age (n = 10–16). b Body weight (n = 10–48). c Plasma GH levels (n = 8–15). d Relative fat mass, assessed by MRI (n = 10–48). e Relative lean mass, assessed by MRI. Data presented as mean + SD (n = 10–48). Asterisk, significant difference between WT and ALSKO mice at the indicated age; section sign, significant difference from all other groups; number sign, significant difference from 21 weeks of age
Table 1.
Means values for body and organ weights of WT and ALSKO mice at 52, 80, and 104 weeks of age
| Traits | 52 weeks | 80 weeks | 104 weeks | |||
|---|---|---|---|---|---|---|
| WT (n = 10) | ALSKO (n = 10) | WT (n = 16) | ALSKO (n = 10) | WT (n = 12) | ALSKO (n = 13) | |
| Body weight, g | 34.60 ± 4.78 | 29.93 ± 2.79a | 35.09 ± 3.35 | 31.18 ± 1.88a | 34.23 ± 3.19 | 27.90 ± 1.08a |
| Body length, cm | 10.56 ± 0.37 | 10.12 ± 0.19a | 10.48 ± 0.25 | 10.18 ± 0.14 | 10.33 ± 0.37 | 10.00 ± 0.19a |
| Liver, g (relative to body weight, %) | 1.68 ± 0.17 (4.88 ± 0.35) | 1.54 ± 0.16 (5.17 ± 0.51) | 1.63 ± 0.19 (4.66 ± 0.48) | 1.80 ± 0.09 (5.79 ± 0.38) | 1.74 ± 0.74 (5.13 ± 2.35) | 1.49 ± 0.14 (5.35 ± 0.59) |
| Quadriceps, g (relative to body weight, %) | 0.32 ± 0.05 (0.94 ± 0.14) | 0.30 ± 0.04 (1.01 ± 0.09) | 0.33 ± 0.04 (0.94 ± 0.15) | 0.29 ± 0.04 (0.93 ± 0.15) | 0.29 ± 0.03 (0.85 ± 0.09) | 0.24 ± 0.04a (0.84 ± 0.12) |
| Tibialis, g (relative to body weight, %) | 0.16 ± 0.03 (0.46 ± 0.07) | 0.13 ± 0.02a (0.43 ± 0.06) | 0.15 ± 0.02 (0.44 ± 0.08) | 0.13 ± 0.02a (0.40 ± 0.07) | 0.14 ± 0.02 (0.41 ± 0.05) | 0.11 ± 0.01a (0.41 ± 0.05) |
| Gastrocnemius, g (relative to body weight, %) | 0.30 ± 0.03 (0.87 ± 0.07) | 0.25 ± 0.03a (0.82 ± 0.06) | 0.28 ± 0.02 (0.81 ± 0.10) | 0.25 ± 0.01a (0.79 ± 0.05) | 0.26 ± 0.03 (0.76 ± 0.09) | 0.22 ± 0.02a (0.78 ± 0.05) |
| Gonadal fat, g (relative to body weight, %) | 1.21 ± 0.71 (3.31 ± 1.54) | 0.83 ± 0.16 (2.76 ± 0.45) | 1.18 ± 0.55 (3.27 ± 1.27) | 0.87 ± 0.32 (2.74 ± 0.85) | 1.15 ± 0.57 (3.28 ± 1.53) | 0.64 ± 0.22 (2.28 ± 0.74) |
| Subcutaneous fat, g (relative to body weight, %) | 0.75 ± 0.43 (2.07 ± 0.97) | 0.35 ± 0.05a (1.17 ± 0.15a) | 0.72 ± 0.54 (1.96 ± 1.26) | 0.37 ± 0.14a (1.18 ± 0.35) | 0.56 ± 0.28 (1.60 ± 0.73) | 0.25 ± 0.07a (0.90 ± 0.24) |
| Brown fat, g (relative to body weight, %) | 0.12 ± 0.03 (0.33 ± 0.07) | 0.1 ± 0.03 (0.33 ± 0.09) | 0.11 ± 0.05 (0.30 ± 0.10) | 0.09 ± 0.01 (0.28 ± 0.04) | 0.09 ± 0.02 (0.26 ± 0.05) | 0.08 ± 0.02 (0.28 ± 0.06) |
| Spleen, g (relative to body weight, %) | 0.09 ± 0.02 (0.26 ± 0.08) | 0.08 ± 0.02 (0.27 ± 0.04) | 0.07 ± 0.01 (0.21 ± 0.04) | 0.10 ± 0.04 (0.31 ± 0.12) | 0.10 ± 0.04 (0.29 ± 0.14) | 0.11 ± 0.06 (0.41 ± 0.24) |
| Kidney, g (relative to body weight, %) | 0.48 ± 0.05 (1.39 ± 0.11) | 0.5 ± 0.09 (1.65 ± 0.17a) | 0.46 ± 0.04 (1.30 ± 0.11) | 0.48 ± 0.02 (1.55 ± 0.10a) | 0.46 ± 0.06 (1.34 ± 0.10) | 0.44 ± 0.04 (1.59 ± 0.11a) |
aSignificantly different from WT mice at the indicated age
Studies of ALSKO mice during growth and development revealed that despite significant reductions in serum IGF-1 levels, serum GH levels, taken randomly from fed mice, were not elevated. Likewise, through 80 weeks of age, we did not detect GH elevations in plasma of ALSKO mice (Fig. 1c). However, samples randomly taken from 104-week-old ALSKO mice showed a significant increase in GH levels compared to all other animals (Fig. 1c). We should also note that at 104 weeks of age 7 out of 13 (54 %) ALSKO mice harbored hepatic or gastric tract tumors, while only 2 out of 12 (17 %) WT mice showed small lesions in the liver. The increases in tumor incidence in ALSKO mice may be related to increases in GH action; however, GH measurements should be interpreted cautiously as they are taken at one time point and in a random fashion.
Body composition of WT and ALSKO mice was evaluated, as measured by MRI, and revealed significant reductions in fat mass and increases in lean (fat-free) mass in ALSKO mice at 52 and 80 weeks of age as compared to WT (Fig. 1d, e), while at 104 weeks, we saw similar tendencies, but they were not statistically significant. However, the observed changes were not totally correlated with fat pad weights (since we only measured a few fat pad weights while MRI measures whole body fat content) or different muscle weights (since MRI measures all fat-free tissues in its lean mass calculations) and therefore may reflect changes in other organs such as the liver, kidney, and spleen (Table 1). We found that relative fat mass (evaluated by MRI) increased and relative lean mass (fat-free mass) decreased significantly from 21 to 52 weeks of age for both WT and ALSKO mice, but remained constant through 104 weeks (Fig. 1d, e). Interestingly at 104 weeks, the relative weight of the gonadal fat pad in ALSKO mice was 2.28 ± 0.21 % while in WT 3.28 ± 0.46 % (p = 0.046) and subcutaneous fat decreased by ~40 % in ALSKO (0.9 ± 0.07 % in ALSKO versus 1.6 ± 0.22 % in WT).
Decreases in serum IGF-1 levels are associated with decreased intracortical porosity during aging
Micro-CT measurements of cortical bone from the femoral mid-diaphysis indicated that from 52 to 80 weeks of age, the mean trait values were statistically indistinguishable in both ALSKO mice and WT mice, but progressive changes were evident from 80 to 104 weeks of age (Fig. 2). In WT mice, we found significant increases in total cross-sectional area (Tt.Ar) and cortical bone area (Ct.Ar) (Fig. 2a, b), while cortical thickness (Ct.Th) decreased (Fig. 2c) and marrow area (Ma.Ar) increased (Fig. 2d), resulting in significant reductions in relative cortical bone area (RCA) at 104 weeks (Fig. 2e). Polar moment of inertia (Jo) (Fig. 2f) increased in WT mice as compared to ALSKO at 104 weeks. However, this was associated with a simultaneous and significant increase in the intracortical porosity (Pc) (Fig. 2g, i). In contrast, ALSKO mice had significant decrease in cortical thickness (Ct.Th) (Fig. 2c) and a marked decrease in RCA (Fig. 2e). Most surprisingly, ALSKO mice showed no age-related changes in intracortical porosity (Fig. 2g, i). TMD did not differ significantly between WT and ALSKO bones during the course of the study (Fig. 2h). Our results indicated no difference in lacunae number between WT (796 ± 77 osteocytes/mm2, n = 6) and ALSKO (737 ± 55 osteocytes/mm2, n = 9) mice at 104 weeks. However, since lacunae number does not indicate osteocyte viability, we also analyzed DAPI-stained sections (nuclear stain) and counted “cell-occupied” lacunae per bone area. Although insignificant, we found that osteocyte number per bone area in ALSKO mice was slightly increased (420 ± 55 cells per mm2, n = 3), as compared to controls (348 ± 81 cells per mm2, n = 3).
Fig. 2.
Decreases in serum IGF-1 due to igfals gene inactivation are associated with elevations in serum GH and decreased intracortical porosity at advanced age. a Tt.Ar; b Ct.Ar; c Ct.Th; d Ma.Ar; e RCA; fJo; g intracortical porosity; h TMD; i transverse micro-CT images of three 104-week WT and ALSKO femora. Note the substantial intracortical porosity in WT femora and the lack of porosity in ALSKO femora. Bar = 0.5 mm; j Stiffness and maximum force at 104 weeks of age assessed by four-point bending assays; asterisk, significant difference between WT and ALSKO mice at the indicated age. Dagger, WT mice are significantly different from WT values at 52 weeks. Double dagger, ALSKO mice are significantly different from ALSKO values at 80 weeks. Number sign, WT mice are significantly different from WT values at 80 weeks. n = 10–16 per group for each measured trait
While increases in Tt.Ar and J0 in control mice appear to have a positive compensatory effect on the structural integrity of these bones, the most dramatic difference between groups was the presence of intracortical porosities in WT but not ASLKO bones. This was reflected in our mechanical tests which showed that WT bones, when compared to ALSKO bones, displayed markedly reduced stiffness and max force values at 104 weeks of age (Fig. 2j). Post-yield displacement (0.15 ± 0.06 mm in controls and 0.10 ± 0.07 mm in ALSKO) and work-to-fracture (5.13 ± 2.4 N/mm in controls and 6.0 ± 3.0 N/mm in ALSKO) did not differ between controls and ALSKO at 104 weeks.
Trabecular bone traits were evaluated at the distal metaphysis and did not vary between WT and ALSKO mice, except in the case of trabecular spacing (Tb.Sp), which was significantly greater in ALSKO mice as compared to WTs at both 80 and 104 weeks of age (Table 2). Also, from 80 to 104 weeks of age, Tb.Sp was significantly increased in both WT and ALSKO mice (Table 2).
Table 2.
Means values for trabecular bone traits of the femoral distal metaphysis
| Traits | 52 weeks | 80 weeks | 104 weeks | |||
|---|---|---|---|---|---|---|
| WT (n = 10) | ALSKO (n = 10) | WT (n = 16) | ALSKO (n = 10) | WT (n = 12) | ALSKO (n = 13) | |
| Bone volume/total volume, % | 6.90 ± 5.46 | 6.90 ± 2.73 | 6.60 ± 4.84 | 5.89 ± 1.22 | 4.13 ± 1.04 | 2.24 ± 0.84 |
| Trabecular Number, 1/mm | 1.32 ± 0.73 | 1.29 ± 0.40 | 1.22 ± 0.82 | 1.01 ± 0.2 | 0.78 ± 0.18 | 0.43 ± 0.14 |
| Trabecular thickness, mm | 0.05 ± 0.007 | 0.05 ± 0.006 | 0.05 ± 0.004 | 0.06 ± 0.004 | 0.05 ± 0.004 | 0.052 ± 0.006 |
| Trabecular spacing, mm | 0.25 ± 0.03 | 0.27 ± 0.02 | 0.30 ± 0.03 | 0.35 ± 0.03a, b | 0.34 ± 0.02 | 0.42 ± 0.06a, b |
| Tissue mineral density, g/cm3 | 0.90 ± 0.02 | 0.92 ± 0.02 | 0.94 ± 0.09 | 0.96 ± 0.03 | 0.92 ± 0.03 | 0.908 ± 0.045 |
aSignificant difference from WT mice at the indicated age
bWT and ALSKO values significantly different than their counterparts at the next younger age group
Effects of decreases in serum IGF-1 on bone remodeling during aging
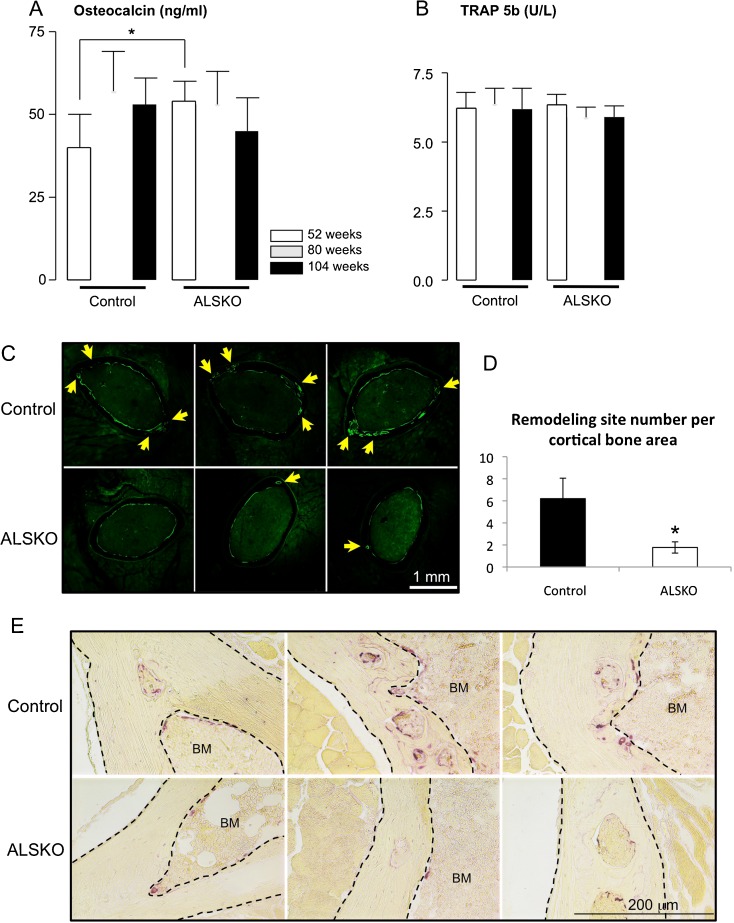
To determine whether the differences in skeletal integrity between WT and ALSKO mice could be explained by changes in bone remodeling, we first measured circulating markers of bone formation and resorption. Plasma levels of the bone formation marker, osteocalcin, did not indicate any age effect for WT or ALSKO mice, though ALSKO levels were significantly greater than WT levels at 52 weeks (Fig. 3a). Likewise, serum levels of TRAP5b, a marker of bone resorption, were statistically indistinguishable among all ages and both groups (Fig. 3b).
Fig. 3.
Igfals gene inactivation associates with reduced intracortical remodeling and reduced TRAP staining. a Plasma osteocalcin levels (n = 10–16). b Serum TRAP5b levels (n = 8–16). c Fluorescent images of thick sections from WT and ALSKO femora at 104 weeks of age. Arrows indicate bone formation at sites of intracortical porosities. d Quantification of intracortical remodeling; e Bright field images of WT and ALSKO femoral cortical sections from 104-week-old mice stained for TRAP
Thick sections of 104-week WT and ALSKO femoral mid-diaphyses revealed calcein labeling as expected (Fig. 3c). However, label clarity was reduced due to the reduction in Ct.Ar and increase in porosity. Further, periosteal double labels were not resolvable (even with a ~30-day inter-label interval) and were seldom found on endosteal surfaces. Thus, periosteal measurements were restricted to relative-labeled perimeter (L.Pm, in percent) and endosteal measurements had a small sample size for calculation of mineral apposition rate (MAR) and bone formation rate (BFR) due to a general absence of double labels. As such, values for L.Pm, MAR, and BFR were found to be statistically indistinguishable between WT and ALSKO mice on a given cortical bone surface (Table 3). As a final note, it was found that the intracortical porosities of WT mice were nearly always undergoing bone formation, as calcein labeling was apparent in virtually all such porosities. We therefore counted the remodeling sites found in each section and normalized them to cortical bone area. We found that WT mice had significantly more intracortical remodeling sites than ALSKO femoral diaphyses (Fig. 3d).
Table 3.
Mean histomorphometry values for 104-week WT and ALSKO mice
| Groups | Periosteal surface | Endosteal surface | ||
|---|---|---|---|---|
| L.Pm (%) | L.Pm (%) | MAR (μm/day) | BFR/B.Pm (μm/day × 100) | |
| WT (n = 10) | 28.2 ± 17.4 | 37.3 ± 7.9 | 0.77 ± 0.28 (n = 6) | 28.7 ± 15.0 |
| ALSKO (n = 11) | 26.9 ± 20.0 | 42.5 ± 8.4 | 0.65 ± 0.32 (n = 4) | 31.5 ± 21.0 |
No significant differences between WT and ALSKO mice were found
Given that osteoclasts were often observed in intracortical porosities of WT mice (Fig. 3d), to address whether their activity was part of the increased remodeling in WT bones, we stained paraffin thin sections with TRAP. The results indicated increased TRAP staining in sections from WT mice (Fig. 3e) both within the cortex and the endosteal surface. It should also be noted that in the very rare cases where intracortical porosities were observed in ALSKO mice (Fig. 3d), lamellar bone infilling was also evident. In contrast, lamellar bone formation was rarely observed within the intracortical porosities of aged WT mice, suggesting both increased resorption and diminished formation in aging WT B6 mice. We further assessed osteoclast number in cultures of nonadherent cells from bone marrow extracted from 104-week-old mice seeded in osteoclast differentiation media. We found that cultures derived from ALSKO mice showed an almost twofold decrease in the number of osteoclasts formed in vitro as compared to WTs (Fig. 4). These findings are in agreement with our previous study, showing decreased osteoclastogenesis in ALSKO mice during growth (Fritton et al. 2010).
Fig. 4.
Igfals gene inactivation associates with reduced osteoclast formation in vitro. Number of osteoclasts per well in primary osteoclast cultures from WT (n = 3) and ALSKO (n = 2) mice at 104 weeks of age
Discussion
In our previous study examining WT and ALSKO mice during growth and development, we observed that deficits in body and skeletal size appeared as early as 4 and 8 weeks of age, respectively, and continued into adulthood (16 weeks) (Courtland et al. 2010). In the present study, our examination of 52-week (older adult) ALSKO mice revealed that, although body weights remained significantly reduced compared to WT mice, nearly all skeletal traits were identical to those of WT mice. Additionally, from 21 to 52 weeks, both WT and ALSKO mice had a significant increase in body weight accompanied by significant decreases in relative lean mass and significant increases in relative fat mass. Thus, by 52 weeks, both WT and ALSKO mice were disproportionally fatter and less lean. However, WT mice were significantly fatter and less lean than ALSKO mice, thereby explaining their increased body weight. Serum IGF-1 levels remained significantly reduced in ALSKO mice throughout the study as expected. There were also no observable differences in GH levels between WT and ALSKO mice through 80 weeks of age, although at 104 weeks ALSKO mice had significantly increased GH levels, suggesting that GH levels may underlie skeletal differences between WT and ALSKO mice at 104 weeks of age.
A previous study examining age-related bone loss in mice showed that femora from female C57BL/6J mice exhibited decreased cortical thickness, increased marrow cavity area, and increased cortical porosity by 121 weeks (Silbermann et al. 1987). However, in that study, the total amount of bone tissue was not measured and cortical porosity differences were not quantified. A later study in CW-1 mice looked at mice as old as 139 weeks and found decreases in BMD and cortical thickness. Yet, CW-1 is an outbred strain and measurements were taken only from the femoral neck of female mice (Weiss et al. 1991). In a study conducted with aged male C57BL/6J mice, decreases in tibial Ct.Th and Ct.Ar as well as decreases in cancellous BV/TV and Tb.N were observed by 104 weeks (Halloran et al. 2002). Thus, there is precedent for cortical and trabecular bone loss in aging inbred mice. However, in the present study, we found that femora from WT male mice exhibit a pattern of age-related bone loss that differs somewhat from previously reported models. In our WT male femora, we found only minor changes in Tb.Sp, and while Ct.Ar values were stable, Tt.Ar values increased from 80 to 104 weeks. These changes were concurrent with a significant increase in the amount of intracortical porosity at 104 weeks of age. It should be noted that some increase in Tt.Ar with age is expected since periosteal bone formation does continue at low levels throughout adulthood (Courtland et al. 2010; Price et al. 2005; Yakar et al. 2009a) and male mice are likely to add greater amounts of bone periosteally than females, if they follow their human counterparts (Szulc et al. 2006). However, in this study, the increases in Tt.Ar are sizeable and statistically significant from 80 to 104 weeks. Given the magnitude of these changes, with relatively constant Tt.Ar and Ct.Ar values prior to 104 weeks, as well as the drastic increase in porosity, it seemed likely that male WT mice were compensating for a loss of cortical bone (due to intracortical resorption), by adding greater amounts of bone periosteally. However, this compensatory response of WT bones did not increase bone mechanical properties, as WT femora showed decreases in stiffness and max load when compared to bones from ALSKO mice. We show here that a decrease in bone strength of WT relative to ALSKO mice is only partially accounted for by a reduction in absolute bone mass and changes in cross-sectional bone geometry; the lack of age-related increase in intracortical porosity in ALSKO bones is a major contributory factor. Our results are in agreement with a previous study of 29 inbred mouse lines showing that genes affecting strength by varying BMD are different than those that affect strength by altering cross-sectional geometry (Wergedal et al. 2005) and likely from those altering intracortical remodeling. Cortical porosity has been reported in several earlier studies and seems to differ greatly among animal models, with low values for rodents (Bagi et al. 1997; Lauritzen et al. 1993) and higher values for sheep (Chavassieux et al. 2001) and dogs (Sietsema 1995; Wilson et al. 1998). In agreement with the μCT data, showing increased intracortical porosity, we demonstrated an increase in intracortical calcein labeling and TRAP staining and a lack of new lamellar bone, indicating increased intracortical bone remodeling/turnover in WT mice that favors resorption over formation.
The current studies reveal a surprising lack of increased intracortical porosity or remodeling aged ALSKO femora. The precise mechanisms underlying this difference in bone aging compared to WT bones remain obscure. However, a number of recent studies have shown that intracortical bone remodeling initiates with locally impaired osteocyte viability, followed by osteoclastic bone resorption and osteoblastic infilling (Verborgt et al. 2002; Cardoso et al. 2009; Kennedy et al. 2012). Age-induced osteocyte apoptosis was reported previously (Almeida et al. 2007) and has been linked to accumulation of reactive oxygen species (Mann et al. 2007). Animal models with reduced IGF-1 signaling, such as the dw/dw mice with decreased secretion of GH (and reduced IGF-1), thyroid stimulating hormone, or prolactin, show increased resistance to oxidative stress (Hauck and Bartke 2000). Similarly, the long-lived GH-deficient Ames dwarf, Snell, and lit/lit dwarfs, which all exhibit low serum IGF-1 levels, show increased resistance to oxidative stress and increased activities of catalase and Cu/Zn superoxide dismutase (Bartke and Brown-Borg 2004; Salmon et al. 2005). It is therefore reasonable to posit that in ALSKO mice, the lifelong decreases in serum IGF-1 act similarly and confer increased resistance to oxidative stress in bone, thus prolonging osteocyte life span and attenuating intracortical resorption and remodeling activities.
We also observed that ALSKO mice exhibit age-related trabecular bone loss beginning at 104 weeks of age. However, the bone loss pattern differed from those of WT mice. While WT mice lost ~30 % of their trabecular bone volume from 52 to 104 weeks (6.9 % at 52 weeks, 6.6 % at 80 weeks, and 4.1 % at 104 weeks), ALSKO mice lost ~60 % of their BV/TV (6.9 % at 52 weeks, 5.9 % at 80 weeks, and 2.2 % at 104 weeks), suggesting active resorption at the cancellous bone compartment. With respect to cortical bone, we found that WT mice showed a ~35 % increase in Tt.Ar and concomitant increase of ~30 % in Ct.Ar with age (from 52 to 104 weeks), while ALSKO mice increased Tt.Ar by only 9 % and had a 1 % decrease in Ct.Ar with age (from 52 to 104 weeks). Importantly, ALSKO mice have no change in intracortical porosity from 80 to 104 weeks of age, which is in stark contrast to WT mice as noted above.
A prior study examining aging in WT mice showed that increases in the osteoclast progenitor pool occurred in tandem with age-related bone loss (Perkins et al. 1994). Thus, we hypothesized that there would be increased intracortical osteoclastic activity in WT mice. Examination of histomorphometric sections from WT mice revealed increased TRAP staining in close proximity to the intracortical porosities. In ALSKO mice, there was notably more infilling (lamellar bone deposits) in areas of intracortical resorption than in WT mice (that may have occurred early during adulthood), but TRAP staining revealed a significant reductions on resorption fronts. These results were in accordance with our ex vivo data where bone marrow cells from 104-week femora were cultured in osteoclast differentiation media. We found that ALSKO mice had significantly lower osteoclast number formed in vitro than WT cultures. These results are in agreement with our previous studies showing impaired osteoclastogenesis in bone marrow extracted from young ALSKO mice (Fritton et al. 2010; Yakar et al. 2009b).
Conclusions
Similar to humans, inbred mice lose bone mass in a sex- and site-specific manner with increasing age. In this study, we found that male C57Bl/6J (WT) mice demonstrated small but significant increases in Tt.Ar and Ct.Ar at the femoral mid-diaphysis from 80 to 104 weeks of age and that these changes were accompanied by increases in intracortical porosity. These increases in cortical bone size may be an adaptive mechanism to counter the loss of intracortical bone mass by increasing stability geometrically (increasing diametral size). However, whole bone mechanical testing did not indicate equal or greater mechanical properties as compared to mice with minimal porosity (ALSKO). Thus, the compensatory mechanism appears insufficient. We found that the age-related decline in skeletal integrity seen in WT male mice is altered in states of IGF-1 deficiency. Specifically, ALSKO mice, although they exhibited age-related bone loss in the form of small decreases in the amount of cortical bone, had few instances of intracortical porosities and no compensatory increases in Tt.Ar but showed some active resorption in the trabecular bone compartment. Thus, although lifelong reductions in serum IGF-1 compromise skeletal size in development leading to slender bones, they are also associated with decreased intracortical bone remodeling and preservation of bone strength during aging.
Acknowledgments
The authors would like to thank Dr. Boisclair YR (Department of Animal Science, Cornell University, Ithaca, New York 14853, USA) for creation of the original ALSKO line.
References
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi CM, Ammann P, Rizzoli R, Miller SC. Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 1997;61(4):336–344. doi: 10.1007/s002239900344. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res. 1999;14(12):2150–2158. doi: 10.1359/jbmr.1999.14.12.2150. [DOI] [PubMed] [Google Scholar]
- Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14(5):769–778. doi: 10.1016/8756-3282(93)90209-S. [DOI] [PubMed] [Google Scholar]
- Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605. doi: 10.1359/jbmr.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavassieux P, Garnero P, Duboeuf F, Vergnaud P, Brunner-Ferber F, Delmas PD, Meunier PJ. Effects of a new selective estrogen receptor modulator (MDL 103,323) on cancellous and cortical bone in ovariectomized ewes: a biochemical, histomorphometric, and densitometric study. J Bone Miner Res. 2001;16(1):89–96. doi: 10.1359/jbmr.2001.16.1.89. [DOI] [PubMed] [Google Scholar]
- Courtland HW, DeMambro V, Maynard J, Sun H, Elis S, Rosen C, Yakar S. Sex-specific regulation of body size and bone slenderness by the acid labile subunit. J Bone Miner Res. 2010;25(9):2059–2068. doi: 10.1002/jbmr.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtland HW, Elis S, Wu Y, Sun H, Rosen CJ, Jepsen KJ, Yakar S. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS One. 2011;6(3):e14762. doi: 10.1371/journal.pone.0014762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric M, Djonic D, Milovanovic P, Nikolic S, Marshall R, Marinkovic J, Hahn M. Region-specific sex-dependent pattern of age-related changes of proximal femoral cancellous bone and its implications on differential bone fragility. Calcif Tissue Int. 2010;86(3):192–201. doi: 10.1007/s00223-009-9325-8. [DOI] [PubMed] [Google Scholar]
- Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, Majeska RJ, Yakar S. Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties even in the absence of tissue IGF-1. J Bone Miner Res. 2010;25(6):1257–1266. doi: 10.1002/jbmr.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritton JC, Kawashima Y, Mejia W, Courtland HW, Elis S, Sun H, Wu Y, Rosen CJ, Clemmons D, Yakar S. The insulin-like growth factor-1 binding protein acid-labile subunit alters mesenchymal stromal cell fate. J Biol Chem. 2010;285(7):4709–4714. doi: 10.1074/jbc.M109.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcif Tissue Int. 2002;70(1):22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17(6):1044–1050. doi: 10.1359/jbmr.2002.17.6.1044. [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Bartke A. Effects of growth hormone on hypothalamic catalase and Cu/Zn superoxide dismutase. Free Radic Biol Med. 2000;28(6):970–978. doi: 10.1016/S0891-5849(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50(5):1115–1122. doi: 10.1016/j.bone.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83(12):4257–4262. doi: 10.1210/jc.83.12.4257. [DOI] [PubMed] [Google Scholar]
- Lauritzen DB, Balena R, Shea M, Seedor JG, Markatos A, Le HM, Toolan BC, Myers ER, Rodan GA, Hayes WC. Effects of combined prostaglandin and alendronate treatment on the histomorphometry and biomechanical properties of bone in ovariectomized rats. J Bone Miner Res. 1993;8(7):871–879. doi: 10.1002/jbmr.5650080713. [DOI] [PubMed] [Google Scholar]
- Lochmuller EM, Matsuura M, Bauer J, Hitzl W, Link TM, Muller R, Eckstein F. Site-specific deterioration of trabecular bone architecture in men and women with advancing age. J Bone Miner Res. 2008;23(12):1964–1973. doi: 10.1359/jbmr.080709. [DOI] [PubMed] [Google Scholar]
- Mann V, Huber C, Kogianni G, Collins F, Noble B. The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone. 2007;40(3):674–684. doi: 10.1016/j.bone.2006.10.014. [DOI] [PubMed] [Google Scholar]
- McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75(8):1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Rigss BL, Keaveny TM, Achenbach SJ, Hoffmann PF, Camp JJ, Rouleau PA, Bouxsein ML, Amin S, Atkinson EJ, Robb RA, Khosla S. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007;22(12):1885–1892. doi: 10.1359/jbmr.070728. [DOI] [PubMed] [Google Scholar]
- Mezquita-Raya P, Munoz-Torres M, Alonso G, de Luna JD, Quesada JM, Dorado G, Luque-Recio F, Ruiz-Requena ME, Lopez-Rodriguez F, Escobar-Jimenez F. Susceptibility for postmenopausal osteoporosis: interaction between genetic, hormonal and lifestyle factors. Calcif Tissue Int. 2004;75(5):373–379. doi: 10.1007/s00223-004-0187-9. [DOI] [PubMed] [Google Scholar]
- Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, Byrne CD, Wood PJ, Cooper C, Holt RI. Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD) Bone. 2005;37(6):833–841. doi: 10.1016/j.bone.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Gibbons R, Kling S, Kahn AJ. Age-related bone loss in mice is associated with an increased osteoclast progenitor pool. Bone. 1994;15(1):65–72. doi: 10.1016/8756-3282(94)90893-1. [DOI] [PubMed] [Google Scholar]
- Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ. Genetic variation in bone growth patterns defines adult mouse bone fragility. J Bone Miner Res. 2005;20(11):1983–1991. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- Rhee EJ, Oh KW, Lee WY, Kim SW, Oh ES, Baek KH, Kang MI, Park CY, Choi MG, Yoo HJ, Park SW. Age, body mass index, current smoking history, and serum insulin-like growth factor-I levels associated with bone mineral density in middle-aged Korean men. J Bone Miner Metab. 2004;22(4):392–398. doi: 10.1007/s00774-003-0500-0. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, Pols HA. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22(11):1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Hayes WC. Age changes in geometry and mineral content of the lower limb bones. Ann Biomed Eng. 1984;12(6):573–584. doi: 10.1007/BF02371450. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Sietsema WK. Animal models of cortical porosity. Bone. 1995;17(4 Suppl):297S–305S. doi: 10.1016/8756-3282(95)00307-y. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Weiss A, Reznick AZ, Eilam Y, Szydel N, Gershon D. Age-related trend for osteopenia in femurs of female C57BL/6 mice. Compr Gerontol [A] 1987;1(1):45–51. [PubMed] [Google Scholar]
- Simmons ED, Jr, Pritzker KP, Grynpas MD. Age-related changes in the human femoral cortex. J Orthop Res. 1991;9(2):155–167. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res. 2009;24(4):737–743. doi: 10.1359/jbmr.081223. [DOI] [PubMed] [Google Scholar]
- Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21(12):1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Tatton NA, Majeska RJ, Schaffler MB. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17(5):907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- Weiss A, Arbell I, Steinhagen-Thiessen E, Silbermann M. Structural changes in aging bone: osteopenia in the proximal femurs of female mice. Bone. 1991;12(3):165–172. doi: 10.1016/8756-3282(91)90039-L. [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005;36(1):111–122. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Wilson AK, Bhattacharyya MH, Miller S, Mani A, Sacco-Gibson N. Ovariectomy-induced changes in aged beagles: histomorphometry of rib cortical bone. Calcif Tissue Int. 1998;62(3):237–243. doi: 10.1007/s002239900423. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, Leroith D, Rosen CJ. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006;189(2):289–299. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- Yakar S, Canalis E, Sun H, Mejia W, Kawashima Y, Nasser P, Courtland HW, Williams V, Bouxsein M, Rosen C, Jepsen KJ. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24(8):1481–1492. doi: 10.1359/jbmr.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J. 2009;23(3):709–719. doi: 10.1096/fj.08-118976. [DOI] [PMC free article] [PubMed] [Google Scholar]