Abstract
Discovering key cellular and molecular traits that promote longevity is a major goal of aging and longevity research. One experimental strategy is to determine which traits have been selected during the evolution of longevity in naturally long-lived animal species. This comparative approach has been applied to lifespan research for nearly four decades, yielding hundreds of datasets describing aspects of cell and molecular biology hypothesized to relate to animal longevity. Here, we introduce a Comparative Cellular and Molecular Biology of Longevity Database, available at (http://genomics.brocku.ca/ccmbl/), as a compendium of comparative cell and molecular data presented in the context of longevity. This open access database will facilitate the meta-analysis of amalgamated datasets using standardized maximum lifespan (MLSP) data (from AnAge). The first edition contains over 800 data records describing experimental measurements of cellular stress resistance, reactive oxygen species metabolism, membrane composition, protein homeostasis, and genome homeostasis as they relate to vertebrate species MLSP. The purpose of this review is to introduce the database and briefly demonstrate its use in the meta-analysis of combined datasets.
Keywords: Life span, Database, DNA repair, Protein homeostasis, Antioxidant enzymes, Stress resistance
Introduction
Many putative human longevity traits have been identified and characterized initially in short-lived invertebrate model species (e.g., Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila sp.). Candidate traits that emerge from such studies are typically then investigated in tractable mammalian models (e.g., mice). Although this basic approach has provided many insights into the biology of longevity, it is negatively affected by artefacts that can arise from strain inbreeding, the unnatural conditions of laboratory husbandry, and in some instances the difficulties in interpreting the effects of temporally and spatially global genetic manipulations.
A “comparative” approach can be a useful complement to the discovery strategy described above. If important traits associated with longevity have been selected over the tens of millions of years during which particular clades have evolved to become increasingly longevous, it should be possible to identify these in the tissues and cells of longer-lived species. This basic assumption underlies the comparative approach to longevity research, in which investigators seek to gain insight into the role(s) of particular traits by measuring them in both long- and short-lived species. This approach has three main strengths. Firstly, comparisons among mammalian species are aided by extreme differences in lifespan between the shortest- and longest-lived mammals. Even if comparisons are confined to mammals, one can choose species with maximum lifespans (MLSP) ranging from several years (e.g., many rodents) to over two centuries (e.g., bow whale). These differences are far greater than the typically 50 % or less increases in lifespan realized via genetic or dietary manipulations of laboratory mice. Secondly, the comparative approach is not affected by unintended consequences of over- or under-expressing a single gene while leaving expression of important complementary genes unchanged. And thirdly, the measurement of a particular trait in a wide range of species with naturally disparate lifespans is relatively fast and inexpensive compared to the generation and study of genetically modified mice. However, two important limitations of the comparative approach outlined above are that: (1) many traits that may appear to be related to lifespan are in fact driven primarily by body mass, which is itself highly correlated with lifespan among mammals, and (2) it can be difficult to obtain a representative sample of the almost 6,000 mammalian and 10,000 avian species on the planet. Here, we introduce a Comparative Cellular and Molecular Biology of Longevity (CCMBL) Database, available at (http://genomics.brocku.ca/ccmbl/), which will help to address these and some other issues affecting the application of comparative methods in longevity research.
A comparative cell and molecular biology of longevity database
Since the 1970s, dozens of papers with hundreds of individual datasets describing various cellular and molecular traits in the context of species longevity have been published. Interest in aging and lifespan research continues to grow, and the amount of comparative data bearing on vertebrate longevity has reached a point where it is possible and advantageous to combine results from studies focused on a single trait, or several related traits, into a much larger and integrative central data repository containing the totality of such data. The CCMBL database is a compendium of the comparative cellular and molecular longevity data currently available. Its purpose is to collate the extensive (and growing) body of data generated by multiple studies and laboratories into a single comprehensive resource, thus facilitating the amalgamation of datasets, meta-analysis of data, and a more integrative understanding of the cellular and molecular traits associated with naturally evolved longevity.
The inaugural version of CCMBL contains approximately 2,300 cellular and molecular trait data values measured in the context of longevity from 44 studies of 176 vertebrate species. The database contains data only for mammalian and avian species, though it may be expanded to include ectothermic vertebrates in the future. CCMBL is implemented in a MySQL relational database. The organization of data within CCMBL is by species, the tissue, or cell type (e.g., brain, fibroblasts, and blood) in which measurements were made, whether these measurements were made in whole tissue (or cells) or in a subcellular fraction such as isolated mitochondria. For all records, the original data describing measured variables are provided, as is the published source of the data. Users can easily query the data either by data category or by species name. For query by data category (explained in detail below), the search outputs all measured data for all traits under the selected category from all studied species. A query by species name outputs data for all measured traits available for that species. In both cases, the trait, trait value, and information on species, their MLSP and body weight, as well as publication with a hyperlink to PubMed abstract, is provided in table format. In addition, by clicking on the species name, the user can hyperlink to AnAge (http://genomics.senescence.info/species/; de Magalhães and Costa 2009), which contains a variety of relevant data on that species (see below). The table can be sorted alphabetically by clicking any of the column headers. To facilitate the use of the data for customized analyses, a copy of the output in tab-delimited text format is also provided for the user to download. To aid the user in determining whether there are any data for a particular species, a list of all included species can be obtained by clicking the designated link. CCMBL is freely available at http://genomics.brocku.ca/ccmbl/. No restrictions have been placed upon its use. The database will be updated regularly as new data become available or in response to an emailed request.
Description of data included in CCMBL database
MLSP is the sole descriptor of longevity used in the database. To provide standardization, all MLSP data are from AnAge (http://genomics.senescence.info/species/; de Magalhães and Costa 2009). In relatively rare exceptions where AnAge has no lifespan data for a particular species, the MLSP values contained in the original publications have been used. This method for standardizing MLSP values renders the MLSPs associated with most datasets in the CCMBL database subtly different from those presented in original publications. For example, the MLSP values for Bos taurus presented in various papers vary widely. In the CCMBL database, the number associated with each dataset is that provided in AnAge. Standardization to a single MLSP value for each species makes management of the data more straightforward while removing a source of variability between studies.
Adult body mass data have been included for all species. Vertebrate species’ adult body mass and MLSP are themselves highly correlated; thus, the determination of correlations with MLSP must account for possible underlying relationships with body mass (see Speakman 2005 for review). In many comparative studies, the body mass of individual animals sampled from has not been included, so it is often necessary to use mean species values. For the purpose of standardization, species adult body mass data from AnAge are used for all species if available. Where it is not, we have used body mass data from the original publications.
One important note about the database is that only data from studies with four or more species have been included, unless the measurement protocol used in a smaller study was deemed sufficiently similar to that used in another study so that multiple datasets could be amalgamated. The four-species limitation was adopted because it is generally not possible to make inferences about the relationship of a specific trait with longevity using only two or three species (reviewed in Speakman 2005).
Measurements of longevity traits have been made in frozen tissue samples, freshly isolated cells, cultured cells, and isolated organelles (e.g., mitochondria), all of which are represented in the database. There are advantages and disadvantages associated with each approach, but useful insight can be gained from all of these data. Measurements made in snap-frozen tissue samples shed light on the basic cellular and tissue characteristics of longer-lived species. Dynamic differences have been studied in isolated cells, providing information about, for example, stress resistance of fibroblasts (Kapahi et al. 1999; Harper et al. 2007; Ogburn et al. 2001; Harper et al. 2011).
A variety of methods are used to measure the traits represented in the CCMBL database including cell-based assays, in vitro biochemical assays of a specific enzyme activity, HPLC, and immunoblotting. Immunoblotting to measure interspecies differences in the levels of proteins is a relatively new tool in comparative biology, and requires knowing the amino acid sequence of a protein, which is increasingly practical as more and more fully sequenced mammalian (and avian) genomes become available. Providing regions with 100 % conserved amino acid sequence can be identified in the same protein in all species investigated, and an antibody raised to an epitope that falls within this region is available, this approach can be used quantitatively (see Salway et al. 2011a for an example).
Organization of data within the CCMBL
The types of cellular and molecular variables studied in a multispecies comparative longevity context to date have been organized into several broad categories: cellular stress resistance, mitochondria and metabolism, reactive oxygen species (ROS) and antioxidants, membrane composition, protein homeostasis, genome homeostasis, and hormones and growth factors. The individual data categories and their relationships to longevity are briefly described below.
Cellular stress resistance
The relationship between cellular stress resistance and MLSP is represented by over 50 data records in the CCMBL database. Fibroblast stress resistance and species MLSP are positively correlated in comparisons among mammalian species (Table 1; Kapahi et al. 1999; Harper et al. 2007), between mammals and birds (Ogburn et al. 2001) and among avian species (Harper et al. 2011). This evidence for cellular stress resistance as a correlate of species longevity is compelling and has been statistically dissociated from body mass and phylogenetic relationships, but it is not known whether this trend applies to other cell types. The lack of comparative data on stress resistance of important cell types such as cardiomyocytes or neurons in particular is an important deficit that is obvious within the database. Individuals of some metazoan species will live for over 200 years, and (at least in humans, and presumably in other species) many individual cardiomyocytes (Bergmann et al. 2009) and neurons (Bhardwaj et al. 2006) will live the entire adult lifespan. It is thus important to understand what characteristics underlie the exceptional longevity of these particular types of cells in the longest-lived species, and this should be addressed in the future.
Table 1.
Cellular stress resistance as a correlate of MLSP and/or body mass
| Cell type | Comparison | Correlation with MSLP | Correlation with body mass |
|---|---|---|---|
| Lymphocytes | 3 Mammalian species | Positive correlation (tert-butylhydrogen peroxide, sodium arsenite, alkaline pH) (Kapahi et al. 1999) | Unknown |
| Dermal fibroblasts | 8 Mammalian species | Positive correlationa (PQ, H2O2, sodium arsenite, alkaline pH) (Kapahi et al. 1999) | Unknown |
| 10 Mammalian species | Positive correlationb (cadmiuma, H2Oa2, heat, rotenonea) (Harper et al. 2007) | Unknown | |
| 35 Avian species | Positive correlationb (cadmiuma, PQa, H2O2, methyl methanesulfonate, low glucose, UVa) (Harper et al. 2011) | Positive (cadmium, methyl methanesulfonate) | |
| Epithelial | 4 Mammalian and avian species | Positive correlation (95 % O2, H2O2) (Ogburn et al. 2001) | Unknown |
aCorrected for phylogeny
bCorrected for body mass
The molecular mechanisms by which cellular stress resistance of longer-lived species is enhanced are also largely unknown. Many of the stressors tested in fibroblasts would affect cellular homeostasis by increasing intracellular concentrations of reactive oxygen species and damaging key structures and macromolecules including membrane phospholipids, proteins, DNA, and RNA. However, as is evident in the database, there have been few measurements in fibroblasts of capacities to maintain homeostatic function of these macromolecules (e.g., Brown and Stuart 2007). These types of measurements generally have been done in whole tissues.
Metabolism, reactive oxygen species, and antioxidants
Harman’s free radical theory of aging (Harman 1956) has been a driving force in aging research, and much of the published data included in the CCMBL database relates to this theory. The original theory was later refined to recognize that in many cell types mitochondria are the main source of endogenous free radical production (Harman 1972); for this reason, the limited available comparative data on mitochondrial abundance (volume density) and bioenergetics are included in the database in the Metabolism category.
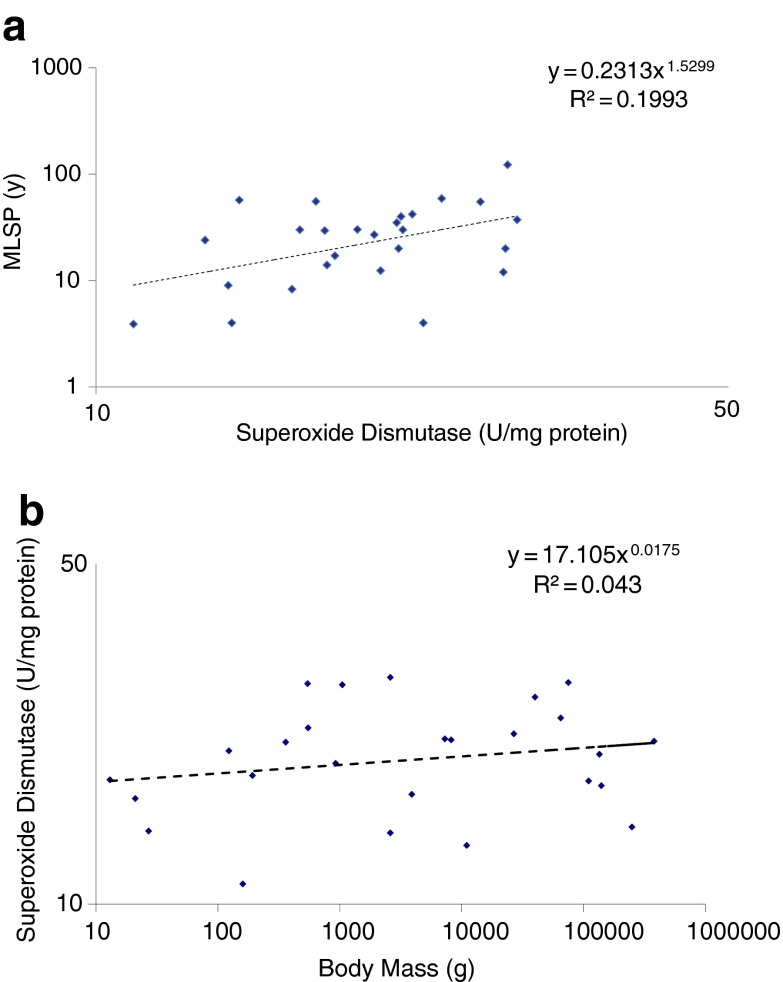
Large datasets from Tolmasoff et al. (1980), Ono and Okada (1984), Lopez-Torres et al. (1993), and (Page et al. 2010) in the Reactive Oxygen Species and Antioxidants category report the activities of antioxidant enzymes, including superoxide dismutases (SODs), catalase, glutathione peroxidase, and glutathione reductase in tissues from a wide range of species. Although there is significant variability in some of the methods used to assess enzyme activities, four studies (Tolmasoff et al. 1980; Ono and Okada 1984; Lopez-Torres et al. 1993; Barja et al. 1994) have measured total tissue SOD activity using essentially the same approach. We pooled the data from these four datasets to obtain SOD activity data in relation to MLSP for 26 mammalian and avian species. For this analysis, we averaged all SOD data for a given species, regardless of tissue in which it was measured, into a single mean value. Analysis of these data, from the brain, heart, and liver, indicated no statistically significant (r2 = 0.20; P > 0.1) relationship between total SOD and either MLSP (Fig. 1a) or body mass (Fig. 1b). This general trend has seen reported for other antioxidant enzymes in some of the larger studies (e.g., Page et al. 2010; 14 species), suggesting that these enzymes are expressed constitutively. While it is possible that the true relationship between the expression of antioxidant genes and MLSP is sufficiently subtle to require an even larger sample to identify, it is interesting that the comparative data complement the conclusions from genetically modified mice, in which no consistent effect on lifespan has been associated with transgenic overexpression of various antioxidant enzymes (reviewed in Perez et al. 2009).
Fig. 1.
No correlation between average tissue superoxide dismutase (SOD) activity and a MLSP or b body mass in 26 mammalian and avian species. For each species (data point), a single mean SOD activity value was calculated from the heart, brain, and liver data reported in four studies (see main text)
Membrane composition
Several investigators have explored the hypothesis that organisms reduce the occurrence of oxidative damage by reducing the number of downstream targets of ROS in cellular membranes, an idea encapsulated by the “membrane pacemaker theory of aging” (e.g., Hulbert 2005). Studies addressing this hypothesis generally produce very large datasets for each individual species, and over a hundred of these data records have been included in CCMBL. The peroxidizability of membranes is described by the “peroxidation index” (PI), calculated using the formula:
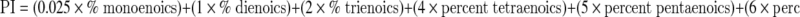 |
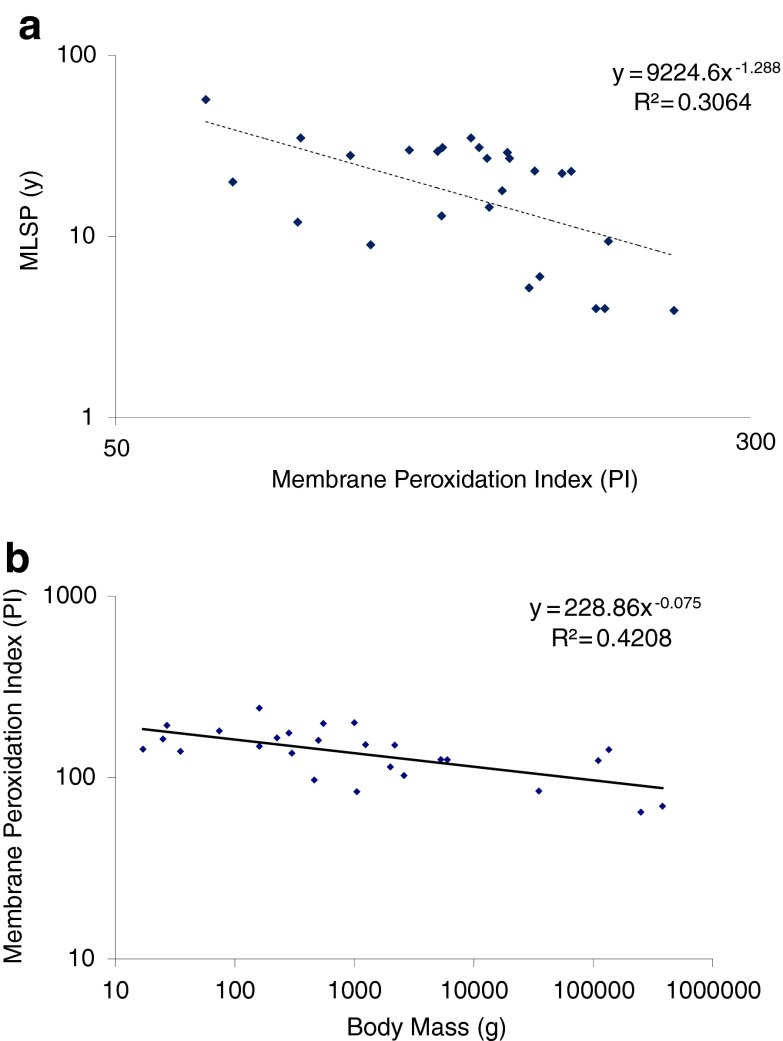
In many original papers, the PI was calculated and provided; where it was not, we have calculated it (if sufficient data were included to make it possible) and included it in the CCMBL. Interestingly, whereas some relatively small datasets support the hypothesis that longer-lived species have membrane phospholipid fatty acyl chain compositions more resistant to peroxidization (e.g., Pamplona et al. 2002; Hulbert 2008), the largest single comparative study of skeletal muscle (Valencak and Ruf 2007) showed no correlation between MLSP and membrane PI (P = 0.236). Since the hypothesis that membrane PI will correlate inversely with MLSP makes essentially the same prediction for any tissue, and for either total cellular or mitochondrial membranes, we pooled data from seven independent studies of 25 mammalian and avian species, merging membrane PI data from the brain, liver, and heart whole tissue or mitochondria into a single species membrane PI value. Figure 2a shows that the correlation between this mean PI value and species MLSP was also nonsignificant (r2 = 0.31; P > 0.5). Thus, as for muscle tissue (Valencak and Ruf 2007), the PI of nonmuscle tissue membranes appears not to be related to MLSP. Interestingly, the correlation between PI and body mass was significant (Fig. 2b; r2 = 0.42; P < 0.025).
Fig. 2.
Membrane phospholipid peroxidation index (PI) is not correlated with a MLSP but is correlated with b body mass. For each species (data point), a single mean PI value was calculated from the heart, brain, and liver whole tissue or mitochondrial PI data reported for 25 mammalian and avian species in seven independent studies (see main text and CCMBL database)
Protein homeostasis
The investigation of cellular proteome maintenance as a correlate of longevity is relatively recent, and so represented by fewer than 50 records in CCMBL. Salway et al. (2011b) measured the activities of enzymes involved in maintaining protein redox homeostasis in the brain, heart, and liver tissues of 15 mammalian and avian species with MLSPs ranging over an order of magnitude and found no evidence of a positive correlation between these activities and MLSP (Table 2). Interestingly, some of these activities were significantly and negatively correlated with species body mass, suggesting that their proportionality may be to cellular metabolic rates, which also declines with increasing body mass (e.g., Porter and Brand 1995). This illustrates again the importance of including body mass data in the database (Speakman 2005).
Table 2.
Correlation of protein homeostasis mechanisms with MLSP and/or body mass
| Trait | Tissue/cell type | Comparison | Correlation with MSLP | Correlation with body mass |
|---|---|---|---|---|
| 20S/26S Proteasome | Heart, brain, and liver | 15 Mammalian and avian species | No correlationa,b (Salway et al. 2011b) | No correlationa (heart and brain) |
| Negative correlationa (liver) | ||||
| Thioredoxin reductase | Heart, brain, and liver | 15 Mammalian and avian species | No correlationa,b (Salway et al. 2011a) | No correlationa (heart) |
| Negative correlationa (brain and liver) | ||||
| Glutaredoxin | Heart, brain, and liver | 15 Mammalian and avian species | No correlationa,b (heart and liver) | No correlationa (all tissues) |
| Negative correlation (brain) (Salway et al. 2011b) | ||||
| Heat shock protein 60 | Heart, brain, and liver | 12–13 Mammalian and avian species | Positive correlationa,b (heart, brain, and liver) (Salway et al. 2011a) | Unknown |
| Heat shock protein 70 | Heart, brain, and liver | 12–13 Mammalian and avian species | Positive Correlationa,b (heart, brain, and liver) (Salway et al. 2011a) | Unknown |
| GRP 78 | Heart, brain, and liver | 12–13 Mammalian and avian species | Positive Correlationa,b (heart, brain, and liver) (Salway et al. 2011a) | Unknown |
| GRP 94 | Heart, brain, and liver | 12–13 Mammalian and avian species | Positive correlationa,b (heart and liver) (Salway et al. 2011a) | Unknown |
aCorrected for phylogeny
bCorrected for body mass
Relative baseline (i.e., unstressed) levels of heat shock proteins (Hsps) are also included in the database. A recent study (Salway et al. 2011a) revealed several highly significant positive correlations of Hsps with longevity (Table 2), including mitochondrial (Hsp60), cytosolic (Hsp70), and endoplasmic reticulum (GRP78 and GRP94) were correlated with MLSP (Salway et al. 2011a). These data suggest that longer-lived organisms have a superior ability to maintain protein homeostasis specifically via protein chaperoning and refolding capacities. This intriguing result indicates a need for further study of the relationships between MLSP and protein homeostasis.
Genome homeostasis, hormones, and growth factors
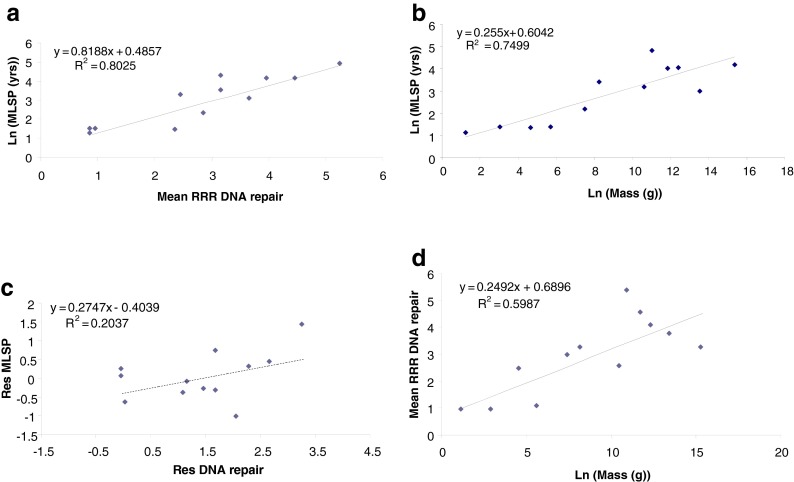
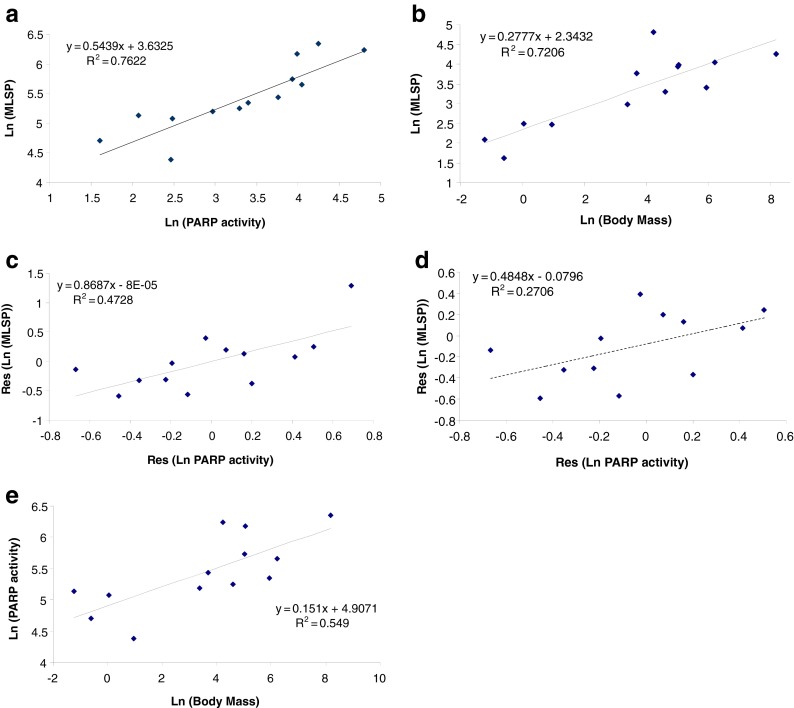
The largest individual data category, Genome Homeostasis, contains almost 250 data records, reflecting the fact that DNA damage in somatic cells has long been considered a major cause of aging (e.g., Gensler and Bernstein 1981). Comparative tests of the hypothesis that superior maintenance of genome integrity underlies increases in longevity within a clade began almost four decades ago, with seminal papers by Hart and Setlow (1974) and later others (e.g., Francis et al. 1981; Hall et al. 1984; Licastro and Walford 1985). These authors showed correlations between MLSP and repair of UV damage-induced lesions by nucleotide excision repair (NER) in dermal fibroblasts. Since then, positive correlations between species MLSP and other DNA repair activities, including poly(ADP-ribose) polymerase (PARP; e.g., Grube and Bürkle 1992) and double-strand break binding proteins (Lorenzini et al. 2009), have been demonstrated (Table 3). However, while these seem to confirm the hypothesis that the robustness of DNA repair is positively correlated with longevity, this interpretation may not be entirely correct. Kato et al. (1980) found no correlation between MLSP and DNA repair in a comparison of 34 species. Francis et al. (1981) suggested a correlation between MLSP and repair of DNA lesions by cultured skin fibroblasts, though in this study donor age was not controlled for. In any case, replotting their DNA repair data for fibroblasts (excluding non-fibroblast data) against the species MLSPs (from AnAge) used in CCMBL (and not the MLSPs provided by the authors) fails to show any significant correlation (r2 = 0.18; 17 species; analysis not shown). Promislow (1994) also showed that the apparent correlations of NER activity with MLSP reported by other authors were actually driven by relationships between NER and body mass. Similarly, reanalysis (Fig. 3) of the combined NER data of Cortopassi and Wang (1996) from five studies shows that an underlying correlation with body mass appears to drive much of the relationship between NER and MLSP. A residual analysis accounting for a contribution of body mass to the leukocyte PARP data (Grube and Bürkle 1992) also indicates that a significant correlation between body mass and PARP activity (Fig. 4) underlies much of the apparent relationship of PARP to MLSP. Thus, reanalysis of relatively large pooled datasets suggests that body mass is at least as strong a correlate of cellular DNA repair activities as is MLSP.
Table 3.
DNA damage and repair as correlates of MLSP and/or body mass
| Trait | Tissue/cell type | Comparison | Correlation with MSLP | Correlation with body mass |
|---|---|---|---|---|
| PARP activity | Mononuclear blood cells | 12 Mammalian species | Positive correlation? (Grube and Burkle 1992) | Positive (Fig. 4) |
| Excretion of DNA repair productions | Urine | 6 Mammalian species | Negative correlation (8oxoGua) | Positive (see Speakman 2005) |
| No correlation (8oxodF, 5-HMUra) (Foksinski et al. 2004) | ||||
| Rate of repair of UV-induced DNA lesions | Fibroblasts | 7 Mammalian species | Positive correlation? (Hart and Setlow 1974) | See combined data reanalysis (Fig. 3) |
| 12 Mammalian species | Positive correlation? (Cortopassi and Wang 1996) | Positive (Fig. 3) | ||
| 8 Primate species | Positive correlation? (Hall et al. 1984) | Unknown | ||
| 21 Mammalian species | Positive correlation? (Francis et al. 1981) | Unknown | ||
| BER | Fibroblasts | 8 Mammalian species | Negative correlation (polymerase beta); no correlation (APE) (Brown and Stuart 2007) | None |
| Double-strand break recognition | Fibroblasts | 13 Mammalian species | Positive correlationa,b (Lorenzini et al. 2009) | No correlationa (Lorenzini et al. 2009) |
aCorrected for phylogeny
bCorrected for body mass
Fig. 3.
The relative rate at which UV-induced lesions are repaired in dermal fibroblasts is correlated with MLSP in 12 mammalian species (a; P < 0.0001). MLSP is highly correlated (P < 0.0005) with adult body mass in the same species (b). The apparent correlation in (a) is considerably weakened (P < 0.1) when effects of body mass are removed in an analysis of residuals (c). The rate of UV-induced lesion repair is correlated (P < 0.05) with species adult body mass (d). Data are from Cortopassi and Wang (1996) and are available in the CCMBL database (http://genomics.brocku.ca/ccmbl/). RRR rat relative repair
Fig. 4.
Poly(ADP-ribose) polymerase (PARP) activity is positively correlated (P < 0.0005) with MLSP in 13 mammalian species (a). In the same species, body mass and MLSP are highly correlated (b; P < 0.001). Residual analysis of the data in (a) to remove the effects of body mass considerably weakens the correlation (c; P < 0.01). Omission of the single human data point in (c) further weakens the correlation (d; P < 0.05). PARP activity is correlated (P < 0.005) with adult body mass (e). Data are from Grube and Burkle (1992) and are available in the CCMBL database (http://genomics.brocku.ca/ccmbl/)
Correlations of DNA repair activities with body mass are actually predicted as a means of addressing the increased probability of developing cancer associated with greater body size. Across the spectrum of mammalian species, adult body mass ranges over a million-fold, with differences in size being attributable primarily to differences in the number of cells within an organism. Thus, the largest mammalian species have several million (or more) times more cells than the smallest. Although this increases the statistical likelihood that any single cell will become cancerous in larger species, the occurrence of cancer is actually quite constant across a range of species whose body masses cover several orders of magnitude (Peto’s paradox; reviewed in Leroi et al. 2003; Caulin and Maley 2011). Given these facts, one should expect between-species differences at the cellular and molecular levels, such as in the rate of mutation accumulation, or the strength of tumor-suppressing mechanisms. Thus, observations that the DNA repair capacity of actively mitotic somatic cells is correlated (positively) primarily with body mass are consistent with expectations: the selective pressures of body mass (body masses range over more than six orders of magnitude among vertebrate species) should be greater than those of lifespan (which range over about two orders of magnitude).
There is evidence that other tumor-suppressive mechanisms also correlate positively with body mass. Downregulation of telomerase activity, which limits the number of cell divisions, is observed in larger species at both the cellular (fibroblasts; Gomes et al. 2011) and tissue (Seluanov et al. 2007) levels (Table 4). In addition, the relative levels of insulin-like growth factor-1, to which many tumors are positively responsive, also correlate negatively with species adult body mass (Stuart and Page 2010). Other tumor-suppressor mechanisms may correlate with MLSP directly however. For example, Gomes et al. (2011) reported shorter telomeres (which would also limit cellular replicative lifespan unless fully opposed by telomerase) in longer-lived species, although two smaller studies did not find this (Seluanov et al. 2007 and Lorenzini et al. 2009). In summary, the majority of published comparative data linking tumor suppression mechanisms with species longevity appear to be actually driven by relationships with body mass. Analysis of larger datasets while accounting for the individual correlations of body mass and MLSP with tumor-suppressive traits is critical to understanding how evolution has solved the cancer problem.
Table 4.
Correlation of telomere length and telomerase activity with MLSP and/or body mass
| Trait | Tissue/cell type | Comparison | Correlation with MSLP | Correlation with body mass |
|---|---|---|---|---|
| Telomerase activity | 7 Tissues | 15 Rodent species | No correlationa,b (Seluanov et al. 2007) | Negative correlationa,b (Seluanov et al. 2007) |
| Fibroblasts | >60 Mammalian species | No correlationa,b (Gomes et al. 2011) | Negative correlationa,b (Gomes et al. 2011) | |
| Telomere length | Liver tissue | 15 Rodent species | No correlationa,b (Seluanov et al. 2007) | No correlationa,b (Seluanov et al. 2007) |
| Fibroblasts | >60 Mammalian species | Negative correlationa,b (Gomes et al. 2011) | No correlationa,b (Gomes et al. 2011) | |
| Fibroblasts | 10 Mammalian species | No correlationa,b (Lorenzini et al. 2009) | No correlationa,b (Lorenzini et al. 2009) | |
| Rate of telomere shortening | Erythrocytes | 5 Avian species | Positive correlation (Haussmann et al. 2003) | Unknown |
aMultiple regression analysis
bPhylogenic independent contrasts
An important caveat in virtually all comparative studies of DNA repair is that the vast majority of data comes from studies of fibroblasts or other cell monocultures. In contrast, there is virtually no information about DNA repair in highly oxidative cells and tissues as it relates to lifespan. The DNA repair requirements of actively mitotic cells, for example, are likely quite different than those of the primarily post-mitotic terminally differentiated cells comprising tissues like the heart and brain. Whereas in the former, reducing the possibility of transformation may be paramount, in the latter, the preservation of genomic integrity may focus on maintaining transcriptional fidelity. Page and Stuart (2011) measured DNA base excision repair enzyme activities in tissues from a wide range of mammals and birds, finding that that the polymerase β-like activity of brain and liver tissues was correlated (negatively) with body mass but not with MLSP. Interestingly, stable end products of DNA repair are also excreted in urine at rates that correlate significantly (and negatively) with body mass (based on data from Foksinski et al. 2004 against body mass; see Page and Stuart 2011). Together, these data are consistent with the interpretation that larger species with lower mass-specific metabolic rates actually repair DNA damage at slower rates than smaller species in the cells of large oxidative tissues like the brain and liver. These results suggest a model where the incidence of oxidative damage in large highly oxidative tissues is lower in larger species (fewer mitochondria per cell, perhaps less ROS produced per mitochondrion), and therefore, the rate of DNA repair required to counter oxidative damage and maintain genomic integrity is concomitantly also reduced.
Summary
Millions of years of evolution have resulted in thousands of individual mammalian and avian species with MLSPs ranging from a few years to over two centuries (documented in AnAge). This wealth of species and lifespan diversity provides an opportunity to discover mechanisms for increasing longevity. Measurements of putative longevity traits in the cells and tissues of species with very different lifespans can provide insight into which cellular and molecular mechanisms are, or are not, key to achieving greater longevity, which may in turn inform approaches to treating aging or age-related disorders in humans. There are, however, some significant limitations inherent in the comparative approach that are perhaps best addressed by analyzing the largest possible datasets while accounting for body mass effects. To help address these limitations, we introduce the CCMBL database as a tool for facilitating the comprehensive study of longevity evolution in vertebrates. The database builds on the success of AnAge (de Magalhães and Cost 2009), which has provided a convenient means to standardize the species MLSP and body mass data used in comparative studies. The use of phylogenetic independent contrasts (Felsenstein 1985; reviewed in Speakman 2005) or other phylogenetic comparative methods have not been addressed here, but these statistical analyses are also facilitated by the organization of data within CCMBL. Regular updating of the CCMBL (by the authors) will maintain the currency of the data while iteratively expanding the analytical power of the database. The CCMBL database should thus provide a comprehensive and current resource for analysis of the cellular and molecular biology of naturally evolved longevity.
References
- Barja G, Cadenas S, Rojas C, López-Torres M, Pérez-Campo R (1994) A decrease of free radical production near critical targets as a cause of maximum longevity in animals. Comp Biochem Physiol Biochem Mol Biol 108(4):501–512 [DOI] [PubMed]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MF, Stuart JA. Correlation of mitochondrial superoxide dismutase and DNA polymerase beta in mammalian dermal fibroblasts with species maximal lifespan. Mech Ageing Dev. 2007;128:696–705. doi: 10.1016/j.mad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Caulin AF, Maley CC. Peto’s Paradox: evolution’s prescription for cancer prevention. Trends Ecol Evol. 2011;26:175–182. doi: 10.1016/j.tree.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi GA, Wang E. There is substantial agreement among interspecies estimates of DNA repair activity. Mech Ageing Dev. 1996;91:211–218. doi: 10.1016/S0047-6374(96)01788-5. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22:D537–D543. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. doi: 10.1086/284325. [DOI] [Google Scholar]
- Foksinski M, Rozalski R, Guz J, Ruszkowska B, Sztukowska P, Piwowarski M, Klungland A, Olinski R. Urinary excretion of DNA repair products correlates with metabolic rates as well as with maximum life spans of different mammalian species. Free Radic Biol Med. 2004;37:1449–1454. doi: 10.1016/j.freeradbiomed.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Francis AA, Lee WH, Regan JD. The relationship of DNA excision repair of ultraviolet-induced lesions to the maximum life span of mammals. Mech Ageing Dev. 1981;16:181–189. doi: 10.1016/0047-6374(81)90094-4. [DOI] [PubMed] [Google Scholar]
- Gensler HL, Bernstein H. DNA damage as the primary cause of aging. Quart Rev Biol. 1981;56:270–303. doi: 10.1086/412317. [DOI] [PubMed] [Google Scholar]
- Gomes NMV, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, Austad SN, Venditti C, Pagel M, Shay JW, Wright WE (2011) Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10(5):761-8 [DOI] [PMC free article] [PubMed]
- Grube K, Burkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992;89:11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KY, Hart RW, Benirschke AK, Walford RL. Correlation between ultraviolet-induced DNA repair in primate lymphocytes and fibroblasts and species maximum achievable life span. Mech Ageing Dev. 1984;24:163–173. doi: 10.1016/0047-6374(84)90068-X. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J Exp Biol. 2011;214:1902–1910. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RW, Setlow RB. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974;71:2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, O’Reilly KM, Huntington CE, Nisbet ICT, Vleck CM. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc R Soc Lond B. 2003;270:1387–1392. doi: 10.1098/rspb.2003.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J Theor Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Explaining longevity of different animals: is membrane fatty acid composition the missing link? Age. 2008;30:89–97. doi: 10.1007/s11357-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/S0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Kato H, Harada M, Tsuchiya K, Moriwaki K. Absence of correlation between DNA repair in ultraviolet irradiated mammalian cells and lifespan of the donor species. Jpn J Genet. 1980;55:99–108. doi: 10.1266/jjg.55.99. [DOI] [Google Scholar]
- Leroi AM, Koufopanou V, Burt A. Cancer selection. Nat Rev Canc. 2003;3:226–231. doi: 10.1038/nrc1016. [DOI] [PubMed] [Google Scholar]
- Licastro F, Walford RL. Proliferative potential and DNA repair in lymphocytes from short-lived and long-lived strains of mice, relation to aging. Mech Ageing Dev. 1985;31:171–186. doi: 10.1016/S0047-6374(85)80028-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G (1993) Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev 70(3):177–199 [DOI] [PubMed]
- Lorenzini A, Johnson FB, Oliver A, Tresini M, Smith JS, Hdeib M, Sell C, Cristofalo VJ, Stamato TD. Significant correlation of species longevity with DNA double strand break recognition but not with telomere length. Mech Ageing Dev. 2009;130:784–792. doi: 10.1016/j.mad.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn CE, Carlberg K, Ottinger MA, Holmes DJ, Martin GM, Austad SN. Exceptional cellular resistance to oxidative damage in long-lived birds requires active gene expression. J Gerontol A Biol Sci Med Sci. 2001;56:B468–B474. doi: 10.1093/gerona/56.11.B468. [DOI] [PubMed] [Google Scholar]
- Ono T, Okada S. Unique increase of superoxide dismutase level in brains of long living mammals. Exp Gerontol. 1984;19:349–354. doi: 10.1016/0531-5565(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Page MM, Stuart JA (2011) Activities of DNA base excision repair enzymes in liver and brain correlate with body mass, but not lifespan. Age (in press) [DOI] [PMC free article] [PubMed]
- Page MM, Richardson J, Wiens BE, Tiedtke E, Peters CW, Faure PA, Burness G, Stuart JA. Antioxidant enzyme activities are not broadly correlated with longevity in 14 vertebrate endotherm species. Age. 2010;32:255–270. doi: 10.1007/s11357-010-9131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum lifespan—a homeoviscous-longevity adaptation? Ann NY Acad Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RK, Brand MD. Cellular oxygen consumption depends on body mass. Am J Physiol. 1995;269:R226–R228. doi: 10.1152/ajpregu.1995.269.1.R226. [DOI] [PubMed] [Google Scholar]
- Promislow DE (1994) DNA repair and the evolution of longevity: a critical analysis. J Theor Biol 170(3):291–300 [DOI] [PubMed]
- Salway KD, Gallagher EJ, Page MM, Stuart JA (2011a) Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev 132(6–7):287–297 [DOI] [PubMed]
- Salway KD, Page MM, Faure PA, Burness G, Stuart JA. Enhanced protein repair and recycling are not correlated with longevity in 15 vertebrate endotherm species. Age (Dordr) 2011;33(1):33–47. doi: 10.1007/s11357-010-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR. Correlations between physiology and lifespan—two widely ignored problems with comparative studies. Aging Cell. 2005;4:167–175. doi: 10.1111/j.1474-9726.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Page MM. Plasma IGF-1 is negatively correlated with body mass in a comparison of 36 mammalian species. Mech Ageing Dev. 2010;131:591–598. doi: 10.1016/j.mad.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Tolmasoff JM, Ono T, Cutler RG. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencak TG, Ruf T. N-3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell. 2007;6:15–25. doi: 10.1111/j.1474-9726.2006.00257.x. [DOI] [PubMed] [Google Scholar]