Abstract
Exercise-induced positive changes in skeletal muscle properties and metabolism decrease the risk for disability, cardiometabolic diseases and mortality. Here, we studied muscle properties and glucose homeostasis in a non-exercise stage in twin pairs with co-twins discordant for physical activity habits for at least 32 years of their adult lives. Isometric knee extension force, MR imaging of midthigh tissue composition and muscle volume, and fasting blood samples were acquired from 16 same-sex (seven monozygotic, nine dizygotic) middle-aged and older twin pairs. The consistently active twins had 20 % higher knee extension forces than their inactive co-twins (p = 0.006) although the active twins had only 4 % higher midthigh muscle cross-sectional areas (p = 0.072). These results were similar in intrapair analysis in which only the seven identical twin pairs were included. The ratio between the area of midthigh fat and muscle tissues was significantly lower among the active twins (0.65 vs. 0.48, p = 0.006). The active twins had also lower fasting plasma glucose levels (5.1 vs 5.6 mmol/l, p = 0.041). The area of midthigh intramuscular (extramyocellular) fat was associated with the markers of glucose homeostasis, especially with glycated hemoglobin, and these associations were emphasized by the diabetic and inactive twins. Regular exercise throughout the adult life retains muscle strength and quality but not necessarily mass. The regular use of muscles also prevents from the accumulation of intramuscular fat which might be related to maintained glucose metabolism and, thus, prevention of metabolic disorders.
Keywords: Exercise, Muscle force, Fat infiltration, Glycated hemoglobin, Glucose metabolism
Introduction
In humans, muscle tissue starts to decrease around the fifth decade of life. The loss of muscle mass is linked to loss of muscle fiber number and size, muscle strength, power, and endurance (Deschenes 2004; Faulkner et al. 2007). Regular physical activity can preserve as well as increase skeletal muscle mass and strength at all ages (PAGAC 2008). Maintenance of muscle properties has notable benefit for the prevention of age-related disability (Rantanen et al. 1999; Janssen 2006), and high muscle strength is associated with lower mortality (Newman et al. 2006; Ruiz et al. 2008). Moreover, exercise-induced positive changes in skeletal muscle structure, function, and metabolism are linked to delay in the progression of multiple diseases (Wolfe 2006; Kujala 2009).
Skeletal muscle volume accounts for the majority of glucose uptake. In individuals with type 2 diabetes mellitus, glucose uptake is reduced by the insulin resistance of skeletal muscle. Exercise training improves the skeletal muscle glucose transport system, but the effects are rather short-lived if they are not followed by another bout of exercise (Henriksen 2002; Boule et al. 2005; Hawley and Lessard 2008). Thus, regular physical activity offers a beneficial way to enhance glucose homeostasis also in insulin resistant muscle. Here, we studied the effects of long-term leisure-time physical activity vs. inactivity on skeletal muscle properties and glucose homeostasis in a non-exercise stage in middle-aged and older co-twins of twin pairs discordant for physical activity habits.
Methods
Subjects
Twin pairs discordant for physical activity were initially identified from the Finnish twin cohort (N = 5,663 healthy pairs) by self-reported physical activity assessments conducted in 1975 and 1981 (Kujala et al. 2002). All pairs who were discordant for physical activity in both assessments were selected for a retrospective physical activity follow-up in which their physical activity history was obtained for each 5-year period, from 2005 back to 1980, by means of a telephone interview (Waller et al. 2008). Recall was anchored to relevant life-events. Twin pairs with consistent discordance in physical activity habits throughout the follow-up were invited to participate in the TWINACTIVE study measurements held in 2007 (Leskinen et al. 2009a). Overall, 16 same-sex middle-aged and older twin pairs (seven monozygotic (MZ) and nine dizygotic (DZ) pairs, five female and 11 male pairs, age range 50–74 years) were able to participate in the measurements and were discordant for physical activity at every time point at which their physical activity habits were assessed. The baseline was set to year 1975 when the physical activity discordance was detected for the first time. Thus, overall, the 16 twin pairs were documented of having continuous physical activity discordance for 32 years of their adult lives. The follow-up’s mean metabolic equivalence task (MET) difference, that is, the average difference in leisure-time activity habits between the inactive and active co-twins, was 8.8 MET h/day (2.2 ± 2.3 vs. 11.0 ± 4.1 MET h/day, p < 0.001, respectively). This is the equivalent of, for example, the exercise volume of a 2-h walk. The most common types of physical activity engaged in by the active twins during the previous 12 months were walking (30 % of total physical activity volume), jogging (11 %), and cross-country skiing (9 %). At the end of the follow-up, the co-twins did not differ in work-related physical activity and alcohol or tobacco use, and they had only minimal difference in their daily energy intake (Leskinen et al. 2009a; Rintala et al. 2011).
Physical activity assessment
Discordance for physical activity was defined on the basis of a series of structured questions on leisure-time activity and physical activity during journeys to and from work. Leisure-time activity was quantified as metabolic equivalent units (average intensity of activity (MET) × average duration of one session (in hours) × (monthly frequency / 30 days)) and expressed as the sum score of leisure-time MET hours per day (Kujala et al. 1998; Waller et al. 2008; Leskinen et al. 2009a).
Muscle force measurements
Maximal isometric left knee extensor force was measured in a sitting position using an adjustable dynamometer chair (Good Strength, Metitur, Palokka, Finland) as described earlier (Sipilä et al. 1996). Briefly, the left knee was set at an angle of 60° from full extension. Overall, four maximal efforts with a 30-s pause between each were conducted. The best performance was accepted as a result. In addition to maximal force, the maximal rate of force development over an interval of 10 ms was recorded. Knee extensor force was measured in 27 co-twins (13 complete pairs). Co-twins with multiple diseases (n = 2), long-standing diabetes (n = 2), and polio (n = 1) were excluded. Left maximal handgrip force was measured with the elbow flexed at 90° using the same adjustable dynamometer chair and the same protocol as described above. Left hand grip force was measured in 31 co-twins (15 complete pairs), excluding the co-twin with polio affecting the left hand.
Midthigh muscle tissue composition acquisition
Nine axial T1-weighted MR images of 10-mm thickness and with a 20-mm slice interval were acquired from the left midthigh using 1.5 T GE-Signa Exite HD CVi with a matrix of 384 × 256, field of view 40 × 28 cm, and gradient echo sequence with TR/TE 550/15.2 ms (FSE-XL PulsSeg). The midslice (fifth image) was positioned at the midpoint lengthwise of the left femur. The midpoint of the femur was skin-marked using the greater trochanter and join line of the knee as anatomical landmarks. The midslice was used for the detailed analyses of the total midthigh cross-sectional area, muscle cross-sectional area, knee extensor (musculus quadriceps femoris) cross-sectional area, and the intramuscular (extramyocellular) and subcutaneous fat areas. Segmentation was made manually using OsiriX software (OsiriX Foundation, Geneva, Switzerland). Muscle tissue was detected by setting a density threshold and using the automatic segmentation parameters. The midthigh volume was calculated from the part of the thigh which all nine slices covered (lengthwise 16 cm) using automatic volume calculation. Fifteen complete pairs were included in the MR analysis because one co-twin was not imaged.
Blood studies
Ten-hour fasting plasma samples were collected by venipuncture after 10 min of supine rest. Plasma glucose was determined using Biosen C-line (EKF-diagnostic, Magdeburg, Germany) and serum insulin by IMMULITE® 1000 Analyzer (Siemens Medical Solution Diagnostics, Los Angeles, CA, USA). The HOMA index was calculated using the formula: (fasting plasma glucose × fasting plasma insulin) / 22.5 (Muniyappa et al. 2008). Glycated hemoglobin (HbA1C) was analyzed using HPLC Variant II (Bio-Rad Laboratories, Munich, Germany). Subjects were advised not to exercise vigorously (except for walking and other routine activities) during the 2 days before their laboratory visit as we were investigating long-term adaptations to exercise.
Other measures
Body composition was determined after an overnight fast using an InBody (720) (Biospace, Korea) eight-point tactile electrode multifrequency impedance plethysmograph body composition analyzer. We also administered symptom-limited maximal clinical exercise tests with a cycle ergometer and estimated VO2peak from the highest load achieved in the test. The zygosity of the co-twins was verified at the Paternity Testing Laboratory (National Public Health Institute, Helsinki, Finland) using DNA extracted from a venous blood sample with a battery of ten highly polymorphic gene markers.
Ethics
The TWINACTIVE study was conducted according to the guidelines for good clinical and scientific practice laid down by the Declaration of Helsinki. The study was approved by the Ethics Committee of the Central Finland Health Care District, and all the participants gave their written informed consent.
Data analysis
Pairwise analyses were used to study differences between the co-twins of the twin pairs. Normality of the means was assessed with Shapiro–Wilk test. Student’s paired t test was used for normally distributed data and the Wilcoxon matched-pair signed-rank test for non-normally distributed data. Ninety-five percent confidence intervals were calculated for the absolute mean differences between the inactive and active co-twins. By studying same-sex twin pairs, all the pairwise analyses were age- and sex-adjusted. The Pearson correlation coefficient was used for the correlation analyses. When calculating individual-based coefficients of determination (R2), the within-pair dependency of twin individuals was taken into account using the cluster option of Stata (svy:regress) (Williams 2000). The level of significance was set at p < 0.05. Data were analyzed using IBM SPSS Statistics 19 and Stata 8.0 software.
Results
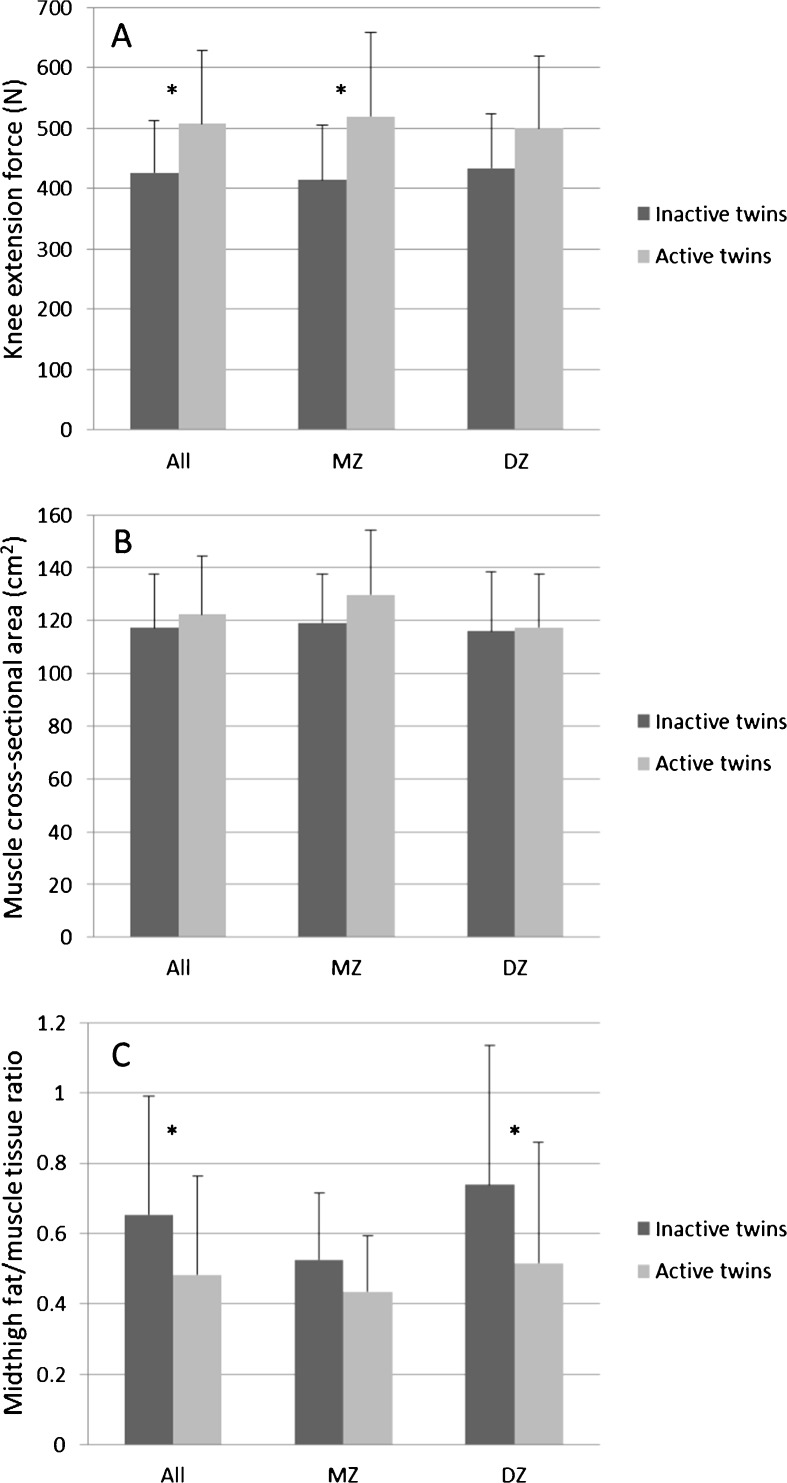
At the end of the follow-up, the active twins were more active, more fit, and leaner than their inactive co-twins (Table 1). The maximal knee extension force was 20 % higher among the active twins (p = 0.006). This finding was consistent for the MZ and DZ pairs (Fig. 1a). The rate of force development was rather similar among the co-twins (pairwise analysis, p = 0.71). No differences in hand grip forces were found (inactive twins, 422.4 N (SD 156.1) vs. active co-twins, 428.1 N (SD 126.5), p = 0.72). The difference in midthigh muscle cross-sectional area between the co-twins was nonsignificant (Fig. 1b), but the active twins had substantially lower fat-to-muscle tissue ratio within their midthighs (Fig. 1c). There was a rather small difference in midthigh muscle volumes between the co-twins (pairwise analysis, p = 0.28) (Table 2).
Table 1.
Baseline and follow-up characteristics in twin pairs discordant for physical activity
| Characteristic | Inactive N = 16 | Active N = 16 | P value |
|---|---|---|---|
| Baseline 1975 | |||
| Sex (n, female:male) | 5:11 | ||
| Age (years) | 28 (range 18–42) | ||
| Leisure-time MET indexa (MET h/day) | 0.2 ± 0.3 | 3.3 ± 2.4 | <0.001 |
| Body heighta (cm) (n = 15) | 173.7 ± 9.8 | 172.9 ± 10.1 | 0.96 |
| Body weighta (kg) | 69.3 ± 16.4 | 66.0 ± 9.4 | 0.57 |
| BMI (kg/m2) (n = 15) | 23.0 ± 4.2 | 22.3 ± 2.0 | 0.88 |
| Follow-up end point 2007 (measured) | |||
| Age (years) | 60 (range 50-74) | ||
| Leisure-time MET index (MET h/day) | 1.6 ± 1.4 | 8.4 ± 4.1 | <0.001b |
| Estimated VO2peak (ml/kg/min) | 26.4 ± 4.9 | 32.5 ± 5.5 | <0.001 |
| Knee extension force (N) (n = 13 pairs) | 425.8 ± 87.3 | 507.8 ± 121.4 | 0.006b |
| Body height (cm) | 171.8 ± 10.4 | 171.1 ± 9.9 | 0.39 |
| Body weight (kg) | 79.5 ± 18.4 | 72.9 ± 11.9 | 0.121 |
| BMI (kg/m2) | 26.7 ± 3.5 | 24.8 ± 2.6 | 0.09 |
| Total body fat percent (%) | 27.0 ± 5.3 | 21.5 ± 6.4 | 0.004b |
| Total body fat mass (kg) | 21.6 ± 7.7 | 15.6 ± 5.0 | 0.015 |
| Total body fat-free mass (kg) | 57.7 ± 12.4 | 57.2 ± 11.1 | 1.00 |
| Fasting plasma glucosec (mmol/l) | 5.6 ± 1.5 | 5.1 ± 1.0 | 0.041 |
| Fasting serum insulinc (μlU/ml) | 10.9 ± 4.8 | 10.6 ± 12.2 | 0.18 |
| HOMA indexc | 2.9 ± 2.2 | 2.6 ± 3.0 | 0.18 |
| HbA1Cc (%) | 5.9d ± 0.6 | 5.7e ± 0.4 | 0.22 |
| Diagnosed type 2 diabetes | 3 | 1 | 0.16f |
| Impaired fasting plasma glucose | 1 | 1 | 1.00f |
Values are means ± SD or frequencies. P values from Wilcoxon matched-pair signed-rank test
BMI body mass index, HOMA the homeostatic model assessment, HbA1C glycated hemoglobin
aSelf-reported
bP value from paired t test (normal distribution)
cDiabetic twins included
d41 mmol/mol
e39 mmol/mol
fSymmetry test, Stata
Fig. 1.
a Knee extensor force, b muscle cross-sectional area, c midthigh fat-to-muscle tissue ratio among inactive and active members for all, MZ, and DZ pairs. Values are means ± SD. The asterisks indicate p < 0.05
Table 2.
Left midthigh muscle properties in twin pairs discordant for physical activity
| Variable | Inactive N = 15 | Active N = 15 | Mean Diff. | 95 % CI | P value |
|---|---|---|---|---|---|
| Total midthigh cross-sectional area (cm2) | 196.2 ± 33.5 | 183.7 ± 22.6 | 12.5 | −6.0 to 31.0 | 0.17 |
| Muscle cross-sectional areaa (cm2) | 117.2 ± 20.5 | 122.4 ± 22.1 | −5.2 | −10.9 to 0.5 | 0.072 |
| Quadriceps Femoris areab (cm2) | 58.7 ± 11.0 | 60.3 ± 11.0 | −1.7 | −4.2 to 0.9 | 0.19 |
| Midthigh fat/muscle ratio | 0.65 ± 0.34 | 0.48 ± 0.28 | 0.17 | 0.04 to 0.30 | 0.006c |
| Muscle volume, left midthigha (dm3) | 1.88 ± 0.38 | 1.94 ± 0.38 | −0.06 | −0.16 to 0.05 | 0.28 |
Values are means ± SD. P values from paired t test
95 % CI 95 % confidence interval for the mean difference
aBone and bone marrow area and intramuscular fat area excluded
bIntramuscular fat included (manual delineation of the muscle group)
cP value from Wilcoxon matched-pair signed-rank test (non-normal distribution)
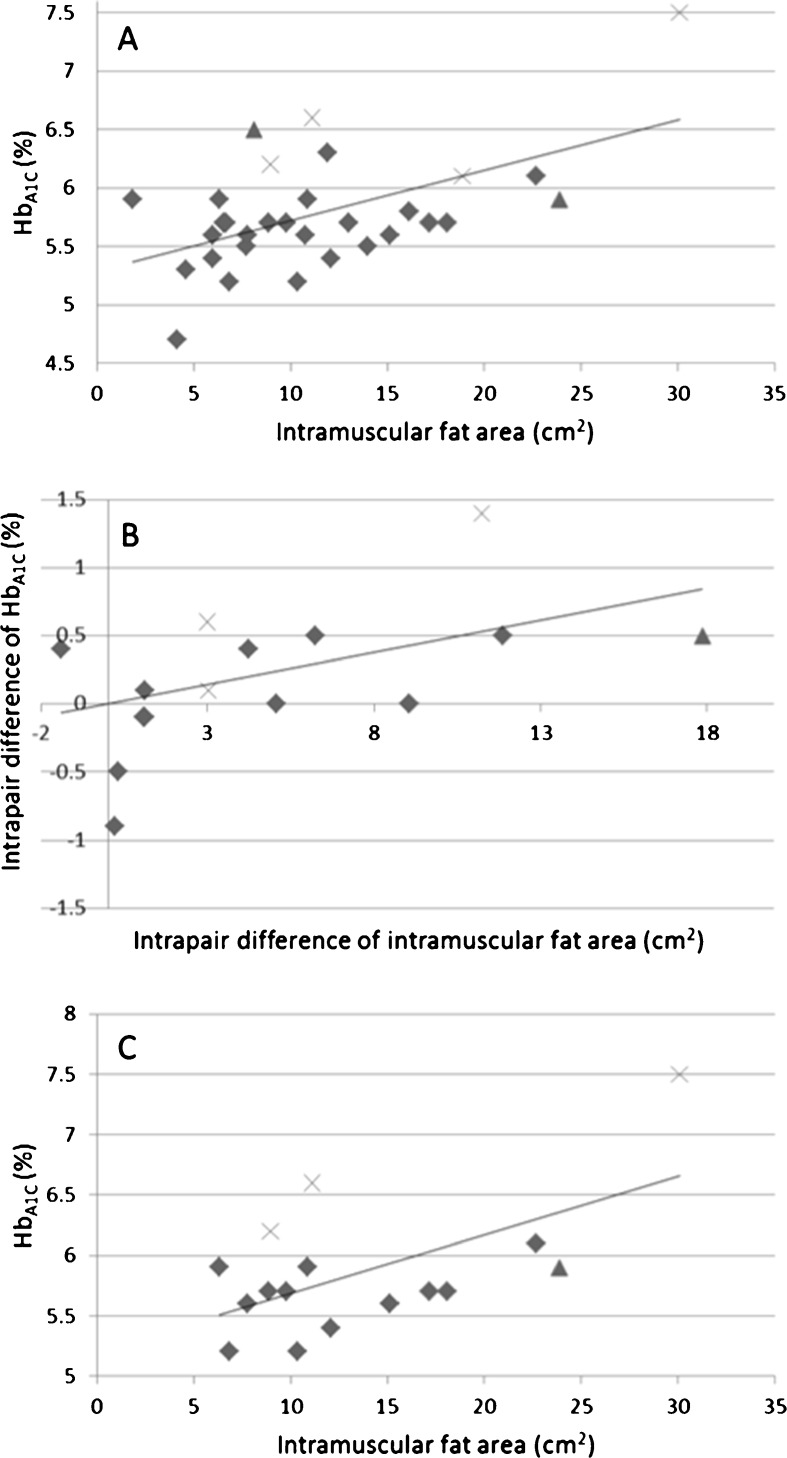
Fasting plasma glucose levels were higher among the inactive co-twins (5.6 vs. 5.1 mmol/l, p = 0.041) and the difference remained significant when the diabetic co-twins (three inactive and one active) were excluded from the pairwise analysis (5.3 vs. 4.8 mmol/l, p = 0.012, n = 13 pairs). The remaining differences in the markers of glucose homeostasis between the co-twins were nonsignificant (see Table 1). We found high associations between the intramuscular (extramyocellular) fat area per se and the markers of glucose homeostasis (R2 = 0.22–0.30), although after excluding the diabetic twins (n = 4), the relationships were more modest (R2 = 0.08–0.10). Furthermore, the inactivity seemed to highlight the association as the correlations coefficients between intramuscular fat and the markers of glucose homeostasis were higher among the inactive twins (r = 0.59–0.69, n = 16) compared to active twins (r = 0.30–0.39, n = 15). The most constant association was found between the intramuscular fat area and HbA1C (R2 = 0.30, p = 0.03, n = 31 twins; R2 = 0.10, p = 0.06, n = 27 non-diabetic twins) (Fig. 2a). This association was also found to be high in the intrapair difference correlation analysis (r = 0.55, p = 0.03, n = 15 pairs; r = 0.54, p = 0.07, n = 12 non-diabetic pairs) (Fig. 2b). The correlation coefficient between intramuscular fat area and HbA1C among the inactive twins was 0.591 (p = 0.016, n = 16) (Fig. 2c).
Fig. 2.
a Individual-based association between intramuscular fat area and HbA1C (r = 0.54, p = 0.03, n = 31 twins), b correlation between the intrapair difference of intramuscular fat area and HbA1C (r = 0.055, p = 0.03, n = 15 pairs), c association between intramuscular fat area and HbA1C among inactive twins (r = 0.59, p = 0.016, n = 16 twins). Twins having diagnosed type 2 diabetes (marked with a multiplication sign); twins having screen detected impaired fasting plasma glucose (marked with black triangle)
Discussion
Physical activity seems to be an efficient way to slow down the loss of muscle strength and muscle atrophy in middle to old age (Sipilä and Suominen 1995; Sipilä et al. 1996; Tarpenning et al. 2004; Goodpaster et al. 2008). Our results add to the existing findings by suggesting that long-term physical activity preserves muscle strength independently of genes (Tiainen et al. 2004). However, we found only rather small intrapair differences in midthigh muscle volumes (intraclass correlation of 0.93 in the 15 pairs) between the co-twins. This is consistent with the idea that genes make a major contribution to body structure and muscle function (Nguyen et al. 1998; Tiainen et al. 2008). Moreover, this finding only reflects the fact that aerobic-type training (e.g., walking and jogging) does not affect lower limb muscle mass or handgrip strength. The difference observed in knee extensor force between co-twins could therefore be explained by a better muscle force-producing system, including neural factors, and by the better muscle quality among the active twins (Tarpenning et al. 2004; Faulkner et al. 2007; Frontera et al. 2008). Hence, exercise-induced retention of muscle strength rather than that of muscle mass could be the key factor in the maintenance of muscle function to avoid age-related mobility limitations (Hughes et al. 2001; Visser et al. 2005; Goodpaster et al. 2006; Newman et al. 2006; Delmonico et al. 2009). Furthermore, regular physical activity is an effective way to slow down the age- and/or inactivity-related infiltration of muscle fat (Goodpaster et al. 2008; Delmonico et al. 2009; Leskinen et al. 2009b). However, the role of muscle fat infiltration in strength loss, muscle weakness, and mobility limitations needs further study (Visser et al. 2005; Goodpaster et al. 2008; Delmonico et al. 2009).
Regular physical activity maintains glucose homeostasis (Henriksen 2002; Boule et al. 2005; Petersen and Shulman 2006; Church et al. 2010; Mikus et al. 2012). Moderate-intensity exercise decreases the risk for type 2 diabetes mellitus (Henriksen 2002; Jeon et al. 2007; PAGAC 2008), even at low levels of activity (<150 min/week) (Waller et al. 2010). Disuse of muscles (due to inactivity) decreases metabolic flexibility and induces lipotoxity, that is, muscles start to fill with fat (Eckardt et al. 2011). This muscle fat infiltration is frequently linked to the insulin resistance (Taube et al. 2009), excluding the findings from endurance athletes who may have high amounts of intramyocellular fat in their insulin sensitive muscles (Goodpaster et al. 2001). In this study, we found the plasma glucose levels to be significantly higher among the persistently inactive co-twins (Table 1). The markers of glucose homeostasis were correlated with the amount of infiltrated fat, and the associations were emphasized when the diabetic and inactive twins were included. Our correlation analysis highlighted the relationship between the amount of intramuscular (extramyocellular) ectopic fat and the level of HbA1C, as shown in Fig. 2. HbA1C, which is the measure of long-term plasma glucose concentration, is one of the mediating factors by which exercise decreases the risk for cardiovascular events (Thomas et al. 2006; Mora et al. 2007; Church et al. 2010). However, the link between the intramuscular lipid accumulation and insulin resistance is not yet fully understood (Goodpaster et al. 2001; Goodpaster and Brown 2005; Dube et al. 2008; Hawley and Lessard 2008; Taube et al. 2009; Eckardt et al. 2011). One of the suggestions for the association could be the reduced aerobic capacity among the inactive co-twins (Leskinen et al. 2010). However, although the number of mitochondria might be reduced in an insulin resistant skeletal muscle, the mitochondrial respiratory capacity can be maintained normal (Larsen et al. 2011).
As such, the accumulation of ectopic “high-risk” fat and adipocyte function are important mechanisms in mediating the prolonged effects of life-long regular exercise on glucose metabolism (Goodpaster et al. 2000; Boule et al. 2005; Petersen and Shulman 2006; Dube et al. 2008; Leskinen et al. 2009b; Taube et al. 2009; Rector and Thyfault 2011). Notably, the co-twins did not differ significantly in either body weight or BMI (see Table 1), and there were no differences in their fat intakes (Rintala et al. 2011), yet the inactive co-twins had significantly more visceral, intramuscular, and liver fat, as we have reported earlier (Leskinen et al. 2009b). Therefore, we can also conclude that the disadvantage of prolonged inactivity is not necessarily seen in body weight status but rather in specific risk factors of metabolic deterioration (Patel et al. 2011).
Our study included an extensive follow-up of leisure-time physical activity habits during adult life (from the thirties to sixties). The baseline was the year 1975, when the first physical activity data were available, but it is probable that the discordance in physical activity had begun earlier. We used structured physical activity questionnaire which has shown high reliability in previous studies (Kujala et al. 1998; Kujala et al. 2002; Waller et al. 2008; Leskinen et al. 2009a). Although we used validated methods to document leisure-time physical activity, subject’s understanding on what leisure-time physical activity is may have changed over time. However, all of our physical activity assessments clearly differentiated inactive and active co-twins in the intrapair analyses. Due to our strict criteria for the physical activity discordance, we ended up with rather a small sample size. However, we are dealing with very unique phenomenon. Despite the fact that different persistent activity levels are common, it is less common that co-twins of a twin pair have persistently different activity levels. According to the classic twin design, MZ co-twins are genetically identical at the sequence level, and the intrapair differences (discordance) in MZ twins are due to environmental factors, taken very broadly. Therefore, we were also able to control for shared genes and childhood environment that may confound the traits studied.
In conclusion, our co-twin control study showed that long-term physical activity preserves knee extensor muscle strength. This finding underlines the role of voluntary, mostly aerobic type of physical activity in maintaining muscle quality, but not necessarily mass. These results were similar in intrapair analysis in which only the seven identical twin pairs were included. Regular exercise, namely, use of muscles, prevents also from the accumulation of intramuscular fat which might be related to maintained glucose metabolism and, thus, prevention of metabolic disorders.
Acknowledgments
We acknowledge support from the EC FP7 Collaborative Project MYOAGE (GA-223576). The TWINACTIVE study was supported by the Academy of Finland (Grant 114 866 and Centre of Excellence in Complex Disease Genetics, (grant numbers: 213506, 129680)) and Finnish Ministry of Education. Tuija Leskinen was supported by the Finnish Cultural Foundation, Juho Vainio Foundation, and Yrjö Jahnsson Foundation. Dr Urho Kujala was supported by the Juho Vainio Foundation.
References
- Boule NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of exercise training on glucose homeostasis. The HERITAGE family study. Diabetes Care. 2005;28:108–114. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes. A randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- Dube JJ, Amati F, Stefanovic-Racic M, Toledo FGS, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12:163–172. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Brown NF. Skeletal muscle lipid and its association with insulin resistance: what is the role for exercise? Exerc Sport Sci Rev. 2005;33:150–154. doi: 10.1097/00003677-200507000-00008. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jc.86.12.5755. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol. 2008;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity and health. J Gerontol. 2001;56A:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med. 2009;43:550–555. doi: 10.1136/bjsm.2009.059808. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;156:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA. 1998;279:2440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Andersen JL, Madsbad S, Worm D, Helge JW, Dela F. Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia. 2011;54:1427–1436. doi: 10.1007/s00125-011-2098-4. [DOI] [PubMed] [Google Scholar]
- Leskinen T, Waller K, Mutikainen S, Aaltonen S, Ronkainen PHA, Alen M, Sipilä S, Kovanen V, Perhonen M, Pietiläinen KH, Cheng S, Suominen H, Kainulainen H, Kaprio J, Kujala UM. Effects of 32-year leisure time physical activity discordance in twin pairs on health (TWINACTIVE study): aims, design and results for physical fitness. Twin Res Hum Genet. 2009;12:108–117. doi: 10.1375/twin.12.1.108. [DOI] [PubMed] [Google Scholar]
- Leskinen T, Sipilä S, Alen M, Cheng S, Pietiläinen KH, Usenius JP, Suominen H, Kovanen V, Kainulainen H, Kaprio J, Kujala UM. Leisure-time physical activity and high-risk fat: a longitudinal population-based twin study. Int J Obes (Lond) 2009;33:1211–1218. doi: 10.1038/ijo.2009.170. [DOI] [PubMed] [Google Scholar]
- Leskinen T, Rinnankoski-Tuikka R, Rintala M, Seppanen-Laakso T, Pöllänen E, Alen M, Sipilä S, Kaprio J, Kovanen V, Rahkila P, Oresic M, Kainulainen H, Kujala UM. Physically active lifestyle, muscle properties and glucose metabolism. PLoS One. 2010;5(9):e12609. doi: 10.1371/journal.pone.0012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012;44:225–231. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritshevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass is associated with mortality in the health, aging and body composition study cohort. J Gerontol A: Biol Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol. 1998;147:2–16. doi: 10.1093/oxfordjournals.aje.a009362. [DOI] [PubMed] [Google Scholar]
- Patel MJ, Slentz CA, Kraus WE. Metabolic deterioration of the sedentary control group in clinical trials. J Appl Physiol. 2011;111:1211–1217. doi: 10.1152/japplphysiol.00421.2011. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:10S–16S. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical activity guidelines advisory committee report. Washington: Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP. Does inactivity cause nonalcoholic fatty liver disease? J Appl Physiol. 2011;111:1828–1835. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- Rintala M, Lyytikäinen A, Leskinen T, Alen M, Pietiläinen KH, Kaprio J, Kujala UM. Leisure-time physical activity and nutrition: a twin study. Pub Health Nutr. 2011;14:846–852. doi: 10.1017/S136898001000090X. [DOI] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjöström M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:92–95. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand. 1996;156:457–464. doi: 10.1046/j.1365-201X.1996.461177000.x. [DOI] [PubMed] [Google Scholar]
- Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–340. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- Tarpenning KM, Hamilton-Wessler M, Wiswell RA, Hawkins SA. Endurance training delays age of decline in leg strength and muscle morphology. Med Sci Sports Exerc. 2004;36:74–78. doi: 10.1249/01.MSS.0000106179.73735.A6. [DOI] [PubMed] [Google Scholar]
- Taube A, Eckardt K, Eckel J. Role of lipid-derived mediators in skeletal muscle insulin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1004–E1012. doi: 10.1152/ajpendo.00241.2009. [DOI] [PubMed] [Google Scholar]
- Thomas D, Elliot EJ, Naughton GA (2006) Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 3:CD002968 [DOI] [PMC free article] [PubMed]
- Tiainen K, Perola M, Kovanen VM, Sipilä S, Tuononen KA, Rikalainen K, Kauppinen MA, Widen EI, Kaprio J, Rantanen T, Kujala UM. Genetics of maximal walking speed and skeletal muscle characteristics in older women. Twin Res Hum Genet. 2008;11:321–334. doi: 10.1375/twin.11.3.321. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Sipila S, Alen M, Heikkinen E, Kaprio J, Koskenvuo M, Tolvanen A, Pajala S, Rantanen T. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004;96:173–180. doi: 10.1152/japplphysiol.00200.2003. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 2008;32:353–361. doi: 10.1038/sj.ijo.0803692. [DOI] [PubMed] [Google Scholar]
- Waller K, Kaprio J, Lehtovirta M, Siventoinen K, Koskenvuo M, Kujala UM. Leisure-time physical activity and type 2 diabetes during a 28-year follow-up in twins. Diabetologia. 2010;53:2531–2537. doi: 10.1007/s00125-010-1875-9. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341X.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]