Abstract
Unexpected changes during gait challenge elderly individuals to a greater degree than young adults. However, the adaptive potential of elderly seems to be retained, and therefore, the training of the mechanisms of dynamic stability as well as muscle strength training may improve the dynamic stability after unexpected perturbations. Thirty-eight subjects (65–75 years) participated in the study, divided into two experimental groups (stability training group, ST, n = 14 and mixed training group, MT, n = 14) and a control group (CG, n = 10). Both experimental groups performed exercises which focused on the mechanisms of dynamic stability. Additionally, the MT group executed a training to improve muscle strength. Session volume and duration were equal for both groups (14 weeks, twice a week, ~1.5 h per session). Pre- and post-intervention, subjects performed a gait protocol with an induced unexpected perturbation. Post-intervention, the margin of stability was significantly increased after the unexpected perturbation in the ST group, indicating an improvement in stability state (pre, −30.3 ± 5.9 cm; post, −24.1 ± 5.2 cm). Further, both intervention groups increased their base of support after the intervention to regain balance after gait perturbation, whereas only the ST group showed a statistically significant improvement (STpre, 90.9 ± 6.6 cm, STpost, 98.2 ± 8.5 cm; MTpre, 91.4 ± 6.2 cm; MTpost, 97.9 ± 12.7 cm). The CG showed no differences between pre- and post-measurements. The exercise of the mechanisms of dynamic stability led to a better application of these mechanisms after an unexpected perturbation during gait. We suggest that the repeated exercise of the mechanisms of dynamic stability contributes to significant improvements in postural stability. Additional strength training for healthy elderly individuals, however, shows no further effect on the ability to recover balance after unexpected perturbations during gait.
Keywords: Dynamic stability, Intervention, Unexpected perturbation, Aging, Recovery performance
Introduction
Human biped locomotion represents a major challenge to the postural system, especially in the elderly, whereas postural stability constitutes the required ability to maintain equilibrium under various static and dynamic conditions (Horak 2006; Shumway-Cook and Woollacott 2006). Individuals have increasing problems with balance and tend to fall more often as they grow older (Lord et al. 1993). Therefore, fall-related injuries in the elderly population are an important economic as well as social problem (Blake et al. 1988; Tinetti et al. 1988; Etman et al. 2012). In Germany, for example, hip fractures of nursing home residents are estimated to cost about 8,160 Euros each, and the overall costs of falls amongst the elderly account for about 2.1–3.4 billion Euros per year (Heinrich et al. 2012).
There are several factors which contribute to an increased fall risk in the elderly population; one of those factors is the experience of unexpected perturbations. Elderly individuals are less able to handle sudden, unexpected changes (Pijnappels et al. 2005; Karamanidis and Arampatzis 2007; Bierbaum et al. 2010). Reasons for the decreased ability to recover balance are the age-related reduction in muscle strength and tendon stiffness (Grabiner et al. 2005; Karamanidis et al. 2008), the delayed generation of propulsive ground reaction forces and joint torques (Robinovitch et al. 2002; Pijnappels et al. 2005; Tseng et al. 2009), and the lower muscular contraction velocities (Hortobágyi et al. 1995; Thelen et al. 1997). If the postural system is unstable, stepping strategies seem to be less successful for the elderly due to reductions in step length and speed (Wojcik et al. 2001; Karamanidis et al. 2008; Bierbaum et al. 2010). Furthermore, decreased recovery performance is attributed to deficits in the application of the mechanisms of dynamic stability (Arampatzis et al. 2008).
However, the control of dynamic stability during locomotion is not a fixed process, but it can adapt to different situations and be modified through exercise. Earlier studies have shown that elderly participants are able to adapt to repeated locomotor perturbations in a predictive as well as in a reactive manner (Bhatt et al. 2006; Heiden et al. 2006; Bierbaum et al. 2010, 2011). These studies demonstrate that locomotor adaptability is still preserved in the elderly. However, although the predictive adjustments of the elderly participants during gait are similar to that of younger ones (Bierbaum et al. 2010), elderly participants showed a somewhat lower adaptive reactive potential after unexpected perturbations (Bierbaum et al. 2011). Therefore, we conclude that the main deficits of stability performance in elderly are the reactive responses after unexpected perturbations during walking (Pijnappels et al. 2005; Bierbaum et al. 2010, 2011). The adaptation potential of the old adults is very important in fall prevention because adequate interventions, aiming to improve the stability performance in the elderly population, may reduce the risk of falls. From a practical point of view, it is impossible for individuals to identify and practice all possible specific perturbations which could lead to falls during daily life. Therefore, it is important to identify underlying mechanisms of dynamic stability which are relevant for the maintenance of the postural stability after gait perturbations. A more general exercise intervention including those mechanisms could allow elderly people to apply the mechanisms in different situations and, therefore, help them to regain balance.
From a biomechanical point of view, there are three mechanisms responsible for maintaining postural stability after perturbations: (a) increase in the base of support, (b) counter-rotating segments around the center of mass (CoM), and (c) the application of an external force (not the ground reaction force) (Hof et al. 2005). A recent publication of our group (Arampatzis et al. 2011) showed that exercise of the mechanisms responsible for dynamic stability increased the stability performance of elderly adults (~30 %) after a simulated forward fall. However, most falls in the elderly population happen during walking (Rubenstein 2006). To the best of our knowledge, no systematic study has been performed to examine the effect of training of the mechanisms of dynamic stability on the recovery performance after an unexpected perturbation during walking in elderly adults. Therefore, elderly participants were trained with a training protocol of various exercise tasks, which integrated the mechanisms responsible for dynamic stability (see Arampatzis et al. 2011). We hypothesized that the exercise of the mechanisms of dynamic stability would contribute to an improvement of the stability performance after an unexpected gait perturbation in the elderly, indicating a transfer of the intervention into diverse situations. However, systematic reviews about the impact of intervention programs on fall risk reveal that multifactorial programs are more effective to reduce the number of falls (Feder et al. 2000; Gillespie et al. 2010). Muscle strength training has shown not only to increase muscle strength but also muscle power and the ability to neurally activate motor units (Hunter et al. 2004). Therefore, we hypothesized that a combined intervention program including exercising the mechanisms of dynamic stability and exercising muscle strength would show a higher improvement in the recovery performance of older adults. Accordingly, the purpose of this study was to examine the effects of specifically exercising the mechanisms of dynamic stability on the ability of elderly adults to regain balance after unexpected perturbations during walking. Furthermore, we wanted to examine the additional effect of increased muscle strength.
Methods
Participants
We examined elderly, non-sport active adults, aged 65 to 75 years. Seventy-five healthy individuals gave their informed consent to the experimental procedure according to the rules of the Declaration of Helsinki. Exclusion criteria included serious neuromuscular or musculoskeletal impairments and any lower limb pain at the ankle, knee, or hip joint. Further exclusion criteria were: any medication during the study (e.g., against joint pain), any history of major trauma or major systemic diseases. Participants were randomly assigned to two experimental groups and one control group. Thirty-eight subjects finished the whole experimental design, whereas 14 participants were included in the stability training group (ST; 10 females and 4 males) and 14 in the mixed training group (MT; 10 females and 4 males). The control group consisted of ten persons (CG; five females and five males) and did not perform any exercise program (Fig. 1).
Fig. 1.
Flow chart of recruitment and participation in the study. The performed gait measurement is named as “GAIT.” MTU refers to the strength measurements of the muscle tendon unit (knee extensors and ankle plantar flexors)
Exercise program
Both intervention groups exercised twice a week, 1.5 h per session for 14 weeks. The ST group performed exercise tasks including mechanisms of dynamic stability. The MT group also performed such exercises, but additionally accomplished a strength training program for the lower extremities.
Exercise tasks for the training of dynamic stability included two mechanisms of dynamic stability (i.e. increase of the base of support and counter-rotating segments around the center of mass; see also Arampatzis et al. 2011). The mechanism “increase of the base of support” was practiced with the following exercises: large and small, fast and slow, single and multiple steps in anterior–posterior and mediolateral direction in order to stabilize the body in different positions. Furthermore, participants were supposed to use large, compensatory steps to regain postural stability after perturbations. According to Maki and McIlroy (1997), compensatory steps are characterized by their reactive nature, an absence of functional anticipatory control, early initiation and rapid execution, and possible adaptive changes in consequence of postural perturbations. The mechanism “counter-rotating segments around the center of mass” was included in the training of arm and leg movements (no compensatory steps) during standing and walking to maintain balance. Participants exercised on narrow support surfaces such as beams, thick ropes, or small bars, as well as on several compliant surfaces. Counter-rotations were initiated by walking with a small base of support, hopping, or landing. Perturbations for the training of both mechanisms were induced by catching or throwing balls during standing or walking on a normal or constricted walkway, by external forces, and by the use of an unstable oscillatory platform on which the participants had to maintain postural stability in a one- or two-legged stance. The difficulty of all exercises was adapted to each individual’s ability (see also Chodzko-Zajko et al. 2009). The ST group performed those exercises including mechanisms of dynamic stability for 1.5 h and the MT group for about 45 min per session. For the remaining 45 min, the MT group performed strength exercises using strength training machines: knee extension and flexion, hip flexion, and ankle extension. The strength training included three sets of 10 to 15 repetitions at 50–70 % of their one-repetition maximum. The intervention program was always supervised by two experienced sport scientists.
Evaluation
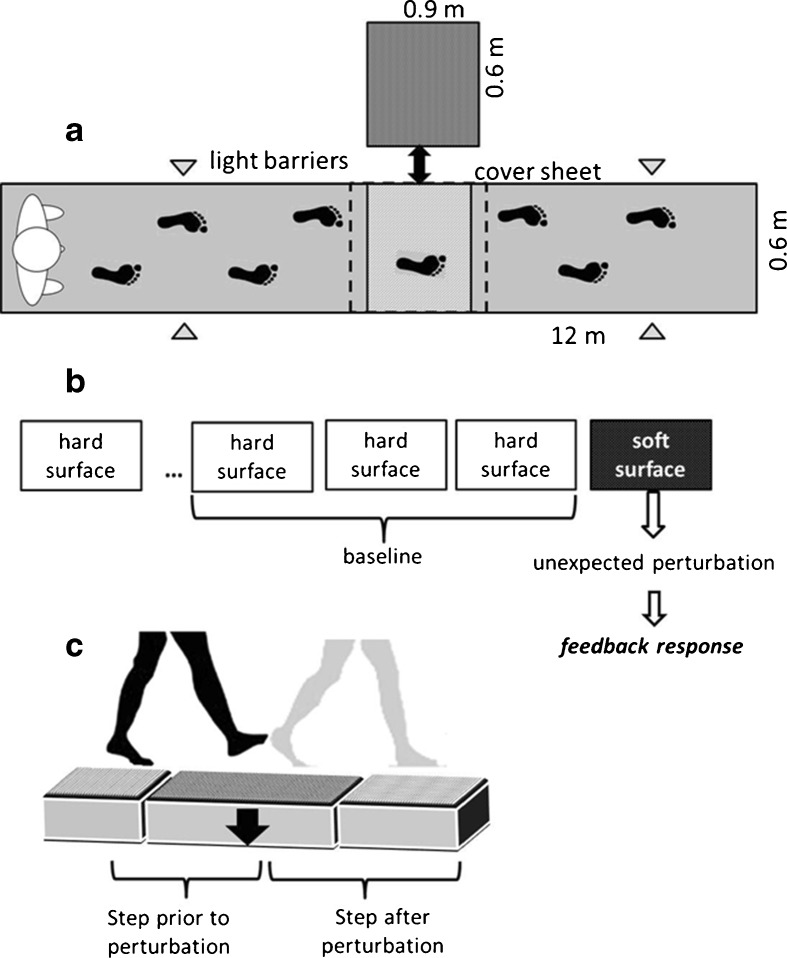
A gait protocol on a gangway was performed before and after the 14 weeks of intervention. The gangway (12 × 0.6 × 0.2 m) included one exchangeable element (0.9 × 0.6 × 0.2 m), which was hidden by a cover sheet to be able to change the surface from hard to soft without the knowledge of the participants (Fig. 2). The soft element was made of foam with an upper surface consisting of relatively hard rubber material (depth = 0.8 cm). The deformation of the soft element during the walking trials was about 10 cm in depth for all groups, whereas the force deformation characteristic was nonlinear. The hard element consisted of the same material as the gangway. To attain the target position of the right leg (middle of the exchangeable element), the participants were advised to walk in a predefined velocity throughout the experimental trials, whereas the practitioners adjusted the starting position if required. Trials with incorrect placement of the right foot were excluded from further analysis. A meta-analysis identified 1.13–1.33 m/s as an average velocity for persons aged 60–79 years (Bohannon and Andrews 2011). Therefore, we chose 1.3 m/s as gait velocity to pretend a moderate gait speed for the participants. The target velocity was controlled by light barriers and a stick which moved 1.3 m/s in front of the subjects along the gangway. The participants were informed that something in the walkway might change, but they were not informed about the point of time and the type of the perturbation. After at least three valid gait trials on the hard surface (baseline), the participants experienced one unexpected soft surface trial. This trial was performed to detect feedback responses because up to this perturbation, the participants were used to the hard surface and had adjusted their behavior accordingly. Only one unexpected perturbation was analyzed due to the influence of expectations and predictive behavior on following trials (Bierbaum et al. 2010, 2011; Marigold and Patla 2002).
Fig. 2.
Walkway (a) and gait protocol (b) for the test of the dynamic stability. The walkway (a) included one covered, exchangeable element, which allowed changing the surface condition from hard to soft without the knowledge of the participants. Three valid gait trials on the hard surface formed the baseline and were followed by one unexpected soft surface trial (b). This soft trial was performed to induce reactive behavior. Analysis was made for the step prior to the perturbation and the step after the perturbation (c)
Quantification of dynamic stability control
Kinematic data were recorded with the Vicon motion capture system (Model 624, Vicon, Oxford, UK) using 12 cameras operating at 120 Hz. The marker model contained 21 reflective markers (diameter, 14 mm), which were fixed at specified positions. The marker trajectories were smoothed out using a Woltring filter routine (Woltring 1986) with a noise level of 10 mm2. Segmental masses, the location of the segment centers of mass, and the center of mass were calculated by a custom-written Matlab model, based on the data reported by Dempster et al. (1959).
Since we wanted to analyze the stability state during single steps, we used the “extrapolated center of mass” concept formulated by Hof et al. (2005) for the quantification of dynamic stability. This concept, based on the approach of Pai and Patton (1997), is also applicable to disturbed movements. The margin of stability as a criterion for the state of postural stability of the human body was calculated as follows:
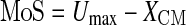 |
MoS indicates the margin of stability in the anterior–posterior direction, Umax the anterior boundary of the base of support, and XCM the position of the extrapolated center of mass in the anterior–posterior direction ( ). PxCM is the horizontal (antero–posterior) component of the projection of the center of mass (CM) to the ground, VxCM is the horizontal CM velocity, and the term
). PxCM is the horizontal (antero–posterior) component of the projection of the center of mass (CM) to the ground, VxCM is the horizontal CM velocity, and the term 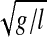 presents the eigenfrequency of a system of length l (inverted pendulum model). Margin of stability was analyzed only in the antero–posterior direction since the extrapolated center of mass shifts primarily in the anterior direction after the perturbation due to the increased velocity of the CM. MoS and the other components of dynamic stability are presented as the average values from touchdown of one leg to touchdown of the other leg to get a more representative stability state during walking. Meandist labels the phase from touchdown left to touchdown of the disturbed, right leg and meanrec labels the phase from touchdown of the disturbed leg to touchdown of the recovery (left) leg.
presents the eigenfrequency of a system of length l (inverted pendulum model). Margin of stability was analyzed only in the antero–posterior direction since the extrapolated center of mass shifts primarily in the anterior direction after the perturbation due to the increased velocity of the CM. MoS and the other components of dynamic stability are presented as the average values from touchdown of one leg to touchdown of the other leg to get a more representative stability state during walking. Meandist labels the phase from touchdown left to touchdown of the disturbed, right leg and meanrec labels the phase from touchdown of the disturbed leg to touchdown of the recovery (left) leg.
For the investigation of the muscle strength of knee extensors (QF) and ankle flexors (TS), the participants performed maximum voluntary isometric contractions (MVIC) on a dynamometer (Biodex-System3, Biodex Medical Systems Inc., Shirley, NY, USA). Based on the torque–angle relation curve, the subjects performed the MIVC’s near the optimal joint angle (i.e., plantar flexion MVIC, ankle angle, 85°; knee angle, 180°; knee extension MVIC, knee angle, 120°; hip angle, 110°), pre- and post-measurement. Resultant joint moments have been calculated according to a previously described method (Arampatzis et al. 2004, 2005). By the use of this method, the antagonistic moment and the displacement of the joint axis in relation to the dynamometer axis are taken into account. Because of technical problems, muscle strength values are preserved for 12 subjects of the ST group, 11 (QF) and 12 (TS) subjects of the MT group, and not for the control group.
A two-factor repeated-measures ANOVA with time (pre vs. post) as inter-subject variables and group as between-subject factors was used to examine the intervention effects on the analyzed dynamic stability parameters. Anthropometric data were analyzed by a one-way ANOVA and Bonferroni post hoc comparisons between the groups. The level of significance was set to α = 0.05.
Results
Age, body height, and body mass showed no differences between the three groups at the pre measurement. Furthermore, no difference between pre- and posttest was found for the body mass (Table 1). The ST group showed no statistically significant differences in the pre/post-comparisons of the maximum ankle plantar flexion moment (TS) as well as maximum knee extension moment (QF) [QF, F(1, 11) = 0.159, p = 0.698, part.η2 = 0.014; TS, F(1, 11) = 4.564, p = 0.056, part.η2 = 0.293]. The MT group, on the other hand, increased significantly [F(1, 10) = 32.582, p < 0.001, part.η2 = 0.765] the maximum isometric knee extension moments after the intervention but not the maximum ankle plantar flexion moment [F(1, 11) = 1.089, p = 0.319, part.η2 = 0.090; Table 1].
Table 1.
Anthropometric data, maximal isometric ankle flexion, and knee extension moment at pre- and post-measurement for the experimental groups (mean ± SD)
| Intervention | ST (n = 14) | MT (n = 14) | CG (n = 10) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Age (years) | 67.4 ± 2.7 | 68.6 ± 3.1 | 68.9 ± 3.2 | |||
| Body height (cm) | 165 ± 7 | 163.5 ± 7.3 | 168.6 ± 9.2 | |||
| Body weight (kg) | 70.7 ± 9.5 | 70.4 ± 9.4 | 73.7 ± 13.0 | 72.8 ± 12.2 | 76.9 ± 13.4 | 76.7 ± 12.9 |
| Max. ankle flexion moment (Nm/kg) | 1.97 ± 0.45 | 2.15 ± 0.45 | 1.94 ± 0.33 | 1.99 ± 0.31 | ||
| Max. knee extension moment (Nm/kg)a | 2.4 ± 0.46 | 2.43 ± 0.39 | 2.19 ± 0.36b | 2.47 ± 0.28b | ||
aStatistically significant interaction between time point of measurement and intervention group [repeated measurements; F(1, 21) = 6.234, p = 0.021, part.η2 = 0.229]
bStatistically significant difference between pre- and post-measurement [F(1, 10) = 32.582, p < 0.001, part.η2 = 0.765]
Baseline (unperturbed trials)
The gait velocity of the baseline trials showed a statistical significant main effect of time [F(1, 35) = 11.205, p = 0.002, part.η2 = 0.243; Table 2]. A closer look reveals that only the CG showed a significant increased gait velocity in the post-measurement [follow-up F(1, 9) = 11.205, p = 0.009, part.η2 = 0.555]. However, the slightly increased gait velocity (~2 %) in the post-measurement did not significantly affect (p > 0.05) the margin of stability and the components of dynamic stability in the pre/post-comparison (Table 2). Therefore, no significant differences between pre- and post-test are observable in the remaining stability parameters for all groups.
Table 2.
Mean values of the stability parameters (mean ± SD) in the three examined groups before (pre) and after (post) the intervention in the last step before the exchangeable element (time frame between touchdown left and touchdown of the right leg, baseline (unperturbed trials)
| Intervention | ST (n = 14) | MT (n = 14) | CG (n = 10) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Margin of stability (meandist) [cm] | −22.9 ± 3.8 | −22.2 ± 3.7 | −21.0 ± 5.3 | −21.6 ± 6.3 | −22.8 ± 5.5 | −24.7 ± 5.0 |
| CoM velocity (meandist) [m/s] a | 1.29 ± 0.04 | 1.31 ± 0.04 | 1.28 ± 0.04 | 1.3 ± 0.05 | 1.29 ± 0.03b | 1.32 ± 0.03b |
| extrapolated CoM (meandist) [cm] | 110.3 ± 6.2 | 110.0 ± 7.2 | 107.4 ± 5.9 | 109.3 ± 8.7 | 112.6 ± 8.2 | 113.8 ± 8.2 |
| Base of support (meandist) [cm] | 87.5 ± 5.4 | 87.8 ± 6.0 | 86.5 ± 3.7 | 87.8 ± 4.3 | 89.8 ± 5.3 | 89.1 ± 5.7 |
| Projected CoM (meandist) [cm] | 72.1 ± 5.0 | 71.3 ± 6.1 | 70.3 ± 4.8 | 71.6 ± 7.1 | 74.3 ± 6.2 | 74.4 ± 6.3 |
| Term (meandist) [s−1] | 3.39 ± 0.09 | 3.39 ± 0.09 | 3.44 ± 0.09 | 3.45 ± 0.09 | 3.37 ± 0.15 | 3.37 ± 0.14 |
aStatistically significant time effect time [F(1, 35) = 11.205, p = 0.002, part.η2 = 0.243]
bStatistically significant differences between pre- and post-measurement [follow-up; F(1, 9) = 11.205, p = 0.009, part.η2 = 0.555]
Perturbed trial—last step prior to the perturbation
In the time frame prior to the unexpected perturbation in the soft surface condition, the parameters of dynamic stability did not show any statistically significant differences between the pre- and post-measurements. This indicates a state of similar gait stability before and after the intervention for all groups in the unexpected trials (Table 3).
Table 3.
Mean values of the stability parameters (mean ± SD) in the three examined groups before (pre) and after (post) the intervention in the last step before the perturbation (time frame between touchdown left and touchdown of the right, disturbed leg, soft surface trial)
| Intervention | ST (n = 14) | MT (n = 14) | CG (n = 10) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Margin of stability (meandist) [cm] | −23.2 ± 5.3 | −23.4 ± 4.3 | −21.1 ± 5.8 | −22.5 ± 6.7 | −23.8 ± 4.6 | −25.1 ± 4.8 |
| CoM velocity (meandist) [m/s] | 1.29 ± 0.06 | 1.31 ± 0.07 | 1.28 ± 0.05 | 1.3 ± 0.07 | 1.3 ± 0.03 | 1.33 ± 0.04 |
| extrapolated CoM (meandist) [cm] | 111.3 ± 6.7 | 110.3 ± 7.4 | 106.8 ± 6.7 | 109.7 ± 9.3 | 114.2 ± 6.8 | 115.1 ± 8.4 |
| Base of support (meandist) [cm] | 88.1 ± 5.2 | 86.9 ± 6.5 | 85.7 ± 5.9 | 87.1 ± 4.5 | 90.4 ± 6.2 | 90.0 ± 6.3 |
| Projected CoM (meandist) [cm] | 73.1 ± 5.4 | 71.8 ± 6.0 | 69.7 ± 5.8 | 72.1 ± 7.0 | 75.6 ± 5.4 | 75.6 ± 6.8 |
| Term (meandist) [s−1] | 3.38 ± 0.09 | 3.39 ± 0.08 | 3.45 ± 0.09 | 3.46 ± 0.09 | 3.37 ± 0.15 | 3.36 ± 0.14 |
Perturbed trial—first step after the perturbation
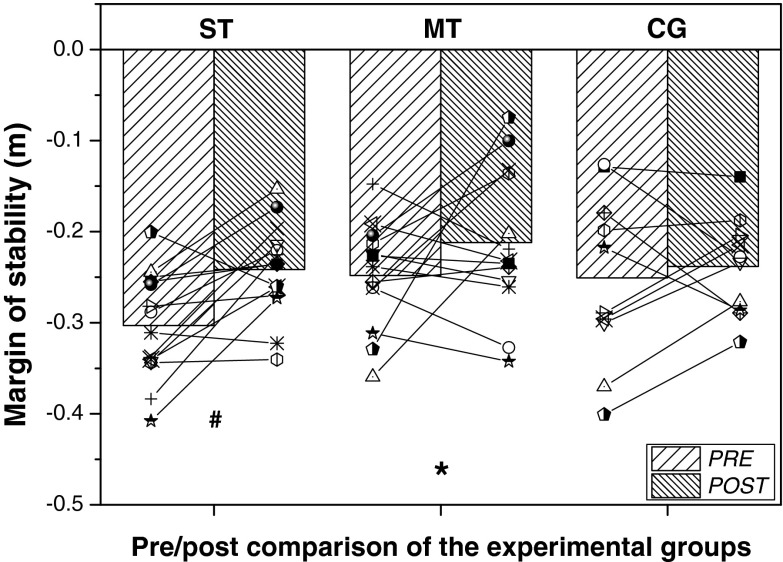
The repeated-measurements ANOVA showed a significant main effect of time [F(1, 32) = 8.046, p = 0.008, part.η2 = 0.187] on the margin of stability at the step after the disturbance. A follow-up comparison revealed a significant increase in the margin of stability after the 14 weeks of training only in the ST group [F(1, 13) = 12.125, p = 0.004, part.η2 = 0.483; Fig. 3].
Fig. 3.
Individual and mean values of margin of stability before and after the intervention at the step after the unexpected perturbation for the stability training group (ST), mixed training group (MT), and control group (CG). Asterisk, statistically significant time effect, F(1, 35) = 8.046, p = 0.008, part.η2 = 0.187); Numbersign, statistically significant differences between pre- and post-measurement [follow up; F(1, 13) = 12.125, p = 0.004, part.η2 = 0.483]
The increase in the margin of stability in the meanrec time frame after the intervention can be explained by a significant increase in the post-intervention base of support at the step after the unexpected perturbation in comparison to the pre-measurements [main effect of time; F(1, 35) = 10.043, p = 0.003, part.η2 = 0.223]. The base of support was significantly increased from pre- to post-measurement in the ST group [F(1, 13) = 15.716, p = 0.002, part.η2 = 0.547] and showed a trend towards an increase in the MT group [F(1, 13) = 3.251, p = 0.095, part.η2 = 0.200; Table 4]. The control group did not show any differences in the base of support before and after the 14 weeks [F(1, 9) = 0.593, p = 0.461, part.η2 = 0.062]. All other parameters remained unaltered for all groups for the time course between touchdown of the disturbed leg and touchdown of the recovery leg.
Table 4.
Mean values of the stability parameters (mean ± SD) in the three examined groups before (pre) and after (post) the intervention in the first step after the unexpected perturbation (time frame between touchdown right, disturbed and touchdown left, recovery leg, soft surface trial)
| Intervention | ST (n = 14) | MT (n = 14) | CG (n = 10) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| CoM velocity (meanrec) [m/s] | 1.42 ± 0.06 | 1.44 ± 0.08 | 1.39 ± 0.08 | 1.40 ± 0.05 | 1.40 ± 0.06 | 1.42 ± 0.06 |
| Extrapolated CoM (meanrec) [cm] | 121.2 ± 7.8 | 122.3 ± 8.7 | 117.4 ± 6.7 | 118.5 ± 7.3 | 123.0 ± 10.0 | 123.6 ± 11.9 |
| Base of support (meanrec) [cm]a | 90.9 ± 6.6 b | 98.2 ± 8.5 b | 91.4 ± 6.2 | 97.9 ± 12.7 | 97.9 ± 9.9 | 99.8 ± 9.3 |
| Projected CoM (meanrec) [cm] | 77.5 ± 5.7 | 78.2 ± 7.0 | 75.3 ± 4.4 | 76.0 ± 5.6 | 79.8 ± 7.6 | 79.8 ± 8.8 |
| Term (meanrec) [s−1] | 3.26 ± 0.08 | 3.26 ± 0.08 | 3.3 ± 0.09 | 3.3 ± 0.08 | 3.25 ± 0.14 | 3.24 ± 0.13 |
aStatistically significant time effect, [F(1, 35) = 10.043, p = 0.003, part.η2 = 0.223]
bStatistically significant differences between pre- and post-measurement [follow-up; F(1, 13) = 15.716, p = 0.002, part.η2 = 0.547]
Discussion
After 14 weeks of exercising the mechanisms of dynamic stability, both experimental groups achieved an average improvement of 18 % in their stability performance after an unexpected gait perturbation. This supports our first hypothesis. The reason for the improvement in the stability state after the perturbation in the post-experiment was a significant increase of the base of support, indicating the importance of this mechanism regarding fall prevention. However, the mixed-exercise intervention group (i.e., training of mechanisms of dynamic stability and muscle strength) did not show clear improvements in the stability state compared to the pre-intervention values, although the base of support after the unexpected perturbation showed a tendency for higher values in the post-condition. Therefore, the second hypothesis had to be rejected.
The rapid generation of high moments is an important factor for successful balance recovery after perturbations (Pijnappels et al. 2005; Hsiao-Wecksler and Robinovitch 2007; Karamanidis et al. 2008; Mademli et al. 2008), and therefore, we expected the MT group to achieve higher improvements in the dynamic stability in comparison to the ST group. The adequate generation of the hip moment seems to be a major contributor to successful balance recovery (Arampatzis et al. 2011). Therefore, since the rectus femoris muscle as a knee extensor partially contributes to the hip moment, we assumed that the observed strength gain in the knee extensors via strength training (~13 %) should possibly be reflected in an increased generation of hip moment. However, this increase in the knee extensor moment did not further increase the post-intervention recovery performance of the MT group. Earlier studies reported a contribution of 30–40 % of muscle strength to the capacity to recover balance with a single step after a forward fall (Wojcik et al. 2001; Grabiner et al. 2005; Karamanidis et al. 2008). Therefore, we suggest that the increase of 13 % of the knee extensor strength in our study is probably too low to achieve an improvement in the stability performance. A certain strength level is necessary to handle perturbations, but maximum leg strength appears not to be the critical factor. The main reason for the enhanced dynamic stability in simulated forward falls after an intervention period, for instance, was not the maximum joint moment but rather the ability to create a joint moment in an appropriate temporal framework (Arampatzis et al. 2011).
Regarding the expected difference between the two training groups, the “less-than-expected” development of the MT group in comparison to the ST group may be a consequence of the increased amount of training of the mechanisms of stability (1.5 h vs. 45 min) for the ST group. Beyond a certain strength level, seated-position strength training seems not to increase the performance in functional tasks such as the behavior after unexpected perturbations (Sherrington et al. 2008). This means that alternative seated strength training for the lower extremities compensates not for the advantage of training the mechanisms of dynamic stability. However, as reduced muscle strength is an important risk factor for falls (Moreland et al. 2004), strength training for the lower extremities still remains an important factor for individuals with muscle weakness (Chandler et al. 1998; Latham et al. 2004).
In the ST group, we found clear improvements in stability performance (~20 %) during the unexpected gait perturbation after the intervention, but without any changes in muscle strength. This finding is in accordance with our earlier results during simulated forward falls (Arampatzis et al. 2011), indicating that exercising the mechanisms of dynamic stability may lead to upgraded neuromuscular coordination and may be sufficient to convey successful strategies for regaining balance after perturbations. Improved capacity of maximal and explosive force production, as well as better neuronal activation, was already found in earlier sensorimotor training studies (Granacher et al. 2007; Schubert et al. 2008) and was explained by increased intermuscular coordination. Further, the repeated application of the mechanisms of dynamic stability seems to facilitate the adequate use of those mechanisms even in unfamiliar situations in order to regain balance. This improvement may be ascribed to a shift from prefrontal activity to a subcortical circuit, involving the cerebellum and basal ganglia, accompanied by increased automatic performance (Floyer-Lea and Matthews 2004; Puttemans et al. 2005; Taube et al. 2007).
Several exercise interventions have so far shown to be effective in reducing the risk of falls in older adults by means of balance, coordination, or strength training (for review see Gillespie et al. 2010). Recently, especially perturbation-based training programs requiring reactive behavior show improvements in balance recovery (Granacher et al. 2011). This is attributed to training specificity. However, our study was performed with the purpose of exercising specific mechanisms of dynamic stability, which are supposed to be fundamental to the regulation of postural stability. The participants did not exercise exact performance tasks, which were then measured, but were trained on exercises focusing on the underlying mechanisms of postural stability. Therefore, the intervention led to an improved application of the mechanisms of dynamic stability after a perturbation during walking, especially to an improved increase of the base of support. The performed perturbation during the measurements differed to the exercises applied in the training, indicating that the application of these mechanisms was transferable to a different situation.
However, we suggest that the imposed perturbation was relatively small because of the chosen gait velocity and the properties of the soft surface; higher effects may potentially be detected by applying more demanding tasks. Further, the demands of the ankle plantar flexion exercises for the MT group were possibly too low for a significant improvement of strength. Only one machine was available for the training of the ankle plantar flexors, and therefore, the participants had to exercise additionally with their own body weight which rather increased strength endurance. Ankle plantar flexion moment, however, is important during gait perturbations (Pijnappels et al. 2004, 2005), and an increase in this maximum moment could potentially support an increase in the base of support. The intervention groups showed no difference in their post values in both muscle groups (which may be caused by different initial strength conditions), but this does not affect the relevance of the performed training of the mechanisms of dynamic stability.
In conclusion, exercising the two mechanisms of dynamic stability (increase of base ofsupport and counter-rotating segmentsaround the center ofmass) led to a better application of these mechanisms after an unexpected perturbation during gait. Significantly better postural stability after the intervention, though, was found only in the stability training group; the training group, which additionally exercised leg strength, did not show any further improvements. Therefore, we suggest that repeatedly exercising the mechanisms of dynamic stability contributes adequately to the accomplishment of improved dynamic stability, but that the performed additional strength training for healthy elderly individuals shows no further effect on the ability to recover balance after unexpected perturbations during gait.
References
- Arampatzis A, Karamanidis K, De Monte D, Stafilidis S, Morey-Klapsing G, Brüggemann GP. Differences between measured and resultant joint moments during voluntary and artificially elicited isometric knee extension contractions. Clin Biomech. 2004;19(3):277–283. doi: 10.1016/j.clinbiomech.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Morey-Klapsing G, Karamanidis K, De Monte G, Stafilidis S, Brüggemann GP. Differences between measured and resultant joint moments during isometric contractions at the ankle joint. J Biomech. 2005;38(4):885–892. doi: 10.1016/j.jbiomech.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Mademli L. Deficits in the way to achieve balance related to mechanisms of dynamic stability control in the elderly. J Biomech. 2008;41(8):1754–1761. doi: 10.1016/j.jbiomech.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Peper A, Bierbaum S. Exercise of mechanisms for dynamic stability control increases stability performance in the elderly. J Biomech. 2011;44(1):52–58. doi: 10.1016/j.jbiomech.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai YC. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006;170(1):61–73. doi: 10.1007/s00221-005-0189-5. [DOI] [PubMed] [Google Scholar]
- Bierbaum S, Peper A, Karamanidis K, Arampatzis A. Adaptational responses in dynamic stability during disturbed walking in the elderly. J Biomech. 2010;43(12):2362–2368. doi: 10.1016/j.jbiomech.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Bierbaum S, Peper A, Karamanidis K, Arampatzis A. Adaptive feedback potential in dynamic stability during disturbed walking in the elderly. J Biomech. 2011;44(10):1921–1926. doi: 10.1016/j.jbiomech.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: prevalence and associated factors. Age Ageing. 1988;17(6):365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998;79(1):24–30. doi: 10.1016/S0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatorone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Dempster WT, Gabel WC, Felts WJ. The anthropometry of the manual work space for the seated subject. Am J Phys Anthropol. 1959;17:289–317. doi: 10.1002/ajpa.1330170405. [DOI] [PubMed] [Google Scholar]
- Etman A, Wijlhuizen GJ, van Heuvelen MJ, Chorus A, Hopman-Rock M. Falls incidence underestimates the risk of fall-related injuries in older age groups: a comparison with the FARE (Falls risk by Exposure) Age Ageing. 2012;41(12):190–195. doi: 10.1093/ageing/afr178. [DOI] [PubMed] [Google Scholar]
- Feder G, Cryer C, Donovan S, Carter Y, et al. Guidelines for the prevention of falls in people over 65. Brit Med J. 2000;321(7267):1007–1011. doi: 10.1136/bmj.321.7267.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol. 2004;92:2405–2412. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community (Review) Cochrane Database Syst Rev. 2010;15(2):CD000340. [Google Scholar]
- Grabiner MD, Owings TM, Pavol MJ. Lower extremity strength plays only a small role in determining the maximum recoverable lean angle in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(11):M1447–M1450. doi: 10.1093/gerona/60.11.1447. [DOI] [PubMed] [Google Scholar]
- Granacher U, Gruber M, Strass D, Gollhofer A. The impact of sensorimotor training in elderly men on maximal and explosive force production capacity. Dtsch Z Sportmed. 2007;58(12):446–451. [Google Scholar]
- Granacher U, Muehlbauer T, Zahner L, Gollhofer A, Kressig RW. Comparison of traditional and recent approaches in the promotion of balance and strength in older adults. Sports Med. 2011;41(5):377–400. doi: 10.2165/11539920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Heiden TL, Sanderson DJ, Inglis JT, Siegmund GP. Adaptations to normal human gait on potentially slippery surfaces: the effects of awareness and prior slip experience. Gait Posture. 2006;24(2):237–246. doi: 10.1016/j.gaitpost.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Heinrich S, Weigelt I, Rapp K, Becker C, Rissmann U, König HH. Sturz- und Frakturprävention auf der Grundlage des Nationalen Expertenstandards Sturzprophylaxe. Z Gerontol Geriatr. 2012;45:128–137. doi: 10.1007/s00391-011-0243-9. [DOI] [PubMed] [Google Scholar]
- Hof AL, Gazendam MGJ, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38(1):1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50(6):B399–B406. doi: 10.1093/gerona/50A.6.B399. [DOI] [PubMed] [Google Scholar]
- Hsiao-Wecksler ET, Robinovitch SN. The effect of step length on young and elderly women’s ability to recover balance. Clin Biomech. 2007;22(5):574–580. doi: 10.1016/j.clinbiomech.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34(5):248–329. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Age-related degeneration in leg-extensor muscle–tendon units decreases recovery performance after a forward fall: compensation with running experience. Eur J Appl Physiol. 2007;99(1):73–85. doi: 10.1007/s00421-006-0318-2. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A, Mademli L. Age-related deficit in dynamic stability control after forward falls is affected by muscle strength and tendon stiffness. J Electromyogr Kinesiol. 2008;18(6):980–989. doi: 10.1016/j.jelekin.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59(1):48–61. doi: 10.1093/gerona/59.1.M48. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Aust J Public Health. 1993;17(3):240–245. doi: 10.1111/j.1753-6405.1993.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Mademli L, Arampatzis A, Karamanidis K. Dynamic stability control in forward falls: postural corrections after muscle fatigue in young and older adults. Eur J ApplPhysiol. 2008;103(3):295–306. doi: 10.1007/s00421-008-0704-z. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997;77(5):488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Patla AE. Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol. 2002;88(1):339–353. doi: 10.1152/jn.00691.2001. [DOI] [PubMed] [Google Scholar]
- Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- Pai YC, Patton J (1997) Center of mass velocity-position predictions for balance control. J Biomech 30(4):347–354 [DOI] [PubMed]
- Pijnappels M, Bobbert MF, van Dieen JH. Contribution of the support limb in control of angular momentum after tripping. J Biomech. 2004;37:1811–1818. doi: 10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. Push-off reactions in recovery after tripping discriminate young subjects, older non-fallers and older fallers. Gait Posture. 2005;21(4):388–394. doi: 10.1016/j.gaitpost.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Putteman V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005;25(17):4270–4278. doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinovitch SN, Helle B, Lui A, Cortez J. Effect of strength and speed of torque development on balance recovery with the ankle strategy. J Neurophysiol. 2002;88(2):613–620. doi: 10.1152/jn.2002.88.2.613. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl. 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- Schubert M, Beck S, Taube W, Amtage F, Faist M, Gruber M. Balance training and ballistic strength training are associated with task-specific corticospinal adaptations. Eur J Neurosci. 2008;27:2007–2018. doi: 10.1111/j.1460-9568.2008.06186.x. [DOI] [PubMed] [Google Scholar]
- Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JCT. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56(12):2234–2243. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M (2006) Motor control—translating research into clinical practice. 3rd edition, Lippincott Williams & Wilkins.
- Taube W, Gruber M, Beck S, Faist M, Gollhofer A, Schubert M. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxford) 2007;189(4):347–358. doi: 10.1111/j.1748-1716.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Wojcik LA, Schultz AB, Ashton-Miller JA, Alexander NB. Age differences in using a rapid step to regain balance during a forward fall. J Gerontol A Biol Sci Med Sci. 1997;52(1):M8–M13. doi: 10.1093/gerona/52A.1.M8. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Stanhope SJ, Morton SM. Impaired reactive stepping adjustments in older adults. J Gerontol A Biol Sci Med Sci. 2009;64(7):807–815. doi: 10.1093/gerona/glp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik LA, Thelen DG, Schultz AB, Ashton-Miller JA, Alexander NB. Age and gender differences in peak lower extremity joint torques and ranges of motion used during single-step balance recovery from a forward fall. J Biomech. 2001;34(1):67–73. doi: 10.1016/S0021-9290(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Woltring HJ. A FORTRAN package for generalized, cross-validatory spline smoothing and differentiation. Adv Engineering Software. 1986;8:104–113. doi: 10.1016/0141-1195(86)90098-7. [DOI] [Google Scholar]