Abstract
Introduction
Prolonged postoperative analgesia cannot be achieved by a single injection of local anesthetic solution. The objective of this study was to optimize the formulation of a ropivacaine hydrochloride (Ropi-HCl) loaded in situ forming implant (ISI) by addition of different co-solvents, and evaluate the in vitro release of Ropi-HCl, and the analgesic effect and toxicity of the optimized formulation in rats.
Material and methods
Triacetin (TA), benzyl benzoate (BB) and polyethylene glycol 400 (PEG 400) were used as additives and added to the solvent of N-methyl-2-pyrrolidone (NMP). Drug release to the surface and inner structural properties of the formed implant were evaluated by scanning electron microscopy (SEM). The analgesic effect was determined by injection near the rat sciatic nerve.
Results
The solvent system added with TA or BB significantly decreased the burst release, whereas PEG 400 increased the Ropi-HCl burst release from the formulation. Over 70% of the incorporated Ropi-HCl was released from all formulations in 14 days in the in vitro assay. The SEM showed that the surface of NMP-BB formulation was less porous and more homogeneous, compared with the other formulations. Compared with Ropi-HCl injection, the optimized formulation (NMP-BB) significantly prolonged the analgesic effect in 48 h (p < 0.05), with a mild degree of motor block from 3 h to 12 h. Histological evaluation of the injection site revealed only mild inflammatory infiltration without obvious pathological nerve alterations.
Conclusions
The biodegradable Ropi-HCl-loaded ISI system with NMP-BB may prove to be an attractive and safe alternative for the delivery of parenteral local anesthetics to prolong pain relief.
Keywords: in situ forming implant, local anesthesia, ropivacaine
Introduction
Post-surgical pain is a major issue in the healing period after surgery. Injection of local anesthetics for reversible nerve block is the site-directed and most effective mainstay in the management of post-surgical pain. A single injection provides a short-term nerve block lasting 4-6 h [1]. To circumvent the short duration of action, viable options have been attempted, including administration of local anesthetics, neurolytic agents or cryoprobes, but all these options have their limitations. For example, catheter infusion may cause infection and migration, requiring constant and meticulous monitoring. Nerve block by neurolytic methods may induce unwanted deficits and new forms of pain due to localized tissue destruction [2].
Over the last decades, several long-acting local anesthetic delivery systems based on biodegradable polymers have been developed. Bioerodible and biodegradable polymer-local anesthetic matrix implants formed by impregnating polyanhydride copolymers with dibucaine and bupivacaine may produce sciatic nerve blocks for 3–5 days in rats [3–5]. Although these solid implants can become useful therapeutic candidates, their use is so far limited to surgical procedures.
Liquid drug delivery systems, mostly based on polyesters (PLA or PLGA), may use vesicles made up of liposomes, lipospheres or microparticles, and have been widely studied in pain management [6–8]. When encapsulated with local anesthetics, these formulations may provide a duration of local analgesia of several hours or days. Although they do not need surgical implantation, the preparations are based on complex and multi-step processes with many formulation parameters to be considered.
As an alternative to conventional solid implants, microparticles or liquid drug-polymer formulations, an injectable implant system has been developed. The in situ implant is formed by injection into the body, where it contacts with the body fluid through precipitation of the polymer [9–11]. This system is composed of insoluble water and biodegradable polymer dissolved in a water-miscible, physiologically compatible solvent such as dimethyl sulfoxide (DMSO), NMP or 2-pyrrolidone. The polymer solidifies at the site of administration and forms an implant upon injection of the drug-loaded polymer solution. Using this technology, other drugs such as doxycycline (Atridox®) for periodontal delivery [12] or leuprolide acetate (Eligard®) for subcutaneously delivery [13] have been commercialized.
The purpose of this study was to optimize the Ropi-HCl loaded into the in situ forming implant (ISI) with different co-solvents. The in vitro release, and surface and cross-sectional morphology of Ropi-HCl were evaluated, and the analgesic effect and toxicity of the optimized formulation were further investigated in rats.
Material and methods
Reagents and animals
Reagents used in this study included poly(D,L-lactide-co-glycolide) (PLGA, 50 : 50, Mw 10000) (Shandong Medical Equipment Research Institute, Jinan, China); Ropi-HCl (Shandong Boyuan Chemical Co., Ltd. Jinan, China); N-methyl-2-pyrrolidone (NMP) (International Specialty Products Limited Corporation Shanghai Representative Office, Shanghai, China); polyethylene glycol 400 (PEG 400) (BASF Limited Corporation Shanghai Representative Office, Shanghai, PR China); and triacetin (TA) and benzyl benzoate (BB) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). All chemicals were of reagent grade quality.
Female Sprague-Dawley (SD) rats weighing 220-280 g were obtained from SLAC laboratory animal Co., Ltd. (Shanghai, China), housed in groups in a 12 h : 12 h light-dark cycle, and cared for in compliance with protocols approved by the Animal Care and Use Committee at the Second Military Medical University in Shanghai, China.
Preparation of the in situ forming implant system
The formulation compositions used in this study are shown in Table I. TA, BB and PEG 400 were the additives in the NMP solvent. ISI was prepared by dissolving PLGA in solvents at room temperature to produce a clear viscous solution. Ropi-HCl powder was added to the polymer solution and mixed completely. The drug concentration was kept constant at a level of 10% (w/w, based on polymer).
Table I.
Compositions of the Ropi-HCl-loaded in situ forming implants
| Ropi-HCl [mg] | PLGA [mg] | NMP [mg] | BB [mg] | TA [mg] | PEG 400 [mg] |
|---|---|---|---|---|---|
| 10 | 100 | 200 | |||
| 10 | 100 | 180 | 20 | ||
| 10 | 100 | 140 | 60 | ||
| 10 | 100 | 200 | 15 |
In vitro drug release assay
In vitro drug release assay was conducted according to the previous report [14]. Briefly, ISI was injected into dialysis tubing (Union Carbide Co. USA, Mw cut-off 14,000 Da) (n = 3), and then immersed into 50 ml of 10 mmol phosphate buffer, pH 7.4 (0.02% w/v sodium azide was added as a preservative) at 37°C with constant shaking (100 rpm). At predetermined time points, a 5 ml sample (which was replaced by fresh buffer solution) was with-drawn and assayed. After 48 h, the complete medium was withdrawn and replaced by a fresh medium at each sampling point. The Ropi-HCl concentration in the medium was determined by a high-performance liquid chromatography (HPLC, L-2200 Auto Sampler [Hitachi, Tokyo, Japan]) system composed of an L-2130 pump, an L-2455 diode array detector, and a D-2000 data analysis program. A 20-µl volume was injected onto a Kromasil 100-5 C18 column (150-µm x 4.6-µm, Sweden) using as the mobile phase a mixture of 20% acetonitrile: 80% 15-mmol buffer phosphate (pH 4.0) at a flow rate of 1.0 ml/min, and DAD was detected at 210 nm.
Drug solubility
An excess amount of Ropi-HCl was added in a 3 ml test tube containing 1 ml of 10-mmol phosphate buffer (pH 7.4). The tube was tightly capped, shaken in a shaking incubator-water bath at 37°C, and centrifuged after 48 h. The supernatant was filtered through a 0.45-µm membrane. The amount of Ropi-HCl in the sample was determined by HPLC as described above after appropriate dilution.
Scanning electron microscopy
The freeze-dried ISI was mounted on a metal stub double-sided tape and coated with gold for 60 s using a plasma sputter (SBC-12, KYKY Tech. Dev. LTD, China). The morphology of the surface and cross-section of the sample was observed with a scanning electron microscope (TS5136MM, TESCAN, Brno, CZ) in a low vacuum condition.
Outcome assessment process
Twenty-four female Sprague-Dawley rats (220-280 g) were randomly divided into four groups and the observers were blinded to the treatment allocation. Prior to nerve block [15], rats were briefly anesthetized (< 2 min) with isoflurane. A 23G needle was introduced posteromedially to the greater trochanter. Upon touching the bone, the needle was withdrawn 1 mm, and the 0.3 ml drug-loaded (40 mg/kg) or the blank formulation was injected.
Hot plate sensory test
Sensory block was measured by the Hargreaves hotplate method [16]. Briefly, the rats were positioned to stand on a glass plate. The infrared beam (adjusting heat temperature to 52°C) was directed through the plate on the plantar surface of the hind paw. Latency to withdraw the hind paw from the glass hotplate was recorded by alternating paws and allowing at least 5 min recovery between plate measurements [17]. If no withdrawal occurred from the hotplate within 15 s, the trial was terminated to prevent injury and the termination time was recorded. The test ended after five measurements, and the mean was calculated. As the withdrawal reflex is from the thigh adductors (femoral not sciatic nerve), the rats can withdraw a paw despite total sciatic nerve block.
Motor block test
Motor block was assessed with a 4-point scale (1): 1, normal; 2, intact dorsiflexion of foot with impaired ability to splay toes when elevated by the tail; 3, toes and foot plantar flexed with no splaying ability; and 4, loss of dorsiflexion, flexion of toes, and impairment of gait. For neural block degree index, partial motor block equals a score of 2 and dense motor block either 3 or 4.
Histology
Rats (n = 6 each group) were sacrificed 7 days after drug injection. The sciatic nerve adjacent to the implant was removed together with the surrounding tissues, preserved in 10% formalin, stained with hematoxylin and eosin (HE), and processed for histology using standard techniques. Histological evaluation was performed by a pathologist from Changhai Hospital (Shanghai, China) as described previously [18]. Briefly, inflammation in the connective tissue was graded as absent, minimal, mild, moderate or severe.
Statistical analysis
The in vitro release data obtained were presented as mean ± SD and analyzed using analysis of variance. In all cases, p < 0.05 was considered to indicate statistical significance.
Results
In vitro release study
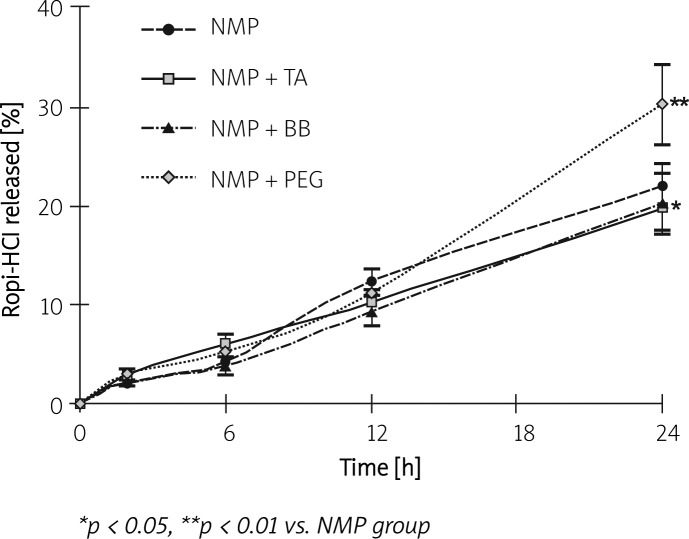
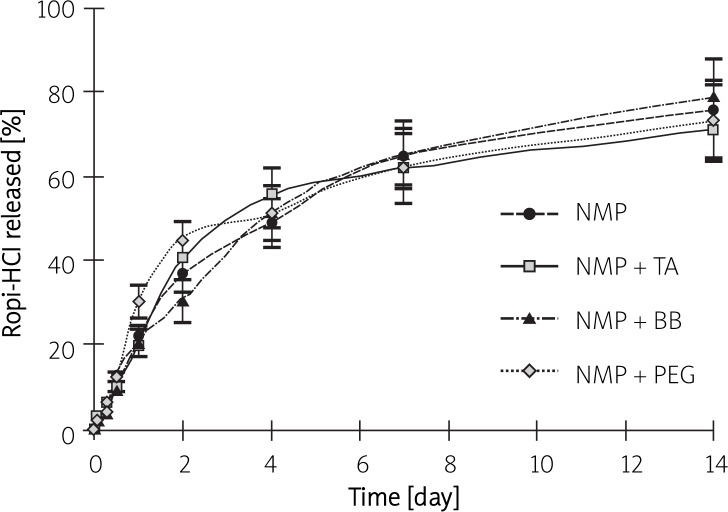
The release profile of Ropi-HCl from ISI prepared with different solvent systems is shown in Figure 1. The initial Ropi-HCl release rate within 24 h was 22.09 ±2.20%, 19.83 ±2.27%, 20.33 ±3.07% and 30.32 ±4.07% for NMP, NMP-TA, NMP-BB and NMP-PEG 400 formulation, respectively. Compared with the NMP-only formulation, the solvent system added with TA or BB formulation significantly decreased the burst release (p < 0.05). However, the initial release of ISI was dramatically enhanced upon addition of PEG 400, which might be related to its composition. In addition, it was found that over 70% of the incorporated Ropi-HCl was released from the four formulations 14 days after incubation in the medium (75.64 ±5.96%, 71.26 ±6.69%, 78.93 ±8.79% and 73.25 ±9.65% for NMP, NMP-TA, NMP-BB and NMP-PEG 400 formulation, respectively) (Figure 2).
Figure 1.
In vitro release of ropivacaine hydrochloride (Ropi-HCl) from the in situ forming implants within 24 h
Figure 2.
In vitro release of Ropi-HCl from the in situ forming implants for 14 days
Micrographs of scanning electron microscopy
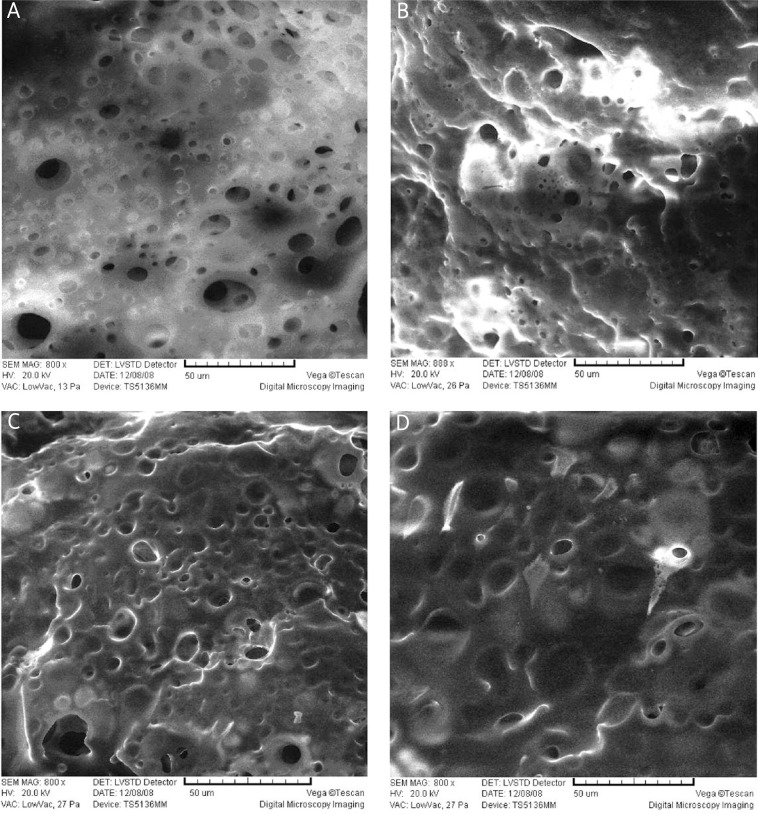
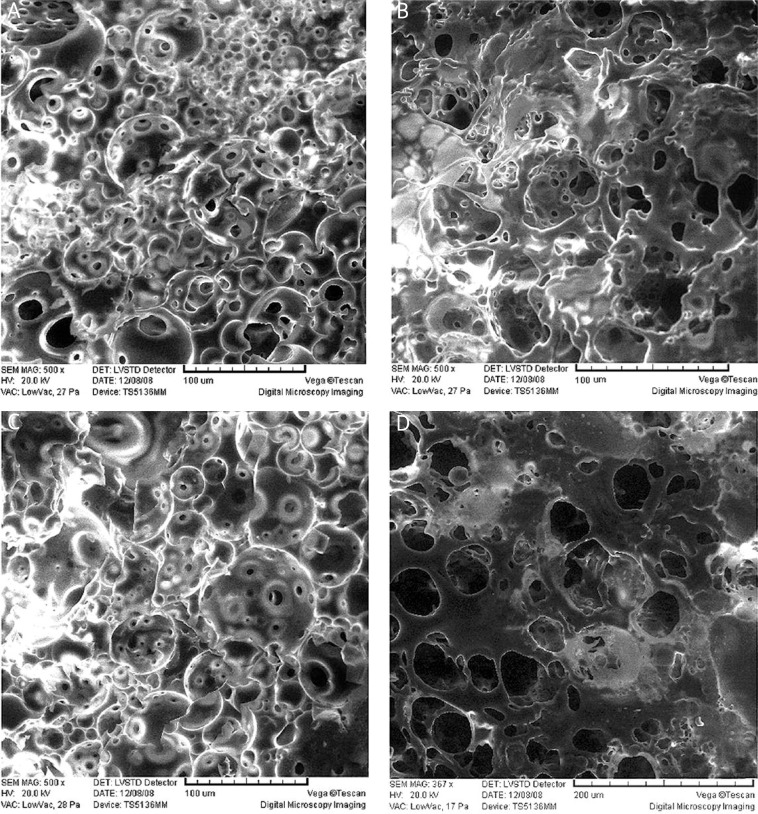
Surface micrographs of the four solidified ISI formulations were detected by SEM 24 h after injection into the release medium. The NMP formulation had a smooth porous surface (Figure 3A), while the surface of the formulation with NMP-TA was relatively rough (Figure 3B). The formulation with NMP-PEG 400 had a loose surface with a higher porosity (Figure 3C). In addition, the pore diameter of the formulation with NMP-PEG 400 was larger than that of NMP and NMP-TA formulations. Compared with the above three formulations, the surface of NMP-BB formulation was less porous and more homogeneous (Figure 3D), suggesting that the formulation with NMP-BB was most optimal. In addition, 14 days after injection, water microchannels penetrated the depots, resulting in higher pore density on the cross-section of PLGA based matrix with polymer erosion. However, there was no significant difference observed between the four formulations (Figure 4).
Figure 3.
Scanning electron micrographs of surface morphology of the in situ forming implants 24 h after injection into the release medium. A – NMP, B – NMP-TA, C – NMP-PEG400, D – NMP-BB
Figure 4.
Scanning electron micrographs of cross-sectional morphology of the in situ forming implants 14 days after injection into the release medium. A – NMP, B – NMP-TA, C – NMP-PEG400, D – NMP-BB
In vivo efficacy
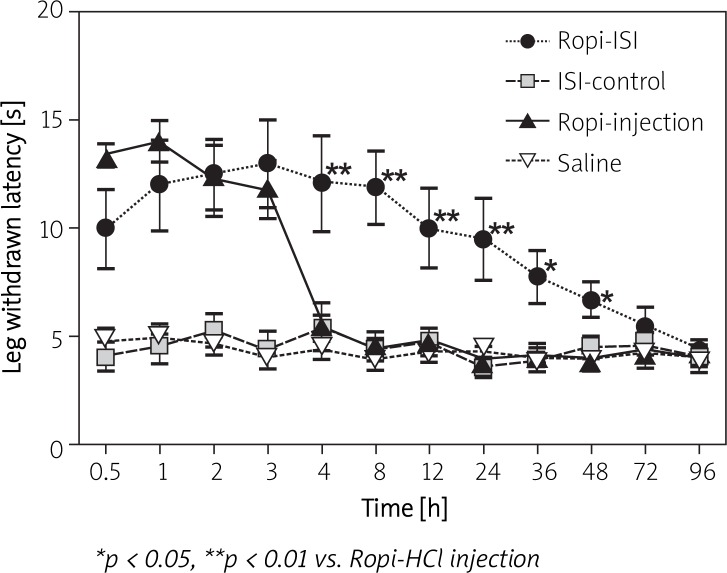
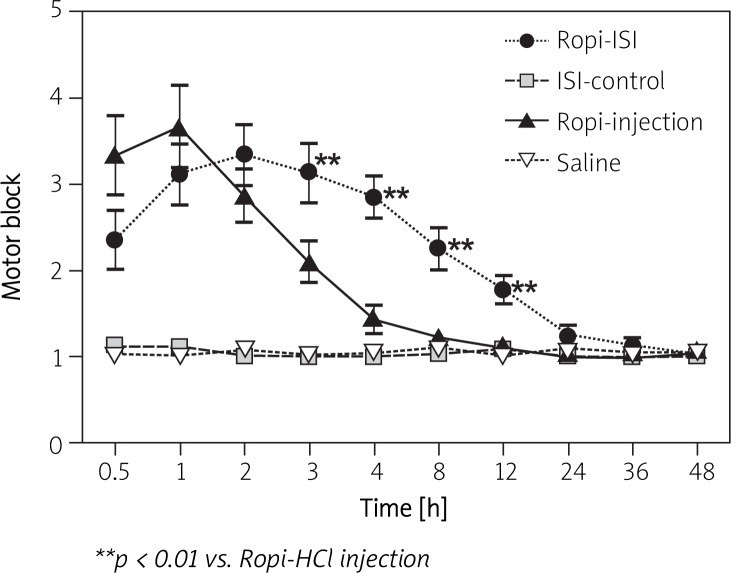
Based on the in vitro results, an in vivo efficacy study was carried out with the most optimized formulation (NMP-BB). Sensory and motor blocks were determined by intramuscular injection near the rat sciatic nerve with saline, Ropi-HCl injection, blank or Ropi-HCl ISI (40 mg/kg). The duration of nerve block was recorded. As shown in Figure 5, sensory block produced by Ropi-HCl injection was obvious in the first 3 h, and soon vanished afterwards due to fast elimination. However, sciatic nerve block produced by Ropi-HCl ISI lasted 72 h. Compared with Ropi-HCl injection, the analgesic effect of Ropi-HCl ISI was significantly prolonged, and all rats returned to the sensory baseline level at 96 h. No sensory block was observed in the saline and blank groups. Motor block was also determined (Figure 6). Rats in the Ropi-HCl ISI group showed signs of slight motor block after injection, as represented by inability to splay their toes properly for 24 h. The same effect was also produced by Ropi injection lasting for 3 h, and no sign of motor deficits was observed thereafter. Rats injected with saline and blank ISI showed no sign of motor block. These results demonstrated that Ropi-HCl ISI could prolong sciatic nerve block.
Figure 5.
Mean leg-withdrawal latency values versus time following the peripheral sciatic nerve injection of 40 mg/kg Ropi-HCl ISI, ISI control, Ropi-HCl injection or saline (n = 6)
Figure 6.
Motor block values versus time following the peripheral sciatic nerve injection of 40 mg/kg Ropi-HCl ISI, ISI control, Ropi-HCl injection or saline (n = 6)
Histological determination
The sciatic nerve and surrounding tissues were evaluated histologically 7 days after injection. No evidence of axonal degeneration or demyelization of the nerve was observed in the rats injected with Ropi-HCl ISI, nor was perineural lymphocyte found in the tissue surrounding the sciatic nerve. Generally, the degree of inflammation was no higher than that of the control group. In addition, no evidence of active inflammatory reaction or tissue irritation extending beyond the local injection site was observed, suggesting that there was no adverse reaction upon application of the formulation (data not shown).
Discussion
The present study explored the feasibility of the PLGA-based ISI system for controlled release of Ropi-HCl. For the preparation of the ISI, Ropi-HCl was chosen as a candidate for the development of a long-acting formulation for the treatment of post-surgical pain. Ropi-HCl is a new member of the long-acting amino amide class of local anesthetics, used as the hydrochloride salt of the S-enantiomer, while bupivacaine is a racemic mixture [19]. Ropivacaine is less lipid soluble than bupivacaine, and possesses less CNS and cardiac toxicity than does bupivacaine [18, 20]. Previous studies also showed that the onset, duration and extent of sensory block of ropivacaine were similar to bupivacaine. However, motor block of ropivacaine was less intense and lasted a short duration compared with bupivacaine [21, 22]. This character is highly desirable for postoperative pain management, since ropivacaine can promote the safety of long-acting sensory block to minimize the potential CNS and cardiac toxicity. In addition, ropivacaine has minimal influence on the mobility of patients.
Our in vitro drug release study was performed in 10 mmol PBS (pH 7.4) at 37°C. The determined saturated solubility of Ropi-HCl was 25.25 mg/ml. To mimic the in vivo physiological sink condition, a large volume of release medium was used, so that the concentration of Ropi-HCl would never reach more than 10% of its maximum solubility [23].
Burst release is the key issue of a sustained drug release system [10]. The purpose of this study was to evaluate the effect of additives on Ropi-HCl release and modify the properties of the formulations. Ropi-HCl ISI is a viscous liquid at room temperature and can be injected using a 23-G needle. After the formulation was injected, the polymer solution solidified as the solvent dissipated into the medium buffer and formed an implant. Solvent/water affinity plays a key role in governing drug release from this system [24]. TA, a hydrophobic solvent with low solvent/water affinity, was reported to decrease the initial burst release due to its ability to slow down the rate of phase separation [10]. In this study, addition of TA decreased the initial release compared with the formulation without TA. It was also reported that BB as the co-solvent could decrease protein burst release from the in situ forming drug delivery system due to its insoluble property and high viscosity (9.57 mPa) [25], thus slowing down the rate of phase separation, resulting in decreased initial release. PEG 400 was reported to decrease the initial burst release of a drug from the polymer matrix through its plasticizing effect [26]. In the present study, PEG 400 was used as an additive in the PLGA/NMP system. It showed a significant difference of initial drug release compared with the formulation without PEG 400. The initial release of Ropi-HCl was faster due to microchannel formation after dissolution of highly hydrophilic PEG 400. In addition, a slow and controlled manner of Ropi-HCl release from the ISI was observed, and the total drug release from the four formulations after 14 days was almost at the same level. The surface and cross-section morphology of ISIs were in good agreement with the in vitro drug release study. Due to lower burst release and a proper 14-day-release profile (78.93%), the formulation with addition of BB was chosen for the next in vivo experiment.
It was found in our study that a single injection of Ropi-HCl ISI near the peripheral sciatic nerve in an SD rat prolonged the reversible nerve block for 72 h. The sustained release property is consistent with the clinical need for managing acute postoperative pain in 72 h. Motor block produced by Ropi-HCl ISI was mild, with a short duration compared with the 10% (w/w) bupivacaine-polymer formulation that lasted more than 30 h [23]. In addition, no evidence of active inflammation or tissue irritation and pathological nerve changes was observed, indicating good tolerability and safety of the in situ forming implant formulations.
In conclusion, this study on controlled release of Ropi-HCl ISI using in situ forming implant systems can safely provide site-specific and prolonged effects of local anesthesia. Future studies should investigate the possibility of using the formulation clinically.
Acknowledgments
The authors are very grateful to Dr. Kexing Fan and Lei Wang for many helpful discussions and technical support. Lei Lu, Wei Zhang and Xin Wu contributed equally to this paper.
References
- 1.Masters DB, Berde CB, Dutta SK, et al. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 1993;79:340–6. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001;20:502–7. doi: 10.1016/s1010-7940(01)00815-6. [DOI] [PubMed] [Google Scholar]
- 3.Masters DB, Berde CB, Dutta SK, Langer R. Prolonged sciatic nerve blockade using sustained release of bupivacaine from a biodegradable polymer matrix. Anesthesiology. 1991;75:A765. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Masters DB, Berde CB, Dutta S, Turek T, Langer R. Sustained local anesthetic release from bioerodible polymer matrices: a potential method for prolonged regional anesthesia. Pharm Res. 1993;10:1527–32. doi: 10.1023/a:1018995913972. [DOI] [PubMed] [Google Scholar]
- 5.Masters DB, Berde CB, Ward JM, Martyn JAJ, Kubsky W. Biochemical and histologic effects of prolonged sciatic nerve blockade with bupivacaine using a biodegradable polymer matrix. Anesthesiology. 1991;75:A680. [Google Scholar]
- 6.Toongsuwan S, Li LC, Erickson BK, Chang HC. Formulation and characterization of bupivacaine lipospheres. Int J Pharm. 2004;280:57–65. doi: 10.1016/j.ijpharm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Görner T, Gref R, Michenot D, Sommer F, Tran MN, Dellacherie E. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J Control Release. 1999;57:259–68. doi: 10.1016/s0168-3659(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 8.Masters DB, Domb AJ. Liposphere local anesthetic timed-release for perineural site application. Pharm Res. 1998;15:1038–45. doi: 10.1023/a:1011978010724. [DOI] [PubMed] [Google Scholar]
- 9.Dunn RL, English JP, Cowsar DR, Vanderbilt DP. Biodegradable in situ forming implants and methods of producing the same. US Patent. 1990;938:763. [Google Scholar]
- 10.Graham PD, Brodbeck KJ, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. J Control Release. 1999;58:233–45. doi: 10.1016/s0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 11.Shah NH, Railkar AS, Chen FC, et al. A biodegradable injectable implant for delivering micro and macromolecules using poly(lactic-co-glycolic) acid (PLGA) copolymers. J Control Release. 1993;27:139–47. [Google Scholar]
- 12.Kranz H, Yilmaz E, Brazeau GA, Bodmeier R. In vitro and in vivo drug release from a novel in situ forming drug delivery system. Pharm Res. 2008;25:1347–54. doi: 10.1007/s11095-007-9478-y. [DOI] [PubMed] [Google Scholar]
- 13.Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25–31. doi: 10.1016/s0090-4295(02)02396-8. [DOI] [PubMed] [Google Scholar]
- 14.Kranz H, Bodmeier R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. Int J Pharm. 2007;332:107–14. doi: 10.1016/j.ijpharm.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 17.Benoliel R, Eliav E, Tal M. Strain-dependent modification of neuropathic pain behaviour in the rat hindpaw by a priming painful trigeminal nerve injury. Pain. 2002;97:203–12. doi: 10.1016/S0304-3959(01)00428-6. [DOI] [PubMed] [Google Scholar]
- 18.Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78:507–14. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 19.Saracoglu A, Saracoglu KT, Eti Z. Comparative study of fentanyl and morphine in addition to hyperbaric or isobaric bupivacaine in combined spinal anaesthesia for caesarean section. Arch Med Sci. 2011;7:694–9. doi: 10.5114/aoms.2011.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsota P, Batistaki C, Apostolaki S, Kostopanagiotou G. Patient-controlled epidural analgesia after Caesarean section: levobupivacaine 0.15% versus ropivacaine 0.15% alone or combined with fentanyl 2 microg/ml: a comparative study. Arch Med Sci. 2011;7:685–93. doi: 10.5114/aoms.2011.24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockway MS, Bannister J, McClure JH, McKeown D, Wildsmith JA. Comparison of extradural ropivacaine and bupivacaine. Br J Anaesth. 1991;66:31–7. doi: 10.1093/bja/66.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Brown DL, Carpenter RL, Thompson GE. Comparison of 0.5% ropivacaine and 0.5% bupivacaine for epidural anesthesia in patients undergoing lower-extremity surgery. Anesthesiology. 1990;72:633–6. doi: 10.1097/00000542-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Shikanov A, Domb AJ, Weiniger CF. Long acting local anesthetic-polymer formulation to prolong the effect of analgesia. J Control Release. 2007;117:97–103. doi: 10.1016/j.jconrel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Brodbeck KJ, DesNoyer JR, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. Part II. The role of solution thermodynamics and bath-side mass transfer. J Control Release. 1999;62:333–44. doi: 10.1016/s0168-3659(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 25.Gao ZH, Shukla AJ, Johnson JR, Crowley WR. Controlled release of a contraceptive steroid from biodegradable and injectable gel formulations: in vitro evaluation. Pharm Res. 1995;12:857–63. doi: 10.1023/a:1016209020160. [DOI] [PubMed] [Google Scholar]
- 26.Tan LP, Venkatraman SS, Sung PF, Wang XT. Effect of plasticization on heparin release from biodegradable matrices. Int J Pharm. 2004;283:89–96. doi: 10.1016/j.ijpharm.2004.06.022. [DOI] [PubMed] [Google Scholar]