Abstract
Introduction
Previous studies have shown that serum p-cresyl sulfate (PCS) and indoxyl sulfate (IS) were significantly related to clinical outcomes in patients on hemodialysis (HD). However, evidence for the relationship in elderly HD patients remains scarce. We explore whether the two toxins can predict clinical outcomes in elderly HD patients.
Material and methods
Fifty stable HD patients more than 65 years old were enrolled from a single medical center. Serum total and free PCS, IS levels and biochemistry were measured concurrently. The clinical outcomes including cardiovascular events and all-cause mortality were analyzed after 38-month follow-up.
Results
Univariate Cox proportional hazard ratio analysis revealed that cardiovascular events were associated with gender (p = 0.02), diabetes (p < 0.01), calcium (p = 0.01), total PCS (p < 0.01), free PCS (p < 0.01) and total IS (p = 0.05). Multivariate analysis showed that diabetes (p = 0.01), total PCS (p = 0.01) and free PCS (p = 0.04) were related to cardiovascular events. For all-cause mortality, only total PCS (p = 0.01) reached significance after adjusting other confounding factors. However, Kaplan-Meier analysis indicated that free PCS (p = 0.02) and total PCS (p < 0.01) were significantly associated with cardiovascular events and total PCS (p = 0.048) was related to all-cause mortality during 38-month follow-up.
Conclusions
Our results indicate that total PCS is a valuable marker in predicting cardiovascular event and all-cause mortality in elderly HD patients.
Keywords: hemodialysis, p-cresyl sulfate, indoxyl sulfate, cardiovascular disease, mortality
Introduction
Increased morbidity and mortality are hallmarks of chronic kidney disease (CKD) and end-stage renal disease (ESRD) patients. Particularly, the mortality risk due to cardiovascular events is dramatically elevated in patients with CKD [1]. Hemodialysis (HD) patients aged > 65 years have exceptionally high mortality rates. A variety of features that include the presence of cardiac disease, underlying disease, age, race, psychosocial status and nutrition are reported to be the cause of increased mortality in HD. A previous investigation found that age, albumin level, pre-albumin level, body mass index and diabetes were significant predictors of mortality in HD patients who were aged > 75 years even though adequate dialysis treatment was performed [2]. Other important reported factors related to survival include late referral, functional dependence, non-ambulatory status, the presence of comorbid conditions and unplanned dialysis [3–5].
p-Cresyl sulfate (PCS) and indoxyl sulfate (IS) are prototypic protein-bound uremic toxin molecules. These two retained solutes are not only biomarkers for renal function but are also involved in the progression of diseases [6]. They contribute to various similarities, including their production by gut bacteria [7], strong albumin binding at the Sudlow II site [8], significant renal metabolism, low dialytic clearance [9, 10] and an emerging role in cardiovascular disease and mortality in renal patients [11]. An increasing body of evidence suggests that there is a significant association of serum PCS [12, 13] and IS [14] with vascular disease and mortality in CKD and HD patients. The precise and detailed roles of serum PCS and IS in the prediction of cardiovascular disease and mortality elderly HD patients warrant further investigation.
Therefore, we conducted a prospective cohort study to evaluate the role of serum total/free PCS and IS in CVD and all-cause mortality in elderly HD patients.
Material and methods
Patients
This study enrolled 50 stable HD patients starting July 2007 from a single medical center. Patients with acute infection, cardiovascular events in the past 3 months, with malignancy, or those younger than 65 years were excluded from this study. All patients had been receiving 4-h maintenance dialysis 3 times a week for 8–100 months using a synthetic dialysis membrane (polysulfone or polyamide). Dialyzers were not reused. Dialysis efficiency was calculated according to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, and single-pool Kt/V of urea nitrogen was calculated [15]. Residual renal function was estimated from an interdialytic urine collection and expressed as weekly renal Kt/V (rKt/V). As a measure of daily protein intake, the normalized protein catabolic rate (g/kg · d) was evaluated. The total and free forms of PCS and IS were measured in each group. This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Mackay Memorial Hospital. Informed consent was obtained from all study patients.
Laboratory assessment
All blood samples were obtained just before the dialysis procedure, and the following tests were performed: blood urea nitrogen (BUN), creatinine, hemoglobin, hematocrit, calcium, phosphate, bicarbonate, i-PTH, albumin, total IS, free IS, total PCS, and free PCS. Serum levels of high sensitivity C-reactive protein (hsCRP) were measured using a Behring Nephelometer II (Dade Behring, Tokyo, Japan) and albumin levels were determined by the bromocresol green method. Blood samples of patients were taken immediately before the HD session two times a week (second and third session). All serum PCS and IS were measured 2 times a week to obtain an average value. Other biochemistries were measured before the second HD session during the same week.
Serum PCS and IS (i.e., combined free and protein-bound fractions) were analyzed with ultra-performance liquid chromatography (UPLC). Serum samples were prepared and deproteinized by a heat denaturation process. The free concentrations of IS and PCS were measured in serum ultrafiltrates, obtained by using Microcon YM-30 separators (Millipore, Billerica, MA, USA), followed by the same sample preparation and analysis that was performed for serum PCS and IS. UPLC (ACQUITY UPLC®) was performed at room temperature using a BEH phenyl column (2.1 mm × 100 mm) and a photodiode array (PDA) detector at 280 nm. The buffers used were (A) 10 mM NH4H2PO4 (pH = 4.0) and (B) 100% acetonitrile. The flow rate was 0.4 ml/min with a 9-min gradient cycling from 82.5% A/17.5% B to 55% A/45% B.
Under these conditions, both PCS and IS were eluted at 2.75 and 1.4 min, respectively. Standard curves for PCS and IS were set at 0.5, 1, 2.5, 5, and 10 mg/l; both were processed in the same manner as the serum samples, and they correlated with the serum samples with average r2 values of 0.999 ±0.001. Quantitative results were obtained and calculated in terms of their concentrations (mg/l). The sensitivity of this assay was 0.425 mg/l for PCS and 0.225 mg/l for IS.
End point evaluation
Study patients were followed up until September 30, 2010. During follow-up, different events, including cardiovascular events and cause of death, were reviewed by 1 independent physician, who was blinded for the study. To control the accuracy of the data, the chart notes were reviewed for all operations due to nephrologic, cardiologic, and vascular defects. Cardiovascular events included death from cardiac causes, myocardial ischemia, non-fatal myocardial infarction, ischemic stroke, or new onset of peripheral vascular disease, whichever developed first. Only one event per subject was included in the analysis. Deaths were accurately recorded and the cause of death was categorized as cardiovascular, infectious, malignancy, or other.
Statistical analysis
The demographic data were expressed as the mean ± standard deviation (SD). A Cox regression analysis model was used to analyze the relationship between independent variables and clinical outcomes including cardiovascular events and all-cause mortality. All variables with a statistically significant p value in the univariate analysis were included in the multivariate analysis. The Kaplan-Meier method (factors were compared using the log-rank test) was used to estimate the cumulative event-free rate for the time to the first cardiovascular event and overall mortality in elderly dialysis patients with PCS level above and below the median (free and total, 1.4 mg/l, 24.3 mg/l, respectively). A value of p less than 0.05 was considered statistically significant. All statistical analyses were conducted by using the SPSS ver. 17.0 software program (SPSS, Chicago, IL).
Results
Table I provides mean ± SD values of the clinical and biochemical characteristics of the whole study population. Mean levels of total serum PCS (21.99 ±12.08 mg/dl) and IS (40.54 ±16.73 mg/dl) were significantly higher in aged HD patients compared to the unbound free PCS (1.59 ±1.12 mg/dl), and IS (4.27 ±2.90 mg/dl). During the study period, 20 patients experienced new cardiovascular events. At the end of the study period, causes of death were recorded as cardiovascular in 9, infectious disease in 4 and other causes in 1 (Table II). Patients were divided into 2 groups according to the levels of free and total PCS: the first, free (23 patients) and total (25 patients) PCS level above the median (> 1.4 mg/l and 24.3 mg/l, respectively); and the second, free (27 patients) and total (25 patients) PCS level below the median (< 1.4 mg/l and 24.3 mg/l, respectively).
Table I.
Clinical and biochemical characteristics of the study population
| Variable | Patients (n = 50) |
|---|---|
| Age [years] | 70.50 ±3.45 |
| Time to HD [months] | 38.55 ±25.87 |
| SBP [mm Hg] | 143.80 ±20.80 |
| DBP [mm Hg] | 84.31 ±12.84 |
| Kt/V | 1.68 ±0.31 |
| rKt/V | 0.05 ±0.08 |
| Albumin [mg/dl] | 4.05 ±0.34 |
| nPCR [g/kg/day] | 1.21 ±0.13 |
| Bicarbonate [mmol/l] | 22.64 ±1.94 |
| Hb [g/dl] | 10.24 ±1.58 |
| Hct [%] | 30.15 ±4.79 |
| Ca [mg/dl] | 8.82 ±0.66 |
| P [mg/dl] | 5.02 ±1.22 |
| i-PTH [pg/ml] | 291.44 ±235.09 |
| ALP [IU/l] | 94.27 ±32.95 |
| Cr [mg/dl] | 10.36 ±2.32 |
| hsCRP [mg/dl] | 0.97 ±1.75 |
| Total IS [mg/l] | 40.54 ±16.73 |
| Total PCS [mg/l] | 21.99 ±12.08 |
| Free IS [mg/l] | 4.27 ±2.90 |
| Free PCS [mg/l] | 1.59 ±1.12 |
SBP – systolic blood pressure, DBP – diastolic blood pressure, Kt/V – a number used to quantify hemodialysis adequacy, rKt/V – renal Kt/V, nPCR – normalized protein catabolic rate, Hb – hemoglobin, Hct – hematocrit, Ca – calcium, P – potassium, i-PTH – intact parathyroid hormone, ALP – alkaline phosphatase, Cr – creatinine, hsCRP – high-sensitivity C-reactive protein
Table II.
Clinical causal association at end of study period
| Clinical causes | Number of patients |
|---|---|
| All-cause mortality: | |
| Cardiovascular death or heart attack | 9 |
| Infections | 4 |
| Other causes | 1 |
| New cardiovascular event | 20 |
Table III shows the relationship among the independent variables with cardiovascular events and all-cause mortality in elderly HD patients. In univariate Cox regression analysis, gender, diabetic mellitus, calcium, total/free PCS and total IS were significantly associated with cardiovascular events. However, multivariate Cox regression analysis conducted by adjusting various confounding factors revealed that total/free PCS and diabetic mellitus are the only factors that reached the maximum significance with cardiovascular events. Univariate analysis was also performed to assess the relationship of all-cause mortality with independent factors and significance was found only with total/free PCS. On the other hand, multivariate analysis showed that total serum PCS was the only factor independently associated with all-cause mortality by various adjustment strategies. However, the serum level of total/free IS was not associated with all-cause mortality in uni- and multivariate analyses. These results strongly suggest that total PCS plays a critical role in all-cause mortality of elderly HD patients.
Table III.
Univariate and multivariate Cox regression analysis for evaluating the relationship between independent variables and clinical outcomes in elderly HD patients
| Variable | Cardiovascular event | All-cause mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||||||||
| HR | 95% CI | Value of p | HR | 95% CI | Value of p | HR | 95% CI | Value of p | HR | 95% CI | Value of p | |
| Gender (female/male) | 3.20 | 1.17–8.94 | 0.02 | 1.08 | 0.06–17.96 | NS | 2.27 | 0.76–7.81 | NS | |||
| Age [years] | 0.89 | 0.78–1.02 | NS | 0.95 | 0.84–1.08 | NS | ||||||
| DM/Non-DM | 0.16 | 0.04–0.62 | 0.01 | 0.02 | 0.01–0.16 | 0.01 | 1.08 | 0.13–8.79 | NS | |||
| Time to dialysis [m] | 1.00 | 0.98–1.01 | NS | 1.01 | 0.98–1.02 | NS | ||||||
| SBP [mm Hg] | 0.99 | 0.97–1.01 | NS | 0.99 | 0.97–1.02 | NS | ||||||
| DBP [mm Hg] | 0.98 | 0.95–1.02 | NS | 0.99 | 0.95–1.03 | NS | ||||||
| rKt/V | 0.51 | 0.11–1.08 | NS | 0.04 | 0.02–0.76 | NS | ||||||
| Kt/V | 0.65 | 0.13–3.17 | NS | 0.67 | 0.12–3.85 | NS | ||||||
| nPCR | 0.97 | 0.77–1.22 | NS | 0.99 | 0.98–1.01 | NS | ||||||
| Creatinine [mg/dl] | 1.03 | 0.83–1.27 | NS | 1.05 | 0.75–1.21 | NS | ||||||
| Hct [%] | 0.91 | 0.67–1.23 | NS | 1.04 | 0.95–1.15 | NS | ||||||
| Albumin [g/dl] | 0.03 | 0.01–1.37 | NS | 2.35 | 0.52–5.13 | NS | ||||||
| Ca [mg/dl] | 1.24 | 1.63–2.43 | 0.01 | 1.03 | 0.97–1.21 | NS | 1.39 | 0.60–3.24 | NS | |||
| P [mg/dl] | 1.04 | 0.73–1.48 | NS | 0.99 | 0.99–1.00 | NS | ||||||
| i-PTH [pg/ml] | 1.00 | 0.99–1.01 | NS | 1.00 | 0.99–1.00 | NS | ||||||
| hsCRP [mg/dl] | 0.79 | 0.49–1.28 | NS | 0.48 | 0.15–1.57 | NS | ||||||
| Total PCS [mg/l] | 1.05 | 1.01–1.08 | < 0.01 | 1.34 | 1.05–1.98 | 0.01 | 1.05 | 1.01–1.10 | 0.01 | 1.06 | 1.01–1.12 | 0.02 |
| Free PCS [mg/l] | 1.47 | 1.10–1.98 | < 0.01 | 1.66 | 1.01–31.90 | 0.04 | 1.45 | 1.01–2.07 | 0.04 | 1.21 | 0.97–2.37 | NS |
| Total IS [mg/l] | 1.02 | 1.00–1.05 | 0.05 | 1.04 | 0.98–1.13 | NS | 1.01 | 0.98–1.04 | NS | |||
| Free IS [mg/l] | 1.08 | 0.94–1.24 | NS | 1.10 | 0.94–1.29 | NS | ||||||
DM – diabetes mellitus, HR – hazard ratios, CI – confidence interval, SBP – systolic blood pressure, DBP – diastolic blood pressure, Kt/V – a number used to quantify hemodialysis adequacy, rKt/V – renal Kt/V, nPCR – normalized protein catabolic rate, Hb – hemoglobin, Hct – hematocrit, Ca – calcium, P – potassium, i-PTH – intact parathyroid hormone, ALP – alkaline phosphatase, Cr – creatinine, hsCRP – high sensitivity C-reactive protein, NS – not significant
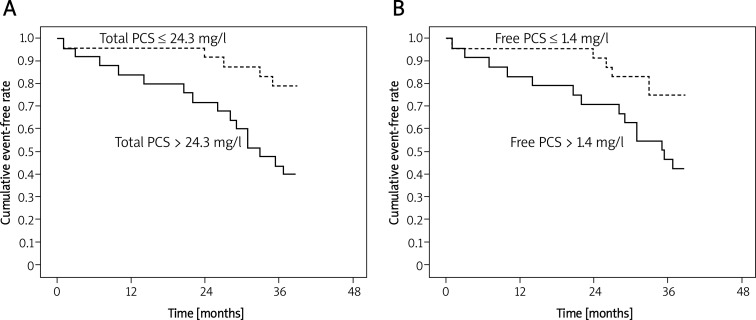
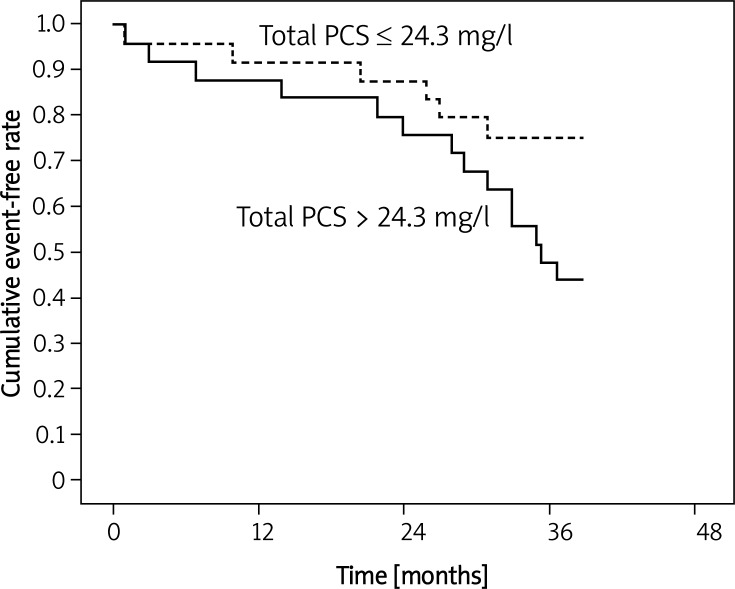
Figure 1 demonstrates the relationship between total serum PCS and cardiovascular events tested by Kaplan-Meier curves. Lower total serum PCS (< 24.3 mg/l) was a better predictor of cardiovascular events than high total serum PCS (> 24.3 mg/l; log-rank, p < 0.01) (Figure 1A). Moreover, lower free PCS (< 1.4 mg/l) also showed better as a predictor of cardiovascular events and survival than high free PCS (> 1.4 mg/l; log-rank, p < 0.02, Figure 1B). On the other hand, Figure 2 also demonstrates that patients with lower total PCS (< 24.3 mg/l) had poorer all-cause mortality than patients with higher total PCS (> 24.3 mg/l). Taken together, these results further confirmed that high levels of total/ free PCS cause cardiovascular events and all-cause mortality in elderly HD patients.
Figure 1.
Kaplan-Meier curves of time to first cardiovascular event. A – Patients with high (> 24.3 mg/l) total PCS concentrations are compared to low (≤ 24.3 mg/l) total PCS concentrations. Log rank p < 0.01. B – Patients with high (> 1.4 mg/l) free PCS concentrations are compared to low (≤ 1.4 mg/l) free PCS concentrations. Log rank p = 0.02
Figure 2.
Kaplan-Meier curves of all-cause mortality. Patients with high (> 24.3 mg/l) total PCS concentrations are compared to low (≤ 24.3 mg/l) total PCS concentrations. Log rank p = 0.048
Discussion
Several in vitro data have suggested a pathophysiological role of the solute in some important aspects of the uremic syndrome. Uremic toxins have gained substantial interest in recent years due to their potential hazardous role in excessive mortality among patients with end stage renal disease (ESRD). These toxins can be considered as nontraditional risk factors in this population. Cardiovascular disease is highly widespread in patients with CKD [16] and PCS and IS have been shown to exert their toxic effects in vitro [17]. Additionally, results obtained in hemodialysis patients with different CKD have identified these two uremic toxins as emerging mortality risk factors [12, 18, 19]. The results of one study suggest that, besides other well-known predictors of survival, serum level of PCS is highly related to mortality and cardiovascular events in patients treated with dialysis [20]. In the present study, we found that although these two uremic solutes acted as good predictors of mortality and cardiovascular events in HD patients, PCS played an essential role in these events.
p-Cresol is reported to affect inflammatory responses by decreasing the reaction of activated polymorphonuclear leukocytes [21] and the endothelial cell response to inflammatory cytokines in vitro [22]. Serum levels of p-cresol are elevated by a factor of around ten in uremic patients, and those of the free, non-protein-bound p-cresol are increased even more significantly [17]. A study conducted in Asian HD patients demonstrated that neither serum p-cresol nor serum IS was associated with mortality [23] and they also demonstrated that higher serum free PCS rather than serum IS is associated with cardiovascular (CV) events and infection-related hospitalization [23]. Hence, the impact of free serum p-cresol concentrations on various outcomes has been studied in hemodialysis patients and this parameter has been shown to be associated with the rate of hospitalization for infectious diseases [20], the occurrence of CV disease [18] and mortality [19]. In many clinical studies, the authors have reported data on p-cresol; however, p-cresol was determined after acidification, so that the concentrations measured were in fact those of the main retention solute, PCS, as each molecule of sulfate which is broken down by hydrolysis will generate one molecule of p-cresol [24].
Recently, Schepers et al. demonstrated that PCS has a pro-inflammatory effect and might, therefore, contribute to the increased susceptibility to vascular damage in renal patients [25]. Effectively, it has recently been demonstrated that PCS induces the detachment of endothelial microparticles even in the absence of overt endothelial damage in hemodialysis patients, suggesting that PCS may alter the endothelial function in this setting [11]. Thus, it makes sense to study PCS rather than p-cresol per se. Our study demonstrated that both total and free PCS were higher in later CKD stages. However, patients with high total serum PCS tended to have a higher rate of CV events and it was also demonstrated that higher total PCS concentrations were associated with all-cause mortality independently of well-known predictors of sex, diabetic mellitus and calcium.
It has been demonstrated that IS plays a role in the progression of CKD by inducing an inflammatory reaction, with enhanced expression of profibrotic cytokines such as transforming growth factor β1 (TGF 1) [26, 27]. In the current study, univariate analyses revealed that serum total IS in HD patients is significantly associated with CVD but not with all-cause mortality. These results corroborate with findings from our previous studies, one in dialysis patients and the other in predialysis patients, where we did not find any association between IS levels and overall mortality [15, 23]. Moreover, in multivariate analysis both CVD and all-cause mortality were not associated with IS. This study also demonstrated that in patient serum high total (> 24.3 mg/l) and free (> 1.4 mg/l) PCS seem to be good predictors of cardiovascular events in HD patients.
In conclusion, there is increasing experimental and clinical evidence in favor of the hypothesis that IS and PCS are involved not only in the progression of CKD, but also in the promotion of cardiovascular disease and mortality. However, although they both belong to the same class of molecules, exert toxic effects on the cardiovascular system and accumulate in the serum of patients with CKD, they may have different impacts in terms of cardiovascular disease.
Acknowledgments
We would like to thank all the patients who were involved in this study. This study was supported in part by a grant from Taiwan National Science Council (NSC 100-2314-B-195-012) and Mackay Memorial Hospital (MMH-102-29).
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–23. [PubMed] [Google Scholar]
- 2.Chauveau P, Combe C, Laville M, et al. Factors influencing survival in hemodialysis patients aged older than 75 years: 2.5-year outcome study. Am J Kidney Dis. 2001;37:997–1003. doi: 10.1016/s0272-6386(05)80016-2. [DOI] [PubMed] [Google Scholar]
- 3.Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching endstage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012–21. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Covinsky KE, Collins AJ, et al. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146:177–83. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Couchoud C, Labeeuw M, Moranne O, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24:1553–61. doi: 10.1093/ndt/gfn698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raff AC, Meyer TW, Hostetter TH. New insights into uremic toxicity. Curr Opin Nephrol Hypertens. 2008;17:560–5. doi: 10.1097/MNH.0b013e32830f45b6. [DOI] [PubMed] [Google Scholar]
- 7.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–25. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 8.Meijers BK, De Loor H, Bammens B, et al. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1932–8. doi: 10.2215/CJN.02940509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanholder R, Meert N, Schepers E, et al. Review on uraemic solutes II-variability in reported concentrations: causes and consequences. Nephrol Dial Transplant. 2007;22:3115–21. doi: 10.1093/ndt/gfm151. [DOI] [PubMed] [Google Scholar]
- 10.Martinez AW, Recht NS, Hostetter TH, et al. Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16:3430–6. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 11.Meijers BK, Van Kerckhoven S, Verbeke K, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis. 2009;54:891–901. doi: 10.1053/j.ajkd.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Liabeuf S, Barreto DV, Barreto FC, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant. 2010;25:1183–91. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 13.Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the protein-bound retention solute p-cresol predicts mortality in hemodialysis patients. Kidney Int. 2006;69:1081–7. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 14.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CJ, Wu CJ, Pan CF, et al. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant. 2010;25:3693–700. doi: 10.1093/ndt/gfq251. [DOI] [PubMed] [Google Scholar]
- 16.Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–56. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 17.Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2010;26:938–47. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijers BK, Claes K, Bammens B, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–9. doi: 10.2215/CJN.07971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijers BK, Bammens B, de Moor B, et al. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73:1174–80. doi: 10.1038/ki.2008.31. [DOI] [PubMed] [Google Scholar]
- 20.Vanholder R, De Smet R, Waterloos MA, et al. Mechanisms of uraemic inhibition of phagocyte reactive species production: characterization of the role of p-cresol. Kidney Int. 1995;47:510–7. doi: 10.1038/ki.1995.64. [DOI] [PubMed] [Google Scholar]
- 21.Dou L, Cerini C, Brunet P, et al. P-cresol, a uraemic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int. 2002;62:1999–2009. doi: 10.1046/j.1523-1755.2002.t01-1-00651.x. [DOI] [PubMed] [Google Scholar]
- 22.De Smet R, Van Kaer J, Van Vlem B, et al. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem. 2003;49:470–8. doi: 10.1373/49.3.470. [DOI] [PubMed] [Google Scholar]
- 23.De Smet R, David F, Sandra P, et al. A sensitive HPLC method for the quantification of free and total p-cresol in patients with chronic renal failure. Clin Chim Acta. 1998;278:1–21. doi: 10.1016/s0009-8981(98)00124-7. [DOI] [PubMed] [Google Scholar]
- 24.Vanholder R, Bammens B, de Loor H, et al. Warning: the unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant. 2011;26:1464–7. doi: 10.1093/ndt/gfr056. [DOI] [PubMed] [Google Scholar]
- 25.Schepers E, Meert N, Glorieux G, et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22:592–6. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 26.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 27.Miyazaki T, Ise M, Hirata M, et al. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int Suppl. 1997;63:S211–4. [PubMed] [Google Scholar]