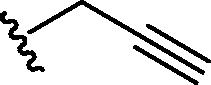
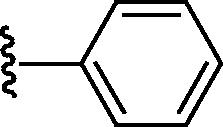
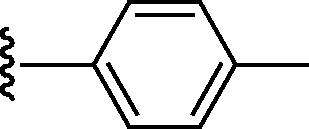
Graphical abstract

Keywords: Bromomaleimides, Thiomaleimides, Pyridazinediones, Reagent synthesis, Reagents for cysteine modification
Abstract
Bromomaleimides are useful building blocks in synthesis and powerful reagents for the selective chemical modification of proteins. A mild new synthesis of these reagents is described, along with the convenient transferability of the approach to dithiomaleimides and bromopyridazinediones.
Bromomaleimides 1 are useful building blocks for synthesis, undergoing a diverse range of reactions including nucleophilic addition and cross-coupling.1–4 For example, dibromomaleimides have been used extensively as starting materials for the synthesis of bisaryl maleimides, as inhibitors of protein kinase C.5,6 Bromomaleimides have also been demonstrated as powerful reagents for the selective chemical modification of cysteine residues in peptides and proteins.7–15 In this context, bromomaleimides represent the parent members of a larger class of reagents for use in bioconjugation. Varying the leaving groups on the 3 and 4 positions of the maleimide allows fine-tuning of the properties of the reagent. Notably, exchanging the bromines with thiophenols affords reagents which can be employed in the in situ bridging of disulfide bonds13 and are tolerant to incorporation into polymers.16 The six-membered ring analogues, bromopyridazinediones 2, offer a similar and yet distinct reactivity profile for cysteine conjugation.17
Bromomaleimides 1 and bromopyridazinediones 2 are commonly synthesised by treating a bromomaleic anhydride 3 with an amine or hydrazine respectively and heating at reflux in AcOH to carry out the dehydrative cyclisation (Scheme 1).8,17 These conditions are extremely harsh, and are not suitable if the amine or hydrazine contains sensitive functionality. Furthermore, to access the dithiophenolmaleimides a subsequent step is required involving the treatment of the dibromomaleimide with thiophenol and a base.13
Scheme 1.

The current synthesis of bromomaleimides 1 and bromopyridazinediones 2.
The reaction of amines with N-methoxycarbonylmaleimide (5, X and Y = H) has been reported as a mild approach to synthesise maleimides.18,19 We report herein on our efforts towards transferring this chemistry to provide a mild and general synthesis of bromomaleimides, dithiophenolmaleimides and bromo-pyridazinediones. Treatment of dibromo-, monobromo- and dithiophenol-maleimides with methyl chloroformate and N-methylmorpholine (NMM) afforded the corresponding N-methoxycarbonyl activated species 5 in excellent yields (Table 1).
Table 1.

| Entry | X | Y | Yield (%) |
|---|---|---|---|
| 1 | Br | Br | 97 |
| 2 | Br | H | 100 |
| 3 | SPh | SPh | 97 |
Stirring N-methoxycarbonyldibromomaleimide (6) with a range of amines at room temperature successfully afforded dibromomaleimides 7 in good yields (Table 2, entries 1–15). This represents an extremely mild synthesis, and is effective for alkyl and aryl amines. It should be noted that aniline itself led to a lower yield (entry 11), due to an undetermined competing degradation pathway, but this could be precluded by substitution in the para position of the aniline (entries 12 and 13). Notable examples include: dibromomaleimide–alkyne12 (entry 3) and dibromomaleimide–azide (entry 6), useful as heterobifunctional cross-linkers, bis-dibromomaleimide (entry 4) as a homobifunctional cross-linker, and dibromomaleimide–aryl fluorides for prospective PET imaging applications (entries 10, 14 and 15). In an analogous fashion N-methoxycarbonylbromomaleimide (8) underwent effective reaction with a number of amines to form monobromomaleimides 9 in moderate yields (entries 16–20). It should be noted that the products in all these reactions are reactive conjugate acceptors, and thus no more than a stoichiometric equivalent of amine should be employed otherwise bis-addition products are observed.
Table 2.

| Entry | X | R | Yield (%) |
|---|---|---|---|
| 1 | Br | 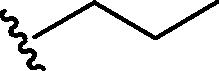 |
79 |
| 2 | Br |  |
79 |
| 3 | Br | 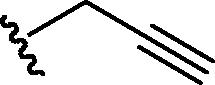 |
82 |
| 4 | Br | 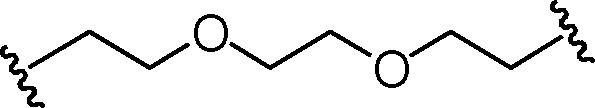 |
70a |
| 5 | Br |  |
69 |
| 6 | Br |  |
80 |
| 7 | Br |  |
60 |
| 8 | Br | 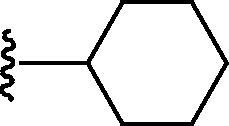 |
78 |
| 9 | Br | 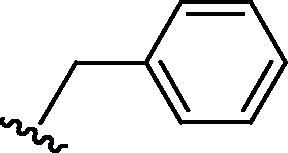 |
86 |
| 10 | Br | 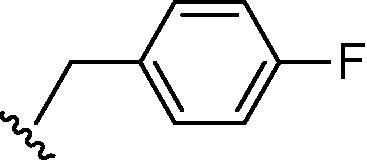 |
72 |
| 11 | Br |  |
19 |
| 12 | Br |  |
78 |
| 13 | Br |  |
99 |
| 14 | Br | 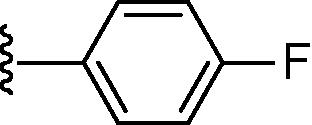 |
64 |
| 15 | Br |  |
46 |
| 16 | H |  |
74 |
| 17 | H | 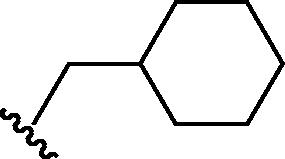 |
62 |
| 18 | H |  |
58 |
| 19 | H | 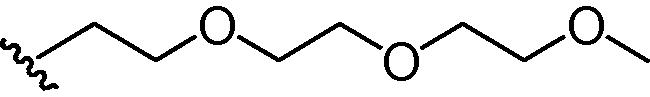 |
44 |
| 20 | H | 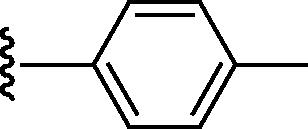 |
63 |
0.4 equiv of diamine used.
The dithiophenolmaleimide variants proved more challenging, and required subtle tuning of the reaction conditions. Addition of amines to activated maleimide 10 led to initial attack to form the acyclic ring-opened intermediates (i.e., cyclisation with release of methyl carbamate was not observed). Upon column chromatography of these intermediates, some cyclisation was observed, implying that the mildly acidic conditions provided by the silica could facilitate the cyclisation. Thus silica was added as a second step in the reaction, and this served to afford the desired dithiophenolmaleimides 11 (Table 3). For simple alkyl amines and benzylamine the yields in this reaction were still disappointing (entries 5–7). In these examples, it was found that the addition of Et3N (1 equiv) at the start of the reaction improved the yield significantly. It could be postulated that the triethylamine was important to prevent protonation, and thus deactivation, of these more basic alkyl amines during the reaction.
Table 3.

Yields in parenthesis indicate the outcome of the reaction when 1 equiv of Et3N was added at the start of the reaction.
Finally, we attempted to transfer this approach to the synthesis of bromopyridazinediones. Thus treatment of activated dibromo- and monobromomaleimides 6 and 8 with N,N′-diethylhydrazine was carried out. Simply stirring the reagents in CH2Cl2 at room temperature led to the desired dibromopyridazinedione 12 and monobromopyridazinedione 13 in moderate yields (Scheme 2).
Scheme 2.

A mild synthesis of bromopyridazinediones.
In conclusion, we have shown that bromomaleimides, dithiophenolmaleimides and bromopyridazinediones can all be accessed by the addition of amines to the appropriate N-methoxycarbonyl activated maleimides. The employed methods are extremely mild and provide a general route to this important class of reagents.
Acknowledgments
We gratefully acknowledge Fundación Alfonso Martín Escudero, RCUK, BBSRC, UCL, Pfizer, and the Wellcome Trust for support of our programme.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2013.04.088.
Contributor Information
Stephen Caddick, Email: s.caddick@ucl.ac.uk.
James R. Baker, Email: j.r.baker@ucl.ac.uk.
Supplementary data
Experimental procedure and spectral data.
References and notes
- 1.Awuah E., Capretta A. J. Org. Chem. 2011;76:3122–3130. doi: 10.1021/jo1025805. [DOI] [PubMed] [Google Scholar]
- 2.Choi D.S., Huang S.L., Huang M.S., Barnard T.S., Adams R.D., Seminario J.M., Tour J.M. J. Org. Chem. 1998;63:2646–2655. doi: 10.1021/jo9722055. [DOI] [PubMed] [Google Scholar]
- 3.Marminon C., Pierre A., Pfeiffer B., Perez V., Leonce S., Joubert A., Bailly C., Renard P., Hickman J., Prudhomme M. J. Med. Chem. 2003;46:609–622. doi: 10.1021/jm0210055. [DOI] [PubMed] [Google Scholar]
- 4.Souffrin A., Croix C., Viaud-Massuard M.-C. Eur. J. Org. Chem. 2012:2499–2502. [Google Scholar]
- 5.Davis P.D., Hill C.H., Lawton G., Nixon J.S., Wilkinson S.E., Hurst S.A., Keech E., Turner S.E. J. Med. Chem. 1992;35:177–184. doi: 10.1021/jm00079a024. [DOI] [PubMed] [Google Scholar]
- 6.Wang J.J., Soundarajan N., Liu N., Zimmermann K., Naidu B.N. Tetrahedron Lett. 2005;46:907–910. [Google Scholar]
- 7.Tedaldi L.M., Smith M.E.B., Nathani R., Baker J.R. Chem. Commun. 2009:6583–6585. doi: 10.1039/b915136b. [DOI] [PubMed] [Google Scholar]
- 8.Smith M.E.B., Schumacher F.F., Ryan C.P., Tedaldi L.M., Papaioannou D., Waksman G., Caddick S., Baker J.R. J. Am. Chem. Soc. 2010;132:1960–1965. doi: 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody P., Smith M.E.B., Ryan C.P., Chudasama V., Baker J.R., Molloy J., Caddick S. ChemBioChem. 2012;13:39–41. doi: 10.1002/cbic.201100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan C.P., Smith M.E.B., Schumacher F.F., Grohmann D., Papaioannou D., Waksman G., Werner F., Baker J.R., Caddick S. Chem. Commun. 2011:5452–5454. doi: 10.1039/c1cc11114k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedaldi L.M., Aliev A.E., Baker J.R. Chem. Commun. 2012:4725–4727. doi: 10.1039/c2cc31673k. [DOI] [PubMed] [Google Scholar]
- 12.Jones M.W., Strickland R.A., Schumacher F.F., Caddick S., Baker J.R., Gibson M.I., Haddleton D.M. J. Am. Chem. Soc. 2012;134:1847–1852. doi: 10.1021/ja210335f. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher F.F., Nobles M., Ryan C.P., Smith M.E.B., Tinker A., Caddick S., Baker J.R. Bioconjugate Chem. 2011;22:132–136. doi: 10.1021/bc1004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathani R.I., Chudasama V., Ryan C.P., Moody P.R., Morgan R.E., Fitzmaurice R.J., Smith M.E.B., Baker J.R., Caddick S. Org. Biomol. Chem. 2013;11:2408–2411. doi: 10.1039/c3ob40239h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher F.F., Sanchania V.A., Tolner B., Wright Z.V.F., Ryan C.P., Smith M.E.B., Ward J.M., Caddick S., Kay C.W.M., Aeppli G., Chester K.A., Baker J.R. Sci. Rep. 2013;3:1525. doi: 10.1038/srep01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones M.W., Strickland R.A., Schumacher F.F., Caddick S., Baker J.R., Gibson M.I., Haddleton D.M. Chem. Commun. 2012:4064–4066. doi: 10.1039/c2cc30259d. [DOI] [PubMed] [Google Scholar]
- 17.Chudasama V., Smith M.E.B., Schumacher F.F., Papaioannou D., Waksman G., Baker J.R., Caddick S. Chem. Commun. 2011:8781–8783. doi: 10.1039/c1cc12807h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L.X., Ni J.H., Singh S. Bioorg. Med. Chem. 2003;11:159–166. doi: 10.1016/s0968-0896(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 19.Heredia K.L., Bontempo D., Ly T., Byers J.T., Halstenberg S., Maynard H.D. J. Am. Chem. Soc. 2005;127:16955–16960. doi: 10.1021/ja054482w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedure and spectral data.