Graphical abstract
Keywords: GABAA receptors, TRPV1 channels, 2-Microelectrode voltage clamp technique, Behavioural pharmacology, Piperine
Abstract
The action of piperine (the pungent component of pepper) and its derivative SCT-66 ((2E,4E)-5-(1,3-benzodioxol-5-yl))-N,N-diisobutyl-2,4-pentadienamide) on different gamma-aminobutyric acid (GABA) type A (GABAA) receptors, transient-receptor-potential-vanilloid-1 (TRPV1) receptors and behavioural effects were investigated.
GABAA receptor subtypes and TRPV1 receptors were expressed in Xenopus laevis oocytes. Modulation of GABA-induced chloride currents (IGABA) by piperine and SCT-66 and activation of TRPV1 was studied using the two-microelectrode-voltage-clamp technique and fast perfusion. Their effects on explorative behaviour, thermoregulation and seizure threshold were analysed in mice. Piperine acted with similar potency on all GABAA receptor subtypes (EC50 range: 42.8 ± 7.6 μM (α2β2)–59.6 ± 12.3 μM (α3β2)). IGABA modulation by piperine did not require the presence of a γ2S-subunit, suggesting a binding site involving only α and β subunits. IGABA activation was slightly more efficacious on receptors formed from β2/3 subunits (maximal IGABA stimulation through α1β3 receptors: 332 ± 64% and α1β2: 271 ± 36% vs. α1β1: 171 ± 22%, p < 0.05) and α3-subunits (α3β2: 375 ± 51% vs. α5β2:136 ± 22%, p < 0.05). Replacing the piperidine ring by a N,N-diisobutyl residue (SCT-66) prevents interactions with TRPV1 and simultaneously increases the potency and efficiency of GABAA receptor modulation. SCT-66 displayed greater efficacy on GABAA receptors than piperine, with different subunit-dependence. Both compounds induced anxiolytic, anticonvulsant effects and reduced locomotor activity; however, SCT-66 induced stronger anxiolysis without decreasing body temperature and without the proconvulsive effects of TRPV1 activation and thus may serve as a scaffold for the development of novel GABAA receptor modulators.
1. Introduction
Piperine (1-piperoylpiperidine) is the pungent component of several pepper species and activates transient receptor potential of the subfamily V member 1 (TRPV1) receptors [1,2]. We have recently shown that piperine modulates γ-aminobutyric acid (GABA) type A (GABAA) receptors [3]. Via TRPV1-activation, piperine affects pain signalling and regulation of the body temperature [4,5], while GABAA receptor modulation is expected to induce fast inhibitory synaptic neurotransmission in the mammalian brain, resulting in, for example, anxiolysis, sedation, hypnosis, muscle relaxation, analgesia and anticonvulsant effects [6–11].
Piperine complies in all respects with Lipinski's “rule of five” and could therefore be a scaffold for the development of novel GABAA receptor modulators [3,12]. However, it is currently unknown whether piperine interacts preferentially with specific GABAA receptor subtypes. Moreover, simultaneous activation of TRPV1 receptors may cause unwanted side effects including changes in pain sensation and body temperature that would be an obstacle to its therapeutic use [5]. Here we analyse the action of piperine and its derivative SCT-66 ((2E,4E)-5-(1,3-benzodioxol-5-yl))-N,N-diisobutyl-2,4-pentadienamide) on nine GABAA receptor subtypes and on TRPV1 receptors. Unlike piperine, SCT-66 did not activate TRPV1 receptors. This compound increased IGABA more potently and more efficaciously than piperine, although with altered subunit dependence. In vivo studies in mice revealed that only piperine affects thermoregulation; that both piperine and SCT-66 have anticonvulsant and anxiolytic effects and reduce locomotor activity; and that SCT-66 has a stronger anxiolytic effect than piperine.
2. Materials and methods
All procedures involving animals were approved by the Austrian Animal Experimentation Ethics Board in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS No. 123). Every effort was made to minimize the number of animals used.
2.1. Reagents
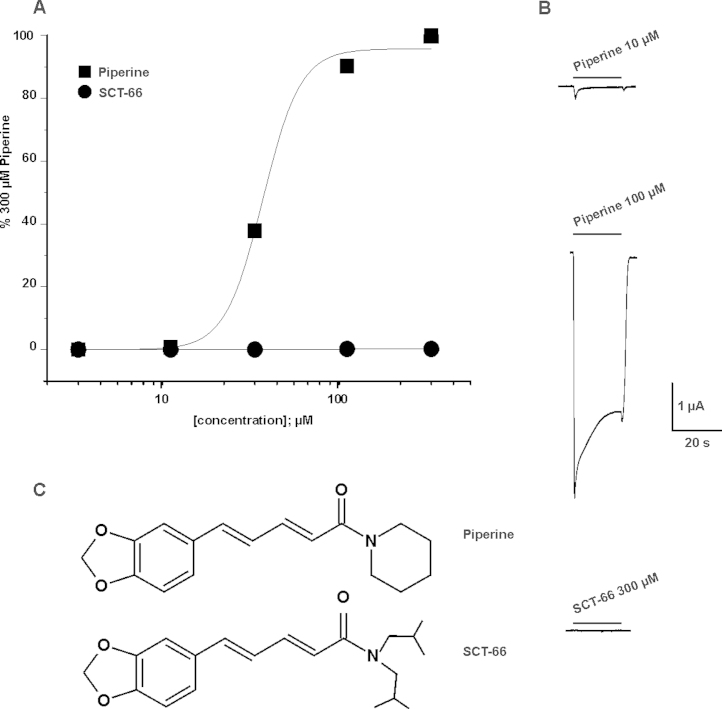
Piperine was obtained from Sigma™ (Vienna, Austria) and the piperine derivative SCT-66 (2E,4E)-5-(1,3-benzodioxol-5-yl))-N,N-diisobutyl-2,4-pentadienamide) was synthesized as described below (for structural formulae see Fig. 1): To a solution of piperic acid chloride (3 mmol, 0.71 g) in 10 mL dry THF, diisobutylamine (10.5 mmol; 1.357 g) was added and stirred overnight. The reaction mixture was evaporated and purified by column chromatography (toluene/ethyl acetate 20:3) to give the compound SCT-66 (0.661 g, 67%) as oil.
Fig. 1.
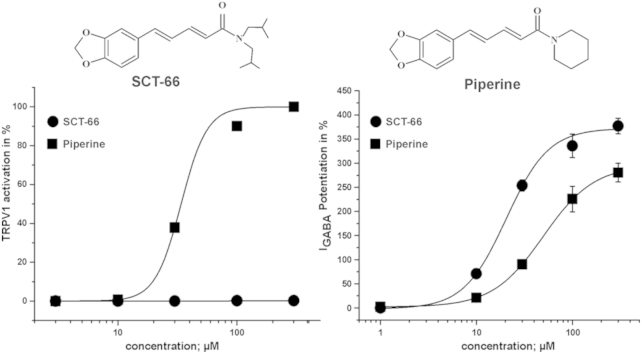
Comparison of TRPV1 activation by piperine and SCT-66. (A) The concentration–response relationship for piperine (■; 3–300 μM) and SCT-66 (●, 3–300 μM) are shown. These normalized data were generated by measuring the net currents evoked in response to a test concentration of agonist and are expressed as a percentage of a preceding 300 μM piperine control response recorded in the same cell. Data are expressed as the mean ± S.E.M with n = 3–10 individual cells. The EC50 for piperine was 33.3 ± 0.1 μM (Hill coefficient of 4.1 ± 0.1; n = 3–10 per concentration). The EC50 value of piperine agrees with [2]. (B) Typical traces showing activation of TRPV1 channels by piperine and the lack of TRPV1 activation by SCT-66 at the indicated concentrations. (C) Structural formulae of piperine and its derivative SCT-66.
1H NMR (200 MHz, CDCl3): δ 7.,54–7.34 (m, 1H), 7,00 (d, J = 1.4 Hz, 1H), 6.90 (dd, J = 8.0, 1.6 Hz, 1H), 6.84–6.71 (m, 3H), 6.39 (d, J = 14.6 Hz, 1H), 5.97 (s, 2H), 3.28 (d, J = 7.5 Hz, 2H), 3.19 (d, J = 7.5 Hz, 2H), 2.12–1.88 (m, 2H), 0.98–0.82 (m, 12H). 13C NMR (50 MHz, CDCl3): δ 167.0, 148.4, 148.3, 142.5, 138.5, 131.2, 125.6, 122.7, 120.8, 108.7, 105.9, 101.5, 56.2, 54.9, 29.2, 27.2, 20.5, 20.3. MS m/z: 329 (12%, M+), 201 (100%), 115 (39%), 57 (17%), 43 (23%). CHN for C20H27NO3: calc.: C 72.92, H 8.26, N 4.25; found: C 72.78, H 8.13, N 4.16.
Stock solutions of piperine and SCT-66 were prepared in 100% DMSO (100 mM for oocyte experiments, 10 mg/μl for animal experiments; Dimethyl Sulfoxide). All chemicals were purchased from Sigma™, Vienna, Austria except where stated otherwise.
2.2. Expression and functional characterization of GABAA receptors and TRPV1 channels
Preparation of stage V-VI oocytes from Xenopus laevis and synthesis of capped off run-off poly(A+) cRNA transcripts from linearized cDNA templates (pCMV vector) were performed as previously described [13]. Briefly, female X. laevis (NASCO™, Fort Atkinson, WI, USA) were anaesthetized by exposing them for 15 min to a 0.2% solution of MS-222 (methane sulfonate salt of 3-aminobenzoic acid ethyl ester) before surgically removing parts of the ovaries. Follicle membranes from isolated oocytes were digested with 2 mg/ml collagenase (Type 1A). Selected stage V-VI oocytes were injected with about 10–50 nl of DEPC- treated water (diethyl pyrocarbonate) containing the different cRNAs at a concentration of approximately 300–3000 pg/nl. The amount of cRNA was determined by means of a NanoDrop ND-1000 (Kisker-biotech™, Steinfurt, Germany).
GABAA receptors: To ensure expression of the gamma-subunit in rat GABAA receptors, cRNAs for expression of α1β2γ2S, α2β2γ2S, α3β2γ2S and α5β2γ2S receptors were mixed in a ratio of 1:1:10. For receptors comprising only α and β subunits (α1β2, α2β2, α1β3, α2β2, α3β2, α5β2), the cRNAs were mixed in a ratio 1:1. cRNAs for α1β1 channels were injected in a ratio 3:1 to avoid formation of β1 homomeric GABAA receptors [14,15].
TRPV1 channels: The rat TRPV1 clone was a gift from Prof. David Julius (Department of Cellular and Molecular Pharmacology, University of California, San Francisco).
After injection, oocytes were stored at 18 °C for 24–48 h in ND96 solution containing penicillin G (10 000 IU/100 ml) and streptomycin (10 mg/100 ml) [16]. Electrophysiological experiments on GABAA receptors and TRPV1 channels were performed using the two-microelectrode-voltage-clamp method at a holding potential of −70 mV (GABAA receptors) and −60 mV (TRPV1), respectively, making use of a TURBO TEC 01 C amplifier (npi electronic™, Tamm, Germany) and an Axon Digidata 1322A interface (Molecular Devices™, Sunnyvale, CA). Data acquisition was done using pCLAMP v.9.2. The bath solution contained 90 mM NaCl, 1 mM KCl, 1 mM MgCl2·6H2O, 1 mM CaCl2 and 5 mM HEPES (pH 7,4). Microelectrodes were filled with 2 M KCl.
2.3. Perfusion system
GABA, piperine and SCT-66 were applied by means of a fast perfusion system [17, ScreeningTool, npi electronic™, Tamm, Germany] to study IGABA modulation and TRPV1 activation. To elicit IGABA, the chamber was perfused with 120 μl of GABA-containing solution at a volume rate between 300 and 1000 μl/s. The IGABA rise time ranged from 100 to 250 ms [13].
To account for possible slow recovery from increasing levels of desensitization in the presence of high GABA or piperine/SCT-66 concentrations, the duration of washout periods was extended from 1.5 min (for 1–10 μM GABA, <10 μM piperine/SCT-66) to 30 min (for ≥30 μM GABA, ≥10 μM piperine/SCT-66). To exclude voltage-clamp errors, oocytes with maximal current amplitudes >3 μA were discarded.
Because of low solubility in the bath solution, piperine and SCT-66 were used up to a concentration of 300 μM. Equal amounts of DMSO were present in all testing solutions. The maximum DMSO concentration in the bath (0.3%) had no observable effects on IGABA or TRPV1.
2.4. Analysing concentration–response curves
Stimulation of chloride currents by modulators of the GABAA receptor was measured at a GABA concentration eliciting between 3 and 7% of the maximal current amplitude (EC3–7). The EC3–7 was determined at the beginning of each experiment.
Enhancement of the chloride current was defined as (I(GABA+Comp)/IGABA) − 1, where I(GABA+Comp) is the current response in the presence of a given compound and IGABA is the control GABA current. Concentration–response curves for activation of TRPV1 channels were generated by comparing the peak response evoked by a test concentration of the compounds at the different concentrations to that evoked by a previous control current recorded in response to 300 μM piperine.
Data were fitted by non-linear regression analysis using Origin software (OriginLab Corporation, USA). Data were fitted to the equation: 1/(1 + (EC50/[Comp])nH), where nH is the Hill coefficient. Each data point represents the mean ± S.E.M. from at least 3 oocytes and ≥2 oocyte batches.
2.5. Behavioural analysis
2.5.1. Animals
Male mice (C57BL/6N) were obtained from Charles River Laboratories™ (Sulzfeld, Germany). For maintenance, mice were group-housed (maximum 5 mice per type IIL cage) with free access to food and water. At least 24 h before the commencement of experiments, mice were transferred to the testing facility, where they were given free access to food and water. The temperature in the maintenance and testing facilities was 23 ± 1 °C; the humidity was 40–60%; a 12 h light–dark cycle was in operation (lights on from 07:00 to 19:00). Only male mice aged 3–6 months were tested. Compounds were applied by intraperitoneal (i.p.) injection of aqueous solutions (either control or compound) 30 min before each test, except for body temperature, which was measured 3 h after injection. Testing solutions were prepared in a solvent composed of saline 0.9% NaCl solution with 10% DMSO and 3% Tween 80. The final DMSO concentration did not exceed 10% (see [18] for effects of DMSO on blood-brain barrier penetration). 1 M NaOH was used to adjust the pH to 7.4. All solutions were prepared freshly on the day of the experiment. Application of the solvent alone did not influence animal behaviour.
2.5.2. Measurement of body temperature
A temperature probe (Type T Thermocouple probe RET-3 connected to a Type T Thermometer, Physitemp Instruments Inc™; Clifton, USA), lubricated with glycerol, was inserted into the rectum of the mouse for a depth of up to 1 cm. The temperature probe remained in the animal till a stable temperature was reached (maximum 10 s).
2.5.3. Open Field Test (OF)
Ambulation was tested over 10 min in a 50 cm × 50 cm × 50 cm field box equipped with infrared rearing detection. Illumination was set to 150 lx. The explorative behaviour of C57BL/6N mice was analysed using the Actimot2 equipment and software (TSE-systems™, Bad Homburg, Germany). Areas were subdivided into border (up to 8 cm from wall), centre (20 cm × 20 cm, i.e. 16% of total area), and intermediate areas according to the recommendations of EMPRESS (European Mouse Phenotyping Resource of Standardized Screens; http://empress.har.mrc.ac.uk). The test was automatically started when the mouse was placed in the centre area.
2.5.4. Elevated Plus Maze Test (EPM)
The animal's behaviour was tested over 5 min on an elevated plus maze 1 m above ground consisting of two closed and two open arms, each 30 cm × 5 cm in size. The height of the closed arm walls was 20 cm. Illumination was set to 180 lx. Animals were placed in the centre, facing an open arm. Analysis was done automatically with Video-Mot2 equipment and software (TSE-systems™, Bad Homburg, Germany) [19].
2.5.5. Seizure threshold
Seizure threshold was determined by pentylentetrazole (PTZ)-tail-vein infusion on freely moving animals at a rate of 100 μl/min (100 mg/ml PTZ in saline). Infusion was stopped when animals displayed generalized clonic seizures. Animals were killed by cervical displacement immediately after the first generalized seizure. The seizure threshold dose was calculated from the infused volume in relation to body weight [20]. Piperine and SCT-66 were applied 30 min before PTZ infusion. Control animals were pre-treated with 10% DMSO in saline containing 3% Tween 80. At the infusion rate of 100 μl/min, generalized seizures are induced within 2 min after beginning infusion of PTZ.
2.5.6. Statistical analysis
Statistical significance of electrophysiological data was calculated using a paired Student t-test with a confidence interval of p < 0.05; for in vivo experiments, one-way ANOVA (Bonferroni Adjustment) was used. Statistical analysis was done with Origin software (OriginLab Corporation; USA). p-values of <0.05 were accepted as statistically significant. All data are given as mean ± S.E.M. (n).
3. Results
3.1. Replacing the piperidine ring by a N,N-diisobutyl-residue prevents activation of TRPV1 receptors
In line with previous studies piperine induced marked inward currents when applied to oocytes expressing TRPV1 receptors (Fig. 1A and B, [2]). A simple structural modification (replacing the piperidine ring by a N,N-diisobutyl residue; Fig. 1C) completely eliminated activation of TRPV1 receptors by SCT-66 (300 μM, Fig. 1A and B).
3.2. Different γ2 subunit dependence of piperine and SCT-66
In order to analyse the interaction of piperine and SCT-66 with different GABAA receptor subtypes, receptors composed of different subunits were heterologously expressed in Xenopus oocytes and IGABA modulation by both compounds was studied by means of the 2-microelectrode voltage-clamp technique and a fast-perfusion system (see Section 2).
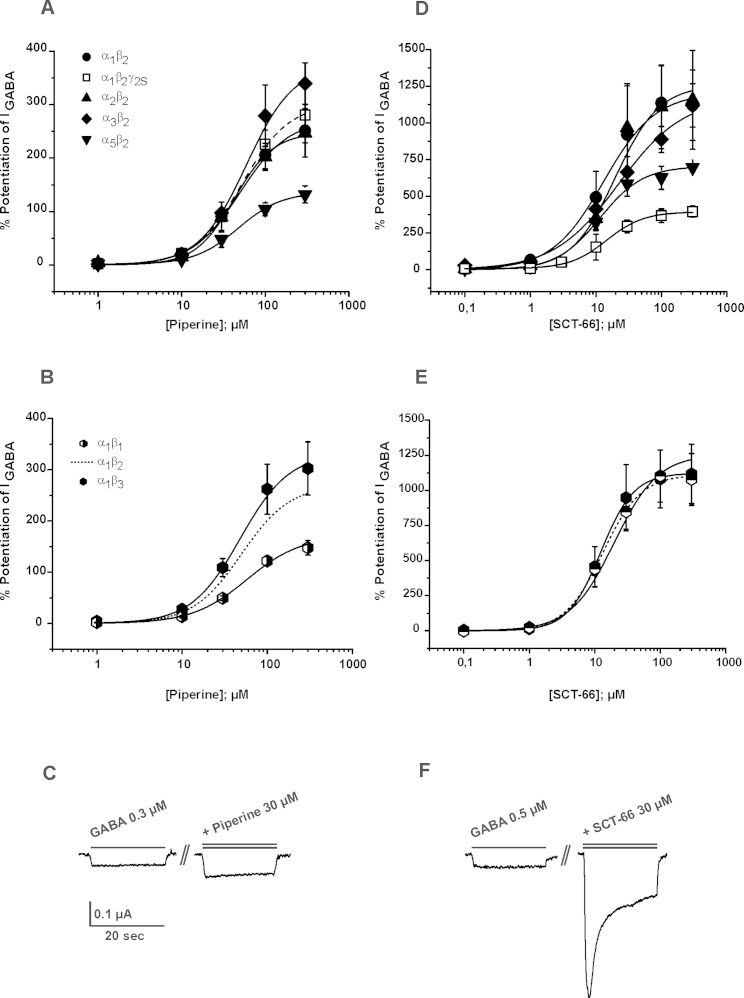
First the enhancement of IGABA by piperine and SCT-66 through α1β2 and α1β2γ2S receptors was compared. As illustrated in Fig. 2A, omitting the γ2S subunit had no significant effect on IGABA enhancement (IGABA,max) or on the potency (EC50) of piperine (α1β2: EC50 = 50.0 ± 7.9 μM, IGABA,max = 271 ± 36%, n = 13 vs. α1β2γ2S: EC50 = 52.4 ± 9.4 μM, IGABA,max = 302 ± 27%; n = 6; p > 0.05; data for modulation of IGABA through α1β2γ2S receptors by piperine taken from [3]). This finding suggests that piperine interacts with a binding site located on α and/or β subunits or the α/β interface. In contrast, co-expression of a γ2S subunit resulted in significant reduction of IGABA enhancement by SCT-66 (α1β2: 1256 ± 292%; n = 4; p < 0.05; α1β2γ2S: 378 ± 15%, n = 6; α2β2γ2S: 572 ± 51%, n = 5; α3β2γ2S: 584 ± 20, n = 5 and α5β2γ2S: 398 ± 26%, see Fig. 2D, Tables 1 and 2) suggesting a role of γ2 in receptor modulation. Co-expression of a γ2S-subunit did, however, not significantly affect the potency of SCT-66 (see Tables 1 and 2).
Fig. 2.
IGABA modulation by piperine and SCT-66 concentration–response curves for IGABA modulation through GABAA receptors of the indicated subunit combinations by piperine (A and B) and SCT-66 (D and E) at a GABA concentration eliciting 3–7% of the maximal GABA response (EC3–7). The enhancement of IGABA by piperine trough α1β2γ2S receptors (dashed line) receptors is taken from [3]. Each data point represents the mean ± S.E.M. from at least five oocytes and at least two oocyte batches. (C and F) Typical traces illustrating IGABA enhancement by 30 μM compound. Control currents (GABA, single bar) and corresponding currents elicited by co-application of GABA and 30 μM piperine/SCT-66 (double bar) are shown.
Table 1.
Potency and efficiency of piperine/SCT-66 enhancement of GABAA receptors with different subunit compositions.
| Subunit combination | EC50 (μM) | Maximum stimulation of-IGABA at EC3–7 | Hill coefficient (nH) | Number of experiments (n) |
|---|---|---|---|---|
| Piperine | ||||
| α1β1 | 57.6 ± 4.2 | 171 ± 22 | 1.4 ± 0.2 | 10 |
| α1β2 | 50.0 ± 7.9 | 271 ± 36 | 1.5 ± 0.3 | 13 |
| α1β3 | 48.3 ± 7.3 | 332 ± 64 | 1.5 ± 0.3 | 7 |
| α2β2 | 42.8 ± 17.6 | 248 ± 48 | 1.9 ± 0.5 | 6 |
| α3β2 | 59.6 ± 12.3 | 375 ± 51 | 1.4 ± 0.2 | 6 |
| α5β2 | 47.5 ± 17.9 | 136 ± 22 | 1.7 ± 0.4 | 6 |
| SCT-66 | ||||
| α1β1 | 13.3 ± 2.9 | 1112 ± 136 | 1.5 ± 0.2 | 4 |
| α1β2 | 19.8 ± 9.7 | 1256 ± 292 | 1.3 ± 0.4 | 4 |
| α1β3 | 12.3 ± 4.5 | 1128 ± 155 | 1.5 ± 0.3 | 3 |
| α1β2γ2S | 21.5 ± 1.7 | 378 ± 15 | 1.8 ± 0.2 | 6 |
| α2β2 | 13.1 ± 9.0 | 1204 ± 233 | 1.1 ± 0.3 | 4 |
| α2β2γ2S | 24.1 ± 7.5 | 572 ± 51 | 1.3 ± 0.3 | 5 |
| α3β2 | 22.2 ± 12.1 | 1169 ± 195 | 0.9 ± 0.2 | 3 |
| α3β2γ2S | 15.1 ± 1.8 | 584 ± 20 | 1.6 ± 0.2 | 5 |
| α5β2 | 11.5 ± 2.7 | 705 ± 24 | 1.3 ± 0.2 | 3 |
| α5β2γ2S | 14.2 ± 1.4 | 398 ± 26 | 2.0 ± 0.3 | 5 |
Table 2.
Comparison of efficiencies for GABAA receptors of different subunit compositions. (*) indicates statistically significant (p < 0.05) differences.
| Piperine | |||||||
|---|---|---|---|---|---|---|---|
| α1β2 | α1β2 | α1β3 | α1β2γ2S1 | α2β2 | α3β2 | α5β2 | |
| α1β1 | * | * | * | ||||
| α1β2 | * | * | |||||
| α1β3 | * | * | |||||
| α1β2γ2Sa | * | * | |||||
| α2β2 | * | ||||||
| α3β2 | * | ||||||
| α5β2 | * | * | * | * | * | ||
| SCT-66 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α1β1 | α1β2 | α1β3 | α1β2γ2S | α2β2 | α2β2γ2S | α3β2 | α3β2γ2S | α5β2 | α5β2γ2S | |
| α1β1 | * | * | * | * | * | |||||
| α1β2 | * | * | ||||||||
| α1β3 | * | * | * | * | * | |||||
| α1β2γ2S | * | * | * | * | * | * | * | * | ||
| α2β2 | * | * | * | * | ||||||
| α2β2γ2S | * | * | * | * | * | * | ||||
| α3β2 | * | * | * | * | ||||||
| α3β2γ2S | * | * | * | * | * | * | * | |||
| α5β2 | * | * | * | * | * | |||||
| α5β2γ2S | * | * | * | * | * | * | * | * | * | |
Emax values for enhancement of IGABA through α1β2γ2S receptors by piperine are taken from [3].
3.3. Piperine potentiates GABAA receptors composed of α1/2/3/5 and β1/2/3 subunits
In order to investigate a potential subunit dependent action of piperine and SCT-66, we studied their interaction with 8 different receptor subtypes (α1β1, α1β2, α1β3, α2β2, α3β2 and α5β2) (Fig. 2A, B, D and E, Table 1). The highest efficacy of piperine was observed for receptors containing α3 subunits, with maximal IGABA potentiation (EC3–7) of 375 ± 51% (n = 6), followed by GABAA receptors composed of α1 and β2 subunits (271 ± 36%, n = 13) and α2 and β2 subunits, respectively (248 ± 48; n = 6) (see also Table 1). Piperine was significantly less efficacious on α5β2 receptors (IGABA,max = 136 ± 22%, n = 6, Fig. 2A, Tables 1 and 2). The potencies of IGABA modulation, however, did not significantly differ with EC50 values ranging from 42.8 ± 17.6 μM (α2β2) to 59.6 ± 12.3 μM (α3β2), Fig. 2B illustrates the effect of piperine on GABAA receptors with three different β-subunits. α1β2 and α1β3 receptors were more efficaciously modulated by piperine than α1β1 receptors (maximal IGABA modulation of α1β2 receptors: 271 ± 36%, α1β3 332 ± 64% vs. α1β1 receptors: 171 ± 22%; (see Fig. 2 C for representative IGABA through GABAA receptors composed of α3 and β2 subunits in the absence and presence of 30 μM piperine).
3.4. Higher potency and different subunit dependence of SCT-66
SCT-66 displayed a higher potency on all subunit compositions tested (Fig. 2E and F, Tables 1 and 2 e.g. on α1β2γ2S receptors: EC50(SCT-66): 21.5 ± 1.7 μM, n = 6 compared to EC50(piperine):57.6 ± 4.2 μM, n = 6, p < 0.01 and IGABA was more efficaciously modulated by SCT-66 than by piperine. Stronger maximal IGABA enhancement by SCT-66 ranged from 1.2-fold (α1β2γ2S receptors) to 6.5-fold (α1β1) (Tables 1–2). Taken together, the stronger IGABA enhancement by SCT-66 was accompanied by an apparent change in receptor subtype dependence (SCT-66 was e.g. equally efficacious on receptors comprising different β-subunits compared to piperine that was more efficacious on β2/3 incorporating receptors, compare Fig. 2B to Fig. 2E).
3.5. Piperine and SCT-66 shift the GABA concentration–response curve
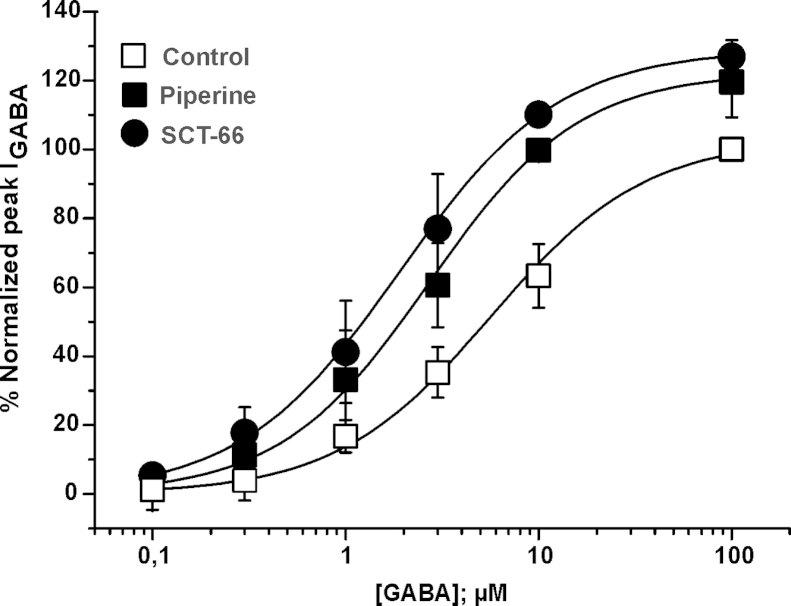
GABA concentration–response curves in the presence of piperine and SCT-66 for α3β2 receptors are compared in Fig. 3. Almost-saturating concentrations of piperine and SCT-66 (100 μM, Fig. 2A, B, D and E) shifted the curves to the left (5.7 ± 1.9 μM and nH = 1.1 ± 0.1 (control); 2.7 ± 0.8 μM and nH = 1.1 ± 0.2 (piperine), and 1.9 ± 0.4 μM and nH = 1.1 ± 0.1 (SCT-66). Enhancement of IGABA,max by piperine and SCT-66 was statistically not significant (IGABA; max-piperine = 123 ± 3; n = 4 and IGABA; max-SCT-66= 129 ± 6%, n = 3; p > 0.05). Neither piperine nor SCT-66 (up to 300 μM) activated GABAA receptors when applied in the absence of GABA.
Fig. 3.
Piperine and SCT-66 shift the GABA concentration–response curve towards higher GABA sensitivity GABA concentration–response curves for α3β2 GABAA receptors in the absence (control, □) and in the presence of 100 μM piperine (■), and 100 μM SCT-66 (●) are compared. The corresponding EC50 values and Hill-coefficients were 5.7 ± 1.9 μM and nH = 1.1 ± 0.1 (control) and 2.7 ± 0.8 μM and nH = 1.1 ± 0.2 (piperine), and 1.9 ± 0.4 μM and nH = 1.1 ± 0.1 (SCT-66), respectively. Each data point represents the mean ± S.E.M. from at least four oocytes and at least two oocyte batches.
3.6. Effects of piperine and SCT-66 on thermoregulation
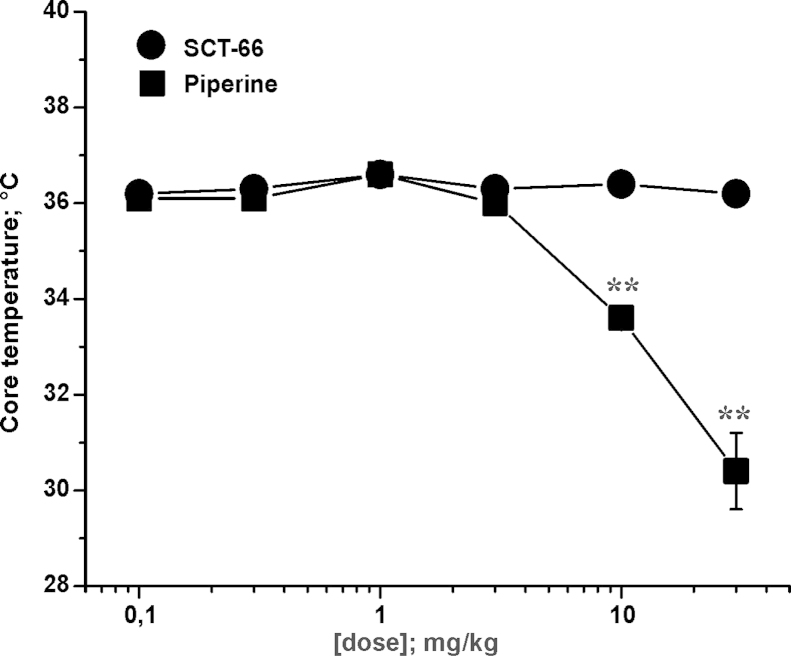
Changes in body temperature might indicate activation of TRPV1 channels in vivo [21]. Core body temperature of male C57BL/6N mice was measured rectally shortly before application of saline, piperine or SCT-66. Basal values did not differ between the groups, averaging 36.80 ± 0.04 °C (n = 184). This temperature measurement was repeated 3 hours after injection of compound (to avoid interference from stress-induced hyperthermia early after injection). As illustrated in Fig. 4, a dramatic drop of body temperature was observed after injection of piperine at doses higher than 3 mg/kg bodyweight: application of 10 mg/kg bodyweight piperine significantly (p < 0.01) reduced body temperature of mice (Control: 36.10 ± 0.10 °C; n = 38 vs. 10 mg/kg bodyweight piperine 34.86 ± 0.29 °C; n = 16). An even more pronounced effect was observed upon application of 30 mg/kg bodyweight: body temperature was lowered to 30.37 ± 0.84 °C (n = 9; p < 0.01). In contrast, no significant changes in body temperature were observed after application of SCT-66 at all tested doses (see Fig. 4), thereby resulting in a statistically significant difference between the two drugs as analysed by one-way ANOVA (p < 0.01).
Fig. 4.
SCT-66 does not reduce body temperature in mice Effects of piperine and SCT-66 on body temperature 3 h after injection of (■) piperine or (●) SCT-66 at the indicated doses (mg/kg bodyweight) are illustrated. Each data point represents the mean ± S.E.M. of at least 9 mice. (**) indicates statistically significant (p < 0.01) differences to control (ANOVA with Bonferroni).
3.7. Piperine and SCT-66 reduce locomotor activity
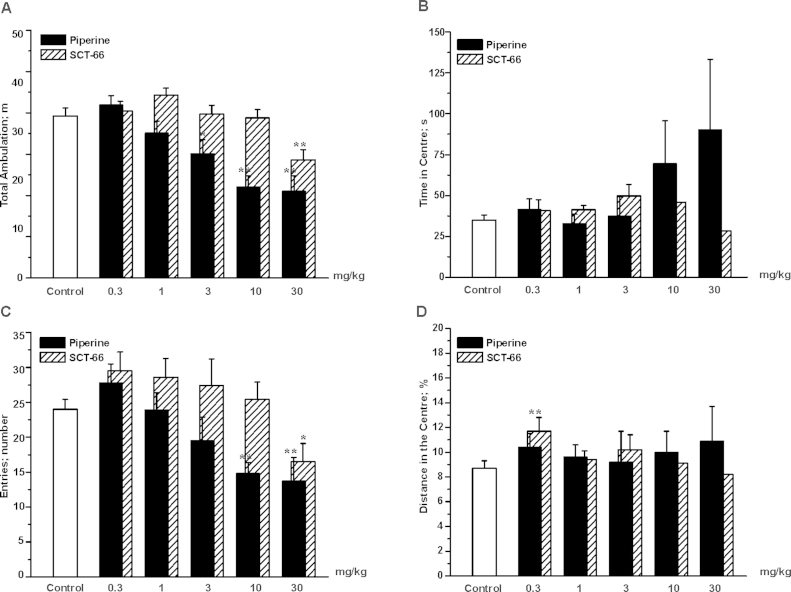
In the Open-Field-Test (OF, see Section 2), control mice covered a distance of 39.3 ± 1.9 m, (n = 20; Fig. 5; white bar). Injection of piperine resulted in a dose-dependent reduction of ambulation: significant reductions were apparent from doses ≥3 mg/kg bodyweight, and the highest dose of 30 mg/kg reduced ambulation by approximately 50% compared to control littermates (control: 39.3 ± 1.9 m; n = 20 vs. 30 mg/kg bodyweight piperine 21.0 ± 3.7 m; n = 13; p < 0.01; see Fig. 5A; black bars for piperine). Unlike piperine, SCT-66 did not affect ambulation over a broad range (0.3–10 mg/kg bodyweight; see Fig. 5A, SCT-66 shaded bars). Only at a dose of 30 mg/kg bodyweight SCT-66 significantly reduced locomotor activity (Control: 39.3 ± 1.9 m; n = 20 vs. 30 mg/kg bodyweight SCT-66: 28.6 ± 2.5 m, n = 10, p < 0.01), however, this effect was still weaker than with piperine at the same dose.
Fig. 5.
Piperine and SCT-66 dose-dependently reduce locomotor activity in the OF test. Bars indicate in (A) the total distance travelled, in (B) the time spent in the centre, in (C) the number of entries to the centre and in (D) the distance travelled in the centre as % of the total distance after application of the indicated dose (mg/kg bodyweight) of piperine (black bars), SCT-66 (shaded bars) or control (white bars). Bars always represent means ± S.E.M. from at least 8 different mice. (*) indicates statistically significant differences with p < 0.05, (**) p < 0.01 to control (ANOVA with Bonferroni).
3.8. Piperine and SCT-66 influence anxiety-related behaviour in the OF test
The marked influence of even low doses of piperine (≥3 mg/kg) on the locomotor activity of mice makes it difficult to analyse anxiolytic properties in activity-based testing conditions. At lower doses, the only difference observed was an increase in distances travelled in the centre area (control: 8.8 ± 0.6%, n = 20 vs. SCT-66 0.3 mg/kg bodyweight: 10.7 ± 1.1%, n = 12; p < 0.05) in mice treated with SCT-66 at a dose of 0.3 mg/kg bodyweight.
3.9. Piperine and SCT-66 reduce anxiety-related behaviour in the EPM test
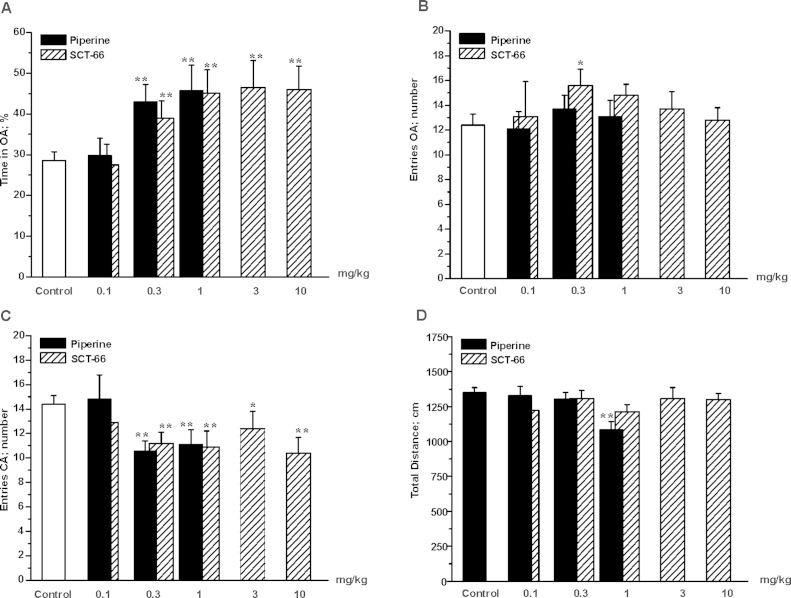
In order to analyse the impact of piperine and SCT-66 on anxiety-related behaviour, male C57BL/6N mice were tested 30 min after i.p. injection in the Elevated-Plus-Maze-test (EPM, see Materials and Methods section). As illustrated in Fig. 6A, control mice (treated with saline; white bar) spent 28.6 ± 2.1% of the total test time in the open arms (OA) of the EPM (n = 27). While the behaviour of mice treated with 0.1 mg/kg bodyweight of piperine did not significantly differ from saline-treated control littermates, upon application of higher doses (i.e. 0.3 and 1 mg/kg bodyweight) mice spent significantly (p < 0.01) more time in the OA (0.3 mg/kg bodyweight: 43.0 ± 4.2%, n = 22 and 1 mg/kg bodyweight: 45.7 ± 6.3%, n = 16, black bars). At a dose of 1 mg/kg bodyweight piperine significantly reduced ambulation (see Fig. 6D), thus, higher doses were not investigated. Unlike piperine, SCT-66 did not significantly influence overall ambulation at the tested doses (0.3–10 mg/kg bodyweight; see Fig. 6D shaded bars). As shown in Fig. 6A, a significant increase in the time spent in the OA was observed with increasing doses of SCT-66, reaching a maximum at a dose of 1 mg/kg bodyweight (control: 28.6 ± 2.1, n = 27 vs. 1 mg/kg bodyweight SCT-66: 45.1 ± 5.7%, n = 14, p < 0.01). This effect remained stable and did not change even when applying higher doses (3–10 mg/kg bodyweight). Moreover, mice treated with 0.3 mg/kg bodyweight SCT-66 visited the OA more frequently than control mice (control: 12.4 ± 0.9, n = 27 vs. 0.3 mg/kg bodyweight SCT-66: 13.7 ± 1.1, n = 22, p < 0.05), while the number of visits to the OA did not differ at the other doses of piperine and SCT-66, respectively (see Fig. 6B). Accordingly, the number of closed arm (CA) entries also dropped significantly at doses ≥0.3 mg/kg bodyweight piperine and SCT-66, respectively (Fig. 6 C).
Fig. 6.
Piperine and SCT-66 display anxiolytic-like effects in the EPM test. Bars indicate in (A) the time spent in the open arms (OA) in % of the total time, in (B) the number of OA entries, in (C) the number of closed arm (CA) entries and in (D) the total distance after application of the indicated dose in mg/kg bodyweight of either piperine (black bars) or SCT-66 (shaded bars), respectively. White bars illustrate the behaviour of control mice. Bars represent means ± S.E.M. from at least 9 different mice. (*) indicates statistically significant differences with p < 0.05, (**) p < 0.01 to control (ANOVA with Bonferroni).
3.10. Piperine and SCT-66 modulate seizure threshold
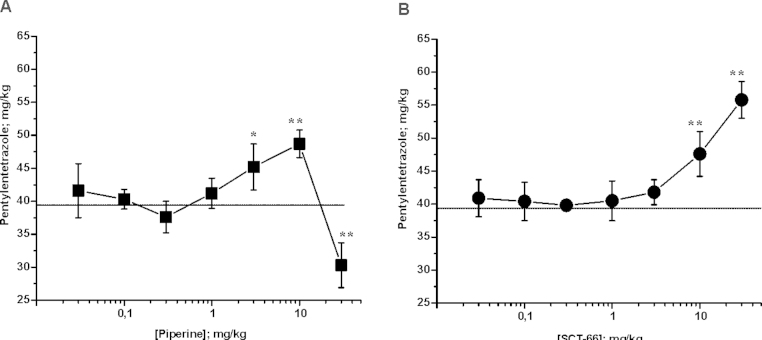
The seizure threshold as assessed using pentylentetrazole (PTZ) tail vein infusions was significantly increased 30 min after i.p. injection of piperine at 3 or 10 mg/kg bodyweight (Control: 39.4 ± 2.8 mg/kg bodyweight PTZ; n = 7; vs. 3 mg/kg bodyweight piperine: 46.2 ± 5.4 mg/kg bodyweight PTZ; n = 4; p < 0.05 and 10 mg/kg bodyweight piperine, respectively: 48,7 ± 2.1 mg/kg bodyweight PTZ; n = 4; p < 0.01). A dose of 30 mg/kg bodyweight, however, resulted in a significant drop in seizure threshold (30.3 ± 3.4 mg/kg bodyweight PTZ; n = 4; p < 0.01; Fig. 7A). Doses below 3 mg/kg bodyweight did not affect seizure threshold.
Fig. 7.
Piperine and SCT-66 affect seizure threshold differently. Changes in seizure threshold upon PTZ-infusion of the indicated dose (mg/kg bodyweight) of piperine (A) and SCT-66 (B) are depicted. Each data point represents the mean ± S.E.M. of a least 3 mice. (*) indicates statistically significant differences with p < 0.05, (**) p < 0.01 to control (ANOVA with Bonferroni).
Unlike piperine, SCT-66 did not display any observable effects on the seizure threshold up to 3 mg/kg bodyweight. Only higher doses significantly raised the seizure threshold (10 mg/kg bodyweight SCT-66: 47.6 ± 3.4 mg/kg bodyweight PTZ; n = 4; p < 0.01 and 30 mg/kg bodyweight SCT-66: 55.8 ± 2.8 mg/kg bodyweight PTZ, n = 4, p < 0.01; Fig. 7B).
4. Discussion
Natural products from distinct structural classes including flavonoids [22–25], terpenoids [26–28], sesquiterpenes [29–31], diterpenes [32], triterpene glycosides [33], polyacetylenes [34], (neo)lignans [28,35], alkaloids [3] or (furano)coumarins [36,37] have been shown to modulate GABAA receptors.
We have recently reported that besides activating TRPV1 receptors [2] piperine modulates GABAA receptors [3]. Here we report that replacing the piperidine ring by a N,N-diisobutyl-residue prevents activation of TRPV1 (Fig. 1A and B). In order to get insights into their therapeutic potentials we subsequently characterized the actions of piperine and its derivative SCT-66 in vitro and in vivo.
4.1. Subunit-dependent modulation of GABAA receptors by piperine
Comparable enhancement of IGABA through α1β2 receptors as through the α1β2γ2S [3] and the similar potencies on the two receptor subtypes suggests that piperine interacts with a binding site located on α and/or β subunits. This hypothesis is in line with our previous findings that GABAA receptor modulation by piperine is not blocked by flumazenil [3].
IGABA enhancement by piperine was most efficacious for GABAA receptors with α3 subunits, weakest for GABAA receptors incorporating α5 subunits (Fig. 2A) and dependent on the β-subunit (Fig. 2B). While there was no significant difference in enhancement of IGABA through GABAA receptors with either β2 or β3 subunits, incorporation of β1 subunits reduced enhancement of IGABA (see also Fig. 2B).
4.2. SCT-66 modulates GABAA receptors with higher potency and efficiency
A principle finding was that replacing the piperidine ring by a N,N-diisobutyl-residue did not only diminish interaction with TRPV1 receptors but additionally increased potency and efficacy of GABAA receptor modulation and affected subunit dependency (Figs. 2E, D and Table 1). Replacing the piperidine ring by a N,N-diisobutyl-residue not only diminished the β2/3 subunit dependence (Fig. 2F), but also induced γ-subunit dependence. Hence, IGABA stimulation in α1β2γ2S receptors was about four times smaller than in α1β2 receptors. These data suggest differences in the binding pockets of the two molecules and/or the existence of an additional binding site for SCT-66 involving the γ-subunit.
4.3. Consequences of different receptor specificity on anxiety, locomotor activity and seizure threshold
In order to analyse the consequences of the structural changes in the piperine scaffold we compared the in vivo action of piperine and SCT-66. However, before analyzing behavioural effects of piperine and SCT-66, the consequences of different TRPV1 activity were studied: since TRPV1 channels are involved in a variety of physiological processes including thermoregulation [38], measuring changes in body temperature is one way to detect their activation. In agreement with the literature, piperine at doses ≥ 10 mg/kg bodyweight drastically lowered body temperature of mice (compare to similar results in rats in [39]). In contrast, SCT-66 did not affect thermoregulation even at high doses (see Fig. 4). Our data derived on TRPV1 channels expressed on oocytes indicate that SCT-66, unlike piperine, does not interact with TRPV1 channels. While the in vivo effects of piperine are thus likely to include a TRPV1-related component, it seems that the in vivo effects of SCT-66 do not.
First insights into the behavioural effects of piperine and SCT-66 were obtained from the OF and the EPM test. Though both compounds reduced animals’ locomotor activity, SCT-66 did so only at higher doses (see Fig. 5A). Considering the higher potency and efficiency of SCT-66 on GABAA receptors in vitro (Fig. 2D and E and Table 1) we speculate that the reduced locomotor activity induced by piperine at doses ≥10 mg/kg reflects interactions with vanilloid receptors. A plausible explanation would be that the alterations in pain sensation and thermoregulation result in depressed ambulation as discomfort and pain may well interfere with the exploratory drive. In contrast, reduced ambulation upon application of high doses of SCT-66 may indeed reflect sedation resulting from an enhancement of IGABA. This is further supported by our finding of relatively subtype-independent, strong modulation of GABAA receptors by SCT-66 that did not differ between receptors containing α1, α2 or α3 subunits, which is seen as a prerequisite for sedative actions of drugs [40,41].
As both tests depend on motor activity, potential anxiolytic effects of piperine could be observed only in one parameter of the EPM test, where mice treated with low doses of either piperine spent significantly more time in the open arms of the maze (see Fig. 6A). In contrast, clear anxiolytic effects were observed for SCT-66, which agrees with the stronger enhancement of IGABA (see Fig. 2D and E) and the lack of TRPV1 activation observed in vitro.
Beside influences on emotional behaviour, positive allosteric modulators of GABAA receptors also influence the seizure threshold. Thus, enhancing GABAergic signalling was shown to significantly increase seizure threshold in mice. Importantly, the seizure threshold is independent of motor activity. Consistent with the data obtained from behavioural testing, the effects of piperine on the PTZ-induced seizure threshold suggest the involvement of more than just one receptor/target in vivo. Thus, piperine revealed a biphasic dose-response curve displaying increased thresholds at doses of 3–10 mg/kg bodyweight, which reverts to decreased thresholds at a dose of 30 mg/kg (Fig 7A). In contrast SCT-66 significantly increased the threshold at a dose of 10–30 mg/kg (Fig. 7B). Little information is available on the effects of TRPV1 activation on seizure threshold. The proposed effects of TRPV1 on epilepsy are controversial: while some groups suggest TRPV1 agonists as potential candidates for antiepileptics [42], others have shown increased glutamate release from hippocampal granule cells as a consequence of TRPV1 activation [43]. We can also not exclude the involvement of receptors other than GABAA and TRPV1. However, TRPV1 activation has been shown to cause vasodilation [44], and we observed vasodilatory effects during the PTZ tail-vein infusion experiments with piperine at doses of 10–30 mg/kg (data not shown), but not with SCT-66.
4.4. Conclusions and outlook
Replacing the piperidine ring by the N,N-diisobutyl residue of piperine diminished interaction with TRPV1 receptors, enhanced potency and efficacy of IGABA modulation, diminished the higher efficacy of piperine on α3-subunit and/or β2/3-subunit containing receptors (compare Fig. 2A and B with Fig. 2D and E) and induced a γ2 subunit dependence (Fig. 2 D). Piperine and SCT-66 induced anxiolytic-like, anticonvulsant action with SCT-66 and less depression of locomotor activity compared to piperine (Figs. 5–7). Its higher receptor specificity (lack of interaction with TRPV1) and higher potency and efficacy of IGABA modulation and its in vivo action suggest that SCT-66 may represent a suitable scaffold for development of novel GABAA receptor modulators with anxiolytic and anticonvulsant potential. The addition of 2 extra methyl groups in SCT-66 significantly increased flexibility in the side chain and almost doubled the molecular volume of this part of the molecule. The generation of further piperine derivatives and studies on different GABAA receptor subtypes will help to clarify the structural basis of the receptor selectivity (TRPV1 vs. GABAA) and changes in IGABA modulation.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF P21241, TRP10, P22395 and the doctoral programme “Molecular drug targets” W1232 to S.H.) and the Swiss National Science Foundation (Project 31600-113109 to M.H.)
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Szallasi A. Piperine: researchers discover new flavor in an ancient spice. Trends Pharmacol Sci. 2005;26:437–439. doi: 10.1016/j.tips.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.McNamara F.N., Randall A., Gunthorpe M.J. Effects of piperine, the pungent componentof black pepper, at the human vanilloid receptor (TRPV1) Br J Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaugg J., Baburin I., Strommer B., Kim H.J., Hering S., Hamburger M. HPLC based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site. J Nat Prod. 2010;73:185–191. doi: 10.1021/np900656g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Marzo V., Gobbi G., Brain Szallasi A. TRPV1: a depressing TR(i)P down memory lane. Trends Pharmacol Sci. 2008;29:594–600. doi: 10.1016/j.tips.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Gunthorpe M.J., Chizh B.A. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14:56–67. doi: 10.1016/j.drudis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Olsen R.W., Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savic M.M., Majumder S., Huang S., Edwankar R.V., Furtmüller R., Joksimović S. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph U., Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Trincavelli M.L., Da Pozzo E., Daniele S., Martini C. The GABAA-BZR complex as target for the development of anxiolytic drugs. Curr Top Med Chem. 2012;12:254–269. doi: 10.2174/1568026799078787. [DOI] [PubMed] [Google Scholar]
- 11.Knabl J., Witschi R., Hosl K., Reinold H., Zeilhofer U.B., Ahmadi S. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 12.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 13.Khom S., Baburin I., Timin E.N., Hohaus A., Sieghart W., Hering S. Pharmacological properties of GABAA receptors containing gamma1 subunits. Mol Pharmacol. 2006;69:640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- 14.Boileau A.J., Baur R., Sharkey L.M., Sigel E., Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Krishek B.J., Moss S.J., Smart T.G. Homomeric beta 1 gamma-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol Pharmacol. 1996;49:494–504. [PubMed] [Google Scholar]
- 16.Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 17.Baburin I., Beyl S., Hering S. Automated fast perfusion of Xenopus oocytes for drug screening. Pflugers Arch. 2006;453:117–123. doi: 10.1007/s00424-006-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broadwell R.D., Salcman M., Kaplan R.S. Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. Science. 1982;217:164–166. doi: 10.1126/science.7089551. [DOI] [PubMed] [Google Scholar]
- 19.Khom S., Strommer B., Ramharter J., Schwarz T., Schwarzer C., Erker T. Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands – in vitro and in vivo characterization. Br J Pharmacol. 2010;161:65–78. doi: 10.1111/j.1476-5381.2010.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loacker S., Sayyah M., Wittmann W., Herzog H., Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effectvia kappa opioid receptors. Brain. 2007;130:1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- 21.Rawls S.M., Benamar K. Effects of opioids, cannabinoids, and vanilloids on body temperature. Front Biosci (Schol Ed) 2011;3:822–845. doi: 10.2741/190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui K.M., Huen M.S., Wang H.Y., Zheng H., Sigel E., Baur R. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol. 2002;64:1415–1424. doi: 10.1016/s0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- 23.Huen M.S., Hui K.M., Leung J.W., Sigel E., Baur R., Wong J.T. Naturally occurring 2′-hydroxyl-substituted flavonoids as high-affinity benzodiazepine site ligands. Biochem Pharmacol. 2003;66:2397–2407. doi: 10.1016/j.bcp.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Marder M., Viola H., Wasowski C., Fernandez S., Medina J.H., Paladini A.C. 6- methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharmacol Biochem Behav. 2003;75:537–545. doi: 10.1016/s0091-3057(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 25.Yang X., Baburin I., Plitzko I., Hering S., Hamburger M. HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root) Mol Divers. 2011;15:361–372. doi: 10.1007/s11030-010-9297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger R.E., Campbell E.L., Johnston G.A. (+)- And (−)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Garcia D.A., Bujons J., Vale C., Sunol C. Allosteric positive interaction of thymol with the GABAA receptor in primary cultures of mouse cortical neurons. Neuropharmacology. 2006;50:25–35. doi: 10.1016/j.neuropharm.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Zaugg J., Ebrahimi S.N., Smiesko M., Baburin I., Hering S., Hamburger M. Identification of GABA A receptor modulators in Kadsura longipedunculata and assignment of absolute configurations by quantum-chemical ECD calculations. Phytochemistry. 2011;72:2385–2395. doi: 10.1016/j.phytochem.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khom S., Baburin I., Timin E., Hohaus A., Trauner G., Kopp B. Valerenic acid potentiates and inhibits GABA(A) receptors: molecular mechanism and subunit specificity. Neuropharmacology. 2007;53:178–187. doi: 10.1016/j.neuropharm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Benke D., Barberis A., Kopp S., Altmann K.H., Schubiger M., Vogt K.E. GABA A receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56:174–181. doi: 10.1016/j.neuropharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Zaugg J., Eickmeier E., Ebrahimi S.N., Baburin I., Hering S., Hamburger M. Positive GABA(A) receptor modulators from Acorus calamus and structural analysis of (+)-dioxosarcoguaiacol by 1D and 2D NMR and molecular modeling. J Nat Prod. 2011;74:1437–1443. doi: 10.1021/np200181d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaugg J., Khom S., Eigenmann D., Baburin I., Hamburger M., Hering S. Identification and characterization of GABA(A) receptor modulatory diterpenes from Biota orientalis that decrease locomotor activity in mice. J Nat Prod. 2011;74:1764–1772. doi: 10.1021/np200317p. [DOI] [PubMed] [Google Scholar]
- 33.Cicek S.S., Khom S., Taferner B., Hering S., Stuppner H. Bioactivity-guided isolation of GABA(A) receptor modulating constituents from the rhizomes of Actaea racemosa. J Nat Prod. 2010;73:2024–2028. doi: 10.1021/np100479w. [DOI] [PubMed] [Google Scholar]
- 34.Baur R., Simmen U., Senn M., Sequin U., Sigel E. Novel plant substances acting as beta subunit isoform-selective positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2005;68:787–792. doi: 10.1124/mol.105.011882. [DOI] [PubMed] [Google Scholar]
- 35.Taferner B., Schuehly W., Huefner A., Baburin I., Wiesner K., Ecker G.F. Modulation of GABAA-receptors by honokiol and derivatives: subtype selectivity and structure-activity relationship. J Med Chem. 2011;54:5349–5361. doi: 10.1021/jm200186n. [DOI] [PubMed] [Google Scholar]
- 36.Zaugg J., Eickmeier E., Rueda D.C., Hering S., Hamburger M. HPLC-based activity profiling of Angelica pubescens roots for new positive GABAA receptor modulators in Xenopus oocytes. Fitoterapia. 2011;82:434–440. doi: 10.1016/j.fitote.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Singhuber J., Baburin I., Ecker G.F., Kopp B., Hering S. Insights into structure-activity relationship of GABAA receptor modulating coumarins and furanocoumarins. Eur J Pharmacol. 2011;668:57–64. doi: 10.1016/j.ejphar.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieto-Posadas A., Jara-Oseguera A., Rosenbaum T. TRP channel gating physiology. Curr Top Med Chem. 2011;11:2131–2150. doi: 10.2174/156802611796904870. [DOI] [PubMed] [Google Scholar]
- 39.Jancso-Gabor A., Szolcsanyi J., Jancso N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol. 1970;206:495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Löw K., Crestani F., Keist R., Benke D., Brünig I., Benson J.A. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 41.Dias R., Sheppard W.F., Fradley R.L., Garrett E.M., Stanley J.L., Tye S.J. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu M., Xie Z., Zuo H. TRPV1: a potential target for antiepileptogenesis. Med Hypotheses. 2009;73:100–102. doi: 10.1016/j.mehy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Bhaskaran M.D., Smith B.N. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp Neurol. 2010;223:529–536. doi: 10.1016/j.expneurol.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baylie R.L., Brayden J.E. TRPV channels and vascular function. Acta Physiol (Oxf) 2011;203:99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]