Abstract
Background
We investigated risk factors for inflammatory breast cancer (IBC), a rare, aggressive, and poorly understood breast cancer that is characterized by diffuse breast skin erythema and edema.
Methods
We included 617 IBC case subjects in a nested case–control study from the Breast Cancer Surveillance Consortium database (1994–2009). We also included 1151 noninflammatory, locally advanced, invasive breast cancers with chest wall/breast skin involvement (LABC), 7600 noninflammatory invasive case subjects without chest wall/breast skin involvement (BC), and 93 654 control subjects matched to case subjects on age and year at diagnosis and mammography registry. We present estimates of rate ratios (RRs) and 95% confidence intervals (CI) from conditional logistic regression analyses for each case group vs control subjects based on multiply imputed datasets.
Results
First-degree family history of breast cancer and high mammographic breast density increased risk of IBC, LABC, and BC. High body mass index (BMI) increased IBC risk irrespective of menopausal status and estrogen receptor (ER) expression; rate ratios for BMI 30 and greater vs BMI less than 25 were 3.90 (95% CI = 1.50 to 10.14) in premenopausal women and 3.70 (95% CI = 1.98 to 6.94) in peri/postmenopausal women not currently using hormones. BMI 30 and greater slightly increased risk of ER-positive BC (RR = 1.40; 95% CI = 1.11 to 1.76). Statistically significant reductions in risk of ER-negative IBC with older age at first birth and of ER-positive IBC with higher education were not seen for LABC and BC of the same ER status.
Conclusions
Different associations with BMI, age at first birth, and education between IBC and/or LABC and BC suggest a distinct etiology for IBC.
Inflammatory breast cancer (IBC) is a rare, poorly understood, and very aggressive form of breast cancer (1). It is defined as a “clinical-pathologic entity that is characterized by diffuse erythema and edema (peau d’orange) of the breast, often without an underlying tumor mass. These clinical findings should involve the majority of the skin of the breast”(2). Other forms of locally advanced, noninflammatory breast cancer with direct invasion of the dermis or ulceration of the skin of the breast (LABC) appear to be a distinct biologic entity from IBC with respect to clinical presentation, demographics, and tumor characteristics (3).
The etiology of IBC has been studied in only a few small case–case studies (1), with only one such study of 68 case subjects conducted in the United States (4). Previous studies have been too small to examine risk factors for IBC by estrogen receptor (ER) expression. The primary focus of this nested case–control study was to evaluate associations between standard breast cancer risk factors and IBC. For comparison, we also evaluated risk factors for LABC and noninflammatory invasive breast cancer without direct extension to the chest wall and/or skin of the breast (BC).
Methods
Data Source
We used data from the Breast Cancer Surveillance Consortium (BCSC) (5) (http://breastscreening.cancer.gov), which was established in 1994 and consists of seven population-based mammography registries that include mammography examinations performed in defined catchment areas. Information on breast cancer and tumor characteristics was obtained from state cancer/Surveillance Epidemiology and End Results (SEER) registries and/or through linkage to pathology laboratories or databases (5). A Statistical Coordinating Center is the repository of data from all sites. Institutional review board approval was obtained for each registry and the Statistical Coordinating Center for either an active or passive consent process or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are compliant with the Health Insurance Portability and Accountability Act. All registries and the Statistical Coordinating Center received a Federal Certificate of Confidentiality.
The BCSC collected demographic, health, and screening history data from women through self-administered questionnaires when they came to a BCSC mammography facility for a mammogram, as well as radiologic-reported breast imaging–reporting data system (BI-RADS) breast density (6). All variables included in this analysis, except age at first birth and Human Epidermal Growth Factor Receptor 2 (HER2) status, were collected by all registries, although collection of some variables began at various time periods. Five registries collected information on age at first birth and six collected information on HER2 status (7). Census data based on zip code tabulation areas from the year 2000 were matched to subject addresses based on zip code (8).
Case Definitions
This study includes three case groups: 1) IBC (identified by morphology code 8530; Extent of Disease Codes 070-073; Tumor Node Metastasis (TNM) Pathologic and Clinical T codes 4D; and Derived American Joint Committee on Cancer (AJCC) T code 44) (9–12); 2) LABC (identified by Extent of Disease Codes 040–062; TNM Pathologic and Clinical T codes 4, 4A-C; derived AJCC T codes 40–43; and AJCC stage IIIB) (9–12); and 3) BC. The definition of the codes used to define the case groups (9–12) are shown in Supplementary Table 1 (available online).
Study Subjects
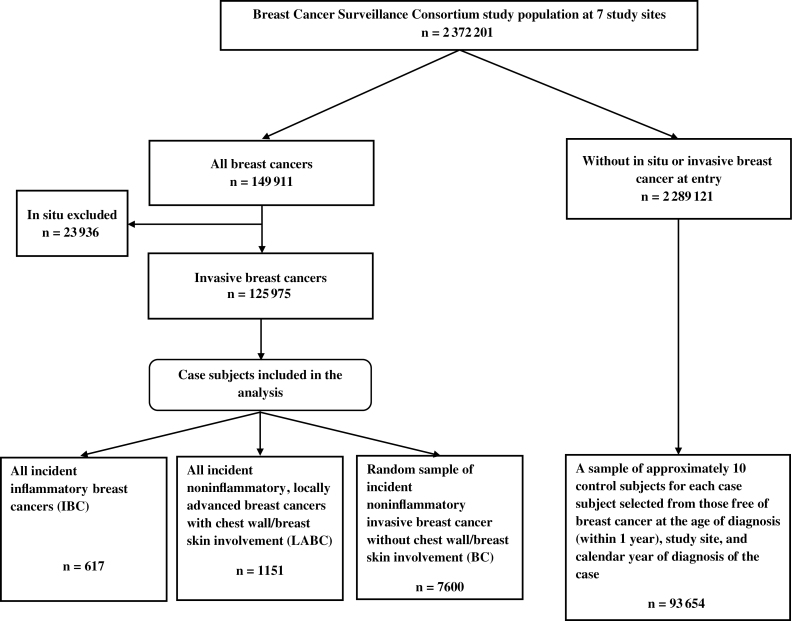
Among 2 372 201 participants, 149 911 breast cancers were diagnosed (some in the same woman), of which 125 975 were invasive. Among those with invasive breast cancer, we identified all cases of IBC (n = 1221), LABC (n = 3360), and BC (n = 121 394). We then excluded cases in which the cancer was diagnosed before January 1, 1994, or after 2005 to 2009, depending on study site; there was a prior breast cancer diagnosis, there was no covariable data; and there were no valid dates before which we know the person did not have breast cancer. After all exclusions, 617 IBC case subjects and 1151 LABC case subjects were included in the analysis. From the remaining 49 636 BC case subjects, we selected a random sample of 7600 (10 times the originally predicted number of IBC case subjects) to lessen the computational burden.
We also selected approximately 10 control subjects per case subject from women who were free of breast cancer at the age of diagnosis (within 1 year) and calendar year of diagnosis of the case subject and were from the same mammography registry, for a total of 93 654 control subjects (Figure 1).
Figure 1.
Study design.
ER status was available for 75.9% of IBC case subjects, 72.7% of LABC case subjects, and 79.6% of BC case subjects. The comparable percentages for progesterone receptor (PR) status were 75.0%, 71.8%, and 78.2% and for HER2 status were 25.6%, 33.4%, and 26.2%. Those classified as having borderline readings for ER (n = 13) and PR (n = 36) were considered hormone receptor positive. Those with HER2 borderline readings (n = 127) were considered negative for HER2.
Exposure Variable Definitions
We obtained information on race, ethnicity, and level of education from any available questionnaire within the BCSC database. We first retrieved BI-RADS mammographic density (6) (coded as 1) almost entirely fat, 2) scattered fibroglandular densities, 3) heterogeneously dense, 4) extremely dense) from the closest screening mammogram before diagnosis or the comparable age for control subjects, then from routine bilateral views associated with diagnosis or up to 30 days after diagnosis, and if no other information was available from a unilateral diagnostic mammogram (up to 30 days after diagnosis). Seventy-nine percent of case subjects with a BI-RADS density reading after the date of diagnosis had a start of treatment date. Of these, 64 case subjects had a density reading after the date of diagnosis, and of these, 11 case subjects started treatment before the density reading (1 IBC case subject, 1 LABC case subject, and 9 BC case subjects).
We obtained information on self-reported height, weight, history of breast cancer in a first-degree female relative, prior breast biopsy or fine needle aspiration, menopausal status, current postmenopausal hormone use, and age at first birth from the questionnaire completed closest in time before or on the date of diagnosis of case subjects and the comparable age for control subjects. We calculated body mass index (BMI) as weight (in kg) divided by height (in meters) squared (kg/m2). We then classified participants as normal weight (<25kg/m2), overweight (25–29.9kg/m2), or obese (≥30kg/m2) (13,14).
The percentage of study subjects with unknown values for the exposure variables ranged from 0 for menopausal status to 48.7 for body mass index among the case and control subjects.
Statistical Analysis
We addressed the missing data by multiple imputations of the missing values, implemented by the sequential regression imputation method (15) using IVEware (http://www.isr.umich.edu/src/smp/ive). Five imputations were obtained with the imputation models, including the following variables without missing values: case–control status (three case groups and controls), study registry, age at diagnosis (continuous), year of diagnosis (continuous), and menopausal status (pre, peri/post); and the following variables with missing values: race/ethnicity (non-Hispanic white, non-Hispanic black, other, Hispanic), self-reported education (less than high school, high school or GED, some college/technology school, college graduate or postcollege education), geocoded high school education from Census data (continuous), geocoded college education from Census data (continuous), geocoded median family income from Census data (continuous), geocoded poverty level from Census data (continuous), breast mammographic density (almost entirely fat, scattered fibroglandular densities, heterogeneously dense, extremely dense), first-degree family history of breast cancer (never, ≥1 relatives), prior breast surgery (no prior breast surgery, ≥1 prior breast procedures), current hormone therapy (yes or no), ER status (positive or negative), age at first live birth (<20 years, 20–24 years, 25–29 years, ≥30 years, nulliparous, <30 years), height (continuous), and body mass index (BMI) (continuous). The imputation models included interaction terms between the outcome and exposure variables. Missing values for ER status are only imputed for the three case groups.
For each of the five imputed datasets, we calculated odds ratios to approximate rate ratios (RRs) and 95% confidence intervals (CIs) for risk factors in relationship to case types (IBC, LABC, BC) compared with control subjects, ER-positive case types compared with control subjects, and ER-negative case types compared with control subjects using conditional logistic regression models. Rate ratios from the five imputed datasets were averaged, and the variance estimated by the average of the variance estimates from the five analyses with an additional between-dataset variance. The same control subjects were used in each model.
Most analyses include premenopausal and peri/postmenopausal case subjects together with BMI categorized as follows: less than 25, 25–29.9, 30 and greater in premenopausal women, less than 25, 25–29.9, 30 and greater in peri/postmenopausal women not currently using menopausal hormone therapy and less than 25, 25–29.9, 30 and greater in peri/postmenopausal women currently using hormone therapy.
We also did case–case comparisons using unconditional logistic regression, adjusting for study registry, age at diagnosis, and year of diagnosis (data not shown). The statistical significance of differences in case–case comparisons was determined by whether the 95% confidence interval around a parameter excluded 1.0.
The missing indicator method, in which a dummy variable was used as the indicator of missingness, generally produced similar results, which are shown in the Supplementary Tables (available online). We also did analyses using the missing indicator method according to ER-positive/PR-positive and ER-negative/PR-negative status, and results were similar to those presented in the Supplementary Tables (available online). HER2 status was unknown for too many case subjects to provide meaningful results. All statistical tests were two-sided.
Results
Tumor characteristics for the three case groups are shown in Table 1. Among those with known ER and PR status, a considerably lower proportion of IBC cases were ER positive and PR positive. The vast majority of IBC and LABC cases were stage III or stage IV, as expected by definition. The stage I or II designations for 5 IBC cases and 28 LABC cases were most likely coding errors. A large majority of BC were stage I and stage II.
Table 1.
Tumor characteristics of inflammatory breast cancer (IBC), noninflammatory, locally advanced breast cancer with chest wall/skin involvement (LABC), and noninflammatory invasive breast cancer without chest wall/skin involvement (BC)
| Characteristic | IBC (n = 617) No. (%)* | LABC (n = 1151) No. (%)* | BC (n = 7600) No. (%)* |
|---|---|---|---|
| ER/PR status†,‡—premenopausal§ | |||
| ER+/PR+ | 64 (45.4) | 124 (61.4) | 935 (68.4) |
| ER+/PR− | 15 (10.6) | 22 (10.9) | 93 (6.8) |
| ER−/PR+ | 6 (4.3) | 5 (2.5) | 43 (3.1) |
| ER−/PR− | 56 (39.7) | 51 (25.3) | 297 (21.7) |
| Unknown ER and/or PR | 41 (22.5) | 53 (20.8) | 376 (21.6) |
| ER/PR status†,‡—peri/postmenopausal║ | |||
| ER+/PR+ | 118 (36.9) | 378 (61.2) | 3220 (70.9) |
| ER+/PR− | 46 (14.4) | 76 (12.3) | 536 (11.8) |
| ER−/PR+ | 13 (4.1) | 11 (1.8) | 79 (1.7) |
| ER−/PR− | 143 (44.7) | 153 (24.8) | 705 (15.5) |
| Unknown ER and/or PR¶ | 115 (26.4) | 278 (31.0) | 1316 (22.5) |
| HER2#—premenopausal§ | |||
| Positive | 18 (37.5) | 28 (30.1) | 98 (21.2) |
| Negative | 30 (62.5) | 65 (69.9) | 365 (78.8) |
| Unknown¶ | 134 (73.6) | 162 (63.5) | 1281 (73.5) |
| HER2#peri/postmenopausal║ | |||
| Positive | 47 (42.7) | 76 (26.0) | 246 (16.1) |
| Negative | 63 (57.3) | 216 (74.0) | 1283 (83.9) |
| Unknown¶ | 325 (74.7) | 604 (67.4) | 4327 (73.9) |
| AJCC version 6 stage | |||
| I | 0 (0.0) | 7 (0.6) | 3754 (52.8) |
| II | 5 (0.8) | 21 (1.8) | 2533 (35.6) |
| III | 480 (79.9) | 1005 (87.9) | 669 (9.4) |
| IV | 116 (19.3) | 111 (9.7) | 161 (2.3) |
| Unknown¶ | 16 (2.6) | 7 (0.6) | 483 (6.4) |
* For the unknown category, the percentage is of the entire population; for the other categories, the percentage is of those with known values.
† Borderline included with positive.
‡ Estrogen receptor positive or negative (ER+ or ER−); progesterone receptor positive or negative (PR+ or PR−)
§ Includes those with unknown menopausal status aged <50 years at diagnosis age or comparable age for control subjects.
║ Includes those with unknown menopausal status aged ≥50 years at diagnosis or comparable age for control subjects.
¶ Percentage with missing data is statistically significantly different based on χ2 test with three case groups. P values for unknown ER and/or PR and HER2 among peri/postmenopausal women and American Joint Committee on Cancer version 6 stage are <.001. P value for HER2 in premenopausal women is .004.
# Borderline included with negative.
IBC case subjects on average had an earlier diagnosis age (57.3 years vs 61.4 years for LABC and 60.7 years for BC). Numbers and percentages of case subjects according to other characteristics of the study population are shown in Table 2. Among IBC case subjects, 70.5% were peri/postmenopausal as opposed to 77% to 77.9% for LABC and BC case and control subjects. The vast majority of the study population was non-Hispanic white and had at least a high school education. Mammograms from which BI-RADS density was obtained were more often done for routine screening among control subjects (94.1%) and BC case subjects (81.1%) than among IBC case subjects (60.0%) and LABC case subjects (66.6%).
Table 2.
Characteristics of inflammatory breast cancer (IBC), noninflammatory, locally advanced breast cancer with chest wall/skin involvement (LABC), noninflammatory invasive breast cancer without chest wall/skin involvement (BC), and control subjects
| Characteristic | IBC (n = 617) No. (%)* | LABC (n = 1151) No. (%)* | BC (n = 7600) No. (%)* | Control (n = 93 654) No. (%)* |
|---|---|---|---|---|
| Menopausal status | ||||
| Premenopausal | 182 (29.5) | 255 (22.2) | 1744 (23.0) | 21558 (23.0) |
| Peri/postmenopausal | 435 (70.5) | 896 (77.9) | 5856 (77.0) | 72096 (77.0) |
| Race/ethnicity | ||||
| White non-Hispanic | 413 (76.3) | 747 (71.1) | 5337 (78.8) | 62434 (76.2) |
| Black non-Hispanic | 47 (8.7) | 79 (7.5) | 393 (5.8) | 4467 (5.4) |
| Other | 26 (4.8) | 75 (7.1) | 484 (7.1) | 7164 (8.7) |
| Hispanic | 55 (10.2) | 149 (14.2) | 562 (8.3) | 7864 (9.6) |
| Unknown†,‡ | 76 (12.3) | 101 (8.8) | 824 (10.8) | 11725 (12.5) |
| Education | ||||
| <High school diploma | 53 (13.2) | 123 (17.0) | 543 (10.3) | 6991 (11.5) |
| High school or GED | 110 (27.4) | 203 (28.0) | 1389 (26.4) | 16387 (26.9) |
| Some college | 130 (32.4) | 206 (28.4) | 1466 (27.9) | 16324 (26.8) |
| College/postgraduate | 108 (26.9) | 193 (26.6) | 1865 (35.4) | 21204 (34.8) |
| Unknown†,‡ | 216 (35.0) | 426 (37.0) | 2337 (30.8) | 32748 (35.0) |
| Age at 1st birth, y | ||||
| <20 | 61 (17.4) | 107 (15.6) | 535 (12.2) | 7253 (13.7) |
| 20–24 | 74 (21.1) | 185 (27.0) | 923 (21.0) | 11969 (22.7) |
| 25–29 | 42 (12.0) | 88 (12.9) | 539 (12.3) | 6519 (12.3) |
| ≥30 | 31 (8.8) | 67 (9.8) | 603 (13.7) | 6153 (11.7) |
| Nulliparous | 67 (19.1) | 127 (18.6) | 891 (20.3) | 9764 (18.5) |
| <30§ | 76 (21.6) | 110 (16.1) | 902 (20.5) | 11161 (21.1) |
| Unknown§ | 206 (43.1) | 467 (40.6) | 3207 (42.2) | 40835 (43.6) |
| Height, in | ||||
| ≤ 62 | 105 (28.9) | 262 (33.6) | 1403 (30.3) | 18326 (33.2) |
| 63–64 | 97 (26.7) | 209 (26.8) | 1291 (27.9) | 15376 (27.8) |
| 65–66 | 94 (25.8) | 179 (23.0) | 1054 (22.8) | 12501 (22.6) |
| ≥67 | 68 (18.7) | 130 (16.7) | 884 (19.1) | 9078 (16.4) |
| Unknown†,‡ | 253 (41.0) | 371 (32.2) | 2968 (39.0) | 38373 (41.0) |
| Body mass index, kg of weight/height m2 | ||||
| <25 | 110 (32.9) | 299 (42.5) | 1882 (47.3) | 23223 (48.4) |
| 25–29.9 | 115 (34.4) | 221 (31.4) | 1242 (31.2) | 14332 (29.8) |
| ≥30 | 109 (32.6) | 184 (26.1) | 852 (21.4) | 10477 (21.8) |
| Unknown†,‡ | 283 (45.9) | 447 (38.8) | 3624 (47.7) | 45622 (48.7) |
| BI-RADS mammographic density | ||||
| Almost entirely fat | 22 (5.3) | 57 (7.5) | 288 (5.8) | 6148 (10.2) |
| Scattered fibroglandular densities | 150 (36.1) | 333 (43.5) | 2030 (40.9) | 27640 (45.8) |
| Heterogeneously dense | 199 (48.0) | 295 (38.6) | 2140 (43.1) | 22182 (36.7) |
| Extremely dense | 44 (10.6) | 80 (10.5) | 504 (10.2) | 4409 (7.3) |
| Unknown | 202 (32.7) | 386 (33.5) | 2638 (34.7) | 33275 (35.5) |
| Indication for mammogram used in the analysis | ||||
| Routine screening | 292 (60.0) | 601 (66.6) | 4645 (81.1) | 65678 (94.1) |
| Additional evaluation following recent mammogram | 3 (0.6) | 7 (0.8) | 107 (1.9) | 389 (0.6) |
| Short interval follow-up | 1 (0.2) | 4 (0.4) | 36 (0.6) | 388 (0.6) |
| Evaluation of breast concern | 191 (39.2) | 286 (31.7) | 940 (16.4) | 3259 (4.7) |
| Other | 0 (0.0) | 4 (0.4) | 2 (0.0) | 91 (0.1) |
| Unknown║ | 130 (21.1) | 249 (21.6) | 1870 (24.6) | 23849 (25.5) |
| Breast cancer in female first-degree relative | ||||
| No | 401 (80.4) | 766 (81.8) | 4860 (80.4) | 62565 (85.9) |
| Yes | 98 (19.6) | 171 (18.3) | 1184 (19.6) | 10298 (14.1) |
| Unknown† | 118 (19.1) | 214 (18.6) | 1556 (20.5) | 20791 (22.2) |
| Prior breast biopsy/fine needle aspiration | ||||
| No | 444 (79.3) | 818 (78.4) | 5201 (76.4) | 66055 (79.7) |
| Yes | 116 (20.7) | 225 (21.6) | 1602 (23.6) | 16800 (20.3) |
| Unknown† | 57 (9.2) | 108 (9.4) | 797 (10.5) | 10799 (11.5) |
* For the unknown category, the percentage is of the entire population; for the other categories, the percentage is of those with known values.
† Percentage with missing data is statistically significantly different based on χ2 test with 3 case groups and control subjects. P values for race/ethnicity, education, height, body mass index, breast cancer in female first-degree relative are <.001. P value for age at first birth = .02. P value for prior breast biopsy/fine needle aspiration = .002.
‡ Percentage with missing data is statistically significantly different based on χ2 test with 3 case groups. P values for body mass index, height, and education are <.001; P value for race/ethnicity = .04.
§ At certain centers, age at first birth was collected as <30 years or ≥30 years.
║ Only a small percentage of those with known breast imaging–reporting data system (BI-RADS) density had unknown indication for the mammogram (0.5%, 0.8%. 0.2%, and 0.6% for IBC, LABC, BC, and control, respectively).
Associations of most variables with IBC, including BMI, did not vary by menopausal status (Table 3). Results for premenopausal and peri/postmenopausal women combined are shown in Table 4. Note that there are small differences in the hazard ratios for BMI when premenopausal and peri/postmenopausal women are included in one model as compared with separate models. Risk of IBC decreased with increasing level of education and increased with greater mammographic density, first-degree family history of breast cancer, and overweight and obesity status in both premenopausal and peri/postmenopausal women. For instance, rate ratios for obesity were 3.90 (95% CI = 1.50 to 10.14) in premenopausal women, 3.70 (95% CI = 1.98 to 6.94) in peri/postmenopausal women not currently using hormones, and 2.94 (95% CI = 1.10 to 7.90) in peri/postmenopausal women currently using hormones.
Table 3.
Multivariable rate ratios (95% confidence intervals) by menopausal status for inflammatory breast cancer (IBC), noninflammatory, locally advanced breast cancer with chest wall/skin involvement (LABC), and noninflammatory invasive breast cancer without chest wall/skin involvement (BC) vs control subjects*
| Variables | IBC | LABC | BC | |||
|---|---|---|---|---|---|---|
| Premenopausal (n = 182) | Peri/postmenopausal l (n = 435) | Premenopausal (n = 255) | Peri/postmenopausal l (n = 896) | Premenopausal (n = 1744) | Peri/postmenopausal (n = 5856) | |
| Demographic factors | ||||||
| Race/ethnicity | ||||||
| White non-Hispanic | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Black non-Hispanic | 1.72 (0.87 to 3.43) | 0.71 (0.43 to 1.16) | 1.87 (0.94 to 3.70) | 1.88 (1.35 to 2.63) | 1.01 (0.78 to 1.30) | 0.84 (0.72 to 0.98) |
| Other | 0.84 (0.36 to 1.95) | 0.55 (0.30 to 1.00) | 1.37 (0.75 to 2.51) | 0.89 (0.55 to 1.44) | 0.82 (0.65 to 1.05) | 0.67 (0.59 to 0.76) |
| Hispanic | 1.03 (0.49 to 2.15) | 1.19 (0.55 to 2.54) | 1.69 (0.95 to 3.01) | 1.14 (0.69 to 1.88) | 0.99 (0.78 to 1.25) | 0.77 (0.67 to 0.87) |
| Education | ||||||
| <High school diploma | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High school or GED | 0.71 (0.20 to 2.51) | 0.54 (0.36 to 0.80) | 0.54 (0.24 to 1.23) | 0.79 (0.55 to 1.13) | 1.14 (0.80 to 1.63) | 0.95 (0.81 to 1.12) |
| Some college | 0.56 (0.19 to 1.63) | 0.72 (0.51 to 1.02) | 0.52 (0.26 to 1.05) | 0.89 (0.57 to 1.40) | 1.28 (0.89 to 1.84) | 0.93 (0.75 to 1.16) |
| College/postgraduate | 0.37 (0.14 to 1.03) | 0.47 (0.31 to 0.71) | 0.30 (0.15 to 0.61) | 0.68 (0.42 to 1.09) | 1.07 (0.79 to 1.45) | 0.89 (0.68 to 1.16) |
| Reproductive/ hormonal factors | ||||||
| Age at first birth, y | ||||||
| <20 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 20–24 | 0.73 (0.30 to 1.81) | 0.80 (0.43 to 1.50) | 1.12 (0.46 to 2.74) | 1.09 (0.54 to 2.18) | 0.91 (0.60 to 1.36) | 0.89 (0.67 to 1.18) |
| 25–29 | 0.27 (0.10 to 0.75) | 1.00 (0.50 to 1.89) | 1.27 (0.60 to 2.70) | 0.93 (0.55 to 1.58) | 1.04 (0.75 to 1.46) | 0.98 (0.74 to 1.30) |
| ≥30 | 0.86 (0.32 to 2.34) | 0.43 (0.17 to 1.05) | 0.90 (0.37 to 2.19) | 1.03 (0.62 to 1.69) | 1.07 (0.66 to 1.72) | 1.08 (0.71 to 1.65) |
| Nulliparous | 0.67 (0.25 to 1.84) | 0.61 (0.31 to 1.19) | 1.14 (0.58 to 2.26) | 0.97 (0.49 to 1.91) | 0.72 (0.45 to 1.14) | 0.83 (0.51 to 1.34) |
| <30† | 0.85 (0.22 to 3.31) | 0.76 (0.18 to 3.13) | 1.21 (0.67 to 2.20) | 0.74 (0.41 to 1.35) | 0.91 (0.72 to 1.15) | 0.85 (0.64 to 1.14) |
| Height and body mass index (BMI) | ||||||
| Height, in | ||||||
| ≤62 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 63–64 | 0.95 (0.49 to 1.85) | 1.20 (0.79 to 1.82) | 0.98 (0.64 to 1.52) | 0.96 (0.77 to 1.18) | 0.86 (0.73 to 1.01) | 1.08 (0.97 to 1.21) |
| 65–66 | 0.95 (0.49 to 1.84) | 1.38 (0.83 to 2.30) | 1.44 (0.87 to 2.35) | 0.94 (0.69 to 1.27) | 0.92 (0.76 to 1.11) | 1.06 (0.92 to 1.21) |
| ≥67 | 1.55 (0.55 to 4.36) | 1.50 (0.57 to 3.93) | 1.14 (0.67 to 1.94) | 1.22 (0.73 to 2.03) | 0.94 (0.80 to 1.12) | 1.15 (0.91 to 1.45) |
| BMI—premenopausal | ||||||
| <25 | 1.00 (referent) | — | 1.00 (referent) | — | 1.00 (referent) | — |
| 25–29.9 | 1.82 (0.88 to 3.77) | 1.04 (0.72 to 1.49) | 1.00 (0.81 to 1.23) | |||
| ≥ 30 | 3.62 (1.30 to 10.04) | 1.03 (0.59 to 1.81) | 0.98 (0.69 to 1.39) | |||
| BMI— peri/postmenopausal—noncurrent hormone users | ||||||
| <25 | — | 1.00 (referent) | — | 1.00 (referent) | — | 1.00 (referent) |
| 25–29.9 | 1.53 (0.95 to 2.46) | 1.13 (0.78 to 1.64) | 1.27 (1.11 to 1.45) | |||
| ≥30 | 3.75 (1.92 to 7.34) | 1.34 (0.76 to 2.36) | 1.41 (1.08 to 1.84) | |||
| BMI— peri/postmenopausal—current hormone users | ||||||
| <25 | — | 1.00 (referent) | — | 1.00 (referent) | — | 1.00 (referent) |
| 25–29.9 | 1.96 (1.00 to 3.86) | 1.00 (0.66 to 1.52) | 1.14 (0.94 to 1.39) | |||
| ≥30 | 3.20 (1.27 to 8.03) | 1.19 (0.70 to 2.03) | 1.23 (0.89 to 1.68) | |||
| Mammographic density | ||||||
| BI-RADS density‡ | ||||||
| 1 | 0.83 (0.23 to 2.99) | 0.50 (0.31 to 0.79) | 0.43 (0.10 to 1.85) | 0.70 (0.52 to 0.95) | 0.91 (0.60 to 1.39) | 0.59 (0.51 to 0.68) |
| 2 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 3 | 1.46 (0.91 to 2.36) | 2.14 (1.57 to 2.92) | 1.24 (0.82 to 1.87) | 1.31 (1.10 to 1.55) | 1.50 (1.28 to 1.77) | 1.37 (1.21 to 1.55) |
| 4 | 2.67 (1.41 to 5.05) | 3.23 (1.79 to 5.80) | 2.50 (1.64 to 3.80) | 1.93 (1.23 to 3.04) | 1.78 (1.43 to 2.20) | 1.88 (1.60 to 2.21) |
| Other factors | ||||||
| Breast cancer in female first-degree relative | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.52 (0.93 to 2.50) | 1.59 (1.14 to 2.20) | 1.52 (0.91 to 2.53) | 1.35 (1.06 to 1.72) | 1.48 (1.27 to 1.71) | 1.37 (1.27 to 1.49) |
| Prior breast biopsy/fine needle aspiration | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| yes | 1.33 (0.85 to 2.09) | 1.04 (0.76 to 1.41) | 1.33 (0.91 to 1.94) | 1.05 (0.87 to 1.26) | 1.20 (1.01 to 1.42) | 1.12 (1.05 to 1.20) |
* Using the multiple imputation method and conditional logistic regression models; each risk factor is adjusted for other risk factors in the table. Results for variables with statistically significant differences among the case groups are bolded.
† At certain centers, age at first birth was collected as < 30 years or ≥ 30 years.
‡ BI-RADS = breast imaging–reporting data system; 1 = Almost entirely fat; 2 = Scattered fibroglandular densities; 3 = Heterogeneously dense; 4 = Extremely dense.
Table 4.
Multivariable rate ratios (95% confidence intervals) in premenopausal and peri/postmenopausal women combined for inflammatory breast cancer (IBC), noninflammatory, locally advanced breast cancer with chest wall/skin involvement (LABC), and noninflammatory invasive breast cancer without chest wall/skin involvement (BC) vs control subjects*
| Variables | IBC (n = 617) | LABC (n = 1151) | BC (n = 7600) |
|---|---|---|---|
| Demographic factors | |||
| Race/ethnicity | |||
| White non-Hispanic | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Black non-Hispanic | 1.04 (0.71 to 1.53) | 1.82 (1.35 to 2.47) | 0.89 (0.77 to 1.01) |
| Other | 0.64 (0.41 to 1.01) | 1.00 (0.67 to 1.50) | 0.72 (0.65 to 0.81) |
| Hispanic | 1.20 (0.63 to 2.26) | 1.29 (0.82 to 2.02) | 0.83 (0.74 to 0.93) |
| Education | |||
| <High school diploma | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High school or GED | 0.60 (0.39 to 0.93) | 0.76 (0.55 to 1.04) | 0.98 (0.85 to 1.13) |
| Some college | 0.67 (0.47 to 0.95) | 0.83 (0.57 to 1.22) | 0.99 (0.83 to 1.19) |
| College/post-graduate | 0.44 (0.30 to 0.64) | 0.58 (0.38 to 0.89) | 0.91 (0.72 to 1.14) |
| Reproductive/hormonal factors | |||
| Age at first birth, y | |||
| <20 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 20–24 | 0.76 (0.42 to 1.35) | 1.05 (0.53 to 2.09) | 0.88 (0.66 to 1.17) |
| 25–29 | 0.74 (0.42 to 1.31) | 1.00 (0.63 to 1.59) | 0.99 (0.78 to 1.25) |
| ≥30 | 0.57 (0.25 to 1.31) | 0.93 (0.57 to 1.52) | 1.07 (0.71 to 1.63) |
| Nulliparous | 0.61 (0.28 to 1.31) | 0.99 (0.55 to 1.79) | 0.79 (0.50 to 1.26) |
| <30† | 0.77 (0.21 to 2.86) | 0.80 (0.47 to 1.37) | 0.86 (0.68 to 1.10) |
| Height and body mass index (BMI) | |||
| Height, in | |||
| ≤62 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 63–64 | 1.17 (0.80 to 1.73) | 0.97 (0.80 to 1.17) | 1.04 (0.94 to 1.14) |
| 65–66 | 1.28 (0.80 to 2.03) | 1.05 (0.79 to 1.39) | 1.03 (0.91 to 1.18) |
| ≥67 | 1.67 (0.66 to 4.25) | 1.17 (0.75 to 1.83) | 1.11 (0.92 to 1.33) |
| BMI—premenopausal | |||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.99 (0.99 to 4.01) | 1.01 (0.73 to 1.41) | 1.04 (0.86 to 1.25) |
| ≥30 | 3.90 (1.50 to 10.14) | 1.02 (0.59 to 1.77) | 1.08 (0.78 to 1.48) |
| BMI—peri/postmenopausal—noncurrent hormone users | |||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.54 (0.97 to 2.45) | 1.13 (0.78 to 1.64) | 1.25 (1.10 to 1.42) |
| ≥30 | 3.70 (1.98 to 6.94) | 1.33 (0.74 to 2.37) | 1.36 (1.05 to 1.77) |
| BMI—peri/postmenopausal—current hormone users | |||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.87 (0.95 to 3.67) | 1.02 (0.68 to 1.55) | 1.13 (0.92 to 1.39) |
| ≥30 | 2.94 (1.10 to 7.90) | 1.22 (0.74 to 2.00) | 1.21 (0.88 to 1.65) |
| Mammographic density | |||
| BI-RADS density‡ | |||
| 1 | 0.54 (0.37 to 0.80) | 0.67 (0.49 to 0.92) | 0.62 (0.54 to 0.70) |
| 2 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 3 | 1.92 (1.48 to 2.51) | 1.31 (1.11 to 1.55) | 1.40 (1.26 to 1.57) |
| 4 | 3.13 (2.03 to 4.85) | 2.18 (1.59 to 3.00) | 1.82 (1.55 to 2.13) |
| Other factors | |||
| Breast cancer in female first-degree relative | |||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.52 (1.15 to 2.01) | 1.40 (1.12 to 1.77) | 1.38 (1.29 to 1.48) |
| Prior breast biopsy/fine needle aspiration | |||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.13 (0.87 to 1.45) | 1.12 (0.95 to 1.32) | 1.14 (1.07 to 1.22) |
* Using the multiple imputation method and conditional logistic regression models; each risk factor is adjusted for other risk factors in the table. Results for variables with statistically significant differences among the case groups are bolded.
† At certain centers, age at first birth was collected as <30 years or ≥30 years.
‡ BI-RADS = breast imaging–reporting data system; 1 = Almost entirely fat; 2 = Scattered fibroglandular densities; 3 = Heterogeneously dense; 4 = Extremely dense.
Risk of LABC also declined with increasing level of education. Additionally, risk of both LABC and BC was associated with first-degree family history of breast cancer, higher mammographic density, and prior breast biopsy/fine needle aspiration. Non-Hispanic blacks were at statistically significantly increased risk of LABC compared with non-Hispanic whites. On the other hand, blacks and other races were at lower risk of BC compared with non-Hispanic whites. Notably, BMI was not associated with increased risk of LABC or BC among premenopausal women or peri/postmenopausal current hormone users, and there were only small increases in risk among peri/postmenopausal women not currently using hormone therapy (eg, RR for obesity = 1.33, 95% CI = 0.74 to 2.37 for LABC; RR for obesity = 1.36, 95% CI = 1.05 to 1.77 for BC).
Analyses for ER-positive tumors vs control subjects and ER-negative tumors vs control subjects are shown in Table 5. First-degree family history of breast cancer, greater mammographic density, and higher BMI were associated with increased risk of both ER-positive and ER-negative IBC, whereas older age at first birth was associated with reduced risk of ER-negative IBC and higher education level with reduced risk of ER-positive IBC.
Table 5.
Multivariable rate ratios (95% confidence intervals) in premenopausal and peri/postmenopausal women combined according to estrogen receptor–positive (ER+) and estrogen receptor–negative (ER−) status for inflammatory breast cancer (IBC), noninflammatory, locally advanced breast cancer with chest wall/skin involvement (LABC), and noninflammatory invasive breast cancer without chest wall/skin involvement (BC) vs control subjects.*
| Variables | ER+ tumors | ER− tumors | ||||
|---|---|---|---|---|---|---|
| IBC | LABC | BC | IBC | LABC | BC | |
| Demographic factors | ||||||
| Race/ethnicity | ||||||
| White non-Hispanic | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Black non-Hispanic | 0.78 (0.43 to 1.41) | 1.26 (0.78 to 2.03) | 0.68 (0.58 to 0.81) | 1.27 (0.75 to 2.16) | 3.13 (1.71 to 5.75) | 1.62 (1.31 to 2.00) |
| Other | 0.53 (0.27 to 1.07) | 0.77 (0.48 to 1.23) | 0.70 (0.62 to 0.81) | 0.72 (0.40 to 1.31) | 1.76 (0.93 to 3.31) | 0.80 (0.59 to 1.07) |
| Hispanic | 0.97 (0.44 to 2.18) | 1.20 (0.70 to 2.05) | 0.83 (0.73 to 0.95) | 1.53 (0.71 to 3.33) | 1.59 (0.98 to 2.58) | 0.85 (0.66 to 1.09) |
| Education | ||||||
| <High school diploma | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| High school or GED | 0.43 (0.21 to 0.89) | 0.77 (0.53 to 1.13) | 0.95 (0.80 to 1.14) | 0.91 (0.56 to 1.48) | 0.71 (0.44 to 1.16) | 1.11 (0.84 to 1.47) |
| Some college | 0.38 (0.24 to 0.61) | 0.88 (0.57 to 1.37) | 0.95 (0.76 to 1.18) | 1.25 (0.72 to 2.16) | 0.70 (0.34 to 1.48) | 1.21 (0.92 to 1.60) |
| College/postgraduate | 0.31 (0.18 to 0.52) | 0.60 (0.38 to 0.95) | 0.89 (0.69 to 1.14) | 0.67 (0.32 to 1.40) | 0.55 (0.30 to 1.02) | 0.99 (0.70 to 1.41) |
| Reproductive/hormonal factors | ||||||
| Age at first birth, y | ||||||
| <20 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 20–24 | 0.95 (0.44 to 2.07) | 1.11 (0.74 to 1.66) | 0.88 (0.67 to 1.15) | 0.60 (0.31 to 1.18) | 0.95 (0.19 to 4.76) | 0.92 (0.56 to 1.50) |
| 25–29 | 1.14 (0.63 to 2.06) | 1.12 (0.72 to 1.73) | 0.97 (0.75 to 1.26) | 0.47 (0.18 to 1.25) | 0.72 (0.32 to 1.62) | 1.09 (0.67 to 1.76) |
| ≥30 | 1.09 (0.54 to 2.22) | 0.90 (0.60 to 1.33) | 1.14 (0.80 to 1.64) | 0.24 (0.07 to 0.87) | 0.95 (0.24 to 3.67) | 0.84 (0.37 to 1.92) |
| Nulliparous | 1.06 (0.52 to 2.18) | 1.22 (0.77 to 1.94) | 0.87 (0.56 to 1.36) | 0.33 (0.13 to 0.83) | 0.56 (0.17 to 1.84) | 0.54 (0.28 to 1.06) |
| <30† | 1.15 (0.33 to 4.01) | 0.92 (0.60 to 1.42) | 0.91 (0.72 to 1.14) | 0.52 (0.12 to 2.33) | 0.57 (0.18 to 1.83) | 0.73 (0.46 to 1.16) |
| Height and body mass index (BMI) | ||||||
| Height, in | ||||||
| ≤62 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 63–64 | 1.23 (0.77 to 1.98) | 0.97 (0.78 to 1.21) | 1.06 (0.97 to 1.16) | 1.08 (0.67 to 1.74) | 0.94 (0.61 to 1.44) | 0.96 (0.74 to 1.24) |
| 65–66 | 1.30 (0.76 to 2.20) | 1.00 (0.75 to 1.34) | 1.07 (0.94 to 1.22) | 1.21 (0.70 to 2.10) | 1.26 (0.75 to 2.11) | 0.92 (0.72 to 1.17) |
| ≥67 | 1.81 (0.72 to 4.54) | 1.09 (0.68 to 1.73) | 1.15 (0.96 to 1.38) | 1.52 (0.50 to 4.65) | 1.49 (0.83 to 2.68) | 0.94 (0.70 to 1.27) |
| BMI—premenopausal | ||||||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.91 (0.80 to 4.56) | 0.99 (0.67 to 1.47) | 1.01 (0.81 to 1.25) | 2.23 (1.00 to 4.93) | 1.11 (0.62 to 1.97) | 1.15 (0.85 to 1.57) |
| ≥30 | 3.53 (1.20 to 10.39) | 1.05 (0.56 to 1.97) | 0.94 (0.65 to 1.35) | 4.67 (1.45 to 15.02) | 0.96 (0.38 to 2.44) | 1.48 (1.00 to 2.19) |
| BMI—peri/postmenopausal— noncurrent hormone users | ||||||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.96 (0.89 to 4.31) | 1.18 (0.83 to 1.68) | 1.30 (1.15 to 1.46) | 1.21 (0.65 to 2.25) | 0.98 (0.52 to 1.88) | 1.04 (0.79 to 1.36) |
| ≥30 | 4.21 (1.91 to 9.28) | 1.44 (0.91 to 2.27) | 1.40 (1.11 to 1.76) | 3.35 (1.73 to 6.49) | 1.06 (0.35 to 3.21) | 1.22 (0.75 to 1.98) |
| BMI—peri/postmenopausal— current hormone users | ||||||
| <25 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 25–29.9 | 1.87 (0.78 to 4.50) | 0.98 (0.60 to 1.61) | 1.15 (0.94 to 1.42) | 1.98 (0.85 to 4.62) | 1.16 (0.52 to 2.60) | 1.04 (0.68 to 1.58) |
| ≥30 | 2.48 (0.79 to 7.84) | 1.11 (0.61 to 2.04) | 1.18 (0.84 to 1.67) | 3.70 (1.24 to 11.00) | 1.58 (0.70 to 3.60) | 1.36 (0.88 to 2.12) |
| Mammographic density | ||||||
| BI-RADS density‡ | ||||||
| 1 | 0.40 (0.19 to 0.84) | 0.76 (0.52 to 1.11) | 0.63 (0.55 to 0.72) | 0.68 (0.31 to 1.49) | 0.46 (0.24 to 0.86) | 0.56 (0.40 to 0.79) |
| 2 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 3 | 1.74 (1.10 to 2.76) | 1.34 (1.09 to 1.64) | 1.40 (1.24 to 1.60) | 2.19 (1.45 to 3.31) | 1.24 (0.86 to 1.80) | 1.39 (1.18 to 1.65) |
| 4 | 3.11 (1.33 to 7.26) | 2.26 (1.49 to 3.43) | 1.83 (1.49 to 2.25) | 3.24 (1.58 to 6.65) | 2.01 (1.08 to 3.75) | 1.77 (1.33 to 2.35) |
| Other factors | ||||||
| Breast cancer in female first-degree relative | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.61 (1.11 to 2.33) | 1.49 (1.14 to 1.95) | 1.43 (1.34 to 1.54) | 1.38 (0.86 to 2.22) | 1.18 (0.77 to 1.82) | 1.18 (0.96 to 1.45) |
| Prior breast biopsy/fine needle aspiration | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 0.97 (0.69 to 1.38) | 1.12 (0.89 to 1.41) | 1.13 (1.04 to 1.23) | 1.30 (0.90 to 1.86) | 1.10 (0.75 to 1.61) | 1.17 (0.99 to 1.38) |
* Using the multiple imputation method and conditional logistic regression models; each risk factor is adjusted for other risk factors in the table. Results for variables with statistically significant differences among the case groups are bolded.
† At certain centers, age at first birth was collected as <30 years or ≥30 years.
‡ BI-RADS = breast imaging–reporting data system; 1 = Almost entirely fat; 2 = Scattered fibroglandular densities; 3 = Heterogeneously dense; 4 = Extremely dense.
Higher education level was associated with lower risk of ER-positive and ER-negative LABC. Overweight and obesity statuses were associated with small increases in risk of noninflammatory breast cancer, particularly among peri/postmenopausal women not currently on hormone replacement therapy with ER-positive BC (eg, RR for obesity = 1.40; 95% CI = 1.11 to 1.76). Associations for some levels of these two variables were statistically significantly different in case–case comparisons of ER-positive IBC with ER-positive LABC and BC.
The inverse association with increasing age at first birth was stronger for ER-negative IBC (eg, RR for age at first birth ≥ 30 years was 0.24 [95% CI = 0.07 to 0.87]) than for LABC and BC (RR was at least 0.84). In case–case analyses, we found statistically significant differences for certain levels of this variable.
Discussion
In this nested case–control analysis, first-degree family history of breast cancer and greater mammographic breast density were associated with increased IBC risk in a manner similar to noninflammatory breast cancer (LABC and BC). Contrary to LABC and BC, high BMI was associated with substantially increased risk of ER-positive and ER-negative IBC in both pre- and peri/ postmenopausal women. Higher level of education was associated with reduced risk of ER-positive IBC, more so than for noninflammatory breast cancer (LABC and BC). Later age at first birth was associated with reduced risk of ER-negative IBC; reductions were greater than for ER-negative LABC and BC. The average age of diagnosis for IBC case subjects was 4 years younger than for LABC and BC case subjects.
To our knowledge, this is the first case–control study of IBC and the first etiologic study according to ER status. Similar to our findings, a small study that compared IBC with non-IBC found increased risk in heavier women, regardless of menopausal status (4). In addition, an analysis of SEER data found lower IBC incidence rates with higher socioeconomic position (16), perhaps reflecting the influence of risk factors related to socioeconomic position. Breast density has been associated with most histologic types of breast cancer and tumor subtypes, although IBC was not specifically examined (17,18).
Notably, overweight and obesity statuses were associated with increased IBC risk regardless of the internal hormonal milieu or the ER status of the tumors. IBC is highly angiogenic, which may be related to inflammation and inflammatory cytokines that up-regulate vascular endothelial growth factor (19), the major factor that stimulates new blood vessel formation. Obesity has been related to inflammatory processes (20). In fact, inflammation and immune-related processes characterized the IBC tumor phenotype in an analysis of IBC’s molecular profile (21). Moreover e-cadherin, which is overexpressed in IBC and accounts for the formation of tumor emboli, is increased in inflammation (22).
Strengths of our study include the relatively large number of IBC case subjects and the inclusion of groups of other breast cancer types for comparison. The study was large enough to allow for evaluation of risk factors by menopausal status and tumor ER status. We chose to present analyses by ER status without regard to other tumor markers because analyses of gene expression patterns largely separate the tumor samples into those that are ER positive and those that are ER negative before further defining subtypes (23).
However, molecular analyses of IBC and other breast cancers have further identified a number of intrinsic tumor subtypes that are not adequately defined by hormone receptor status. In fact, all of these subtypes have been identified in IBC, with a smaller proportion of luminal A subtype and a larger proportion of HER2-enriched subtype in IBC than non-IBC (21). After accounting for the influence of molecular subtypes, the largest such analysis to date found 18% of genes remained differentially expressed in IBC, yielding an IBC-specific molecular subtype-specific 79-gene signature (21).
Another potential limitation of this study is the substantial amount of missing data for several covariables. Information on some exposures was not collected for all calendar years or at all study sites, suggesting that the data are missing at random. We used two methods to address the missing data: 1) multiple imputation of the missing values and 2) the missing indicator method, in which we used a dummy variable as an indicator of missing data. Results from the multiple imputation and missing indicator methods were generally similar. We did find the standard associations for other invasive breast cancers with regard to ER status—namely, the differences in risk according to hormone receptor status for age at first birth, nulliparity, and body mass index (24). Finally, we did not have data on some factors known to be associated with breast cancer risk, such as alcohol consumption. Studies in other populations will be needed to address this limitation.
In summary, associations with family history of breast cancer and mammographic breast density were similar for IBC, LABC, and BC. Associations with BMI, education level, and age at first birth differed for IBC and LABC and BC of the same ER status. Varying risk factor associations between inflammatory and noninflammatory breast cancer suggest a distinct etiology for this clinically unique type of breast cancer. Future research on IBC should attempt to account for the differential distribution patterns of molecular subtypes between IBC and non-IBC in an effort to identify risk factors that are IBC specific rather than subtype specific.
Funding
This work was supported by the National Cancer Institute–funded BCSC (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C) and by the Intramural Research Program at the National Cancer Institute, National Institutes of Health.
Supplementary Material
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
We thank the participating women, mammography facilities, and radiologists for the data they provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes is provided at http://breastscreening.cancer.gov. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. A full description of these sources is available at http://www.breastscreening.cancer.gov/work/acknowledgement.html.
References
- 1. Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (IBC). Breast Dis. 2005, 2006;22:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greene FL, Page DL, Fritz A, Balch CM, Haller DG, Morrow M. Breast. In: AJCC Cancer Staging Manual. 6th ed New York: Springer-Verlag; 2002;225–226 [Google Scholar]
- 3. Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol. 2003;21(12):2254–2259 [DOI] [PubMed] [Google Scholar]
- 4. Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16(12):3731–3735 [DOI] [PubMed] [Google Scholar]
- 5. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. Am J Roentgenol. 1997;169(4):1001–1008 [DOI] [PubMed] [Google Scholar]
- 6. American College of Radiology Breast imaging reporting and data system (BI-RADS). 4th ed Reston, VA: American College of Radiology; 2003 [Google Scholar]
- 7. Phipps AI, Buist DS, Malone KE, et al. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012;22(5)340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Census Bureau Census 2000 ZCTAs. http://www.census.gov/geo/ZCTA/zcta_brch_vw.pdf Accessed 17 July 2013
- 9. American Joint Committee on Cancer Manual for Staging of Cancer. 4th ed Philadelphia: J.B. Lippincott; 1992 [Google Scholar]
- 10. American Joint Committee on Cancer Cancer Staging Manual. 5th ed Philadelphia: Lippincott–Raven; 1997 [Google Scholar]
- 11. American Joint Committee on Cancer Cancer Staging Manual. 6th ed New York: Springer-Verlag; 2002 [Google Scholar]
- 12. National Cancer Institute, National Institutes of Health SEER Program: Comparative Staging Guide for Cancer. Version 1.1. NIH Publication No. 93–3640. Bethesda, MD: National Insitutes of Health; 1993 [Google Scholar]
- 13. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults Clinical Guideline on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults–The Evidence Report. Bethesda, MD: National Institutes of Health; 1998 [Google Scholar]
- 14. World Health Organization Physical Status: The Use and Intrepretation of Anthropometry. Geneva, Switzerland: World Health Organization; 1995 [PubMed] [Google Scholar]
- 15. Raghunathan TE, Lepkowski JM, van Hoewyk M, Solenberger PW. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol. 2001;27(1)85–95 [Google Scholar]
- 16. Schlichting JA, Soliman AS, Schairer C, et al. Association of inflammatory and noninflammatory breast cancer with socioeconomic characteristics in the Surveillance, Epidemiology, and End Results Database, 2000–2007. Cancer Epidemiol Biomarkers Prev. 2011;21(1):155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yaghjyan L, Colditz GA, Collins L, et al. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103(15):1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phipps AI, Buist DSM, Malone KE, et al. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012;22(5):340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13(10)2825–2830 [DOI] [PubMed] [Google Scholar]
- 20. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. British J Nutrition. 2004;92(3)347–355 [DOI] [PubMed] [Google Scholar]
- 21. Van Laere S, Ueno N, Finetti P, et al. Uncovering the molecular secrets of inflammatory breast cancer biology: an integrated analysis of three distinct Affymetrix gene expression data sets [published online ahead of print Feburary 8, 2013]. Clin Cancer Res.10.1158/1078-0432.CCR-12–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozturk H, Ozturk H, Guneli E, Yagmur Y, Buyukbayram H. Expression of CD44 and e-cadherin cell adhesion molecules in hypertrophied bladders during chronic partial urethral obstruction and after release of partial obstruction in rats. Urology. 2005;65(5)1013–1018 [DOI] [PubMed] [Google Scholar]
- 23. Perou C, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(17):747–752 [DOI] [PubMed] [Google Scholar]
- 24. Althuis MD, Fergenbaum JH, Garcia-Closas M, et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2001;13(10):1558–1568 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.