Abstract
Evidence that obesity is associated with cancer incidence and mortality is compelling. By contrast, the role of obesity in cancer survival is less well understood. There is inconsistent support for the role of obesity in breast cancer survival, and evidence for other tumor sites is scant. The variability in findings may be due in part to comorbidities associated with obesity itself rather than with cancer, but it is also possible that obesity creates a physiological setting that meaningfully alters cancer treatment efficacy. In addition, the effects of obesity at diagnosis may be distinct from the effects of weight change after diagnosis. Obesity and related comorbid conditions may also increase risk for common adverse treatment effects, including breast cancer–related lymphedema, fatigue, poor health–related quality of life, and worse functional health. Racial and ethnic groups with worse cancer survival outcomes are also the groups for whom obesity and related comorbidities are more prevalent, but findings from the few studies that have addressed these complexities are inconsistent. We outline a broad theoretical framework for future research to clarify the specifics of the biological–social–environmental feedback loop for the combined and independent contributions of race, comorbid conditions, and obesity on cancer survival and adverse treatment effects. If upstream issues related to comorbidities, race, and ethnicity partly explain the purported link between obesity and cancer survival outcomes, these factors should be among those on which interventions are focused to reduce the burden of cancer.
The prevalence of overweight (body mass index [BMI] = 25.0–29.9kg/m2) and obesity (BMI ≥ 30.0kg/m2) in the United States rose from 13.5% in the 1960s to 35.9% in 2010 (1,2). Of the top 10 causes of death in the United States in 2009, five are related to obesity, including heart disease, stroke, cancer, diabetes mellitus, and kidney disease (3). Disparities have been noted in cancer survival and treatment outcomes across ethnic groups and levels of obesity (4–6). Herein we review evidence regarding the potential impact of obesity and race on cancer survival and treatment outcomes.
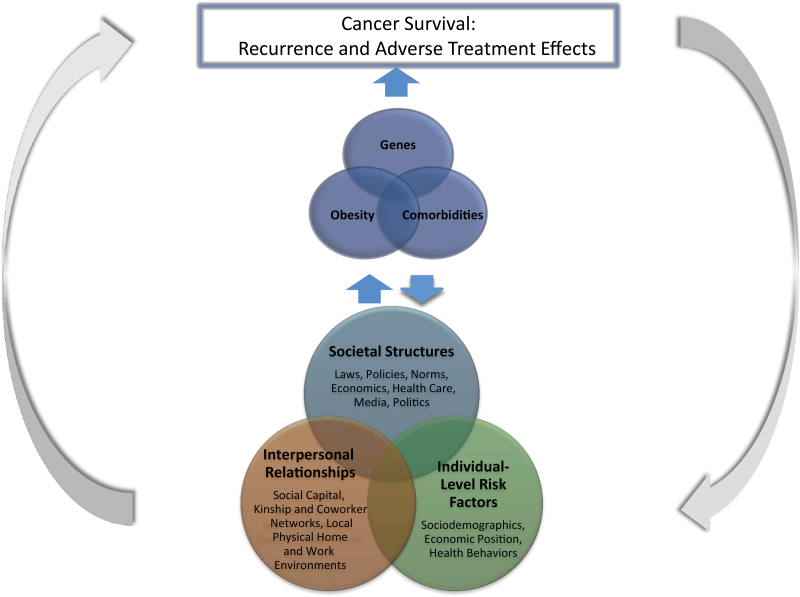
The recently published “Annual Report to the Nation on the Status of Cancer” (4) commented that differences in cancer outcomes across racial and ethnic groups might be explained by differences in risk behaviors and access to and use of screening and cancer treatments. The racial and ethnic groups for whom cancer survival is worse are the same groups in which obesity is more prevalent, including blacks and Native American/Pacific Islanders (1,4). It could be hypothesized that the disparities by race and ethnicity for cancer survival and treatment outcomes are explained, in part, by disparities in obesity and related comorbidities. A theoretical framework for discussing these complexities is suggested in Figure 1. Disparities in underlying social and physical determinants are embodied and expressed through biological responses and genetic pathways, which may lead to disparities in risk for obesity and comorbidities. Differential risk in obesity and comorbidities may then lead to disparities in survivorship outcomes. All of these relationships operate in a feedback loop of biological–environmental interactions. In this review, we evaluate the evidence needed to answer questions regarding these complexities and make recommendations for future research.
Figure 1.
Framework for the combined influence of race and obesity on cancer survivorship. The bottom Venn diagram represents distal determinants of disparate cancer survival outcomes. Disparities in these underlying social and physical determinants are embodied and expressed through biological responses and genetic pathways, which lead to disparities in risk for obesity and comorbidities. In the top Venn diagram, differential genes, obesity, and comorbidities then lead more proximally to disparities in survivorship outcomes. All of these relationships operate in a feedback loop of biological–social–physical environment interactions, making it difficult to disentangle which of the underlying or mediating factors are the greatest contributors to disparities in survivorship.
Obesity, Disease-Free Survival, and Mortality
Nearly 10 years have passed since the publication of the landmark study by Calle et al., which reported that compared with men and women of normal weight, for those who were very obese (BMI > 40.0kg/m2) the relative risk (RR) for death from cancer was 1.52 for women and 1.62 for men (7). Since then, numerous cohort studies on this topic have been published, with the majority focusing on prostate cancer (8–12) and postmenopausal breast cancer (12–14) and only a few focusing on colorectal cancer (12,15).
Although obesity is an established risk factor for incidence of several cancers, the impact of obesity on cancer survival is not well understood. It is unknown whether the greatest risk associated with obesity occurs before diagnosis (reflecting the role of obesity as a risk factor for incident disease) or whether weight gain or loss during or after treatment has a greater influence on prognosis. Clearly, each could be relevant. Understanding the difference is important to developing appropriate interventions to reduce the burden of cancer.
A recent large systematic review of the current evidence linking body adiposity to prognosis in prostate, colorectal, and breast cancer concluded that most of the 33 studies reviewed supported an association between body adiposity and site-specific mortality or cancer progression (16). However, most studies included were not designed to evaluate this association, not all studies controlled for other lifestyle factors that may influence survival, and causal inference from observational research can be limited by measurement error, making it challenging to disentangle the true independent effect of obesity. A randomized controlled trial would offer the strongest design to answer remaining questions, but long-term randomized controlled trials with sufficient sample size to accrue cancer endpoints are challenging both with regard to logistics and financial feasibility.
Several smaller systematic reviews were also identified that evaluated the association of obesity and survival in breast, endometrial, and ovarian cancer (17–19). These studies also supported an association of obesity with poorer survival with the caveat that further studies are needed to confirm the findings, given limitations inherent to observational studies, issues of limited statistical power, and interstudy variation.
A recent systematic review and meta-analysis included six population-based cohorts with data on prediagnosis BMI and prostate cancer mortality (11). The pooled relative risk for prostate cancer mortality was 1.15 (95% confidence interval [CI] = 1.06 to 1.25) for each 5kg/m2 increase in BMI. For an additional six studies with data from prostate cancer patient cohorts, the pooled relative risk for mortality was 1.20 (95% CI = 0.99 to 1.46) for each 5kg/m2 increase in BMI (11). Another study on prostate cancer noted that it is possible that obese patients develop more aggressive tumors (20). A Baltimore area cohort study of prostate cancer patients observed that postdiagnosis weight gain was associated with a twofold increased risk of prostate cancer recurrence, but specific mortality estimates were not reported (10). Overall, although the findings to date are suggestive regarding the association of obesity with prostate cancer survival, additional data are needed, particularly for subgroups known to have poor outcomes, including black men.
Evidence to support obesity as a risk factor for incident colorectal cancer is fairly strong and consistent (12,15,21), but data are relatively sparse with regard to prognosis and survival. Of 1096 incident colorectal cancer case subjects in the Iowa Women’s Health Study, 293 died from the disease (22). All-cause mortality was statistically significantly higher by 30% to 40% among colon cancer case subjects in the highest compared with the lowest category for all anthropometric measures of obesity (including weight, height, BMI, waist–hip ratio, and waist circumference). Colon cancer–specific mortality was also elevated among those in the highest compared with the lowest category for the same anthropometric measures, although the association was slightly lower in magnitude (25% to 30% increased risk). However, none of the anthropometry and colon cancer–specific mortality associations were statistically significant after multivariable adjustment, including first course of cancer treatment (22). In a group of 4381 colon cancer patients participating in adjuvant therapy trials, patients who were obese at study entry (postdiagnosis) had a higher risk of recurrence and mortality when compared with patients who were not obese (23).
The majority of published data on obesity and cancer prognosis comes from breast cancer cohorts. The California Teachers Study (24), the Danish Breast Cancer Cooperative Group (25), the Multiethnic Cohort Study (26), the Collaborative Women’s Longevity Study (27), and the Life After Cancer Epidemiology cohort (28), among others, have all reported a positive association between pre- or postdiagnosis obesity and breast cancer mortality. The relative risks for breast cancer mortality in these cohorts comparing normal weight (BMI < 25.0kg/m2) with obesity (BMI ≥ 30.0 or 35.0kg/m2) have ranged 1.3 to 2.28. Obesity is also associated with poorer disease-free survival (DFS) in breast cancer patients. A systematic review of the literature revealed that when obese women are diagnosed with breast cancer, they can expect poorer breast cancer–specific and overall survival than nonobese women (19).
A recent study examining prediagnosis BMI and survival after breast cancer found that obese women had an increased risk of overall death (hazard ratio [HR] = 1.17; 95% CI = 1.04 to 1.32) and severe obesity was associated with increased risk of non–breast cancer death (HR = 1.40; 95% CI = 1.02 to 1.92) compared with normal-weight women (13). Moreover, morbidly obese (≥40kg/m2) women had the greatest risk for all death outcomes (HR = 1.81; 95% CI = 1.42 to 2.32), non–breast cancer death (HR = 3.01; 95% CI = 2.09 to 4.33), and breast cancer death (HR = 1.40; 95% CI = 1.00 to 1.96) compared with normal-weight women (13).
Despite the current inability to definitively draw the inference that prediagnosis obesity increases risk for poor cancer prognosis, some provocative data are beginning to emerge from patients who have had bariatric surgery (29–31). For some time now, it has been appreciated that obese individuals who undergo bariatric surgery experience immediate benefits to their metabolic and cardiovascular health (31). Longer term follow-up suggests that bariatric surgery may also be associated with reduced cancer incidence, perhaps as a result of the overall improvements in metabolic health. However, in at least one study, bariatric surgery was associated with increased hyperproliferation and decreased apoptosis in colorectal epithelial cells, possibly leading to increased, not decreased, cancer risk (32).
One of the first of these landmark bariatric surgery studies was the Swedish Obese Subjects study, which was a nonrandomized study comparing health outcomes of obese individuals who underwent bariatric surgery (n = 2010) with health outcomes of those who received nonsurgical obesity treatment (n = 2037) (33). At 10 years of follow-up, weight change in the bariatric surgery group averaged −19.9kg (standard deviation [SD] = 15.6), compared with +1.3kg (SD = 13.7) in the nonsurgical obesity treatment group. After a median follow-up time of 10.9 years, the hazard ratio for cancer incidence was lower in the bariatric surgery group than the no-surgery group (HR = 0.67; 95% CI = 0.52 to 0.85), with slightly stronger results for women than men (33). In the same cohort, there were no statistically significant relationships between weight change and cancer incidence. In one of the few studies to examine the association of bariatric surgery with cancer-specific mortality, Adams et al. conducted a retrospective cohort study by linking data from patients who had bariatric surgery with the Utah cancer registry; controls were individuals with BMI ≥ 35kg/m2 as recorded in the Utah driver’s license database [30]. Cancer-related mortality was substantially lower among obese individuals who underwent bariatric surgery (HR for cancer morality = 0.54; 95% CI = 0.37 to 0.78), with stronger associations among women (62% reduced risk in cancer-related deaths) (30). Additional observational and mechanistic data are needed before definitive inferences can be made about the association of bariatric surgery with cancer prognosis and mortality.
Bariatric surgery results reflect rather sudden and usually sustained weight loss. It is also important to examine weight changes (either gain or loss) after a cancer diagnosis and how this might affect development of new primary cancers or overall survival. Thivat et al. (34) examined weight change during chemotherapy treatment in relation to survival among 111 breast cancer patients. Although the sample size was rather small, women who either gained or lost more than 5% of their weight between diagnosis and postchemotherapy treatment had a more than twofold increased risk of death compared with women whose weight remained stable (34). The After Breast Cancer Pooling Project (ABCPP) recently reported results from a pooled analysis of 18333 breast cancer survivors from four US and Chinese cohorts (35). The investigators examined self-reported prediagnosis weight (1 year before diagnosis) and self-reported weight (3 cohorts) or measured weight (1 cohort) on average 2.1 years after diagnosis. After a mean follow-up time of 8.1 years, a modest U-shaped association of weight change with risk of death was observed. Women who experienced large weight loss (≥10%) had an overall mortality hazard ratio of 1.41 and 3.25 in the United States and China, respectively. There was no overall or subgroup (ie, smokers, those with comorbid conditions) association of postdiagnosis weight gain with either breast cancer–specific or total mortality. These ABCPP results are in contrast with previous reports that suggest that weight loss after a breast cancer diagnosis improves prognosis (28,36). Most of these weight change data come from breast cancer cohort studies; data are lacking on whether extremes in weight change affect other cancers. Further, data are needed to differentiate unintentional weight loss, which may reflect cancer cachexia typical of advanced disease or sarcopenia/muscle wasting (37) and intentional weight loss that may be part of a supervised program aimed at overall health benefits. In addition, it may be useful to examine body composition instead of weight alone. In one recent study, breast cancer survivors with sarcopenia had a poorer survival than did women without sarcopenia (P = 0.002). Interestingly, 13% of the patients with sarcopenia were also overweight (BMI = 25.0–29.9kg/m2). Body composition independent of weight or BMI may play an important role in survivorship and should be examined in greater detail (37).
Obesity and Treatment Efficacy
A critical, yet unanswered, question is whether obesity physiologically alters cancer treatment efficacy. In 2012, BMI and breast cancer risk results were published from two large chemoprevention trials (Breast Cancer Prevention Trial testing tamoxifen vs. placebo; STAR Trial comparing tamoxifen vs. raloxifene—both for primary prevention of breast cancer) (14). These two trials were designed for prevention, not DFS or mortality; however, so many patients are prescribed these medications after diagnosis that it is relevant to mention the findings here. One of the provocative findings was that the association of BMI with premenopausal breast cancer risk was higher for women randomly assigned to tamoxifen (HR = 2.33; 95% CI = 1.10 to 4.90) than for women randomly assigned to placebo (HR = 1.41; 95% CI = 0.82 to 2.43) (14). However, the study was not powered to test whether an interaction of BMI with tamoxifen was statistically significant, so the results are inconclusive. It remains unknown why obese premenopausal women taking a Selective Estrogen Receptor Modulator (SERM) would be at elevated risk for breast cancer compared with obese premenopausal women not taking a SERM. Only a few studies have investigated whether obesity influences response to treatment. Premenopausal breast cancer patients with hormone receptor–positive tumors (n = 1803) were randomly assigned to receive goserelin plus tamoxifen or anastrazole with or without zoledronic acid (38). After a median follow-up time of 62.6 months, 64 breast cancer–related deaths occurred. Overweight patients randomized to anastrazol had a more than twofold increased risk of death compared with those on tamoxifen (38). In another trial, breast cancer patients who were obese appeared to have a poorer response to chemotherapy than nonobese patients independent of other prognostic indicators (39). Finally, a small randomized controlled trial tested tamoxifen, fenretimide, both, or placebo for 2 years among women with a prior diagnosis of in situ breast cancer, a small invasive (stage I) breast cancer, or unaffected high-risk women (eg, with a 5-year Gail risk score ≥1.7) (40). Although disease endpoint data were not reported, there were differential effects of tamoxifen by BMI in relation to the surrogate biomarker endpoints of insulin resistance and beta cell function. Understanding the influence of obesity on treatment efficacy should be a high priority for future research, particularly for breast and other hormone-dependent cancers.
Multiple potential mechanistic pathways could be hypothesized through which obesity may biologically influence treatment efficacy. For example, there could be differential reactivation of dormant tumor cells across levels of adiposity. Evidence consistent with this hypothesis includes the positive association of visceral fat with circulating tumor cells and lung cancer metastasis in animals (41) and the observation that receptors for growth factors that are elevated with obesity (eg, insulin-like growth factor 1 receptor) are commonly expressed on circulating tumor cells (42,43). There could also be differences in the pharmacokinetics of cancer drugs by adiposity, particularly for more lipophilic drugs. If the drugs are metabolized at a different rate in the fat tissue, it is possible that both cancer treatment efficacy and toxicities could vary across obesity status. There is a paucity of information on influence of obesity on the pharmacokinetics of most anticancer drugs from properly powered trials (44). Additionally, obesity has been shown to affect multiple molecular pathways relevant to cancer recurrence and progression, including insulin signaling, growth factors, apoptosis, and the tumor microenvironment (45).
An alternate hypothesis could be that obesity does not biologically influence treatment efficacy and that the differential survival outcomes across obesity are explained by differential application of known efficacious treatments by obesity and/or upstream factors noted at the bottom of Figure 1. Intentional reduction of first chemotherapy dose, lower relative dose intensity, and frequency of treatment modifications have been found to be independently associated with obesity and other upstream factors (6,46–50). Recent guidelines recommend dosing of chemotherapy by full body weight or body surface area (44). However, there continues to be ample evidence to suggest that oncologists limit chemotherapy dosing to overweight and obese patients, perhaps because of fears of treatment toxicities (44). There is also evidence that surgery is technically more difficult among obese patients and that surgical recovery and outcomes are worse among obese than lean patients, including evidence specific to cancer surgery (51–54). Radiation treatments are observed to be more difficult to deliver among obese prostate cancer patients (54). Each of these has implications for cancer prognosis as well as the potential for adverse treatment effects.
BMI is clearly associated with multiple chronic conditions, including cardiovascular diseases, type 2 diabetes (55), pulmonary disease (56), renal disease (57), and dementia (51). Multiple studies have observed differences in cancer treatments according to comorbid conditions (58–61). Variability in administration of adjuvant breast cancer therapy has been observed across obesity status, even after adjustment for comorbidities (6). However, it remains possible that the relationship of obesity with cancer survival could be explained, in part, by differences in treatment according to obesity-related comorbidities.
Obesity and Adverse Treatment Effects
In addition to the effects obesity may have on treatment efficacy and the challenges of providing treatment to obese compared with nonobese patients, it is also possible that obesity and associated chronic conditions alter the incidence, severity, or clinical course of common adverse effects of cancer treatment. Herein we review the available evidence regarding the potential influence of obesity on the burden of common adverse cancer treatment effects.
Lymphedema
Lymphedema is a chronic condition characterized by an accumulation of protein-rich fluid and associated swelling of the affected body part. Cancer treatments such as lymph node dissection and radiotherapy can damage the lymphatic drainage routes, leading to fluid build-up, discomfort, and reduced mobility and function (62). Excess adiposity may increase risk of lymphedema by increased inflammation, added stress to the lymphatic system, or slower healing times after surgery (63). Although it may arise as a complication of treatment for several cancers, the majority of lymphedema research has been conducted within breast cancer.
There is convincing evidence that obesity increases risk of lymphedema after treatment for breast cancer. Prospective studies have reported statistically significantly higher lymphedema risk for obese vs normal-weight women, with odds ratios (OR) ranging from 2.93 to 3.60 (63–66). Women who are overweight, but not obese, appear to be at lower but still statistically significantly increased risk, with odds ratios ranging from 2.00 to 2.24 (63,64,67). The dose–response relationship between excess weight and lymphedema risk is further demonstrated by two separate studies, both reporting an odds ratio of 1.08 for each additional BMI unit above the normal weight category [95% CI = 1.05 to 1.12 (64); 95% CI = 1.0004 to 1.165 (65)]. Similarly, a study conducted in Hong Kong observed statistically significantly higher BMI in breast cancer patients with lymphedema compared with matched control subjects; this is notable given that BMI was low in both groups (22.9±3.6kg/m2 for case subjects vs 21.8±3.1kg/m2 for control subjects) (68). This suggests that adiposity is relevant to lymphedema across a wide range of body sizes, not just for those women who are clinically obese. Although it is unclear whether weight gain after treatment may increase the risk of lymphedema (66), weight loss has been shown to reduce lymphedema among overweight breast cancer survivors in a pilot study (69). Further research on the effects of weight loss on breast cancer survivors with lymphedema is ongoing within the Penn TREC Survivor Center.
Beyond breast cancer, the evidence is less consistent regarding the relevance of obesity as a risk factor for lymphedema. Three studies reported no association of BMI and incident lower extremity lymphedema among cervical cancer survivors (70–72). By contrast, a prospective cohort study among cervical cancer survivors found that low BMI (<18.5kg/m2) was associated with increased frequency of lymphedema. However, these results may be affected by higher tobacco use and stage at diagnosis in this group (73). A retrospective chart review of 286 Japanese women with endometrial cancer observed no association of BMI and lymphedema (74), whereas a cross-sectional survey study among 243 Australian women observed a 2.7-fold increased risk for lymphedema among overweight compared with normal-weight endometrial cancer survivors (95% CI = 1.0 to 7.5) (70). Beesley et al. also observed a non-statistically significant increase in lymphedema risk among overweight and obese ovarian cancer survivors compared with normal-weight women (OR = 1.9; 95% CI = 0.8 to 4.5).
Quality of Life
Reduced quality of life, including functional impairment, psychosocial distress, limitations in social functioning, and emotional problems, is frequently reported by cancer survivors (75). Obesity has been associated with lower physical and functional well-being and poorer quality of life among endometrial cancer (76,77), breast cancer (78), prostate cancer (79–81), and colorectal cancer survivors (82). Two other studies with heterogeneous samples of cancer survivors (eg, breast, colorectal, prostate, bladder, uterine, and melanoma) have also demonstrated reduced quality of life among obese vs nonobese participants (83,84). Obesity is also associated with higher prevalence and severity of site-specific symptoms, such as incontinence in prostate cancer survivors (80–85).
Fatigue
Fatigue may be the most commonly reported and distressing symptom among cancer survivors (86,87). The overall prevalence of cancer-related fatigue (CRF) is estimated at 48% among cancer survivors, regardless of tumor type or treatment (88). Obesity has been positively associated with CRF for a number of cancer sites, including breast (89–92), endometrial (93), and childhood leukemia (94). Hypothesized pathways through which obesity might influence CRF include chronic inflammation, insulin resistance, and metabolic factors such as abnormalities of energy production and use (88). Factors predicting clinically significant CRF include a BMI greater than 25kg/m2, weight gain, physical inactivity, and low physical functioning (91,95). Also, the severity of fatigue symptoms is associated with higher BMI (95). Additionally, researchers found that breast cancer survivors with CRF had higher BMIs and were more likely to be obese at baseline than survivors not experiencing CRF at 42 months after treatment (89).
Peripheral Neuropathy
Peripheral neuropathy is a potentially debilitating side effect of neurotoxic chemotherapy regimens and is a frequent reason for early cessation of treatment. Three classes of chemotherapy drugs commonly associated with peripheral neuropathy are taxanes, vinca alkaloids, and platinum compounds. Symptoms include numbness, tingling, burning/stabbing sensations, weakness of the hands or feet, and balance problems (96). Very little information is available regarding the potential relationship between obesity and peripheral neuropathy. In breast cancer, two studies observed no association between obesity and chemotherapy-induced peripheral neuropathy after taxane administration (97,98). Further, a study among multiple myeloma patients found that obesity did not increase the risk of peripheral neuropathy (99). However, given that obesity is also a strong risk factor for diabetes, which can induce neuropathy, it may be difficult to determine the independent effect of obesity on chemotherapy-induced neuropathy (39). One population-based cohort study observed that 9% of participants had comorbid diabetes at cancer diagnosis, with higher rates for some cancers (eg, 19% and 14% among pancreatic and uterine cancer patients, respectively) (100).
Functional Health
The negative effect of cancer and its treatments on functional health among cancer patients, as compared with age-matched adults with no cancer history, has been observed in three large observational cohorts (101–103). The effect of obesity on functional health is also well demonstrated (104–106). No studies that specifically focused on the differential impact of cancer and its treatments on functional status among obese vs nonobese survivors were identified. However, given the evidence that both cancer and obesity are associated with worse functional health, it seems quite likely that functional health would be worse among obese than nonobese survivors.
Cardiotoxicity
Cancer treatments can increase survivors’ risk for adverse cardiac effects, including hypertension, ischemia, left ventricular dysfunction, venous thromboembolism, and brachycardia (107). Because obesity is already a strong risk factor for cardiovascular disease and late effects of chemotherapy may not appear for many years after treatment completion, it is difficult to determine the specific role of obesity in treatment-related cardiac toxicities. Other sources of increased cardiovascular risk, such as weight gain after chemotherapy among breast cancer survivors (108), further complicate the question. The relationships between excess pretreatment weight, weight gain after treatment, and treatment-related cardiovascular outcomes have not been extensively studied.
The Relevance of Race to the Obesity–Cancer Burden Link
Race, Obesity, and Cancer Survival
The 2002–2008 data from the 18 Surveillance Epidemiology and End Results (SEER) geographic areas demonstrate that 5-year cause-specific survival is poorer for blacks and American Indians/Alaskan Natives when compared with non-Hispanic whites, Asian/Pacific Islanders, and Hispanics, regardless of tumor site (109). More recently, it has been reported that blacks are more likely to die from cancer than other racial and ethnic groups (110).
Race and ethnicity are independent predictors of DFS in breast cancer patients. Several studies have documented worse breast cancer–specific DFS and overall survival in black and Hispanic American women compared with non-Hispanic white women (111–113). However, when examining both racial and ethnic variation and obesity’s impact on DFS in breast cancer, the literature appears inconsistent. Within the Multiethnic Cohort Study, obesity was associated with a higher risk for all-cause and breast cancer–specific mortality compared with high-normal BMI. No differences were noted across ethnic groups (26). Another study found that blacks with nonmetastatic triple-negative breast cancer had lower 5-year DFS and a higher 5-year recurrence rate compared with nonblacks (114). In this study, black patients had a higher BMI than nonblack patients; after adjusting for BMI, race remained independently associated with survival (114). Also, a lower DFS was seen in Hispanic American patients with triple-negative breast cancer, independent of obesity (115). Dignam et al. (116) reported similar findings. Research is needed to specifically address the independent and combined contribution of race and obesity on DFS in breast cancer patients, as well as to explore these issues on cancer sites beyond breast cancer.
The colinearity of race and ethnicity with obesity and associated comorbidities creates a tangled web that may require greater sensitivity than is possible in currently available epidemiologic datasets. For example, the large studies that have addressed the relationship of obesity with cancer treatment success may not address quality of or access to cancer care, comorbidities, or ability to take adequate time off of work to receive full treatment and fully recover from treatment. A population based case–control study Contraceptive And Reproductive Experiences (CARE) compared breast cancer mortality across 1604 black and 2934 non-Hispanic white women and within levels of prediagnosis BMI. The results were adjusted for obesity-related comorbidities and showed an effect of obesity on breast cancer only among non-Hispanic white women. However, black women were 33% more likely to die of breast cancer than non-Hispanic white women, even after adjustment for obesity and comorbidities (117). The question of whether this was because of quality of or access to care remains unanswered.
The contribution of race/ethnicity and socioeconomic position on both cancer survival and obesity are difficult to untangle. Cultural, environmental, psychological, and behavioral factors; health-care quality and access; and biological characteristics that are associated with both obesity and survival may partly explain differential outcomes noted by race or ethnicity. Socioeconomic factors that are present before cancer diagnosis may influence survival and mortality after diagnosis. Race-based disparities in survival may, in part, reflect low rates of early detection and screening. Low screening rates and later screening dates for detecting cancer mean that blacks are less likely to survive cancer after 5 years and have overall higher cancer mortality rates than members of other racial and ethnic groups (110,118–121). Several barriers, actual and perceived, contribute to these low rates. Compared with non-Hispanic white women, black women are likely to cite inadequate health insurance and poor access to mammography, as well as psychosocial barriers like mistrust of the health-care system and racial discrimination, as reasons for not completing screening (122). For prostate cancer, individual barriers for black men include lack of health insurance, lack of knowledge, cost, lack of sense of urgency, inconvenient doctor’s office hours, and a lack of information about what type of medical professional to seek and where to find one (123). Notably, many of these barriers of access to screening parallel access to factors that influence obesity, such as lack of access to healthy foods or safe and adequate environments for physical activity. More data are needed to either address or put aside the possibility that obesity and related comorbidities could partly explain racial and ethnic disparities in cancer treatment outcomes. To further complicate this relationship, members of different racial and ethnic groups may be influenced differently, so both within-race and across-race analyses are needed. At present, cohort studies of cancer risk (ie, etiology) and cancer survivorship have too few minorities to be able to adequately address these complex questions. There are no existing cohorts that were designed specifically to address the complex issues of race, ethnicity, and obesity as they relate to cancer survival and survivorship. Novel methods and exposure assessment are needed to measure the upstream variables denoted in Figure 1. It is exceedingly unlikely that existing longitudinal cohorts have collected data with the requisite level of precision to address these issues, as most social and behavioral factors are collected for the purpose of controlling for confounding rather than as causal factors important to cancer control in their own right.
Race, Obesity, and Adverse Treatment Effects
Adverse treatment effects may complicate day-to-day functioning for cancer survivors, deteriorate overall health and ability to fight disease, and affect mental health. The degree to which adverse treatment outcomes influence a cancer survivor may also vary by race and ethnicity, as well as by obesity. Most research in this area focuses on non-Hispanic white cancer survivor populations, and limited data are available to examine the variability by race or ethnicity for these outcomes. Furthermore, there is a lack of data exploring whether variability in the prevalence of comorbidities and response to treatment across race and ethnicity may influence the purported association of obesity and adverse treatment effects. Although the number of studies in this area is limited, herein we highlight the current state of the field in select areas.
Lymphedema.
Most studies of lymphedema in breast cancer survivors either consist largely of non-Hispanic white women or have not reported the racial or ethnic breakdown of their study sample. Therefore, little information is available about how lymphedema risk may vary between population groups. A higher incidence of lymphedema has been observed among black women vs non-Hispanic white women (124). Meeske et al. also investigated risk factors for lymphedema in non-Hispanic White and black breast cancer survivors. The authors included 271 non-Hispanic white and 223 black women with in situ to stage III-A primary breast cancer and a diagnosis of arm lymphedema in their study (64). They concluded that arm lymphedema was associated with younger age at diagnosis, history of hypertension, obesity, and having had surgery that required excision of 10 or more lymph nodes. Although black women (28%) appeared to be more likely to develop arm lymphedema when compared with non-Hispanic white women (21%), the difference was not statistically significant after adjusting for comorbidities, such as hypertension. Both obesity and hypertension have previously been associated with lymphedema onset and progression (125) and are more common among black women than non-Hispanic white women. Given the limited number of studies in this area, additional investigations are warranted in minority populations. No studies have examined whether race or ethnicity modifies the relationship between obesity and lymphedema.
Quality of Life.
As noted earlier, cancer survivors experience detrimental changes in psychological and physiological health that can impact health-related quality of life (HRQOL). The magnitude of these differences in symptoms may vary depending on race or ethnicity and patterns of obesity. Many studies have independently examined the association between race and HRQOL (126–129) and obesity and HRQOL (76,80,84) among cancer survivors; however, few examined the combined effects of race and obesity on HRQOL among cancer survivors. Minority cancer survivors experience lower HRQOL than their non-Hispanic white counterparts (128,129). Black breast cancer survivors report poorer physical functioning and general health and poorer physical and social well-being compared with non-Hispanic white survivors (129–132). Moreover, blacks had low HRQOL outcomes due to higher levels of stress and worry related to recurrence and financial concerns (133–135). Hispanic American breast cancer survivors report lower HRQOL outcomes compared with other racial groups (126). Less acculturated Hispanic American cancer survivors are observed to have statistically significantly lower functional well-being, emotional well-being, and breast cancer concerns compared with non-Hispanic white survivors (136). The associations between obesity and lower quality of life are observed across racial groups (81); however, more research is needed to assess whether the relationship between obesity and reduced quality of life is stronger among some groups than others.
Fatigue.
Few studies have reported on CRF specifically within racial or ethnic minority groups. It is unknown whether race and ethnicity modify the relationship between obesity and CRF. One study observed no statistically significant differences in CRF between black and non-Hispanic white breast cancer survivors (134).
Peripheral Neuropathy and Cardiotoxicity.
Two studies observed statistically significantly higher incidence of peripheral neuropathy among black cancer patients as compared with non-Hispanic white breast cancer patients treated with taxanes (98,137). By comparison, one other study did not (97). Beyond this, no studies were identified regarding the potential for race or ethnicity to mediate or moderate the relationship of obesity with peripheral neuropathy or cardiotoxicity of cancer treatments or to examine whether these factors may differ across racial and ethnic groups.
Functional Health.
One study was identified that examined physical health and obesity in black, Hispanic American, Asian American, and non-Hispanic white cancer survivors (138). Black and Hispanic American survivors reported substantially lower physical health scores compared with Asian American and non-Hispanic white survivors (138). Black survivors had the highest rates for obesity, which was associated with lower physical function scores than for nonobese survivors (138).
Pressing Questions
Unwinding the tangle of obesity, comorbidities, race and ethnicity, and cancer survival and adverse treatment outcomes will require studies specifically designed with these goals in mind. A scaffolding to frame the complexity of these relationships is depicted in Figure 1. As reviewed above, the effects of obesity on cancer recurrence and adverse treatment outcomes show some variability. It could be hypothesized that some of this variability may be due to upstream variability across research cohorts with regard to homogeneity of comorbid health conditions and race or ethnicity. Throughout the review, we have noted unanswered questions specific to understanding the role of obesity in cancer survival and adverse treatment effects. Additional pressing questions appear below. These questions address the potential for the relationships of obesity, chronic disease, and race or ethnicity to confound, alter, or mediate relationships among obesity, cancer recurrence, and adverse treatment effects.
What are the combined and independent contributions of race, comorbidities, and obesity on DFS, recurrence, and overall mortality in cancer patients?
What are the combined and independent contributions of race, comorbidities, and obesity on HRQOL and other adverse treatment effects?
Is there variability in the effect of obesity on cancer survival across racial and ethnic minority groups (both within-race and across-race analyses are needed)?
To what extent does race or ethnicity modify the effect of obesity or comorbid health conditions on adverse treatment effects such as lymphedema and CRF?
Possible methodological approaches could include meta-analysis or pooling projects of prospective cohort studies with the aim of exploring these complex relationships and how they impact survival. Further, large national databases (SEER, National Cancer Registry, or pooling large registries [ie, California, Texas, NY]) that include comorbidities, obesity subgroups, treatment, pathology race/ethnicity, and socioeconomic status could be leveraged to study relationships between obesity and related comorbidities on cancer treatment outcomes/survival by race/ethnicity. Ultimately, it may be necessary to design and conduct large observational cohort studies with the primary aim of determining the relative importance of race and ethnicity in the link between obesity and cancer survival.
Conclusion
Since the publication of the landmark study by Calle et al. 10 years ago (7), research on the effects of obesity on cancer survival has increased substantially. In this review, we have focused on the role of obesity in cancer recurrence and adverse treatment effects. This area is ripe for further investigation, and we urge researchers to consider whether obesity may be a marker for upstream issues that vary across race and ethnic groups and which factors covary with obesity, including comorbid chronic diseases, access to care, health behaviors, and screening practices. If the role of obesity on cancer survival is due, in part, to these upstream factors, then these issues, along with obesity, should be the target of interventions to reduce the burden of cancer.
Funding
This work was supported by grants from the National Institutes of Health (U54-CA155850 at University of Pennsylvania [KHS, LTD]; U01CA116850 at the Fred Hutchinson Cancer Research Center [MLN]; 1U54CA155435 at University of California at San Diego [LC-B]; and U54 CA155496 at Washington University [BFD]).
References
- 1. National Center For Health Statistics Prevalence of Overweight, Obesity and Extreme Obesity Among Adults: United States, Trends 1976–80 Through 2005–2006. Hyattsville, MD: National Center for Health Statistics; 2008. [Google Scholar]
- 2. Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497 [DOI] [PubMed] [Google Scholar]
- 3. Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: Final Data for 2009. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 4. Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–2527 [DOI] [PubMed] [Google Scholar]
- 6. Griggs JJ, Culakova E, Sorbero ME, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25(3):277–284 [DOI] [PubMed] [Google Scholar]
- 7. Calle R, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New Engl J Med. 2003;348(17):1625–1638 [DOI] [PubMed] [Google Scholar]
- 8. Chamberlain C, Romundstad P, Vatten L, et al. The association of weight gain during adulthood with prostate cancer incidence and survival: a population-based cohort. Int J Cancer. 2011;129(5):1199–1206 [DOI] [PubMed] [Google Scholar]
- 9. Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684 [DOI] [PubMed] [Google Scholar]
- 10. Joshu CE, Mondul AM, Menke A, et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila). 2011;4(4):544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4(4):486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126(3):692–702 [DOI] [PubMed] [Google Scholar]
- 13. Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132(2):729–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cecchini R, Costantino J, Cauley J, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP-P1 and STAR Breast Cancer Prevention Trials. Cancer Prev Res (Phila). 2012;5(4):583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oxentenko AS, Bardia A, Vierkant RA, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer Prev Res (Phila). 2010;3(12):1608–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;32:311–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang HS, Yoon C, Myung SK, et al. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2011;21(9):1525–1532 [DOI] [PubMed] [Google Scholar]
- 18. Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5(7):901–910 [DOI] [PubMed] [Google Scholar]
- 19. Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–635 [DOI] [PubMed] [Google Scholar]
- 20. Williams SB, Salami S, Regan MM, et al. Selective detection of histologically aggressive prostate cancer: an Early Detection Research Network Prediction model to reduce unnecessary prostate biopsies with validation in the Prostate Cancer Prevention Trial. Cancer. 2012;118(10):2651–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Cancer Research Fund/AICR Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 22. Prizment AE, Flood A, Anderson KE, et al. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2229–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16(6):93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bessonova L, Marshall SF, Ziogas A, et al. The association of body mass index with mortality in the California Teachers Study. Int J Cancer. 2011;129(10):2492–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ewertz M, Jensen MB, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31 [DOI] [PubMed] [Google Scholar]
- 26. Conroy SM, Maskarinec G, Wilkens LR, et al. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129(2):565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg. 2009;208(6):1093–1098 [DOI] [PubMed] [Google Scholar]
- 30. Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring). 2009;17(4):796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117(9):1788–1799 [DOI] [PubMed] [Google Scholar]
- 32. Sainsbury A, Goodlad RA, Perry SL, et al. Increased colorectal epithelial cell proliferation and crypt fission associated with obesity and roux-en-Y gastric bypass. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1401–1410 [DOI] [PubMed] [Google Scholar]
- 33. Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–662 [DOI] [PubMed] [Google Scholar]
- 34. Thivat E, Therondel S, Lapirot O, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caan BJ KM, Shu XO, Pierce JP, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epi Bio Prev. 2012;21(8):1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101(9):630–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villasenor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 Trial. J Clin Oncol. 2011;29(19):2653–2659 [DOI] [PubMed] [Google Scholar]
- 39. de Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119(1):145–153 [DOI] [PubMed] [Google Scholar]
- 40. Johansson H, Gandini S, Guerrieri-Gonzaga A, et al. Effect of fenretinide and low-dose tamoxifen on inuslin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68(22):9512–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le TT, Huff TB, Cheng JX. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer. 2009;9:42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hellawell GO, Turner GD, Davies DR, et al. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62(10):2942–2950 [PubMed] [Google Scholar]
- 43. de Bono JS, Attard G, Adjei A, et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer Res. 2007;13(12):3611–3616 [DOI] [PubMed] [Google Scholar]
- 44. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553–1561 [DOI] [PubMed] [Google Scholar]
- 45. Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895 [DOI] [PubMed] [Google Scholar]
- 46. Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31 [DOI] [PubMed] [Google Scholar]
- 47. Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165(11):1267–1273 [DOI] [PubMed] [Google Scholar]
- 48. Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–4531 [DOI] [PubMed] [Google Scholar]
- 49. Smith K, Wray L, Klein-Cabral M, et al. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin Breast Cancer. 2005;6(3):260–266; discussion 267–269 [DOI] [PubMed] [Google Scholar]
- 50. Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95(20):1545–1548 [DOI] [PubMed] [Google Scholar]
- 51. Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benoist S, Panis Y, Alves A, et al. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179(4):275–281 [DOI] [PubMed] [Google Scholar]
- 53. Eichenberger A, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95(6):1788–1792 [DOI] [PubMed] [Google Scholar]
- 54. Choban PS, Flancbaum L. The impact of obesity on surgical outcomes: a review. J Am Coll Surg. 1997;185(6):593–603 [DOI] [PubMed] [Google Scholar]
- 55. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321(4):249–279 [DOI] [PubMed] [Google Scholar]
- 57. Munkhaugen J, Lydersen S, Wideroe TE, et al. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis. 2009;54(4):638–646 [DOI] [PubMed] [Google Scholar]
- 58. Cronin DP, Harlan LC, Potosky AL, et al. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101(10):2308–2318 [DOI] [PubMed] [Google Scholar]
- 59. Cronin DP, Harlan LC, Clegg LX, et al. Patterns of care in a population-based random sample of patients diagnosed with non-Hodgkin’s lymphoma. Hematol Oncol. 2005;23(2):73–81 [DOI] [PubMed] [Google Scholar]
- 60. Harlan LC, Klabunde CN, Ambs AH, et al. Comorbidities, therapy, and newly diagnosed conditions for women with early stage breast cancer. J Cancer Surviv. 2009;3(2):89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gross CP, McAvay GJ, Guo Z, et al. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109(12):2410–2419 [DOI] [PubMed] [Google Scholar]
- 62. Honnor A. Classification, aetiology and nursing management of lymphoedema. Br J Nurs. 2008;17(9):576–586 [DOI] [PubMed] [Google Scholar]
- 63. Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130(3):981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in black women and white women. Breast Cancer Res Treat. 2009;113(2):383–391 [DOI] [PubMed] [Google Scholar]
- 65. Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54 [DOI] [PubMed] [Google Scholar]
- 66. Ridner SH, Dietrich MS, Stewart BR, et al. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19(6):853–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM. 2005;98(5):343–348 [DOI] [PubMed] [Google Scholar]
- 68. Mak SS, Yeo W, Lee YM, et al. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res. 2008;57(6):416–425 [DOI] [PubMed] [Google Scholar]
- 69. Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110(8):1868–1874 [DOI] [PubMed] [Google Scholar]
- 70. Beesley V, Janda M, Eakin E, et al. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109(12):2607–2614 [DOI] [PubMed] [Google Scholar]
- 71. Kim JH, Choi JH, Ki EY, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. 2012;22(4):686–691 [DOI] [PubMed] [Google Scholar]
- 72. Ohba Y, Todo Y, Kobayashi N, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol. 2011;16(3):238–243 [DOI] [PubMed] [Google Scholar]
- 73. Kizer NT, Thaker PH, Gao F, et al. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer. 2011;117(5):948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Todo Y, Yamamoto R, Minobe S, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol. 2010;119(1):60–64 [DOI] [PubMed] [Google Scholar]
- 75. Valdivieso M, Kujawa AM, Jones T, et al. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 2012;9(2):163–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fader AN, Frasure HE, Gil KM, et al. Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet Gynecol Int. 2011;2011:308609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Courneya KS, Karvinen KH, Campbell KL, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430 [DOI] [PubMed] [Google Scholar]
- 78. Milne HM, Gordon S, Guilfoyle A, et al. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16(12):1059–1068 [DOI] [PubMed] [Google Scholar]
- 79. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261 [DOI] [PubMed] [Google Scholar]
- 80. Dieperink KB, Hansen S, Wagner L, et al. Living alone, obesity and smoking: important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncol. 2012;51(6):722–729 [DOI] [PubMed] [Google Scholar]
- 81. Anast JW, Sadetsky N, Pasta DJ, et al. The impact of obesity on health related quality of life before and after radical prostatectomy (data from CaPSURE). J Urol. 2005;173(4):1132–1138 [DOI] [PubMed] [Google Scholar]
- 82. Jansen L, Koch L, Brenner H, et al. Quality of life among long-term (≥5 years) colorectal cancer survivors—systematic review. Eur J Cancer. 2010;46(16):2879–2888 [DOI] [PubMed] [Google Scholar]
- 83. Mosher CE, Sloane R, Morey MC, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer. 2009;115(17):4001–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc. 2010;42(4):665–671 [DOI] [PubMed] [Google Scholar]
- 85. Wolin KY, Luly J, Sutcliffe S, et al. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol. 2010;183(2):629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Curt GA. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000;37(4 Suppl 6):14–17 [DOI] [PubMed] [Google Scholar]
- 87. Lawrence DP, Kupelnick B, Miller K, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;32:40–50 [DOI] [PubMed] [Google Scholar]
- 88. Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–2269 [DOI] [PubMed] [Google Scholar]
- 89. Andrykowski MA, Donovan KA, Laronga C, et al. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reinertsen KV, Cvancarova M, Loge JH, et al. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4(4):405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer. 2011;19(10):1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Winters-Stone KM, Bennett JA, Nail L, et al. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35(5):815–821 [DOI] [PubMed] [Google Scholar]
- 93. Basen-Engquist K, Scruggs S, Jhingran A, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200(3):288.e1–288.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meeske KA, Siegel SE, Globe DR, et al. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol. 2005;23(24):5501–5510 [DOI] [PubMed] [Google Scholar]
- 95. Meeske K, Smith AW, Alfano CM, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16(6):947–960 [DOI] [PubMed] [Google Scholar]
- 96. Stubblefield MD, McNeely ML, Alfano CM, et al. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(8 Suppl):2250–2260 [DOI] [PubMed] [Google Scholar]
- 97. Schneider BP, Zhao F, Wang M, et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J Clin Oncol. 2012;30(25):3051–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Speck RM, Sammel MD, Farrar JT, et al. Racial disparities in the incidence of dose-limiting chemotherapy induced peripheral neuropathy. In: San Antonio Breast Cancer Symposium; San Antonio, TX, December 4–8, 2012. [Google Scholar]
- 99. Dimopoulos MA, Mateos MV, Richardson PG, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31 [DOI] [PubMed] [Google Scholar]
- 100. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120(9):1986–1992 [DOI] [PubMed] [Google Scholar]
- 101. Ness KK, Wall MM, Oakes JM, et al. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16(3):197–205 [DOI] [PubMed] [Google Scholar]
- 102. Sweeney C, Schmitz KH, Lazovich D, et al. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98(8):521–529 [DOI] [PubMed] [Google Scholar]
- 103. Michael Y, Kawachi I, Berkman L, et al. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000;89(11):2176–2186 [DOI] [PubMed] [Google Scholar]
- 104. Fontaine KR, Cheskin LJ, Barofsky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract. 1996;43(3):265–270 [PubMed] [Google Scholar]
- 105. Stewart AL, Brook RH. Effects of being overweight. Am J Public Health. 1983;73(2):171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Trakas K, Oh PI, Singh S, et al. The health status of obese individuals in Canada. Int J Obes Relat Metab Disord. 2001;25(5):662–668 [DOI] [PubMed] [Google Scholar]
- 107. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–2247 [DOI] [PubMed] [Google Scholar]
- 108. Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(8 Suppl):2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Howlader NNA, Krapcho M, Neyman N. et al. eds. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) http://seer.cancer.gov/csr/1975_2009_pops09/ Accessed April 1, 2012 [Google Scholar]
- 110. American Cancer Society Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 111. Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27(13):2157–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mukhtar RA, Moore AP, Nseyo O, et al. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res Treat. 2011;130(2):635–644 [DOI] [PubMed] [Google Scholar]
- 113. Sarker M, Jatoi I, Becher H. Racial differences in breast cancer survival in women under age 60. Breast Cancer Res Treat. 2007;106(1):135–141 [DOI] [PubMed] [Google Scholar]
- 114. Christiansen N, Chen L, Gilmore J, et al. Association between African American race and outcomes in patients with nonmetastatic triple-negative breast cancer: a retrospective analysis by using results from the Georgia Cancer Specialist Database. Clin Breast Cancer. 2012;12(4):270–275 [DOI] [PubMed] [Google Scholar]
- 115. Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658–3669 [DOI] [PubMed] [Google Scholar]
- 116. Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–254 [DOI] [PubMed] [Google Scholar]
- 117. Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29(25):3358–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Merkin SS, Stevenson L, Powe N. Geographic socioeconomic status, race, and advanced-stage breast cancer in New York City. Am J Public Health. 2002;92(1):64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Myers RE, Wolf TA, McKee L, et al. Factors associated with intention to undergo annual prostate cancer screening among African American men in Philadelphia. Cancer. 1996;78(3):471–479 [DOI] [PubMed] [Google Scholar]
- 120. Shen Y, Dong W, Esteva FJ, et al. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at M.D. Anderson Cancer Center? Breast Cancer Res Treat. 2007;102(3):347–356 [DOI] [PubMed] [Google Scholar]
- 121. Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553 [DOI] [PubMed] [Google Scholar]
- 122. Gerend MA, Pai M. Social determinants of black–white disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2913–2923 [DOI] [PubMed] [Google Scholar]
- 123. Weinrich SP, Reynolds WA, Jr., Tingen MS, et al. Barriers to prostate cancer screening. Cancer Nurs. 2000;23(2):117–121 [DOI] [PubMed] [Google Scholar]
- 124. Kwan ML, Darbinian J, Schmitz KH, et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg. 2010;145(11):1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kocak Z, Overgaard J. Risk factors of arm lymphedema in breast cancer patients. Acta Oncol. 2000;39(3):389–392 [DOI] [PubMed] [Google Scholar]
- 126. Ashing-Giwa KT, Tejero JS, Kim J, et al. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413–428 [DOI] [PubMed] [Google Scholar]
- 127. Paskett ED, Alfano CM, Davidson MA, et al. Breast cancer survivors’ health-related quality of life: racial differences and comparisons with noncancer controls. Cancer. 2008;113(11):3222–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Powe BD, Hamilton J, Hancock N, et al. Quality of life of African American cancer survivors. A review of the literature. Cancer. 2007;109(2 Suppl):435–445 [DOI] [PubMed] [Google Scholar]
- 129. Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. J Cancer Surviv. 2011;5(2):191–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rao D, Debb S, Blitz D, et al. Racial/Ethnic differences in the health-related quality of life of cancer patients. J Pain Symptom Manage. 2008;36(5):488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Russell GV, Pierce CW, Nunley L. Financial implications of obesity. Orthop Clin North Am. 2011;42(1):123–127, vii [DOI] [PubMed] [Google Scholar]
- 133. Ashing-Giwa KT, Padilla G, Tejero J, et al. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13(6):408–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gill KM, Mishel M, Belyea M, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31(3):633–639 [DOI] [PubMed] [Google Scholar]
- 135. Shelby RA, Lamdan RM, Siegel JE, et al. Standardized versus open-ended assessment of psychosocial and medical concerns among African American breast cancer patients. Psychooncology. 2006;15(5):382–397 [DOI] [PubMed] [Google Scholar]
- 136. Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schneider BP, Li L, Miller K, et al. Genetic associations with taxane-induced neuropathy by a genome-wide association study (GWAS) in E5103. J Clin Oncol. 2011;29(15 Suppl):abstract 1000 [Google Scholar]
- 138. Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]