Abstract
Previous investigations showing that polydisperse biguanide (PDBG) molecules have activity against human immunodeficiency virus type 1 (HIV-1) also suggested a relationship between PDBG biologic activity and the lengths of hydrocarbon linkers surrounding the positively charged biguanide unit. To better define structure-activity relationships, PDBG molecules with select linker lengths were evaluated for cytotoxicity, anti-HIV-1 activity, and in vivo toxicity. Results of the in vitro experiments demonstrated that increases in linker length (and, therefore, increases in compound lipophilicity) were generally associated with increases in cytotoxicity and antiviral activity against HIV-1. However, a relationship between linker length asymmetry and in vitro therapeutic index (TI) suggested structural specificity in the mechanism of action against HIV-1. Polyethylene hexamethylene biguanide (PEHMB; biguanide units spaced between alternating ethylene and hexamethylene linkers) was found to have the highest in vitro TI (CC50/IC50) among the compounds examined. Recent improvements in PEHMB synthesis and purification have yielded preparations of PEHMB with in vitro TI values of 266 and 7000 against HIV-1 strains BaL and IIIB, respectively. The minimal toxicity of PEHMB relative to polyhexamethylene biguanide (PHMB; biguanide units alternating with hexamethylene linkers) in a murine model of cervicovaginal microbicide toxicity was consistent with considerable differences in cytotoxicity between PEHMB and PHMB observed during in vitro experiments. These structure-activity investigations increase our understanding of PDBG molecules as agents with activity against HIV-1 and provide the foundation for further preclinical studies of PEHMB and other biguanide-based compounds as antiviral and microbicidal agents.
Keywords: HIV-1, Biguanide, Antiviral
1. Introduction
The global spread of human immunodeficiency virus type 1 (HIV-1), driven increasingly by viral transmission from the male to female partner during heterosexual intercourse [1] has necessitated efforts on many levels to decrease or eliminate the risk of transmission. Because campaigns to promote abstinence, initiatives to educate people about the risks of HIV-1 and other sexually transmitted pathogens, and programs to promote the use of condoms and other barrier methods have proven to be only partially effective against the continued spread of HIV-1, the need remains urgent for other, more direct means of intervention [1]. In response to this need, an increasing number of agents with activity against HIV-1 and other sexually transmitted disease agents are being developed for use as topical vaginal or rectal microbicides. At present, there are numerous compounds in various stages of preclinical and clinical development that differ widely in both their physical attributes and their mechanisms of action [2–4].
Our efforts in this direction have focused on the development of biguanide-based antiviral compounds that are unique among other potential microbicides with respect to chemistry, physical characteristics, and potential mechanism of action. Polydisperse biguanides (PDBGs) are cationic molecules comprised of biguanide repeat units separated by defined linkers of similar or variable length. PDBGs used in these studies contained biguanide repeat units separated by hydrocarbon linkers (methylene groups) of variable length (Fig. 1). PDBGs are typically associated with chloride anions but can be associated with other anions as dictated by their intended use. The negative decadic logarithm of the acid-ionization constant (pKa) of the biguanide group is approximately 10.5, a property that ensures protonation (fully cationic) of the PDBG molecule at pH values typical of the cervicovaginal environment (pH 4–4.5) [5]. Additional advantages of PDBGs under investigation include the low cost of synthesis and the ease with which these compounds can be produced.
Fig. 1.
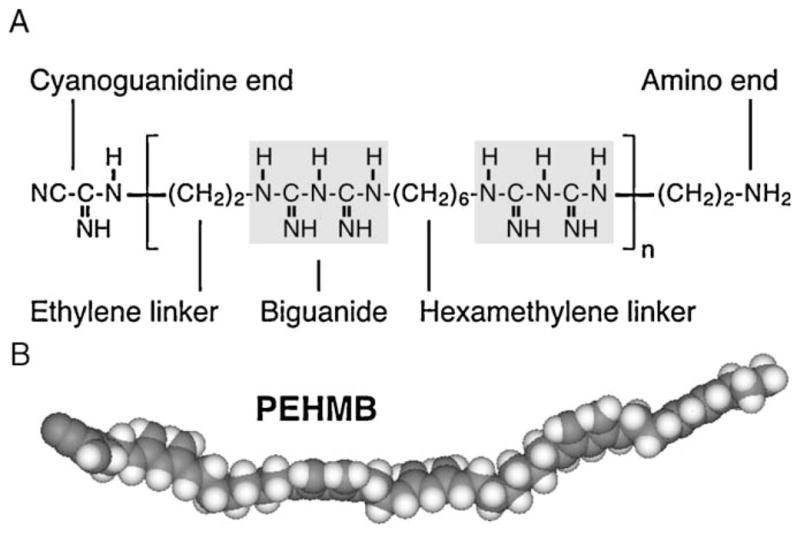
Structure of the polybiguanide polyethylene hexamethylene biguanide (PEHMB). PEHMB consists of biguanide subunits flanked by alternating ethylene and hexamethylene linkers (a 2–6 backbone configuration). The chemical structure (A) and space-filling model (B) of PEHMB are shown. Derivatives of PEHMB were synthesized by varying the n1-biguanide-n2 backbone structure, where n1 and n2 indicate the number of methylene units in the hydrocarbon chains flanking the biguanide repeat unit.
PDBGs are part of a unique family of biguanide-based compounds characterized by safe and effective use in humans. Chlorhexidine gluconate is a bis-biguanide (two biguanide groups) that has been used safely and effectively as a vaginal disinfectant [6,7] and as an antimicrobial mouth rinse [8]. Chlorhexidine gluconate has also been shown in nonhuman primate studies to be protective against chlamydial infection [9]. Polyhexamethylene biguanide (PHMB), which is a longer-chain PDBG, is used as the broad-spectrum bactericidal ingredient in contact lens solutions [10], as a treatment for Acanthamoeba keratitis [11], as an oral antiseptic agent [8,12], and as a swimming pool disinfectant [13]. Particularly relevant to the use of PDBGs as microbicidal agents were the demonstration that bacterial vaginosis can be safely and effectively treated using PHMB [14] and the finding that PHMB has potent in vitro activity against herpes simplex virus type 1 [15]. Although PHMB also has antiviral activity against HIV-1, its in vitro cytotoxicity precluded its further development as a potential microbicide candidate [16].
A considerable strength of the biguanide-based family of molecules is the ease and flexibility with which different molecules having diverse characteristics and activities can be synthesized. Biguanide-based molecules can be tailored by (i) making alterations in the hydrocarbon chains that link the biguanide repeats in the backbone of the molecule, which in turn alter electrostatic charge density, hydrophobicity, and chain flexibility; (ii) adding caps to one or both ends of the molecule to supplement or augment the activity of the parent molecule; (iii) changing the molecular weight (i.e., the number of biguanide repeat units); and (iv) changing the identity of the accompanying anion. These modifications, when introduced alone or in combination, permit the synthesis of a large number of different PDBG molecules that can be screened for characteristics desirable in a topical vaginal microbicide, including low cytotoxicity, potent anti-HIV-1 activity, spermicidal activity, and broad-spectrum effectiveness against other sexually transmitted pathogens.
Previous investigations demonstrated that length changes in the hydrocarbon linkers within the PDBG backbone affected the in vitro cytotoxicity and anti-HIV-1 activity of the resulting PDBG molecules [16]. Those studies concluded with the demonstration that polyethylene hexamethylene biguanide (PEHMB), which consists of biguanide subunits flanked by alternating ethylene and hexamethylene linkers (a 2–6 backbone configuration), was characterized by low in vitro cytotoxicity and considerable in vitro anti-HIV-1 activity. The experiments described here were designed to better define relationships between backbone configuration and biologic activity using a broader panel of PDBGs with variations in backbone structure. The results of these experiments validate the course of our empiric design, synthesis, purification, and evaluation, and further advance the development of biguanide-based molecules, such as PEHMB, as microbicidal agents.
2. Materials and methods
2.1. Synthesis of biguanide-based compounds
The PDBG compounds used in these studies were synthesized using a two-step protocol [17,18]. The first step included the preparation of the co-monomer, alkylene-bis-cyanoguanidine. As an example, synthesis of PHMB (biguanide subunits flanked by hexamethylene linkers; a 6-6 backbone configuration) began by reacting hexamethylenediamine with sodium dicyandiamide to produce hexamethylene bis-cyanoguanidine. In the second step, the latter compound was then reacted at 160 to 185 °C for 4 to 6 h with hexamethylene diamine hydrochloride (the second co-monomer) to form polydisperse oligomeric molecules containing an average of six to eight biguanide units at a mean molecular weight of 2610 (US patent #5942218). PDBG molecules synthesized in this manner (as a chloride salt) were readily soluble in water. N-9 and PDBG solutions were prepared at % (vol/vol) and % (wt/vol) concentrations, respectively.
Backbone variants of PEHMB (2-6) (Fig. 1) were synthesized by varying the hydrocarbon chain lengths within the bis-cyanoguanidine or diamine co-monomers used in the synthesis reactions. The first series of polydisperse compounds, in which the ethylene (n1) linker was lengthened in the context of a constant hexamethylene (n2 = 6) linker, included polypropylene hexamethylene biguanide (PPHMB; 3-6), polybutylene hexamethylene biguanide (PBHMB; 4-6), and PHMB (6-6). Additional polydisperse compounds, which included polyethylene octamethylene biguanide (PEOMB; 2-8), polyethylene decamethylene biguanide (PEDMB; 2-10), and polyethylene dodecamethylene biguanide (PEDDMB; 2-12), were synthesized to examine the effect of length changes in the n2 linker in combination with a constant ethylene (n1 = 2) linker. The numbers that follow each compound name (n1-n2) indicate the number of methylene groups in the linkers that flank the biguanide units in the PDBG molecule.
2.2. Cell culture
P4-R5 MAGI cells (National Institutes of Health AIDS Research and Reference Reagent Program, catalog #3580) were maintained in Dulbecco’s modified Eagle medium supplemented with 0.1 μg/ ml puromycin [19]. This HeLa-based cell line, which expresses CD4, CXCR4, and CCR5, produces β-galactosidase subsequent to HIV-1 infection under the direction of an integrated copy of the HIV-1 long terminal repeat. The Sup-T1 T-lymphocyte cell line (American Type Culture Collection [ATCC] catalog #CRL-1942) was cultured in RPMI 1640 (Roswell Park Memorial Institute medium). Media for both cell lines were supplemented with 10% fetal bovine serum, L-glutamine (0.3 mg/ml), antibiotics (penicillin, streptomycin, and kanamycin at 0.04 mg/ml each), and 0.05% sodium bicarbonate.
2.3. Assays of in vitro cytotoxicity
P4-R5 MAGI cells used to assess in vitro cytotoxicity were seeded in 96-well culture plates (4 × 104 cells per well) and exposed to compounds at the indicated concentrations 16 to 18 h after plating. At the conclusion of the exposure period, the presence of viable cells was quantified using a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) cytotoxicity assay, as described previously [16,20].
2.4. Assessment of viral infection inhibition
To assess the ability of each compound to interfere with HIV-1 infection, P4-R5 MAGI cells (plated at 8 × 104 cells per well in 12-well culture plates) were incubated with cell-free preparations of HIV-1 strains IIIB (X4 phenotype) or BaL (R5 phenotype) (Advanced Biotechnologies, Columbia, MD) with each compound at the desired concentration (300 μl total volume in triplicate wells). Cells were washed once with phosphate-buffered saline (PBS) after the exposure period and supplied with new media (2 ml). After a 48-h incubation period, cells were harvested and assayed for β-galactosidase activity using the Galacto-Star β-Galactosidase Reporter Gene Assay System for Mammalian Cells (Applied Biosystems, Bedford, MA).
2.5. Determining inactivation of cell-free HIV-1
Cell-free preparations of HIV-1 strain IIIB were mixed in a 1:1 ratio with dilutions of each compound (10 μl total volume) to achieve the desired final concentrations. Following a 10-min incubation at 37 °C, the mixtures were diluted 1:100 in RPMI 1640 (with 10% fetal bovine serum) and added (300 μl per well to duplicate wells) to P4-R5 MAGI cells for 2 h. After a wash with PBS and a 48-h incubation period in new media, the cells were harvested and assayed for β-galactosidase activity as described above. IC50 values (the concentration at which virus infection was reduced to 50% of the control value) shown in Table 1 were calculated with respect to compound concentrations achieved during the 10-min incubation.
Table 1.
In vitro cytotoxicity and antiviral activities of PDBGs and control compounds.
| Compound (linkers) | Cytotoxicity: CC50 (%)
|
Antiviral activity: IC50 (%)
|
|||||
|---|---|---|---|---|---|---|---|
| 10-min exposure | 2-h exposurea | 6-h exposure | VII-IIIBb | VII-BaLb | CFI-IIIB | CAI-IIIB | |
| PEHMB (2-6) | 1.0930 | 0.7738 | 0.6995 | 0.0099 | 0.0147 | 0.0795 | 0.0128 |
| PPHMB (3-6) | 0.4116 | 0.1995 | 0.2046 | 0.0028 | 0.2032 | 0.1646 | 0.0054 |
| PBHMB (4-6) | 0.4221 | 0.0707 | 0.0534 | 0.0023 | 0.0042 | 0.0749 | 0.0085 |
| PEOMB (2-8) | 0.1624 | 0.0657 | 0.0274 | 0.0011 | 0.0013 | 0.0300 | 0.0028 |
| PHMB (6-6) | 0.0461 | 0.0094 | 0.0088 | ND | ND | ND | ND |
| PEDMB (2-10) | 0.0301 | 0.0163 | 0.0131 | ND | ND | ND | ND |
| PEDDMB (2-12) | 0.0169 | 0.0080 | 0.0044 | ND | ND | ND | ND |
| N-9 | 0.0645 | 0.0061 | 0.0056 | ND | ND | ND | ND |
| DS | > 2 | > 1 | > 1 | < 0.0001 | 0.0008 | 0.0034 | 0.0009 |
| PEHMB (2-6)c | ND | 2.1 | ND | 0.0003 | 0.0079 | ND | ND |
2.6. Assaying inactivation of cell-associated HIV-1
Sup-T1 cells were infected with HIV-1 IIIB (1 μl virus per 106 cells). After 72 h, HIV-1-infected cells were then pelleted and resuspended in RPMI 1640 (1 × 106 cells per 95 μl) and mixed with 5 μl of compound to achieve the desired final concentrations. After a 10-min incubation at 37 °C, the mixture was diluted 1:10 and added (300 μl per well in triplicate wells) to P4-R5 MAGI cells. Following a 2-h adsorption period, the P4-R5 MAGI cells were washed once with PBS and supplied with new media (2 ml). After a 48-h incubation, the cells were harvested and assayed for β-galactosidase activity as described above. IC50 values (Table 1) were calculated with respect to compound concentrations achieved during the 10-min incubation.
2.7. In vivo analysis of cervicovaginal toxicity using a Swiss Webster mouse model
Assessments of in vivo toxicity were performed as previously described [21,22]. Six- to 10-week old female Swiss Webster mice (Charles River Laboratories, Wilmington, MA), hormonally synchronized prior to the experiment, were anesthetized and inoculated intravaginally with 60 μl of unformulated PHMB (6-6), PEHMB (2-6), N-9, or the control diluent. Mice were sacrificed at 10 min, 2 h, 4 h, or 8 h following application, and the entire reproductive tract was surgically excised. Tissues were formalin-fixed and embedded in paraffin prior to staining with hematoxylin and eosin. Tissue sections from all animals within a treatment group were visually examined using an Olympus IX81 microscope (Center Valley, PA) to assess the gross morphologic condition of the cervicovaginal mucosa. Following the overall assessment of the tissue, representative fields from the vaginal and cervical epithelium from each treatment group were photographed using a high-resolution digital camera.
Immune cell recruitment following compound exposure was evaluated by reacting harvested tissues with a monoclonal rat antimouse antibody specific to CD45, which identifies all cells of hematopoietic origin (except erythrocytes). Histologic and immunohistochemical procedures have been described previously [21,22]. Tissue sections reacted with secondary antibody alone or a purified rat isotype control (BD Biosciences, San Jose, CA) served as controls for each antibody reaction, and visualization was performed using an Olympus IX81 microscope with a high-resolution digital camera.
3. Results
3.1. Increased hydrocarbon linker length is associated with greater in vitro cytotoxicity compared with PEHMB
PDBGs with different backbone structures relative to PEHMB (Fig. 1) were characterized initially to determine relationships between compound structure and in vitro cytotoxicity and to eliminate from further analyses compounds with less than desirable toxicity profiles. In these experiments, N-9 was used as a reference standard because the cytotoxicity of this compound, which serves as an example of a “failed” microbicide [23] had been previously established [16,20,24].
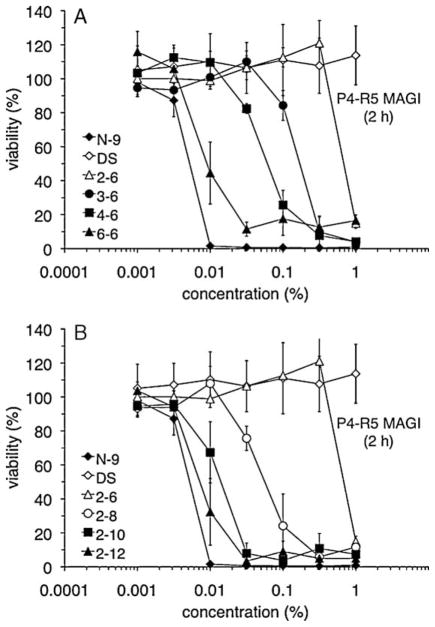
P4-R5 MAGI cell sensitivity to each compound was assessed after a 2-h exposure (Fig. 2). Increases in the length of the n1 linker (Fig. 2A) or the n2 linker (Fig. 2B) were clearly associated with greater in vitro cytotoxicity. The same relationship between increased linker length and cytotoxicity was apparent in CC50 values (the concentration at which cell viability was reduced to 50% of the control value) calculated from these results (Table 1). Although PDBGs do not have surfactant properties, reductions in cell viability following exposure to PHMB (6-6), PEDMB (2-10), and PEDDMB (2-12) were similar to those caused by N-9 exposure. Similar observations were made during experiments that examined cytotoxicity after 10-min and 6-h exposures (Table 1). In all experiments, PEHMB (2-6) was the least cytotoxic of the PDBG-based compounds evaluated.
Fig. 2.
Variations in the backbone n1 (A) or n2 (B) chain length alter the in vitro cytotoxicity of biguanide-based compounds. P4-R5 MAGI cells were exposed to N-9, dextran sulfate (DS), or the indicated PDBG compounds for 2 h and subsequently assayed for viability, which is expressed as a percentage relative to mock-exposed cells. Combined results are depicted from two independent cytotoxicity assays, in which each concentration was examined in duplicate. Error bars indicate the standard deviation for each data point.
3.2. Greater activity against HIV-1 is generally associated with longer linker lengths relative to PEHMB
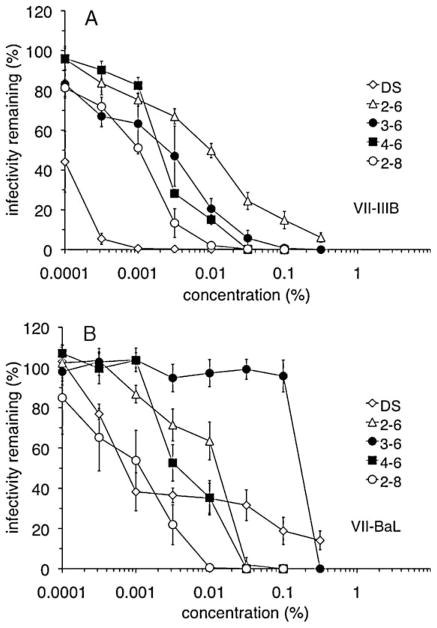
Because the suitability of a candidate microbicide is measured by its effect on cell viability as well as anti-HIV-1 activity, only the following compounds, which had low relative cytotoxicity (2-h CC50 ≥ 0.05%), were assessed for their anti-HIV-1 activity: PEHMB (2-6), PPHMB (3-6), PBHMB (4-6), and PEOMB (2-8). In assays using either the HIV-1 X4 strain IIIB (Fig. 3A) or the R5 strain BaL (Fig. 3B), the most effective PDBG was PEOMB (2-8). In both experiments, the relative order of activity was PEOMB (2-8) > PBHMB (4-6) > PEHMB (2-6). Interestingly, however, PPHMB (3-6) was dramatically less effective against HIV-1 BaL (0.2032% IC50,) compared with its activity against HIV-1 IIIB (0.0028% IC50). These experiments demonstrated through concentration-dependent antiviral activity and calculated IC50 values (Table 1) that the presence of longer linkers relative to PEHMB (2-6) was associated with a greater capacity for inhibiting HIV-1 infection relative to PEHMB (2-6).
Fig. 3.
Backbone linker length changes affect the ability to interfere with infection by HIV-1 strains IIIB or BaL. In a VII assay, dextran sulfate (DS) or the indicated compounds were incubated with cell-free HIV-1 strains (A) IIIB or (B) BaL in the presence of P4-R5 MAGI cells for 2 h at 37 °C. Infectivity remaining after compound exposure is expressed as a percentage relative to cells infected in the absence of compound. Combined results are depicted from two independent assays, in which each concentration was examined in duplicate. Error bars indicate the standard deviation for each data point.
Experiments were also performed to determine the effects of backbone changes on activity against cell-free and cell-associated forms of HIV-1. All four compounds were less active against cell-free HIV-1 IIIB relative to their respective activities in the viral infection inhibition (VII) assay (Table 1). In contrast, antiviral activities determined in assays using HIV-1 IIIB-infected Sup-T1 cells were higher and more comparable to the levels of activity obtained in the VII assay (Table 1). With respect to activity against cell-free and cell-associated virus, PEOMB (2-8) again had the greatest antiviral activity. These results demonstrated backbone-dependent activity with respect to cytotoxicity and several forms of HIV-1 infectivity.
3.3. PEHMB provides the best combination of antiviral activity and low cytotoxicity among the PDBGs examined
Previous investigations [16] suggested that PEHMB (2-6) was worthy of further exploration as a microbicidal compound when both in vitro cytotoxicity and anti-HIV-1 activity were considered. In the present studies, similar comparisons were made using in vitro therapeutic indices (TIs) to provide relative indicators of performance in assays of cytotoxicity and antiviral activity (TI = CC50/IC50). Comparisons of TI values across all four antiviral assays (Table 2) revealed that PEHMB (2-6) had the highest TI values among the four PDBG compounds evaluated. TI values of other PDBGs (Table 2) assessed in previous studies [16] also supported the conclusion that PEHMB (2-6) represented an optimal combination of antiviral activity and low cytotoxicity. Improvements in PEHMB synthesis and purification have yielded preparations of PEHMB (also known as NB325) with lower cytotoxicity, higher anti-HIV-1 activity (Table 1), and much greater in vitro TI values (Table 2; 266 and 7000 against HIV-1 strains BaL and IIIB, respectively).
Table 2.
Therapeutic indices calculated for variant PBGs.
| Compound | Linkers | Methylenes per repeat | Therapeutic index
|
|||
|---|---|---|---|---|---|---|
| VII-IIIBa,b | VII-BaLa | CFI-IIIBc | CAI-IIIBc | |||
| PEB | 2-2 | 4 | 14 | ND | ND | ND |
| PEHMB | 2-6 | 8 | 78 | 53 | 14 | 86 |
| PTMB | 4-4 | 8 | 17 | ND | ND | ND |
| PPHMB | 3-6 | 9 | 72 | 1 | 3 | 77 |
| PBHMB | 4-6 | 10 | 31 | 17 | 6 | 50 |
| PEOMB | 2-8 | 10 | 62 | 52 | 5 | 59 |
| PHMB | 6-6 | 12 | 10 | ND | ND | ND |
| PEHMBd | 2-6 | 8 | 7000 | 266 | ND | ND |
ND: not determined.
An in vitro TI was calculated for each compound by dividing its CC50 value (2-h exposure) by the IC50 value determined in the VII-IIIB or VII-BaL assay.
In vitro TIs for PEB, PTMB, and PHMB were calculated using data from previous studies [16].
An in vitro TI was calculated for each compound by dividing its CC50 value (10-min exposure) by the IC50 value determined in the CFI-IIIB or CAI-IIIB assay.
PEHMB (2-6) (also known as NB325) produced using an improved synthesis protocol and partial purification.
3.4. Differences in calculated PDBG charge density and hydrophobicity result from changes in hydrocarbon linker length
We previously hypothesized that the cytotoxicity and antiviral activities of PDBG molecules [16] might be attributed, at least in part, to interactions between PDBGs and anionic phospholipids within the cell and viral membranes [25,26]. These interactions, which are likely both electrostatic and hydrophobic in nature, should be affected by changes in hydrocarbon linker length that result in alterations in both charge density and hydrophobicity of the PDBG molecule. To determine the effect of linker length on these physical attributes, the Molecular Operating Environment molecular modeling program (Chemical Computing Group, Montreal, Quebec, Canada) was used to calculate the surface area, charge distribution, hydrophobicity, and the logarithm of the octanol-water partition coefficient ([log P (o/w)], which is an indicator of lipophilicity) of select PDBG variants. Each PDBG was represented by a model compound composed of a three repeat-unit oligomer capped by a biguanide. As expected, changes in both charge density and hydrophobicity accompanied increases in hydrocarbon linker length (Table 3). Increases in total van der Waals surface area (vdW SA) and total solvent accessible surface area (SASA) were consistent with increases in the number of methylene units within each repeat. Increases in methylene content also increased PDBG hydrophobicity and lipophilicity, as indicated by increases in the percent hydrophobic SASA and log P (o/w), respectively. PDBG charge density was decreased by the addition of methylene units, however, as shown by decreases in the positive vdW SA relative to the total vdW SA (% positive SA).
Table 3.
Calculated values of key physical properties for select PBG structuresa.
| PBG (linkers) | Methylenes per repeat | Total vdW SA (Å2)b | Positive vdW SA (Å2)c | Positive SA (%)d | Total SASA (Å2)e | Hydrophobic SASA (Å2)f | Hydrophobic SASA (%)g | log P (o/w)h |
|---|---|---|---|---|---|---|---|---|
| PEB (2-2) | 4 | 950.7 | 799.1 | 84 | 1493.6 | 315.34 | 21 | −2.45 |
| PEHMB (2-6) | 8 | 1206.1 | 901.5 | 75 | 1892.4 | 707.11 | 37 | 2.85 |
| PTMB (4-4) | 8 | 1196.3 | 901.5 | 75 | 1880.3 | 695.17 | 37 | 2.85 |
| PPHMB (3-6) | 9 | 1258.0 | 927.1 | 74 | 1991.3 | 815.35 | 41 | 4.18 |
| PBHMB (4-6) | 10 | 1318.6 | 952.7 | 72 | 2064.0 | 889.33 | 43 | 5.51 |
| PEOMB (2-8) | 10 | 1317.3 | 952.7 | 72 | 2031.2 | 864.58 | 43 | 5.51 |
| PHMB (6-6) | 12 | 1428.7 | 1003.9 | 70 | 2243.8 | 1102.51 | 49 | 8.16 |
Values were calculated for a three repeat-unit oligomer capped by a biguanide.
Total vdW SA.
Total positively charged vdW SA.
Percent positively charged vdW SA (relative to total SA).
Total SASA; the solvent refers to water.
Total hydrophobic SASA.
Percent hydrophobic SASA (relative to total SASA).
The log P (o/w) values refer to charge-neutral species, calculated using the Molecular Operating Environment molecular modeling program (Chemical Computing Group, Montreal, Quebec, Canada). Higher positive values indicate increasing lipophilicity, whereas a negative value indicates that the molecule is hydrophilic.
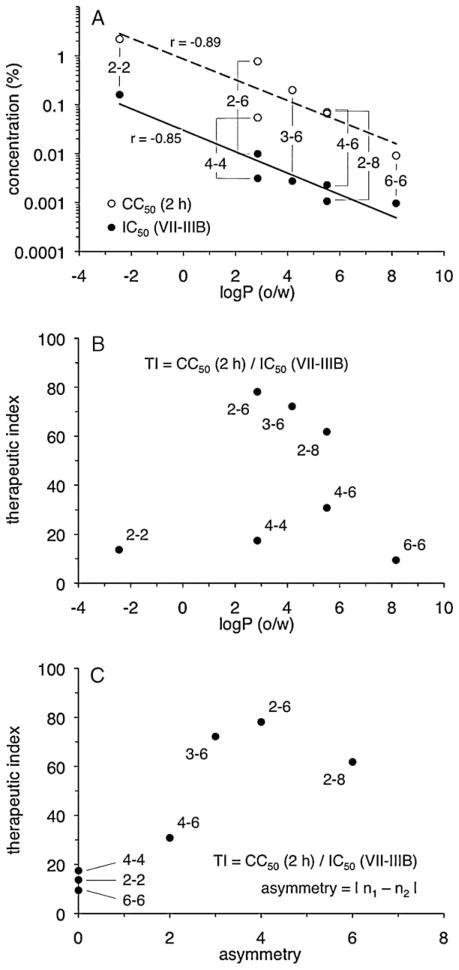
3.5. Compound lipophilicity and hydrocarbon linker asymmetry are associated with changes in biologic activity
Comparisons between linker length and PDBG activity in the above experiments indicated that the presence of more methylene units resulted in increases in both cytotoxicity and antiviral activity. Because lipophilicity is a measure of methylene content in the PDBG molecule, relationships were examined between log P (o/w) (Table 3) and compound activity in assays of cytotoxicity and antiviral activity (Table 1). Decreases in CC50 (i.e., increases in cytotoxicity) were found to associate with increases in calculated lipophilicity (Fig. 4A). Similarly, decreases in IC50 (i.e., increases in inhibitory activity against HIV-1 IIIB) were associated with increases in PDBG lipophilicity (Fig. 4A). A similar relationship between lipophilicity and activity against HIV-1 BaL was also apparent (data not shown). No correlations were noted between lipophilicity and antiviral activity when the results from the cell-free (CFI) and cell-associated (CAI) VII assays were analyzed (data not shown).
Fig. 4.
Compound lipophilicity and hydrocarbon linker asymmetry are associated with changes in biologic activity. The log of the octanol-water partition coefficient [log P (o/w)] for each compound was plotted against (A) cytotoxicity (CC50 value, 2-h exposure) and anti-HIV-1 activity (IC50 value, VII-IIIB) (Table 1) or (B) the VII-IIIB therapeutic index (Table 2). (C) The VII-IIIB therapeutic index for each compound (Table 2) was plotted against its asymmetry value, which is the absolute value of the difference between n1 and n2. Trendlines were determined by least squares regression (Microsoft® Excel). Pearson product moment correlation coefficients (r) are shown.
The preceding analyses also suggested that factors other than compound lipophilicity affected PDBG biologic activity. PEHMB (2-6) and PTMB (4-4) had the highest and lowest calculated TI values, respectively, despite their identical log P (o/w) values. PBHMB (4-6) and PEOMB (2-8) were also equally lipophilic but differed somewhat in their capacities for inhibiting infection by HIV-1 IIIB or BaL. As suggested by these examples, there was no apparent association between log P (o/w) and TI (Fig. 4B). However, because lipophilicity calculations reflected total methylene content without accounting for the distribution of methylene groups in each repeat unit, the effect of hydrocarbon linker length asymmetry across each biguanide unit was investigated. These analyses suggested a positive relationship between linker asymmetry (defined as the absolute difference between n1 and n2) and compound TI (Fig. 4C). Because compounds with asymmetry greater than PEOMB (2-8) were not examined for antiviral activity nor considered in these analyses, however, it was not possible to determine whether the relationship between linker asymmetry and compound TI was strictly linear, or if the TI of PEHMB (2-6) represented a peak value along a nonlinear curve.
3.6. In vivo exposure to PEHMB for up to 8 h results in negligible damage to murine cervicovaginal tissues
Results of in vitro cytotoxicity experiments demonstrated that PEHMB (2-6) was the least cytotoxic of the PDBG compounds evaluated. These experiments also showed that PHMB (6-6), which is distinguished from PEHMB (2-6) by a different linker configuration and greater methylene content, was one of the most cytotoxic PDBGs examined. These two compounds were evaluated in the murine model of cervicovaginal toxicity [21,22] (i) to confirm the hypothesis that low in vitro cytotoxicity of PEHMB (2-6) was predictive of its low in vivo toxicity and (ii) to demonstrate PDBG structure-function relationships in vivo by showing PDBG backbone-dependent differences in toxicity between PEHMB (2-6) and PHMB (6-6). A mouse model of toxicity that can be used to evaluate cervicovaginal tissue integrity and immune cell recruitment subsequent to compound exposure was selected for these studies because it was predictive of clinical outcomes when characterizing a single compound (N-9) for toxicity [21] or when distinguishing between two formulations with different concentrations [22] of the same active ingredient (C31G).
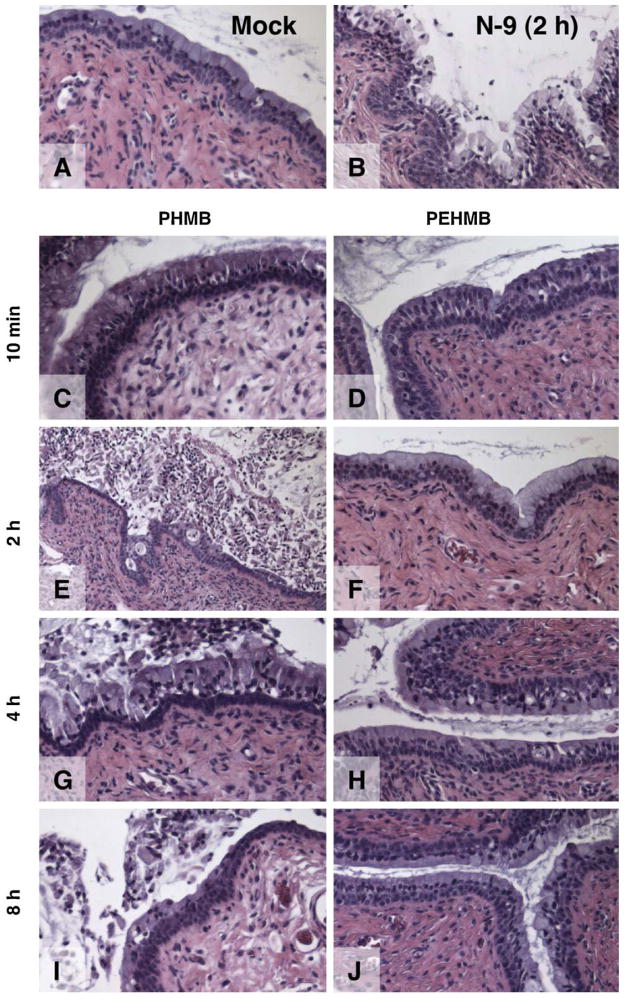
In these experiments, vaginal and cervical epithelial tissues were examined following exposure to PEHMB (2-6), PHMB (6-6), or N-9 introduced as aqueous, unformulated solutions. Consistent with previous findings [21], damage to the vaginal epithelium was negligible following 10 min, 2 h, 4 h, or 8 h of N-9 exposure (data not shown). Comparable results were obtained using PEHMB (2-6) or PHMB (6-6). Time- and compound-dependent changes in cervical epithelial integrity were observed, however (Fig. 5). As shown previously [21], exposure to N-9 (1%) for 2 h caused extensive fragmentation and sloughing of the upper layer of the cervical epithelium (Fig. 5B) relative to mock (water)-exposed tissues (Fig. 5A). Exposure to 1% PHMB (6-6) for 10 min resulted in indications of epithelial damage, including the loss of the overlying mucus (Fig. 5C). After a 2-h exposure to PHMB (6-6), severe fragmentation of 50% to 75% of the cervical epithelium was observed (Fig. 5E). Exposures of 4 h (Fig. 5G) caused even greater losses in epithelial integrity. Considerable epithelial damage was still apparent at 8 h after application, but there was evidence that epithelial regeneration had begun (Fig. 5I).
Fig. 5.
PEHMB (2-6) does not adversely affect the integrity of the mouse cervical epithelium after exposures up to 8 h. Female Swiss Webster mice were inoculated intravaginally with (A) diluent (water) only or 1% solutions of unformulated (B) N–9, (C, E, G, I) PHMB, or (D, F, H, J) PEHMB. Mice were sacrificed at 10 min, 2 h, 4 h, or 8 h following application, and the entire reproductive tract was surgically excised. Formalin-fixed, paraffin-embedded tissues were stained with hematoxylin and eosin. Representative fields from the cervical epithelium of each treatment group are shown.
In contrast, tissues exposed to 1% PEHMB (2-6) were generally intact. After 10 min of exposure to PEHMB (2-6), no epithelial damage was apparent (Fig. 5D). After exposures of 2 h or longer (Figs. 5F, H, and J), most of the epithelium was indistinguishable from the mock-treated tissue; mild to moderate damage was limited to less than 10% of the epithelium examined. These results clearly demonstrated that, in contrast to N-9 and PHMB (6-6), PEHMB (2-6) at 1% was well tolerated in vivo and had little or no unfavorable impact on the integrity of the cervical epithelium.
3.7. In vivo exposure to PEHMB does not result in recruitment of CD45-positive immune cells
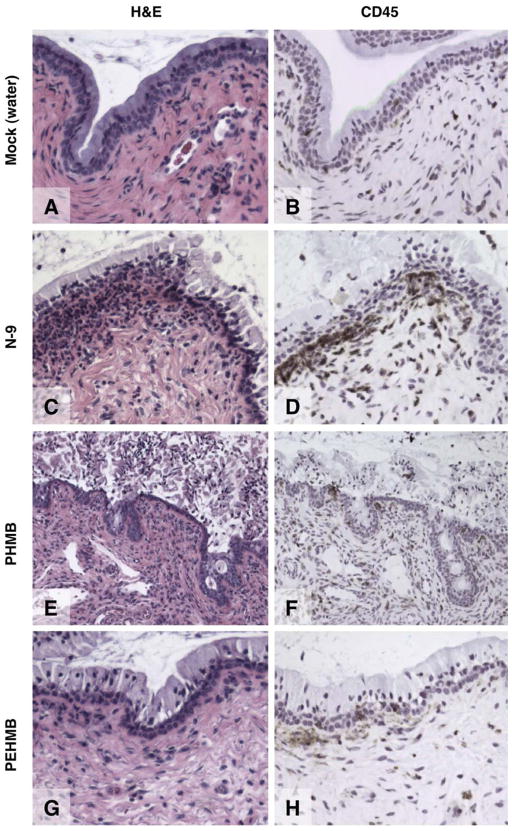
Previous mouse model experiments demonstrated that cervical epithelial disruption caused by N-9 was associated with the recruitment of large numbers of CD45-positive immune cells to the cervical epithelium [21]. Because 1% PEHMB (2-6) was well tolerated by the cervical epithelium, whereas PHMB (6-6) was not, it was hypothesized that these compounds might also differ in induced immune cell recruitment. To examine this hypothesis, mouse cervicovaginal tissues were exposed to N-9, PHMB (6-6), or PEHMB (2-6) for 2 h (Fig. 6) and harvested for comparisons of epithelial integrity and recruitment of cells positive for CD45, which has been shown to be expressed on all cells of hematopoietic origin (except erythrocytes). Again, N-9 exposure caused moderate to severe disruption of the epithelial surface (Fig. 6C) and severe inflammation in more than 50% of the visible epithelium, as indicated by the subepithelial recruitment of CD45-positive cells (Fig. 6D). The presence of PHMB (6-6), which caused severe epithelial fragmentation (Fig. 6E), resulted in mild to moderate levels of inflammation (Fig. 6F). In contrast, exposure to PEHMB (2-6) for 2 h resulted in little or no epithelial damage (Fig. 6G; < 10% of the visible epithelium characterized as having mild to moderate damage) and little or no recruitment of CD45-positive cells (Fig. 6H), with pockets of moderate inflammation limited to the few areas where epithelial damage was noted. These results demonstrated that, unlike N-9, exposure to PEHMB (2-6) did not cause extensive inflammation involving immune cell recruitment to the cervical epithelium.
Fig. 6.
In vivo application of PEHMB does not result in the recruitment of CD45-positive immune cells to the cervical epithelium. Tissues harvested from Swiss Webster mice that were (A and B) mock exposed or exposed to (C and D) N-9, (E and F) PHMB, or (G and H) PEHMB for 2 h were reacted with a monoclonal rat antimouse antibody specific to CD45, which identifies all cells of hematopoietic origin (except erythrocytes). Representative fields from the cervical epithelium of each treatment group are shown. H&E: hematoxylin and eosin.
4. Discussion and conclusion
These studies extended initial compound discovery and development efforts [16] through a methodical approach to documenting relationships between backbone linker length and bioactivity. These results offered initial insights into factors that affect biguanide-based compound cytotoxicity and provided indications that compounds such as PEHMB (2-6) use a specific mechanism of action to inhibit HIV-1 infection. Additionally, the mouse model studies validated in vitro observations regarding structure-dependent differences in compound cytotoxicity and provided further indications of PEHMB (2-6) safety.
Changes in biguanide-based oligomer cytotoxicity were shown to be associated with alterations in compound backbone structure. Increases in the number of methylene units within a single repeat, which result in decreased charge density, increased hydrophobicity, and increased lipophilicity (Table 3), should facilitate interactions with lipophilic elements of the plasma membrane. Mammalian cell cytotoxicity and antibacterial activity may be consequences of the ability of PDBGs to interact with and perturb the structure or integrity of the plasma membrane. The effectiveness of PHMB (6-6) as an antibacterial agent [12,14] and its relatively high cytotoxicity are consistent with this hypothesis, as PHMB (6-6) had the lowest charge density and the highest hydrophobicity of the compounds analyzed (Table 3). In addition, PDBGs with fewer methylene groups relative to PHMB (6-6) had lower cytotoxicity and, by extension, may have lower or limited antibacterial activity. This conclusion suggests that PEHMB (2-6), which was shown to have much lower cytotoxicity compared with PHMB (6-6), may have little or no impact on normal vaginal flora if it is used as a topical microbicide.
The association between greater antiviral activity and increased methylene content may have also been a consequence of nonspecific interactions with the viral and cellular membranes, which could potentially decrease the integrity (and infectivity) of the virus and interfere with virus-cell interactions necessary for viral binding and entry. Results of experiments involving preincubation of each compound with cell-free virus (Table 1) indicated that all four compounds were able to inactivate the virus, presumably by direct interactions with anionic phospholipid components of the viral membrane [25,26]. However, these compounds had considerably greater activity when incubated with both virus and target cell (8- to 59-fold lower IC50 concentrations in the VII-IIIB assay compared with the CFI-IIIB assay; Table 1), suggesting at least one of three possibilities: (i) greatly increased antiviral activity as a consequence of longer compound exposure (10 min in the CFI-IIIB assay compared with 2 h in the VII-IIIB assay); (ii) cooperative antiviral activity during virus binding and entry caused by simultaneous interactions with membrane components of virus and target cell; or (iii) specific interactions with viral and cellular proteins involved in virus binding or entry.
Although the first two mechanisms could not be discounted by the current results, several observations pointed to a specific mechanism of action. First, compounds that were structurally different yet identical with respect to total methylene content and lipophilicity—PEHMB (2-6) and PTMB (4-4), as well as PBHMB (4-6) and PEOMB (2-8)—had distinctly different TI values. The implication of this result was that the mechanism of action has structural specificity. Second, analyses of structure-activity relationships demonstrated that changes in TI were not associated with changes in the lipophilicity (a characteristic that would be expected to affect nonspecific biologic activity) but were associated with specific arrangements of the backbone linkers. Third, whereas PPHMB (3-6) and PBHMB (4-6) were equally effective against HIV-1 IIIB (Fig. 2), PPHMB (3-6) was a less potent inhibitor (approximately 48-fold higher IC50 concentration) of strain BaL compared with PBHMB (4-6). This latter observation implied that the removal of a single methylene group from alternating repeat units was sufficient to specifically abrogate antiviral activity against R5 virus without affecting the compound’s efficacy against X4 virus.
These results also suggested the hypothesis that the specific mechanism of biguanide-based molecules may be HIV-1 co-receptor dependent. Mechanism-of-action experiments have confirmed this hypothesis, demonstrating that PEHMB (2-6) specifically interacts with the second extracellular loop of CXCR4 [27]. This interaction is sufficient to inhibit HIV-1 infection by X4 HIV-1 IIIB, as well as inhibit immune cell chemotaxis induced by the CXCR4 ligand CXCL12 [27]. Furthermore, this interaction persists even after the compound has been removed from the media, providing antiviral activity attributed to prolonged interactions with CXCR4 [28]. This specific mechanism of action, which likely involves the cationic biguanide motifs, is consistent with the participation of the cationic “basic-basic-any-basic” (BBXB) domains in inhibition of HIV-1 infection by the CXCR4 ligand CXCL12 [29,30].
With respect to PDBG antiviral activity against R5 virus, the mechanism of action may be different, because the BBXB amino acid motif was not required for inhibition of HIV-1 infection mediated by the CCR5 ligand RANTES [31]. Initial mechanism-of-action experiments involving PEHMB (2-6) and R5 BaL virus also support this conclusion. In preliminary experiments in which flow cytometry was used to determine the effect of PEHMB (2-6) on the detection of cell surface CCR5, interactions between PEHMB (2-6) and specific extracellular CCR5 epitopes were not detected (N. Thakkar, B. Wigdahl, and F. C. Krebs, unpublished results), despite the clear demonstration of the antiviral activity of PEHMB (2-6) against HIV-1 BaL. Investigations into the mechanism of action of PEHMB (2-6) specific to R5 HIV-1 strains are under way.
Assessments of PEHMB (2-6) in a mouse model of cervicovaginal toxicity [21,22] provided a clear in vivo validation of our observation that simple changes in PDBG structure have a dramatic effect on compound cytotoxicity. Specifically, PEHMB (2-6), which was much less cytotoxic in vitro compared with either PHMB (6-6) or N-9, was clearly less toxic in vivo compared with these compounds. Because the murine model of toxicity can predict clinical outcomes when assessing a single compound (N-9) for toxicity [21] or when distinguishing between two formulations with different concentrations [22] of the same active ingredient (C31G), the present studies suggest that PEHMB (2-6) may have a favorable toxicity profile in future clinical safety trials.
Using these observations as a foundation, current developmental efforts involving PEHMB (2-6) and its biguanide-based derivatives are focused on further optimization of compound synthesis and purification protocols, defining mechanisms of action, and determining the breadth of activity with respect to clinical isolates and virus subtypes. Recent advances in rationally identifying and modifying variables in PEHMB (2-6) synthesis have yielded a product in a partially purified state that combines decreased in vitro cytotoxicity and greater antiviral activity against both virus co-receptor phenotypes, with TIs of 266 and 7000 against HIV-1 BaL (R5) and IIIB (X4), respectively (Tables 1 and 2). Greater gains in compound TI across co-receptor phenotype and virus subtype will likely be achieved by further improvements in synthetic strategies and postsynthesis purification, which will advance the development of biguanide-based inhibitors of HIV-1 infection toward clinical trials and their eventual use as microbicidal agents.
Acknowledgments
We gratefully acknowledge Catherine Stiller, Patricia Welsh, and Lynn Budgeon for their technical support, as well as Dr. Richard Stockel for his contributions to the synthesis of PDBG compounds used in these studies. These studies were supported by Public Health Service grants P01 AI37829 and U19 AI076965.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) AIDS epidemic update: November 2009. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2009. [Google Scholar]
- 2.Buckheit RW, Jr, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85:142–58. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antivir Chem Chemother. 2009;19:143–50. doi: 10.1177/095632020901900401. [DOI] [PubMed] [Google Scholar]
- 4.Minces LR, McGowan I. Advances in the development of microbicides for the prevention of HIV infection. Curr Infect Dis Rep. 2010;12:56–62. doi: 10.1007/s11908-009-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen B. Vaginal flora in health and disease. Clin Obstet Gynecol. 1993;36:107–21. doi: 10.1097/00003081-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Shubair M, Stanek R, White S, Larsen B. Effects of chlorhexidine gluconate douche on normal vaginal flora. Gynecol Obstet Invest. 1992;34:229–33. doi: 10.1159/000292767. [DOI] [PubMed] [Google Scholar]
- 7.Stray-Pedersen B, Bergan T, Hafstad A, Normann E, Grogaard J, Vangdal M. Vaginal disinfection with chlorhexidine during childbirth. Int J Antimicrob Agents. 1999;12:245–51. doi: 10.1016/s0924-8579(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosin M, Welk A, Kocher T, Majic-Todt A, Kramer A, Pitten FA. The effect of a polyhexamethylene biguanide mouth rinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque regrowth. J Clin Periodontol. 2002;29:392–9. doi: 10.1034/j.1600-051x.2002.290503.x. [DOI] [PubMed] [Google Scholar]
- 9.Patton DL, Sweeney YT, McKay TL, DeMers SM, Clark AM, Rabe LK, et al. 0.25% chlorhexidine gluconate gel. A protective topical microbicide. Sex Transm Dis. 1998;25:421–4. doi: 10.1097/00007435-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Keeven J, Wrobel S, Portoles M, DeCicco BT. Evaluating the preservative effectiveness of RGP lens care solutions. Clao J. 1995;21:238–41. [PubMed] [Google Scholar]
- 11.Larkin DF, Kilvington S, Dart JK. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology. 1992;99:185–91. doi: 10.1016/s0161-6420(92)31994-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosin M, Welk A, Bernhardt O, Ruhnau M, Pitten FA, Kocher T, et al. Effect of a polyhexamethylene biguanide mouth rinse on bacterial counts and plaque. J Clin Periodontol. 2001;28:1121–6. doi: 10.1034/j.1600-051x.2001.281206.x. [DOI] [PubMed] [Google Scholar]
- 13.Millis NF, Eager E, Hay AJ, Kasian PA, Pickering WJ, Tan MA. Survey of bacteria in private swimming pools. Med J Aust. 1981;1:573–5. doi: 10.5694/j.1326-5377.1981.tb135836.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerli S, Rossetti D, Di Renzo GC. A new approach for the treatment of bacterial vaginosis: use of polyhexamethylene biguanide. A prospective, randomized study. Eur Rev Med Pharmacol Sci. 2003;7:127–30. [PubMed] [Google Scholar]
- 15.Valluri S, Fleming TP, Laycock KA, Tarle IS, Goldberg MA, Garcia-Ferrer FJ, et al. In vitro and in vivo effects of polyhexamethylene biguanide against herpes simplex virus infection. Cornea. 1997;16:556–9. [PubMed] [Google Scholar]
- 16.Krebs FC, Miller SR, Ferguson ML, Labib M, Rando RF, Wigdahl B. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed Pharmacother. 2005;59:438–45. doi: 10.1016/j.biopha.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.East GC, McIntyre JE, Shao J. Polybiguanides: synthesis and characterization of polybiguanides containing hexamethylene groups. Polymer. 1997;38:3973–84. [Google Scholar]
- 18.Zhang Y, Jiang J, Chen Y. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer. 1999;40:6189–98. [Google Scholar]
- 19.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241:651–62. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 20.Krebs FC, Miller SR, Malamud D, Howett MK, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antiviral Res. 1999;43:157–73. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 21.Catalone BJ, Kish-Catalone TM, Budgeon LR, Neely EB, Ferguson M, Krebs FC, et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48:1837–47. doi: 10.1128/AAC.48.5.1837-1847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalone BJ, Miller SR, Ferguson ML, Malamud D, Kish-Catalone T, Thakkar NJ, et al. Toxicity, inflammation, and anti-human immunodeficiency virus type 1 activity following exposure to chemical moieties of C31G. Biomed Pharmacother. 2005;59:430–7. doi: 10.1016/j.biopha.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 24.Krebs FC, Miller SR, Catalone BJ, Welsh PA, Malamud D, Howett MK, et al. Sodium dodecyl sulfate and C31G as microbicidal alternatives to nonoxynol 9: comparative sensitivity of primary human vaginal keratinocytes. Antimicrob Agents Chemother. 2000;44:1954–60. doi: 10.1128/aac.44.7.1954-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broxton P, Woodcock PM, Heatley F, Gilbert P. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol. 1984;57:115–24. doi: 10.1111/j.1365-2672.1984.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda T, Ledwith A, Bamford CH, Hann RA. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim Biophys Acta. 1984;769:57–66. doi: 10.1016/0005-2736(84)90009-9. [DOI] [PubMed] [Google Scholar]
- 27.Thakkar N, Pirrone V, Passic S, Zhu W, Kholodovych V, Welsh W, et al. Specific interactions between the viral coreceptor CXCR4 and the biguanide-based compound NB325 mediate inhibition of human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 2009;53:631–8. doi: 10.1128/AAC.00866-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thakkar N, Pirrone V, Passic S, Keogan S, Zhu W, Kholodovych V, et al. Persistent interactions between biguanide-based compound NB325 and CXCR4 result in prolonged inhibition of human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 2010;54:1965–72. doi: 10.1128/AAC.00934-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altenburg JD, Broxmeyer HE, Jin Q, Cooper S, Basu S, Alkhatib G. A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities. J Virol. 2007;81:8140–8. doi: 10.1128/JVI.00268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dettin M, Pasquato A, Scarinci C, Zanchetta M, De Rossi A, Di Bello C. Anti-HIV activity and conformational studies of peptides derived from the C-terminal sequence of SDF-1. J Med Chem. 2004;47:3058–64. doi: 10.1021/jm031067a. [DOI] [PubMed] [Google Scholar]
- 31.Proudfoot AE, Fritchley S, Borlat F, Shaw JP, Vilbois F, Zwahlen C, et al. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J Biol Chem. 2001;276:10620–6. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]