Abstract
Objectives
This study explored how advertising affects demand for cigarettes and potential substitutes, including snus, dissolvable tobacco, and medicinal nicotine.
Methods
A web-based experiment randomized 1062 smokers to see advertisements for alternative nicotine products or soft drinks, then complete a series of purchase tasks, which were used to estimate demand elasticity, peak consumption, and cross-price elasticity (CPE) for tobacco products.
Results
Lower demand elasticity and greater peak consumption were seen for cigarettes compared to all alternative products (p < .05). CPE did not differ across the alternative products (p ≤ .03). Seeing relevant advertisements was not significantly related to demand.
Conclusions
These findings suggest significantly lower demand for alternative nicotine sources among smokers than previously revealed.
Keywords: tobacco, smokeless, nicotine, demand, elasticity, substitute
Promotion of reduced-harm alternatives to cigarettes has increased since the 1990s. Initially, these products were modified cigarettes that were poorly received by consumers and offered relatively little health risk reduction potential.1 Since the early 2000s there has been a proliferation of novel types of smokeless tobacco (ST) products, including snus, a product patterned on low-nitrosamine products available in Sweden. This marketing coincided with the emergence of Swedish research suggesting reductions in smoking rates and cancer mortality could be attributed to use of snus in lieu of cigarettes.2–4 Manufacturers such as Star Tobacco have marketed other, more processed oral tobacco products like Ariva, which is comprised of finely milled low-nitrosamine tobacco compressed into a lozenge. This concept was adopted by RJ Reynolds with its line of dissolvable products, including Camel Orbs, Sticks, and Strips. Philip Morris introduced a variant on this concept in 2011 with Marlboro and Skoal Sticks, where finely ground tobacco was applied to toothpick-like sticks for oral use. In spite of this proliferation of novel ST products, little is known about consumer perceptions and use of these products.
Some public health advocates have pointed to oral ST products as having the potential for harm reduction among smokers who might otherwise not quit tobacco, but who would replace smoking cigarettes with oral ST.5–8 However, others have raised concerns that any individual health benefits incurred by these “switchers” could be offset by increased use of ST among youth and former smokers.9 There is also concern that promoting alternative tobacco products could lead to a renormalization of tobacco use with unknown consequences. A basic question arises regarding the promotion and public health benefits of ST products: Would smokers who have not quit smoking despite rising prices, increasing social isolation, and clear health risks be willing to adopt an alternative product on a permanent basis? Thus far, ads for these novel alternative products have tended to endorse the convenience of the product and ability to use the product in places where smoking is no longer permitted, but ads have avoided making explicit health claims associated with use.10 There is no debate that exposure to tobacco advertising influences product choice,11 but it is less clear whether and how specific marketing messages will influence current cigarette smokers to switch to ST as an alternative nicotine delivery mechanism.
Previous surveys have found that smokers are interested in using alternatives to cigarettes.12–14 However, self-reported interest in ST product trial may not predict use, and, indeed, in test markets where novel ST products have been made available, trial and adoption rates appear to be low.15 Perceived relative risk may play a role. Despite the evidence of lower individual-level risks, evidence shows that smokers misperceive the risks of ST, nicotine, and smoking. Borland et al.16 found that only 1 in 6 smokers believed ST to be less harmful than cigarettes, while only 1 in 3 believed nicotine replacement therapy (NRT) was much less harmful than cigarettes, with no changes in the prevalence of these misperceptions over the period from 2002 to 2009. Many smokers wrongly believe nicotine to be a cause of cancer.16–18 Both Cummings 19 and Kozlowski & Edwards 20 note that smokers require accurate, science-based information about the relative risks of tobacco and nicotine products; information that could then possibly drive them toward less harmful products.21 Another factor may be self-identification with smoking per se – that is, smokers may not see themselves as the ‘type of person’ who uses smokeless tobacco, even novel forms, and prefer the perceived flexibility of the cigarette.22 So while smokers may express interest in less harmful alternatives to cigarettes in the abstract, this interest may not translate into actual use, and simply asking questions about intentions and interest may not provide strong evidence of the extent to which smokers would be willing to move from cigarettes to a safer alternative nicotine delivery product.
One way to estimate smokers’ demand for ST products is through the use of purchase tasks, which allow estimation of the reinforcing properties of different products.23–25 In these tasks, subjects are asked to project how much of a given product they would use across a range of unit prices, assuming it were the only product available to them. Few and colleagues 26 have shown this measure is stable in the short term as an estimate of cigarette demand. The simulated purchase task method generates estimates of demand elasticity and maximum consumption for each product in isolation – indeed, the task instructs participants to imagine they “have no access to cigarettes or nicotine products other than those offered…”.23 So, while the tasks give insight into the demand for each product individually, the ability of a purchase task to directly address the questions of whether smokers would substitute ST for cigarettes is limited, especially given the reality that both products are concurrently available.
To address the issue of concurrent availability, economists employ a measure referred to as cross-price elasticity of demand (CPE), which captures change in use of one product given the change in price of another. As used in the drug abuse liability literature, CPE has typically been assessed by offering an alternative reinforcer at a fixed cost while varying the cost of the primary reinforcer.27–30 An alternative (ie, oral tobacco) would be considered a substitute if its consumption rises in response to increasing cost of the primary product (ie, cigarettes). An alternative would be considered a complement if its consumption drops in response to increasing cost of the primary product.
Both the demand and CPE tasks have been found valid as well as temporally stable in small-sample studies.23,26 Bidwell et al also demonstrates behavioral validation of these methods among adolescent smokers31. MacKillop further adds to these implications highlighting that demand curves, in particular, are sensitive to price32 and support craving and motivation patterns for addictive drugs 33.
This study first tests whether exposure to current examples of ST advertising influence demand for ST products in a group of current smokers. If the ads appeal to smokers and encourage them to consider switching from cigarettes to ST products, this would suggest that marketing campaigns that do not explicitly reference the potential health benefits of ST might nonetheless promote harm reduction. Second, the study compares smoker demand for four types of nicotine products – cigarettes, snus, dissolvables, and nicotine lozenges – using purchase task-based estimates of demand elasticity, peak consumption, and cross-price elasticity.23 In this way, we may be able to estimate the extent to which smokers viewed different oral tobacco products as acceptable substitutes for the cigarettes they smoke.
METHODS
Participants
In July 2010, current cigarette smokers were recruited from a pre-existing U.S.-based Internet panel maintained by Global Market Insite (GMI; Bellevue, WA). More detailed information about panel characteristics, quality control, recruitment, and retention methods can be found elsewhere (http://www.gmi-mr.com/global-panel/index.php). Panelists were eligible if they were a current cigarette smoker of at least 5 cigarettes per day, aged 18 or older, and able to read and write in English. GMI invited 24,608 panelists self-identified as smokers. The American Association for Public Opinion Research response rate (RR4) for this survey was 9.5%, with a cooperation rate (CR4) of 83%, achieving a final N of 1062, at which point the survey was closed.
Methods and Materials
Potential participants were recruited by e-mail, and completed a screening questionnaire to determine eligibility (≥ 5 cigarettes per day; ≥ 18 years old; able to read and write English). All eligible participants were led to a screen describing the study, then completed the “core questionnaire” including demographics, tobacco knowledge, and beliefs, nicotine dependence (Fagerström Test for Nicotine Dependence - FTND), craving (Questionnaire of Smoking Urges -QSU), smoking expectancies (Brief Smoking Consequences Questionnaire-Adult - BSCQ-A), delay discounting (Monetary Choice Questionnaire - MCQ),34–37 and previous use of ST/NRT. The participants were randomized into two groups. One group viewed ads for and then rated three smoking alternatives (Camel Snus, Camel Dissolvable Tobacco, & Commit medicinal nicotine lozenges) and a control group viewed ads for and then rated non-tobacco products (ie, beverages: Coca-Cola, Vitamin Water, & Minute Maid Orange Juice). The ads featured in this study were unaltered and though appeared similar in imagery and messaging, were not specifically coded for these factors. The specific ads used are shown in Supplemental Materials A. After viewing each randomized ad (six total, two per product), participants completed questionnaires about their reactions to the messaging.38 Participants rated their willingness to try each product on a scale of 1–4 (definitely not – definitely yes). After seeing all ads, participants were asked to select one of the products presented as the most likely to try and one of the products as the least likely to try. After viewing and rating the different assigned advertisements, all participants completed four single-product purchase tasks (cigarettes, snus, dissolvables, lozenge) with sequence of presentation counterbalanced across subjects by an orthogonal Latin Square; a randomization approach by which each individual condition is repeated only once in a series.
Before completing the task, all participants received brief descriptions of each of the products to ensure a base level of knowledge about the products:
Cigarettes: For purposes of the study, assume that these are your favorite brand.
Dissolvables: These are tablets, strips, and sticks made from powdered, compressed, flavored tobacco, and are placed in the mouth. Camel is a common brand name.
Snus: This is a smokeless tobacco product packaged in small pouches that are held under the upper lip. Camel is a common brand name.
Lozenges: These are nicotine lozenges available in pharmacies. Commit is a common brand name.
The task wording is identical to that employed by Mackillop et al.,23 with the exception that the word cigarette was replaced with snus, dissolvables, or lozenge as appropriate. The participant responded to the purchase task by entering the number of individual products (ie,: one cigarette, one dissolvable tablet, one sachet of snus, and one lozenge) they would use if the product would cost $0, $0.01, $0.05, $0.13, $0.25, $1, $2, $3, $4, $5, $6, $11, $35, $70, $140, $280, $560, and $1120 (in that order).
After completing the four single-product purchase tasks, participants completed three comparative purchase tasks to estimate cross-product elasticities. These were counterbalanced across subjects using a Latin Square to manage effects of presentation order. It should be noted that these were independent Latin Squares, so a participant did not necessarily rate products in the same order for single-product and cross-price tasks. The price of each of the alternative oral tobacco products (ie, snus, dissolvables, and lozenge) was fixed at $1, while the cigarette prices ranged across the same values as above. The task wording was altered to ask participants to assume that the alternative was concurrently available at $1, regardless of the price of cigarettes.
Now imagine another TYPICAL DAY during which you smoke. The following questions ask how many cigarettes you would consume if they cost various amounts of money. The available cigarettes are your favorite brand. Assume that you have the same income/savings that you have now, but this time, assume that [Dissolvables/Commit/Snus] are also available at a fixed price of $1 each. In addition, assume that you would consume the products that you request on that day. That is, you cannot save or stockpile products for a later date. Please respond to these questions honestly. How many cigarettes and/or [Dissolvables/Commit/Snus] would you smoke/use if cigarettes were [PRICE]?
Following the purchase tasks, participants were debriefed and received $25 for completing the 60 minute study. The study protocol was reviewed and approved by the Roswell Park Cancer Institute Institutional Review Board.
Data Analysis
Data were initially examined using descriptive statistics (means and percentages), chi-squares, and t-tests. Individual and average demand elasticities for cigarettes, snus, dissolvables, and nicotine lozenge were estimated from purchase task data in Prism 5 (GraphPad, La Jolla, CA) using the exponential demand equation,39 expressed as logQ = logQ0 + k(e−αPs − 1), where α is the rate of change in demand (elasticity), Q0 is peak consumption (at price 0), Ps is price (normalized to Q0), and k is a constant (1.756 in this case, based on best fit to sample average consumption). Hursh and Silberberg propose α as a measure of ‘essential value’ of a reinforcer (ie, product), and in this study is used as an indicator of elasticity.39 To allow logarithmic transformation of the data, the first zero consumption value was recoded as 0.001 and subsequent 0 values were ignored.40 Cross-price elasticities (CPE) were calculated in SPSS 16.0 (SPSS, Chicago, IL) as the slope of the regression line (B1) fit to log-transformed consumption of each alternative product (C; snus, dissolvables, lozenges when offered at a fixed price of $1) versus log-transformed cigarette price (P): logC = B0 + B1logP.28 Positive values provide evidence for substitution (ie, consumption of the alternative increases as a function of increasing cigarette price), while negative values indicate complementarity (i.e, consumption of the alternative decreases as a function of increasing cigarette price). Per prior publications,28 CPEs with an absolute value greater than 0.20 were taken as evidence for significant substitution or complementarity. Individual differences in α, Q0, and CPE were assessed using GEE to account for the repeated measures nature of the data (Normal or Gamma distribution, log link function, unstructured correlation matrix).41
RESULTS
Demographics Data
To check for fidelity of randomization, we compared the two groups on a number of demographic and smoking behavior variables. Results are presented in Table 1. Overall, the two groups are equivalent, though the Soft Drink Group was slightly larger. Those randomized to the Tobacco group were somewhat more likely to be heavy or light smokers, and to report no interest in quitting, though neither achieved statistical significance.
Table 1.
Characteristics of Participants Randomly Assigned to View Soft Drink or Tobacco Product Advertisements.
| Tobacco (N = 492) % | Soft Drink (N = 569) % | |
|---|---|---|
| Sex | ||
| Male | 54.0 | 55.9 |
| Female | 46.0 | 44.1 |
| Age Category (Years) | ||
| 18–24 | 14.0 | 13.2 |
| 25–39 | 31.1 | 30.8 |
| 40–54 | 35.4 | 33.0 |
| 55+ | 19.5 | 23.0 |
| Race | ||
| White | 81.1 | 83.0 |
| Black | 12.8 | 11.8 |
| Other | 6.1 | 5.3 |
| Education | ||
| HS or less | 26.2 | 20.9 |
| Some college | 41.9 | 50.0 |
| College grad or more | 31.9 | 29.1 |
| Cigarettes per Day | ||
| 0–5 | 41.1 | 36.0 |
| 6–10 | 41.1 | 47.8 |
| 11–20 | 12.4 | 13.0 |
| 21+ | 5.3 | 3.2 |
| Time to First Cigarette (Minutes) | ||
| 61+ | 11.2 | 9.8 |
| 31–60 | 15.9 | 14.9 |
| 6–30 | 49.9 | 50.4 |
| 0–5 | 23.0 | 25.0 |
| Ever Used Smokeless or Oral NRT | ||
| No | 45.3 | 42.6 |
| Yes | 54.7 | 57.4 |
| Thinking about quitting | ||
| Yes, 30 days | 11.6 | 14.7 |
| Yes, 6 months | 20.9 | 20.6 |
| Yes, in the future | 43.7 | 47.2 |
| No | 23.8 | 17.6 |
Demand for cigarettes and alternatives
Based on purchase tasks among all participants (N = 1062), 1.1% of participants reported they would consume no cigarettes at the price of $0 (called “zero demand” here for brevity), compared to 61.5% for snus, 34.5% for lozenge, and 56.2% for dissolvables. Essentially this reflects lack of demand. A logistic regression model examined effect of product, ad condition, and demographic factors on reporting zero demand. We found that compared to cigarettes, dissolvables [OR = 148.6, 95% CI: 84.9, 260.2], lozenge [OR = 52.3, 95% CI: 30.2, 90.5], and snus [OR = 182.6, 95% CI: 104.1, 320.3] all had substantially greater odds of zero demand. Those who saw soft-drink ads were more likely to report zero demand [OR = 1.74, 95% CI: 1.41, 2.15]. Those who had previously used oral NRT [OR = 0.52, 95% CI: 0.42, 0.64] or ST [OR = 0.29, 95% CI: 0.22, 0.38] were less likely to report zero demand. Older participants were slightly more likely [OR = 1.01, 95% CI: 1.01, 1.02] to report zero demand.
Those who expressed any demand for any of the products were included in the demand curve fitting procedures. Overall, the exponential demand equation provided good fit to the sample average consumption data for cigarettes and the three alternatives among those expressing any demand (R2 > 0.93). The demand curves shown in in Figure 1 reveal higher peak consumption and lower demand elasticity for cigarettes relative to the snus, dissolvables, and lozenges. For analysis of individual-level data, outliers (> 3 SD) and cases with poor fit to the exponential demand model (R2 < 0.3) were excluded, reducing the total number of observations across all products by 4.0% (N=98) for Q0 and 5.1% (N=127) for alpha.
Figure 1.
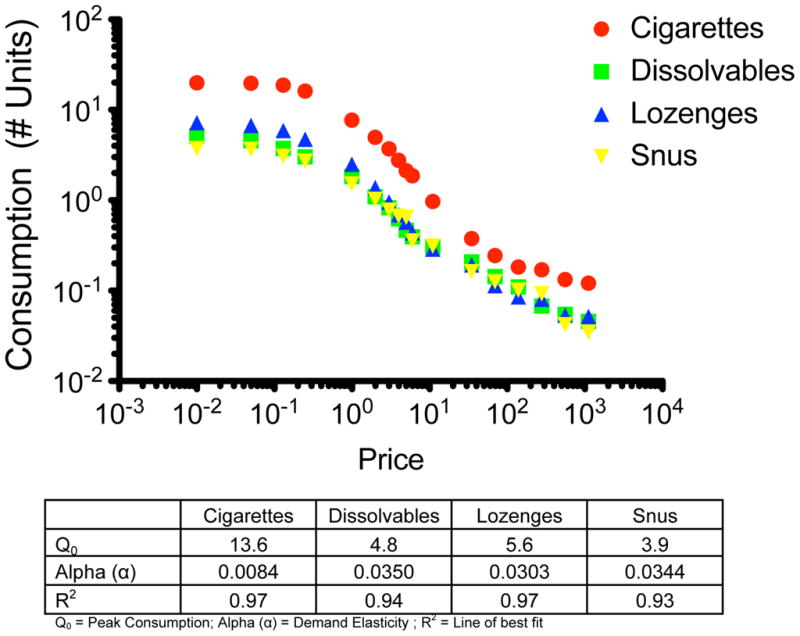
Peak Consumption
GEE modeling results are presented in Table 2. There was no significant main effect of seeing ads for smokeless tobacco or the nicotine lozenge compared to control ads on overall peak consumption (p = .64). However, as shown in Table 2, peak consumption did vary by product type (p < .001). Mean values of peak consumption are reflected in Table 3. Relative to cigarettes, peak demand for snus (B = −.75, p < .001), dissolvables (B = −.65, p < .01), and lozenges (B = −.55, p < .01) were each lower. According to sequential Bonferroni tests, the differences among products were statistically significant (p’s ≤ .03). Age, FTND score, and craving scores were all positively associated with peak consumption across products (p ≤ .01). We noted no significant main effects in peak consumption by sex, race, education, income, and prior use of ST or NRT.
Table 2.
GEE Parameter Estimates for Models Predicting Individual Estimates of Peak Consumption and Demand Elasticity. Bolded values are statistically significant.
| Variable Name | Peak Consumption | Elasticity | ||||
|---|---|---|---|---|---|---|
| B | Std. Error | p-value | B | Std. Error | p-value | |
| Intercept | 1.71 | .09 | .00 | −.55 | .42 | .19 |
| Control Ads | −.01 | .02 | .64 | .04 | .11 | .73 |
| ST & NRT Ads | REF | REF | ||||
| Dissolvables | −.65 | .03 | < .01 | 1.58 | .10 | < .01 |
| Lozenges | −.55 | .03 | < .01 | 1.45 | .09 | < .01 |
| Snus | −.75 | .04 | < .01 | 1.72 | .11 | < .01 |
| Cigarettes | REF | REF | ||||
| Female | .01 | .03 | .77 | .10 | .13 | .44 |
| Male | REF | REF | ||||
| Other | .00 | .06 | 1.00 | −.33 | .20 | .10 |
| Black/African American | −.11 | .07 | .11 | .14 | .22 | .54 |
| White | REF | REF | ||||
| College Grad | −.03 | .04 | .42 | −.05 | .16 | .77 |
| Some College | .04 | .03 | .19 | −.10 | .13 | .44 |
| High School or Less | REF | REF | ||||
| Prefer not to disclose income | .07 | .12 | .55 | −.40 | .31 | .20 |
| Do not know income | .11 | .21 | .60 | .17 | .56 | .77 |
| $100,000 or more | −.05 | .07 | .42 | .36 | .23 | .12 |
| $75,001 – $100,000 | −.04 | .04 | .33 | −.23 | .17 | .17 |
| $50,001 – $75,000 | −.08 | .04 | .04 | .20 | .17 | .24 |
| $25,001 – $50,000 | −.03 | .03 | .32 | .13 | .16 | .42 |
| $25,000 or less | REF | REF | ||||
| Age (continuous) | .00 | .00 | .01 | .00 | .00 | .38 |
| FTND (Nicotine Dependence) | .09 | .01 | < .01 | −.15 | .03 | < .01 |
| QSU Factor 1 (Cigarette Craving) | .05 | .01 | < .01 | −.14 | .04 | < .01 |
| Ever Used NRT | −.02 | .03 | .46 | −.02 | .12 | .86 |
| Never Used NRT | REF | REF | ||||
| Ever Used ST | −.03 | .03 | .36 | −.09 | .12 | .47 |
| Never Used ST | REF | REF | ||||
Table 3.
Estimated Peak Consumption (Number of Units) by Product, Adjusted for Covariates
| Product | Mean | Std. Error | 95% Wald Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Dissolvables | 6.16 | 3.96 | 1.74 | 21.73 |
| Lozenges | 6.77 | 4.34 | 1.93 | 23.80 |
| Snus | 5.56 | 3.58 | 1.58 | 19.64 |
| Cigarettes | 11.74 | 7.53 | 3.34 | 41.26 |
Covariates include Age, FTND, QSU
Own-Price Demand Elasticities
We saw no significant main effect of seeing ST or NRT ads compared to control ads on overall demand elasticity among those with any demand (p = .73; see Table 2). We noted significant main effects of product type with greater demand for cigarettes compared to snus, dissolvables or lozenges (B ≥ 1.45, p < .01). We also saw a significant difference in demand for both dissolvables and lozenges compared to snus such that elasticity was greater for snus (p < .05). Income, nicotine dependence, and reported cigarette cravings also were related to product demand. Nicotine dependence and craving were negatively associated with demand elasticity, such that higher dependence and craving scores were associated with lower demand elasticity (B ≤ −.15, p < .01). We noted no significant differences between demand elasticity by sex, race, education, age, and prior use of ST or NRT.
Cross Price Elasticity (CPE)
GEE parameter estimates for models predicting individual estimates of cross-price elasticity are found in Table 4. When sample average alternative product consumption (where price is fixed at $1) is regressed onto cigarette price, positive slopes are seen, suggesting that snus, dissolvables and nicotine lozenges on average serve as weak substitutes for cigarettes: CPE for Camel Snus was .26 (Range: −1.00, 1.09), for Camel Dissolvables was .33 (−1.06, 1.12), and for Commit Lozenge was .32 (−1.14, 1.17). The per-cigarette prices at which average consumption of the alternative overtook that of cigarettes were $11 for snus, $4 for lozenge, and $5 for dissolvables. Multivariate analysis showed that CPE did not differ significantly by exposure to product advertising. CPE was not significantly associated with neither peak consumption nor FTND as well as sex, education, age, and prior use of NR. CPE were negatively associated with demand elasticity (p = .019) as well as prior use of ST (B = −.12; p = .04), and race (p = .002). Here, black participants showed lower CPE compared to whites (B = −.33, p = .001) and participants who we unsure of their income revealed higher CPE compared to those in the lowest income bracket (B = .44, p < .001). Craving scores were also positively associated with CPE (B = .07, p < .001).
Table 4.
GEE Parameter Estimates for Models Predicting Individual Estimates of Cross-Price Elasticity. Bolded values are statistically significant.
| Variable Name | B | Std. Error | p-value |
|---|---|---|---|
| Intercept | −.09 | .17 | .61 |
| Dissolvables | .02 | .02 | .40 |
| Lozenges | .06 | .03 | .03 |
| Snus | REF | ||
| Soft Drink Ads | .05 | .04 | .31 |
| Tobacco Product Ads | REF | ||
| Female | .03 | .05 | .55 |
| Male | REF | ||
| Other | −.13 | .11 | .23 |
| Black/African American | −.33 | .10 | < .01 |
| White | REF | ||
| College Grad or more | .04 | .07 | .57 |
| Some College | .05 | .06 | .39 |
| High School or below | REF | ||
| Preferred not to disclose their income | .24 | .13 | .07 |
| Did not know their income | .44 | .09 | < .01 |
| $100,000 or more | .10 | .09 | .27 |
| $75,001 – $100,000 | .13 | .08 | .10 |
| $50,001 – $75,000 | .03 | .07 | .69 |
| $25,001 – $50,000 | .06 | .07 | .39 |
| $25,000 or less | REF | ||
| Age - Continuous | .00 | .00 | .96 |
| FTND (Nicotine Dependence) | .00 | .01 | .74 |
| QSU Factor 1 (Cigarette Craving) | .07 | .02 | < .01 |
| Q0 (Peak Consumption of cigarettes) | .02 | .09 | .78 |
| Alpha (Demand Elasticity of cigarettes) | −.56 | .24 | .02 |
| Ever Used NRT | −.07 | .05 | .13 |
| Never Used NRT | REF | ||
| Ever Used ST | −.12 | .06 | .04 |
| Never Used ST | REF |
DISCUSSION
The current study first sought to examine how exposure to ST advertising would impact current smokers’ interests in using three potential substitutes for cigarettes, ie snus, dissolvable tobacco, and nicotine lozenges. This study found a difference in the demand elasticity between cigarettes, snus, dissolvables and nicotine lozenges. As we would expect current smokers showed a stronger demand for cigarettes compared to the other three oral tobacco or nicotine products we tested. Our results also suggest that snus, dissolvables and nicotine lozenges are relatively modest substitutes for cigarettes, as our CPEs show that a 10% increase in cigarette prices would lead to between a 2.6 – 3.3% increase in ST consumption, depending on the product. In general, the CPEs of the different oral tobacco products were similar to each another. Differences in these indices among the alternative products suggested that snus was the least attractive of a set of largely unattractive alternatives. This could be due to the novelty of the oral products compared to cigarettes, especially as the ST products were relatively new to the market. We also found that viewing advertising for oral tobacco products relative to control ads had no impact on how participants performed on the purchase tasks except at the zero price point – exposure to the tobacco ads increased odds of having some demand, but beyond this initial nudge, they did not have a systematic effect on level of demand or demand elasticity. Hence, brief exposure to an ad for a novel product may not substantially influence smokers’ demand for that product, although this not rule out the possibility that repeated exposures to advertising could impact demand for a product. Currently, cigarette use is portrayed in retail and online marketing as acceptable, thus encouraging use among adolescents.11 It can be inferred that repeated, more positive exposure to novel products in place of smoking ads may impact the market in such a way that would encourage use of these products over cigarettes. Here, ad condition also did not influence average single product elasticities or CPE. We did not present any information about relative risks, instead using examples of current product messaging along with very neutral product descriptions. So, in the absence of health-relevant information smokers may not be really motivated to find alternatives to tobacco products.
Some of the observed preference for lozenge could be attributed to familiarity effects – lozenge is well known and widely advertised, and so this could contribute to participant decision making. It would be interesting to test these same responses over a period of time; as novel ST products become more familiar to the general public. While prior work about product trials suggested that smokers who had used NRT and/or ST in the past might be more receptive to them as substitutes,42–45 we saw that those with prior use of oral NRTs showed lower estimates of CPE, particularly for nicotine lozenge and dissolvables, suggesting they were less acceptable substitutes. This could be interpreted in one of two ways. First, prior use of NRT is likely to have been in the context of a cessation attempt, and this failed attempt may cloud their impression of the NRT and similar products. Second, their direct experience with the oral NRT could have been unpleasant (eg, aversive, insufficient nicotine), making them less willing to use such products in the future. We did not see an effect, positive or negative, of any prior ST use.
Interestingly, craving for cigarettes (as assessed by QSU Factor 1) appeared to have a significant underlying relationship with demand across products, such that higher craving was associated with lower alpha estimates (ie, more inelastic demand). This makes sense since smokers with higher cigarette cravings should be less price-sensitive. Similarly, we noted a main effect for craving on cross-price elasticity, with higher craving associated with higher substitutability. This is interesting inasmuch as it suggests that demand and cross-price elasticity obtained from simulation tasks may be state-dependent, arguing for measurement of craving particularly when assessed outside the laboratory, where deprivation can be better controlled.
There were several limitations to this study. Novel products were examined, and unfamiliarity with these products may have adversely affected product demand. We assessed lifetime use of any smokeless or oral nicotine replacement product, but we were unable to examine the level of familiarity beyond this relatively crude indicator of familiarity, as we did not assess participants’ prior knowledge or experiences with Camel Snus, Camel Dissolvables, or Commit Lozenge before presenting the ads. We assessed only a single exposure to these ads for novel products, for which repeated ad exposure under naturalistic conditions may be necessary to stimulate demand. Ad viewing time was not controlled or measured (participants had to hit a key to continue to the questions), so it is possible that some participants may have had far greater exposure to the ads than others. However, allowing participants to advance on their own accord simulates a more realistic ad viewing experience, since viewers of magazine ads would spend varying times assessing the contents and messages of an ad on their own time. Another limitation concerns how our web-based design may not adequately represent actual behavior-- the nature of the particular tasks is purely hypothetical and based on self-report. Furthermore, these reports may not represent the broader population of smokers, due to lower coverage of socially disadvantaged populations where smoking is concentrated, as this convenience sample was drawn from an opt-in internet panel. Thus, it is unclear the extent to which these findings reflect real-world scenarios. That being said, the findings here of relatively low appeal of snus and dissolvable tobacco parallel the field experience in test markets of relatively low snus trial and adoption 15 and studies presenting such products as alternatives to cigarettes.42–44
Additionally, due to the use of unaltered magazine ads which, by nature of current regulation, did not focus specifically on harm reduction, we were unable to test whether or not participants seemed more interested in switching products for the purposes of harm reduction. An ongoing study implemented by our group tests this question in more detail.
Our findings suggest that from purchase and cross price elasticity tasks can be used to determine demand for alternative products compared to cigarettes. Despite smokers expressed interest in substituting other potentially less harmful alternatives for cigarettes, demand and substitutability of those products in the continued presence of cigarettes is low. In terms of tobacco control policy, a new look at product access and price may be in order. For example, some have suggested a ‘risk-based’ tax structure that would make cigarettes far more expensive than other forms of nicotine delivery in order to redirect demand.46 These data provide some support for this approach, though the price differentials at which alternative use outstripped cigarette use may be politically unrealistic as determined by projections from the cost of a single unit in the package (11:1 in the case of snus, or the equivalent of $44 per pack of cigarettes versus $4 per tin of snus). Still, this approach is likely worthwhile to explore.
Supplementary Material
Footnotes
Human Subjects Statement
A total of 1062 current smokers recruited from a preexisting on-line panel completed this study. The Institutional Review Board at Roswell Park Cancer Institute reviewed and approved the study protocol.
Conflict of Interest Statement
RJO has served as a consultant to the Food and Drug Administration (Tobacco Products Scientific Advisory Committee, Tobacco Constituents Subcommittee), and was supported by the Centers for Disease Control and Prevention is preparing a monograph chapter on novel smokeless tobacco product marketing. KMC has provided expert testimony on behalf of plaintiffs in cases against the tobacco industry. This work was supported by a grant from the National Cancer Institute (R01CA141609 to RJO).
References
- 1.Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.Fagerström KO, Schildt EB. Should the European Union lift the ban on snus? Evidence from the Swedish experience. Addiction. 2003;98:1191–5. doi: 10.1046/j.1360-0443.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.Foulds J, Ramstrom L, Burke M, Fagerström K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control. 2003;12:349–59. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henningfield JE, Fagerstrom KO. Swedish Match Company, Swedish snus and public health: a harm reduction experiment in progress? Tob Control. 2001;10:253–7. doi: 10.1136/tc.10.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodu B, Godshall WT. Tobacco harm reduction: an alternative cessation strategy for inveterate smokers. Harm Reduct J. 2006;21:37. doi: 10.1186/1477-7517-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips CV, Heavner KK. Smokeless tobacco: the epidemiology and politics of harm. Biomarkers. 2009;14 (Suppl 1):79–84. doi: 10.1080/13547500902965476. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski LT. Effect of smokeless tobacco product marketing and use on population harm from tobacco use policy perspective for tobacco-risk reduction. Am J Prev Med. 2007;33 (Suppl 6):S379–86. doi: 10.1016/j.amepre.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Zeller M, Hatsukami D. Strategic Dialogue on Tobacco Harm Reduction Group. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–32. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomar SL, Fox BJ, Severson HH. Is smokeless tobacco use an appropriate public health strategy for reducing societal harm from cigarette smoking? Int J Environ Res Public Health. 2009;1:10–24. doi: 10.3390/ijerph6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romito LM, Saxton MK, Coan LL, Christen AG. Retail promotions and perceptions of R.J. Reynolds’ novel dissolvable tobacco in a US test market. Harm Reduct J. 2011;8:10. doi: 10.1186/1477-7517-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Rockville: Office of the Surgeon General, Public Health Service; 2012. [Google Scholar]
- 12.Timberlake DS. Are smokers receptive to using smokeless tobacco as a substitute? Prev Med. 2009;49:229–32. doi: 10.1016/j.ypmed.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Heavner KK, Rosenberg Z, Phillips CV. Survey of smokers’ reasons for not switching to safer sources of nicotine and their willingness to do so in the future. Harm Reduct J. 2009;2:6–14. doi: 10.1186/1477-7517-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gartner CC, Jimenez-Soto EV, Borland R, et al. Are Australian smokers interested in using low nitrosamine smokeless tobacco for harm reduction? Tob Cont. 2010;19:541–6. doi: 10.1136/tc.2009.033670. [DOI] [PubMed] [Google Scholar]
- 15.Biener L, McCausland K, Curry L, Cullen J. Prevalence of trial of snus products among adult smokers. Am J Public Health. 2011;101:1874–6. doi: 10.2105/AJPH.2010.200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borland R, Cooper J, McNeill A. Trends in beliefs about the harmfulness and use of stop-smoking medications and smokeless tobacco products among cigarettes smokers: Findings from the ITC four-country survey. Harm Reduct J. 2011;8:21. doi: 10.1186/1477-7517-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings KM, Hyland A, Giovino GA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res. 2004;6 (Suppl 3):S333–S340. doi: 10.1080/14622200412331320734. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman S, Ferguson SG, Rohay J, Gitchell JG. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: relationship with use and compliance. Addiction. 2008;103:1371–1378. doi: 10.1111/j.1360-0443.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 19.Cummings KM. Programs and policies to discourage the use of tobacco products. Oncogene. 2002;21:7349–7364. doi: 10.1038/sj.onc.1205810. [DOI] [PubMed] [Google Scholar]
- 20.Kozlowski LT, Edwards BQ. “Not safe” is not enough: smokers have a right to know more than there is no safe tobacco product. Tob Control. 2005;14(Suppl 2):ii3–ii7. doi: 10.1136/tc.2004.008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson SG, Gitchell JG, Shiffman S. Providing accurate safety information may increase a smoker’s willingness to use nicotine replacement therapy as part of a quit attempt. Addict Behav. 2011;36:713–716. doi: 10.1016/j.addbeh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Sami M, Timberlake DS, Nelson R, et al. Smokers’ perceptions of smokeless tobacco and harm reduction. J Public Health Policy. 2012;33:188–201. doi: 10.1057/jphp.2012.9. [DOI] [PubMed] [Google Scholar]
- 23.Mackillop J, Murphy JG, Ray LA, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16:57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- 24.Murphy JG, MacKillop J, Tidey JW, et al. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113:207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine Tob Res. 2010;12:416–422. doi: 10.1093/ntr/ntq018. Epub 2010 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Few LR, Acker J, Murphy C, MacKillop J. Temporal stability of a cigarette purchase task. Nicotine Tob Res. 2012;14:761–765. doi: 10.1093/ntr/ntr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: The concurrent presence of a substitute and an independent reinforcer. Behav Pharmacol. 2003;14:137–144. doi: 10.1097/00008877-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006;85:73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74:253–264. doi: 10.1016/j.drugalcdep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Shahan TA, Odum AL, Bickel WK. Nicotine gum as a substitute for cigarettes: a behavioral economic analysis. Behav Pharmacol. 2000;11:71–79. doi: 10.1097/00008877-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Bidwell LC, MacKillop J, Murphy JG, et al. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addictive Behav. 2012;37:1257–1263. doi: 10.1016/j.addbeh.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKillop J, Few LR, Murphy JG, et al. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addict. 2012;107:2191–2200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKillop J, Brown CL, Stojek MK, et al. Behavioral economic analysis of withdrawal-and-cue-elicited craving for tobacco: an initial investigation. Nicotine Tob Res. 2012;14:1426–1434. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 35.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 36.Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633–1643. doi: 10.1080/14622200802409990. [DOI] [PubMed] [Google Scholar]
- 37.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 38.Smith P, Bansal-Travers M, O’Connor R, et al. Correcting over 50 years of tobacco industry misinformation. Am J Prev Med. 2011;40:690–698. doi: 10.1016/j.amepre.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7:412–426. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- 41.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 42.O’Connor RJ, Norton KJ, Bansal-Travers M, et al. US smokers’ reactions to a brief trial of oral nicotine products. Harm Reduct J. 2011;8:1. doi: 10.1186/1477-7517-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borland R, Li L, Mortimer K, et al. The acceptability of nicotine containing products as alternatives to cigarettes: findings from two pilot studies. Harm Reduct J. 2011;8:27. doi: 10.1186/1477-7517-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpenter MJ, Gray KM. A pilot randomized study of smokeless tobacco use among smokers not interested in quitting: changes in smoking behavior and readiness to quit. Nicotine Tob Res. 2010;12:136–43. doi: 10.1093/ntr/ntp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider NG, Cortner C, Justice M, et al. Preferences among five nicotine treatments based on information versus sampling. Nicotine Tob Res. 2008;10:179–186. doi: 10.1080/14622200701767837. [DOI] [PubMed] [Google Scholar]
- 46.Sweanor D, Alcabes P, Drucker E. Tobacco harm reduction: how rational public policy could transform a pandemic. Int J Drug Policy. 2007;18:70–74. doi: 10.1016/j.drugpo.2006.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


