Abstract
A comprehensive prompting strategy designed to maximize the rate of Brief Intervention (BI) for “heavy drinking” was implemented from 2001 to 2003 for a randomized controlled trial of a post-BI treatment enhancement. Thirty-one internists at four out-patient practices in a county of 150,000 in a rural US state documented their BI’s using an intervention checklist. The prompting procedures implemented in this study yielded documented BI for 39% of identified cases, but participation rates varied by physician and clinic and over time. The overall rate was lower than expected. Implications and recommendations for future BI research and training are offered; the paper’s limitations are discussed.
Keywords: primary health care, alcohol drinking, physician’s practice patterns, preventive health services, heath services research, alcohol use screening method, physician prompting, at-risk drinking, hazardous drinkers, problem drinking
Introduction
The primary care office is considered an appropriate arena for screening and identification of at-risk alcohol consumption and for potential intervention (Institute of Medicine, 1990; US Preventive Services Task Force, 2004). Prevalence rates of at-risk drinking in US primary care populations have been estimated at between 11% – 20% (Adams, Barry, and Fleming, 1996; Curry et al., 2000; Fleming, Manwell, Barry, and Johnson, 1998). Patients seeking ongoing medical care presumably are interested in their health and predisposed to accept health advice; furthermore, the familiar health care setting may bestow a cachet that enhances efficacy. Indeed, the short- and long-term effectiveness of brief intervention (BI) for reducing alcohol consumption, achieving abstinence, reducing or eliminating alcohol consumption-related problems in multiple life areas, and/or reducing biomarkers of alcohol use or abuse (such as GGT activity) in a primary care setting has been demonstrated in a number of studies (Bien, Miller, and Tonigan, 1993; Chick, Ritson, Connaughton, Steward, and Chick, 1988; Manwell, Fleming, Mundt, Stauffacher, and Barry, 2000; Moyer, Finney, Swearingen, and Vergun, 2002). Furthermore, cost-benefit ratios in favor of BI have been reported for both medical and societal outcomes up to 48 months following the intervention (Mundt, 2006). Thus, there is little disagreement that an office-based BI by a Primary Care Provider (PCP) for alcohol misuse is efficacious and should be standard practice.
However, implementing screening and intervention for alcohol consumption-related problems in primary care settings has been problematic (Andreasson, Hjalmarsson, and Rehnman, 2000; Beich, Gannik, and Malterud, 2002; CASA, 2000). For example, there is evidence that only about one-third of primary care providers screen their patients for alcohol use during an annual visit (Spandorfer, Israel, and Turner, 1999). In a survey of problem drinkers, only 24% reported that their drinking had been addressed at a recent medical visit (Weisner and Matzger, 2003). While lack of training may account for some of the failure to screen, one major survey found that among physicians who had completed CME substance abuse training within the past five years, only 7.6% appropriately identified substance abuse as a possible diagnosis for a hypothetical male patient with early signs of alcohol abuse (CASA, 2000).1
Time constraints present a significant barrier to BI implementation. A single behavioral intervention may require only a small amount of provider attention, but collectively, if providers did all the screening and preventive intervention that is recommended by the US Preventive Services Task Force,2 (USPSTF), it is estimated they would have to spend an additional 1,773 hours annually, or 7.4 hours each working day (Yarnall, Pollak, Ostbye, Krause, and Michener, 2003). The USPSTF rates screening and behavioral counseling interventions to reduce alcohol misuse as a “B Recommendation,” meaning that the strength of evidence and magnitude of net benefit are high, but not as high as an “A Recommendation.” BI may fall off the priority list if a patient has a condition requiring one or more A-Level services. Providers trained to do BI in one study later indicated they considered the addition to their workload onerous, and could not recommend provider-initiated screening and BI, even though they had endorsed the importance and desirability of counseling patients on drinking (Beich et al., 2002).
In addition, BI may be under-utilized because the mechanisms for identifying appropriate patients are inadequate, inconsistently implemented, and typically have no demonstrated reliability and validity. To aid providers in the identification and management of medical concerns, many practices have instituted pre-encounter screening systems, such as waiting-room questionnaires. Use of such screening tools in isolation may be an inefficient means of promoting effective BI (Beich, Thorsen, and Rollnick, 2003), but more intensive prompting of physicians and/or patients through reminders, tags or notices on front of charts, general checklists or questionnaires, highlighted screening results, or additional counseling recommendations can improve rates of BI and other preventive care intervention (Austin, Balas, Mitchell, and Ewigman, 1994; Balas et al., 2000; Buchsbaum, Buchanan, Lawton, Elswick, and Schnoll, 1993; Hung et al., 2006; Saitz, Horton, Sullivan, Moskowitz, and Samet, 2003). In a review of strategies for increasing screening procedures, Fleming (1997) indicated the following were efficacious: group education sessions, education by respected colleagues/opinion leaders, performance feedback, educational outreach to individual physicians (i.e., academic detailing), and financial incentives or penalties. For alcohol screening, in particular, Fleming recommended comprehensive clinic-based programs that include patient questionnaires, assessment tools for providers, computerized reminder systems, and lists of community resources and support agencies.
In the context of a larger trial (Helzer et al., 2008), we implemented several of the empirically based recommendations for physician prompting in order to increase screening rates. The prompting techniques themselves were not the focus of the trial; rather, they were embedded in the procedures. In this paper, we describe the systematic measures taken to encourage BI in a university-affiliated outpatient primary care practice, and the outcome of these efforts, for the purpose of illustrating a “real world” application of these techniques. In light of existing literature showing the efficacy of various physician prompting techniques, it was expected that the procedures employed would result in a high rate of BI.
Methods
Design
The methods used to facilitate and encourage BI (summarized in Table 1) were undertaken in the context of a large randomized controlled trial of a post-BI treatment enhancement intervention (Helzer et al., 2008). Participation in the trial required physician BI and referral. The procedures reported here were implemented to maximize the identification of patients eligible for the randomized trial. The University of Vermont Committee on Human Research in the Medical Sciences (IRB) approved the trial and all recruitment procedures.
Table 1.
Prompting techniques
| Group and individual brief intervention training for physicians |
| Patient-targeted exam room posters and brochures |
| Pre-screening of charts for eligibility |
| Notices to physicians on front of chart |
| Thank you notes and chocolates for each referral |
| Notice to provider after enrollment of patient |
| Financial incentive to physician or office (discontinued after first year) |
| Bi-weekly site visits by study personnel |
| Monthly feedback charts |
| Periodic BI booster sessions and preliminary results presentations |
Participants
Participants were 31 Primary Care Internal Medicine (PCIM) clinicians (17 male and 14 female) located at one of four outpatient office practices affiliated with the University of Vermont College of Medicine. Twenty-three of the 31 were MD faculty members of the Department of Medicine, one was a nurse practitioner and seven were medical residents. All the clinicians at each site participated and were included in the sample automatically, because the study procedures were applied within each site and affected the practice as a whole. Prior to the onset of recruitment, several formal workshops were conducted to enhance clinicians’ existing knowledge and skills. BI training was conducted by an expert consultant and closely followed the NIAAA guide, Training Physicians in Techniques for Alcohol Screening and Brief Intervention (Fleming, Cotter, and Talboy, 1997). Trainings included lecture, role-play, and discussion components pertinent to screening for alcohol consumption-related problems and counseling techniques for individuals meeting criteria for hazardous drinking, abuse, or dependence. All collaborating clinicians received training by a specialist in one or more of the following formats: (1) department-wide workshop, (2) on-site, small-group instruction, and/or (3) individual tutorial. Further details about trainings are available from the authors upon request. Each physician’s exam rooms were subsequently stocked with patient-oriented study posters, NIAAA “How to Cut Down on Your Drinking” brochures, and Frequently Asked Questions study summary sheets.
Procedures
The intervention targets were patients scheduled for a Health Maintenance Exam (HME), an extensive “physical” recommended every three years, for which PCPs are allotted one hour. This visit type was chosen for two reasons. First, collaborating physicians indicated that the generous time allotment would enable them to adequately address preventive health concerns such as heavy drinking. Second, patients routinely completed a comprehensive self-assessment screening questionnaire, the General Health Questionnaire (GHQ),3 prior to this visit. The questionnaire asked patients to provide detailed medical, surgical, and medication history; identify current health problems; complete a review of systems checklist; indicate family history of major diseases; and, most importantly, describe their typical day and health habits, including alcohol consumption and problems. This screening questionnaire was highly advantageous because it enabled advance identification of eligible patients and, therefore, provided a denominator of cases from which to derive eligibility and referral rates. Furthermore, no alteration of the clinic’s standard assessment practices was necessary.
To facilitate the identification of hazardous drinkers, a research assistant (RA)4 reviewed charts prior to the actual HME visit. Specifically, the RA examined the alcohol questions on the GHQ, which included the CAGE (Mayfield, McLeod, and Hall, 1974), average number of daily alcoholic drinks, alcohol consumption within the previous 24 hours, and previous history of alcohol consumption-related problems. The following criteria were considered clinical indicators of at-risk drinking: (1) CAGE score of two or more, (2) three or more daily drinks for men (two or more daily drinks for women), and (3) any drinking in patients with a history of alcohol consumption-related problems.
If a patient endorsed one or more of these criteria on the GHQ, the RA attached a study referral form and an NIAAA brochure to the chart, along with a personalized handwritten note to the doctor highlighting relevant answers on the GHQ and recommending BI and referral to the trial. Referral forms were designed in collaboration with participating physicians and included: (1) an outline/checklist for alcohol consumption screening and intervention, with space for the physician to indicate results and plan; (2) an introductory statement about the research study that the physician could read to the patient, and (3) space to indicate permission for the researchers to contact the patient to provide more information about the study. For descriptive data purposes, physicians were asked to return all referral forms to a designated bin, regardless of the results of the BI or the patient’s willingness to be contacted. Names were omitted for patients who did not agree to be contacted.
The RA made bi-weekly visits to each collaborating clinic to check for referrals. Thank you notes with chocolate enclosed were delivered to physicians following each referral. If the patient enrolled in the study, the physician was notified by e-mail and was encouraged to followup on the BI at the next clinic visit. Financial incentives, via contribution to the physician’s individual Continuing Medical Education fund for each referral received, were offered to compensate for the lack of insurance reimbursement for preventive care. These donations were later directed to a general office fund to support perquisite expenses (such as a water cooler), then eliminated altogether in the final stages of the study due to budget constraints.
To maintain interest, the RA regularly brought bagels and other treats for staff to each clinic when she came to review the charts. Halfway through the recruitment period, study posters were revised for novelty. Each month, colored feedback graphs were distributed to all clinic personnel summarizing the number of referrals made by each physician and each clinic, also with chocolate attached. Periodically, the Project Director visited each site to re-orient staff to the study protocol. BI “booster sessions” and preliminary results presentations were made to physicians and staff. These presentations were made in a variety of forums, including on-site staff and/or faculty meetings, specially scheduled lunchtime workshops, and departmental Grand Rounds. At each presentation, copies of the NIAAA “Physicians Guide to Helping Patients with Alcohol Problems” were distributed, along with customized pens with the study’s logo and contact information. To facilitate access to BI training, a self-directed Web-based training program that physicians could review for CME credit was developed and made available through the university’s Continuing Medical Education office.
Results
Brief Intervention and Referral
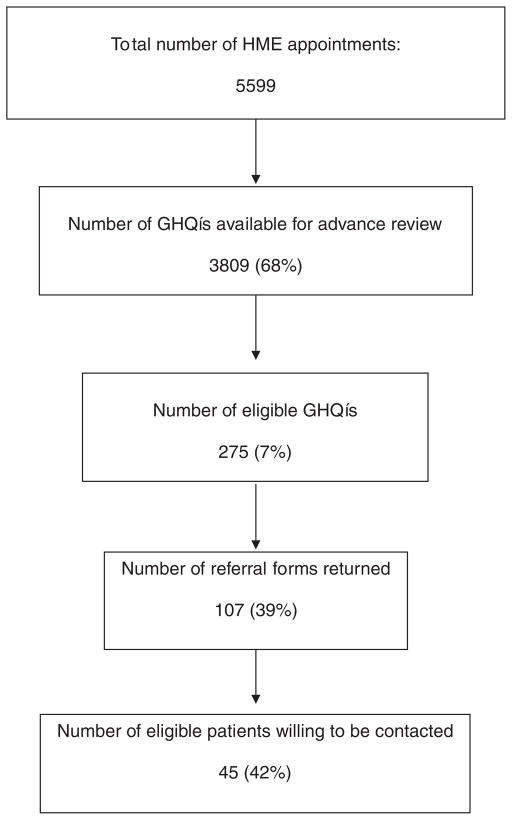
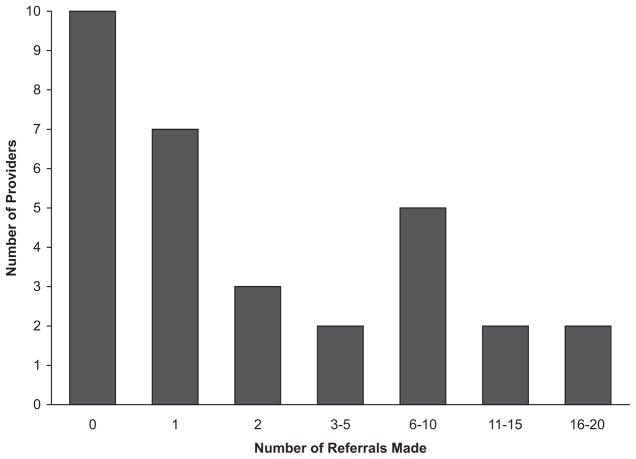
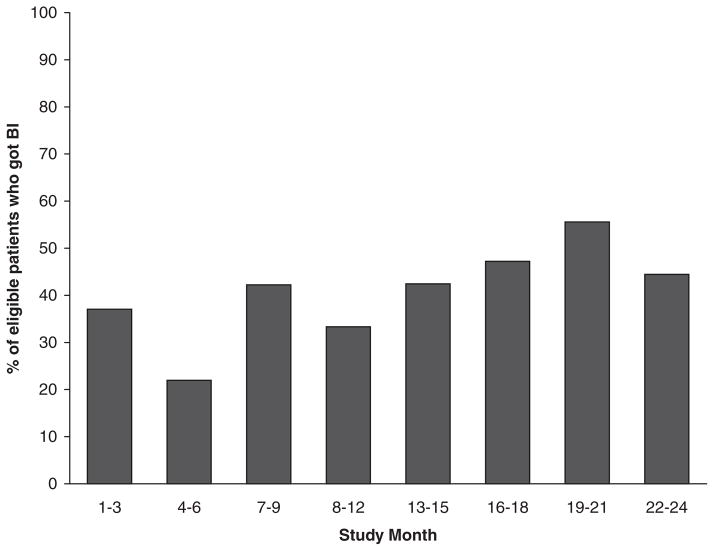
A flow diagram representing outcome of the recruitment process is displayed in Figure 1. As illustrated, 5,599 patients were scheduled for health maintenance appointments during the two-year recruitment period. For 3,809 (68%) of these appointments, the corresponding GHQ was completed by the patient, mailed back to the clinic, and made available for advance review by the RA. Of the GHQs reviewed, 275 (7%) met our screening criteria and were highlighted by the RA; physicians returned a referral form for 107 (39%) of these. Participation in the recruitment process varied by physician and clinic and over time. As illustrated in Figure 2, the modal number of referrals per physician was 0 (range: 0 –18). About half of the physicians who submitted referral forms did so for only one or two patients during two years of recruitment. Over half of all referrals (52%) came from only four of the 31 clinicians. While the overall referral rate was 39% of patients screened positive, rates in the four individual clinics were 17%, 34%, 50%, and 51%. Finally, the referral rate increased over time, from 34% for year 1 compared to 47% for year 2; see Figure 3.
Figure 1.
Recruitment flow diagram.
Figure 2.
Number of referrals made by providers.
Figure 3.
Referral rate over time.
Willingness to Participate in Research
Of the 107 eligible patients for whom a referral form was returned, 45 of them (42%) agreed to be contacted by a researcher. Thus, the case identification procedures implemented in this study yielded a pool of potential research participants that represents about 0.8% of all the patients presenting for health maintenance exams at these practices (or 1.2% of those with completed pre-screening forms). Notably, there was an inverse relationship between the number of referrals generated and the willingness of those referrals to be contacted. The clinic with the lowest rate of referral (i.e., 17%) had the highest rate of patients consenting to researcher contact (83%). Rates of consenting patients at the other clinics were 50%, 31%, and 37%, respectively.
Discussion
The purpose of this report was to describe the application of specific techniques for increasing alcohol screenings in primary care and the outcome of this effort. Despite intensive measures by research personnel, documented BI was obtained for about one-third of the patients who met study criteria. The rate of referral was significantly higher in the second year, suggesting the effect of this type of recruitment effort may build instead of fade over time.
While the recruitment protocol was consistent across the four clinics, variability in referral rates (17% – 51%) suggests the procedures were adopted more systematically and effectively in some clinics than in others. For example, in spite of instructions to return a referral form for any patient with whom they had conducted BI, it is clear that some physicians returned referral forms only for patients who expressed interest in the study. This anecdote is supported by the finding that the total referral rate was inversely related to the consenting referral rate (i.e., the number of patients willing to be contacted by researchers). While the clinics were all part of the same University-affiliated department, they differed in a number of ways, such as location (urban vs. suburban), demographics of patients served, number of clinicians and consistency of their schedules, etc. These and other factors may have influenced referral rates. For example, there may be practice culture differences with respect to receptivity to research protocols and concerns about patient anonymity. Furthermore, individual differences undoubtedly influence which clinicians utilize available tools (such as questionnaires, referral resources, etc.). Some “early adopter” physicians, to borrow a phrase from the marketing literature, might have carried the weight of referrals in this project. Unfortunately, with a sample size of only four clinics and 31 providers, we are unable to make any definitive statements about the influence of these practice or person characteristics.
Because some BIs performed with patients not interested in the study were not documented, it is likely that our estimated BI rate (39%) is a lower bound. However, the actual rate of BI (including those that were undocumented) during this study recruitment period likely was higher than otherwise would have occurred under normal clinic procedures. This is because the techniques described here truly maximized the identification of heavy drinkers and the support for BI delivery in the context of a 60-minute physician encounter and minimized any additional effort on the part of the caregiver.
Various factors might account for the low overall referral rate. For example, Clinical Inertia (CI) is a phenomenon observed in the management of diabetes, hypertension, and dyslipidemia; it refers to the failure of health care providers to initiate or intensify therapy for these conditions even when they are recognized and the treatment is indicated (Phillips et al., 2001). In the context of problem drinking, CI can be defined as the failure to do BI when indicated based on quantity and frequency of alcohol consumed and/or negative consequences of use. CI for the management of diabetes can be overcome through feedback to the provider (Ziemer et al., 2006). Despite intense feedback efforts here, variability in referral rates persisted.
Another clinical phenomenon that may operate in this context is physicians’ lack of comfort and skill in discussing alcohol use with their patients (Beich et al., 2002). Awkward physician-patient communication dynamics have been documented for other behavioral health discussions such as HIV risk, narcotics use, and depression (Epstein et al., 1998; Meredith and Mazel, 2000; Merrill, Rhodes, Deyo, Marlatt, and Bradley, 2002). Regarding alcohol use, Spandorfer et al. (1999) found that a majority (72%) of PCPs preferred not to counsel patients. McCormick et al. (2006) audiotaped clinical encounters between doctors and their patients who had screened positive for harmful drinking. Qualitative analyses indicated that physicians commonly avoided the subject of alcohol use, displayed discomfort in the discussion, and/or offered vague advice. The authors concluded that further educational interventions are needed to “increase provider comfort and effectiveness with this important role” (p. 971). Findings of McCormick et al. suggest that perhaps the BI training conducted at the start of this project was inadequate to completely overcome the psychological barrier of physician discomfort with advising patients about their drinking behavior.
Limitations
The patients included in this sampling frame (i.e., those registering for a HME visit who complied with pre-visit paperwork) are unlikely to be representative of the larger population of primary care patients. Indeed, the rate of heavy/problem drinking in this sample (7%) was lower than expected based on the published literature. For example, Saitz et al. (2003) reported a 14% eligibility rate in their primary care screening study using similar criteria and prompting methodology. Furthermore, samples of patients seen for acute, episodic visits are likely to have, on average, a much higher rate – perhaps in the 30% range noted by other studies (Kaner, Heather, Brodie, Lock, and McAvoy, 2001; Lock and Kaner, 2004). The decision to use the GHQ as the main method of identifying at-risk drinkers allowed for pre-identification of cases and for implementation of additional strategies to support BI. In short, it offered a “best case scenario” for evaluating the effectiveness of these support strategies. It is estimated that the rate of BI among heavy/problem drinkers would be much lower under typical clinic circumstances in which time is limited and there is no pre-screening, identification, incentives, and feedback.
Another limitation is the sample of physicians. Only 31 clinicians were included, and they were from one health care organization in a relatively small geographic area. They varied in the extent of their experience in practice overall and in their level of prior training and experience in brief alcohol intervention. Detailed data on the personal and professional background characteristics of the physicians, which would have proved helpful in interpreting the findings, were not available.
Implications and Recommendations
One approach for improving BI among physicians has been to incorporate education on alcohol screening, referral, and intervention into medical school or post-graduate training curricula. While such efforts have increased knowledge and skills, they have not substantially impacted subsequent intervention rates (Popp, Schwartz, and Schoener, 1998; Walsh, Roche, Sanson-Fisher, and Saunders, 2001). Findings of McCormick et al. (2006) suggest perhaps this training should include an affective component to address physician discomfort, in addition to advancing objective knowledge and skill. It is recommended that physician educators incorporate assessments of self-efficacy into their training procedures, as confidence is essential for the adoption of new skills.
Redesigning office processes and the adoption of an office-based system that includes all staff members may be required to fully incorporate routine preventive health screening into clinical practice (Carpiano, Flocke, Frank, and Stange, 2003; Fleming, 1997; Hung et al., 2006). Comprehensive system change via quality improvement initiatives such as the Robert Wood Johnson Foundation Chronic Care Model (Robert Wood Johnson Foundation, n.d.) is gaining increasing interest. Furthermore, the Center for Medicare and Medicaid Services is pilot testing the Medical Home project whereby primary care offices will receive higher reimbursement rates for providing comprehensive care to patients with chronic medical problems. This better reimbursement will enable primary care offices to develop office systems that support the extra effort involved in delivering important preventive care services.
System redesigns that include automation of the screening and BI process might provide additional information to the health care team while also fostering a more active patient role. For example, Butler, Chiauzzi, Bromberg, Budman, and Buono (2003) tested the effectiveness of a computer-assisted screening and intervention for drinkers in primary care. The technique was effective in generating a significant reduction of drinking and, notably, was implemented in an automated, systematic manner that required no additional staff burden. Automation may conserve clinic staff and provider time and circumvent the problem of provider discomfort with the topic, while simultaneously encouraging or supporting patients to take a more active role in their preventive health. Whether automated BIs are as efficacious as good provider-delivered BIs is an empirical question that our research team is currently investigating.
Conclusion
This paper represents an example of how research on the efficacy of prompting techniques was translated into clinical practice in a real-world setting. The interventions applied in this study, including physician education, patient-oriented prompting materials, patient questionnaires, chart highlighting, counseling recommendations, patient resources, performance feedback, incentives, and positive reinforcement, were costly to implement and not very fruitful. Increasing the rate of BI would seem to require substantial systemic change. Comprehensive quality improvement initiatives and intensive educational campaigns that address physician affective reactions to alcohol and heavy drinkers in addition to their factual knowledge may be required. Perhaps, too, it is time to shift the focus of preventive care delivery from provider-driven to patient-directed and collaborative modalities. There may be effective ways of prompting patients to raise their drinking concerns with their doctors instead of the other way around. For example, investigations into the uses of technology for enhancing self-directed care are underway. Future studies might examine other modalities for promoting self-directed care, or for educating patients about alternative means for obtaining assistance with behavior change.
Acknowledgments
This study was supported by National Institute of Alcohol Abuse and Alcoholism (grant numbers AA11954-01A1 and 1 R21 AA015777-01A1 to Dr. John Helzer, PI.) The authors would like to acknowledge the contribution of expert consultant Robert M. Swift, M.D., Ph.D., who provided the initial BI training to participating physicians.
Glossary
- Brief Intervention
The context of this research is embedded in Western medicine, and we take the perspective of the US National Institute on Alcohol Abuse and Alcoholism (NIAAA) in defining diagnoses, treatments, and treatment goals. Brief intervention, as defined by the NIAAA (Alcohol Alert No. 43, April, 1999), is generally restricted to four or fewer sessions, each session lasting from a few minutes to one hour, and is designed to be conducted by health professionals who do not specialize in the treatment of alcohol dependence. It is most often used with patients whose alcohol consumption-related symptoms do not meet the criteria for alcohol dependence, and its goal may be moderate drinking rather than abstinence. The content and approach of brief intervention vary depending on the level of alcohol consumption and extent of alcohol consumption-related health and psychosocial problems. The approaches used in brief intervention are not unique to alcoholism
- they are applicable across a range of behavioral health concerns
such as smoking, diet, and physical activity
- Clinical Inertia
The failure of health care providers to initiate or intensify therapy for chronic conditions even when they are recognized and the treatment is indicated (Phillips et al., 2001)
Biographies

Gail L. Rose, Ph.D., is a Research Assistant Professor in the Psychiatry Department at the University of Vermont College of Medicine, Burlington, Vermont, U.S.A., and Assistant Professor in the UVM College of Education and Social Services, Burlington, Vermont, U.S.A. For the past 10 years she has been conducting clinical research on alcohol use and misuse as Project Director at the Health Behavior Research Center. She has been involved in continuing medical education/training for identification and treatment of alcohol use disorders using a motivational interviewing approach and in the training of clinical psychology doctoral students in cognitive behavioral therapy for alcohol abuse and dependence. Dr. Rose is also involved in mentoring, doctoral student development, and clinical and research training for doctoral students across disciplines.

Dennis A. Plante, M.D., has been a practicing General Internist at the University of Vermont, College of Medicine, since 1984. He has a special interest in both Medical Decision Making and in the applications of Quality Improvement ideas to clinical medicine. As a faculty member in the College of Medicine, Dr. Plante is involved in teaching both medical students and residents in training in the Department of Medicine Health Care Service. He has been involved in collaborative research with investigators from the University of Vermont and the State of Vermont. He is currently a regional collaborator in the State of Vermont’s Governor’s Blueprint for Health – a new initiative to improve the quality of health care by applying the principles of the Chronic Care Model of Wagner.

Colleen S. Thomas, M.S., is a statistical analyst at the University of Vermont, where she also received her M.S. in Biostatistics in 2003. She has consulted to the Health Behavior Research Center on a number of projects during the past three years. In addition, she has co-authored several journal articles relating to substance use. Her interests include SAS programming, data cleaning techniques, hierarchical linear models, and repeated measures designs.

Laura J. Denton, B.A., has her bachelor’s degree in Psychology and English Literature. She is currently the Study Conduct Support Coordinator at Duke University’s Clinical Research Support Office, where she assists with regulatory training and communications for Duke University’s School of Medicine in Durham, North Carolina. Ms. Denton was a Research Assistant on the project referred to in this article while working at the Health Behavior Research Center at the University of Vermont. She also worked on developing protocols for the North Central Cancer Treatment Group (NCCTG), whose research base is housed in the Cancer Center at the Mayo Clinic in Rochester Minnesota.

John E. Helzer, M.D., is a Professor of Psychiatry at the University of Vermont College of Medicine and the Director of the Health Behavior Research Center at the University. He and Dr. Rose have been research collaborators for the past 10 years. Their research is focused on developing simple technologies to create behavioral tools that patients can use to guide and enhance their own self-care. Dr. Helzer’s research background is in Psychiatric Epidemiology, intervention, and Psychiatric Taxonomy. Currently, he devotes most of his time to research but is also engaged in patient care and teaching at the College of Medicine.
Footnotes
Symptoms included recurrent abdominal pains, intermittently elevated blood pressure and gastritis visible on gastroscopy, irritability and waking up frequently at night. The vignette noted that the patient was married, had job-related anxiety and stress, but reported normal libido and no previous psychiatric history.
As stated in their fact sheet (http://www.ahrq.gov/clinic/uspstfab.htm), “The U.S. Preventive Services Task Force (USPSTF) first convened by the U.S. Public Health Service in 1984, and since 1998 sponsored by the Agency for Healthcare Research and Quality (AHRQ), is the leading independent panel of private-sector experts in prevention and primary care. The USPSTF conducts rigorous, impartial assessments of the scientific evidence for the effectiveness of a broad range of clinical preventive services, including screening, counseling, and preventive medications. Its recommendations are considered the ‘gold standard’ for clinical preventive services.
The mission of the USPSTF is to evaluate the benefits of individual services based on age, gender, and risk factors for disease; make recommendations about which preventive services should be incorporated routinely into primary medical care and for which populations; and identify a research agenda for clinical preventive care.”
The General Health Questionnaire is available by request from the author.
Three RAs worked on this project. All were female. They had bachelor’s degrees in psychology or nursing, and prior experience doing psychological research and/or clinical care.
Declaration of interest: The authors report no conflict of interest. They alone are responsible for the content and writing of this paper.
References
- Adams WL, Barry KL, Fleming MF. Screening for problem drinking in older primary care patients. JAMA. 1996;276:1964–1967. [PubMed] [Google Scholar]
- Andreasson S, Hjalmarsson K, Rehnman C. Implementation and dissemination of methods for prevention of alcohol problems in primary health care: a feasibility study. Alcohol & Alcoholism. 2000;35:525–530. doi: 10.1093/alcalc/35.5.525. [DOI] [PubMed] [Google Scholar]
- Austin SM, Balas EA, Mitchell JA, Ewigman BG. Effect of physician reminders on preventive care: meta-analysis of randomized clinical trials. Proceedings – Annual Symposium on Computer Applications in Medical Care; 1994. pp. 121–124. [PMC free article] [PubMed] [Google Scholar]
- Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Archives of Internal Medicine. 2000;160:301–308. doi: 10.1001/archinte.160.3.301. [DOI] [PubMed] [Google Scholar]
- Beich A, Gannik D, Malterud K. Screening and brief intervention for excessive alcohol use: qualitative interview study of the experiences of general practitioners. BMJ. 2002;325:870. doi: 10.1136/bmj.325.7369.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: systematic review and meta-analysis. BMJ. 2003;327:536–542. doi: 10.1136/bmj.327.7414.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction. 1993;88:315–336. doi: 10.1111/j.1360-0443.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Buchsbaum DG, Buchanan RG, Lawton MJ, Elswick RK, Jr, Schnoll SH. A program of screening and prompting improves short-term physician counseling of dependent and nondependent harmful drinkers. Archives of Internal Medicine. 1993;153:1573–1577. [PubMed] [Google Scholar]
- Butler SF, Chiauzzi E, Bromberg JI, Budman SH, Buono DP. Computer-assisted screening and intervention for alcohol problems in primary care. Journal of Technology In Human Services. 2003;21:1–19. [Google Scholar]
- Carpiano RM, Flocke SA, Frank SH, Stange KC. Tools, teamwork, and tenacity: an examination of family practice office system influences on preventive service delivery. Preventive Medicine. 2003;36:131–140. doi: 10.1016/s0091-7435(02)00024-5. [DOI] [PubMed] [Google Scholar]
- CASA – National Center on Addiction and Substance Abuse at Columbia University. Missed opportunity: national survey of primary care physicians and patients on substance abuse. New York, NY: 2000. [Google Scholar]
- Chick J, Ritson B, Connaughton J, Stewart A, Chick J. Advice versus extended treatment for alcoholism: a controlled study. British Journal of Addiction. 1988;83:159–170. doi: 10.1111/j.1360-0443.1988.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Curry SJ, Ludman E, Grothaus L, Donovan D, Kim E, Fishman P. At-risk drinking among patients making routine primary care visits. Preventive Medicine. 2000;31:595–602. doi: 10.1006/pmed.2000.0754. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Morse DS, Frankel RM, Frarey L, Anderson K, Beckman HB. Awkward moments in patient-physician communication about HIV risk. Annals of Internal Medicine. 1998;128:435–442. doi: 10.7326/0003-4819-128-6-199803150-00003. [DOI] [PubMed] [Google Scholar]
- Fleming MF. Strategies to increase alcohol screening in health care settings. Alcohol Health & Research World. 1997;21:340–347. [PMC free article] [PubMed] [Google Scholar]
- Fleming MF, Cotter F, Talboy E. Training physicians for alcohol screening and brief intervention. Washington, DC: NIAAA Publication Distribution Center; 1997. [Google Scholar]
- Fleming MF, Manwell LB, Barry KL, Johnson K. At-risk drinking in an HMO primary care sample: prevalence and health policy implications. American Journal of Public Health. 1998;88:90–93. doi: 10.2105/ajph.88.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Rose GR, Badger GJ, Searles JS, Thomas CS, Lindberg S, et al. Using interactive voice response to enhance brief alcohol intervention in primary care settings. Journal of Studies on Alcohol and Drugs. 2008;269:251–258. doi: 10.15288/jsad.2008.69.251. [DOI] [PubMed] [Google Scholar]
- Hung DY, Rundall TG, Crabtree BF, Tallia AF, Cohen DJ, Halpin HA. Influence of primary care practice and provider attributes on preventive service delivery. American Journal of Preventive Medicine. 2006;30:413–422. doi: 10.1016/j.amepre.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Broadening the base of treatment for alcohol problems. Washington, DC: National Academy Press; 1990. [PubMed] [Google Scholar]
- Kaner EF, Heather N, Brodie J, Lock CA, McAvoy BR. Patient and practitioner characteristics predict brief alcohol intervention in primary care. British Journal of General Practice. 2001;51:822–827. [PMC free article] [PubMed] [Google Scholar]
- Lock CA, Kaner EFS. Implementation of brief alcohol interventions by nurses in primary care: do non-clinical factors influence practice? Family Practice. 2004;21:270–275. doi: 10.1093/fampra/cmh310. [DOI] [PubMed] [Google Scholar]
- Manwell LB, Fleming MF, Mundt MP, Stauffacher EA, Barry KL. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcoholism: Clinical and Experimental Research. 2000;24:1517–1524. [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. American Journal of Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- McCormick KA, Cochran NE, Back AL, Merrill JO, Williams EC, Bradley KA. How primary care providers talk to patients about alcohol: a qualitative study. Journal of General Internal Medicine. 2006;21:966–972. doi: 10.1111/j.1525-1497.2006.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LS, Mazel RM. Counseling for depression by primary care providers. International Journal of Psychiatry in Medicine. 2000;30:343–365. doi: 10.2190/T0YP-U28Q-52Q4-G28M. [DOI] [PubMed] [Google Scholar]
- Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “Narc cabinet. Journal of General Internal Medicine. 2002;17:327–333. doi: 10.1046/j.1525-1497.2002.10625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- Mundt MP. Analyzing the costs and benefits of brief intervention. Alcohol Research & Health. 2006;29:34–36. [PMC free article] [PubMed] [Google Scholar]
- Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Annals of Internal Medicine. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- Popp SM, Schwartz KL, Schoener EP. Brief intervention in hazardous drinking: an important adjunct to medical school ATOD curriculum. Substance Abuse. 1998;19:1–6. doi: 10.1080/08897079809511368. [DOI] [PubMed] [Google Scholar]
- Robert Wood Johnson Foundation. Improving Chronic Illness Care. n.d Retrieved December 28, 2009 from http://www.improvingchroniccare.org.
- Saitz R, Horton NJ, Sullivan LM, Moskowitz MA, Samet JH. Addressing alcohol problems in primary care: a cluster randomized, controlled trial of a systems intervention: the Screening and Intervention in Primary Care (SIP) Study. Annals of Internal Medicine. 2003;138:372–382. doi: 10.7326/0003-4819-138-5-200303040-00006. [DOI] [PubMed] [Google Scholar]
- Spandorfer JM, Israel Y, Turner BJ. Primary care physicians’ views on screening and management of alcohol abuse: inconsistencies with national guidelines. The Journal of Family Practice. 1999;48:899–902. [PubMed] [Google Scholar]
- US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendations statement. Annals of Internal Medicine. 2004;140:555–557. doi: 10.7326/0003-4819-140-7-200404060-00016. [DOI] [PubMed] [Google Scholar]
- Walsh RA, Roche AM, Sanson-Fisher RW, Saunders JB. Interactional skills of students from traditional and non-traditional medical schools before and after alcohol education. Medical Education. 2001;35:211–216. doi: 10.1046/j.1365-2923.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Weisner C, Matzger H. Missed opportunities in addressing drinking behavior in medical and mental health services. Alcoholism: Clinical & Experimental Research. 2003;27:1132–1141. doi: 10.1097/01.ALC.0000075546.38349.69. [DOI] [PubMed] [Google Scholar]
- Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? American Journal of Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer DC, Doyle JP, Barnes CS, Branch WT, Jr, Cook CB, El-Kebbi IM, et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting: improving Primary Care of African Americans with Diabetes (IPCAAD) 8. Archives of Internal Medicine. 2006;166:507–153. doi: 10.1001/archinte.166.5.507. [DOI] [PubMed] [Google Scholar]