Abstract
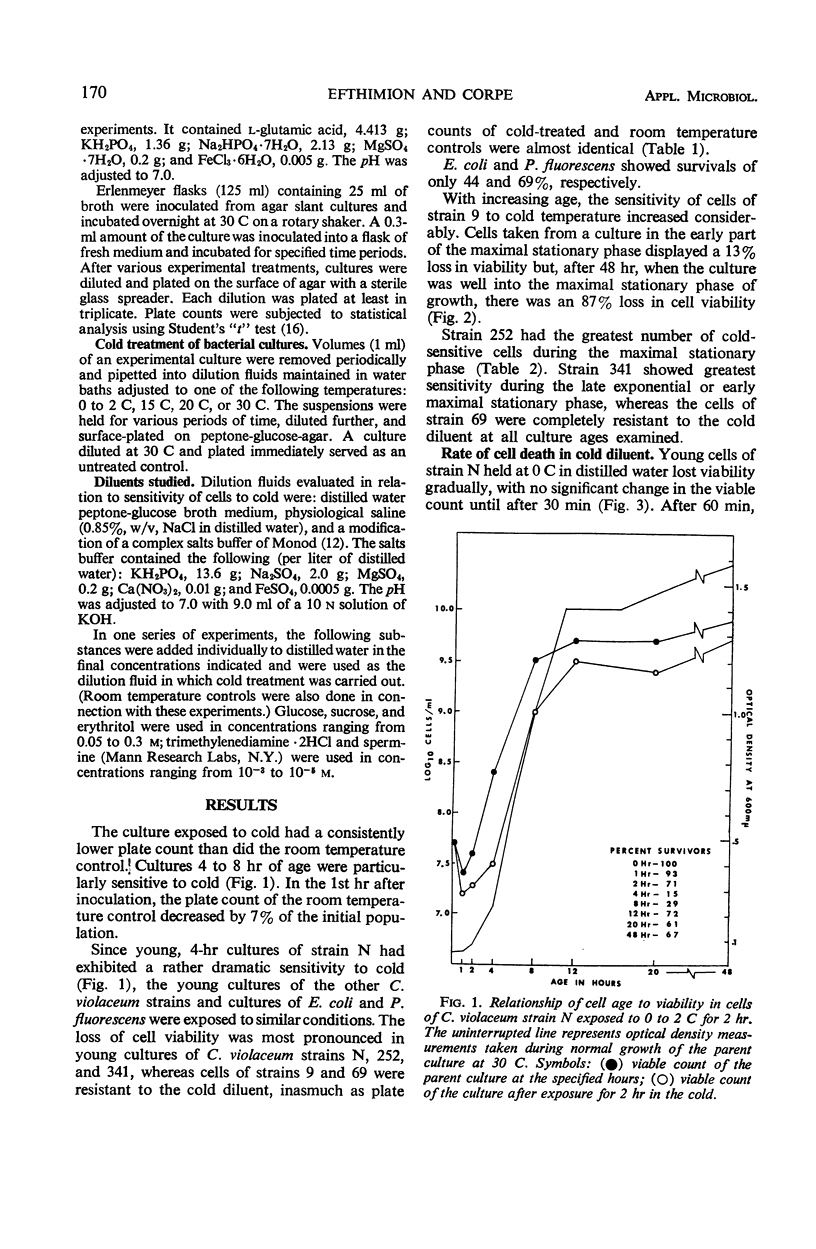
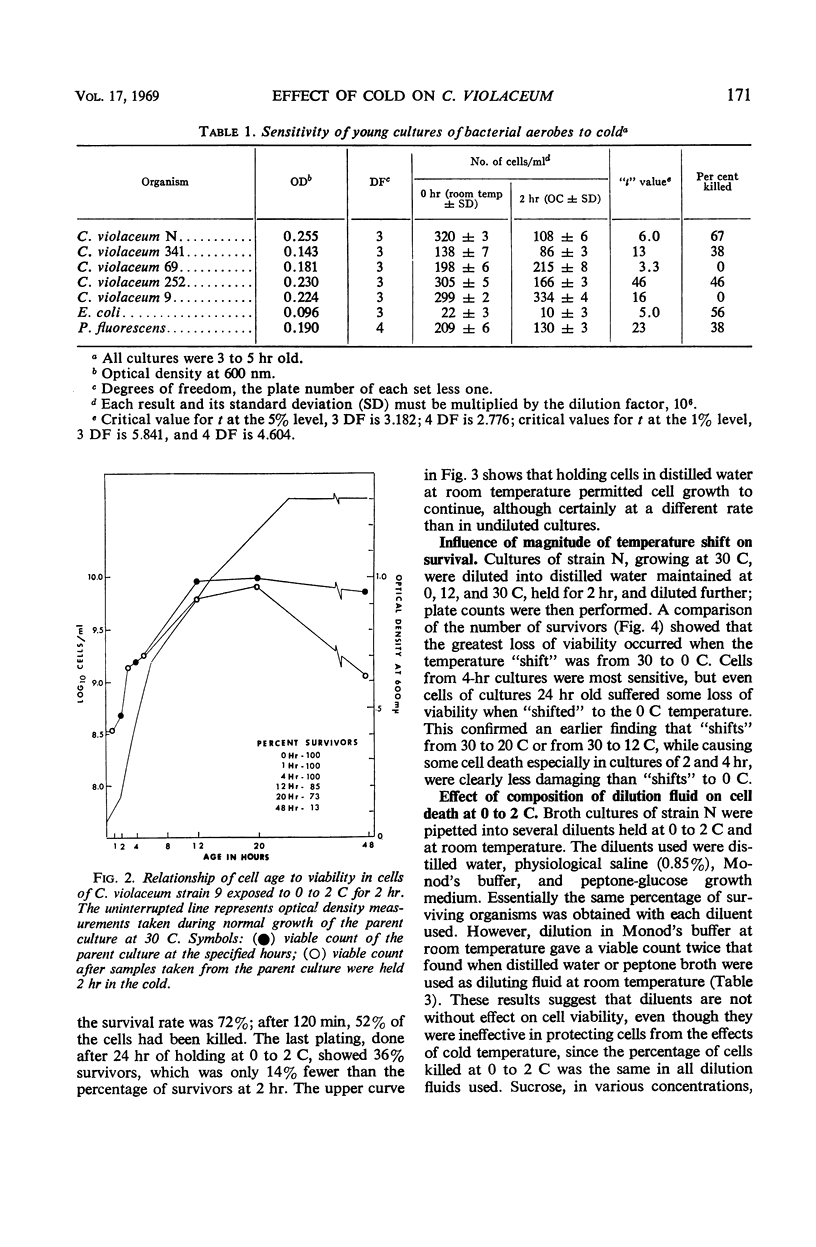
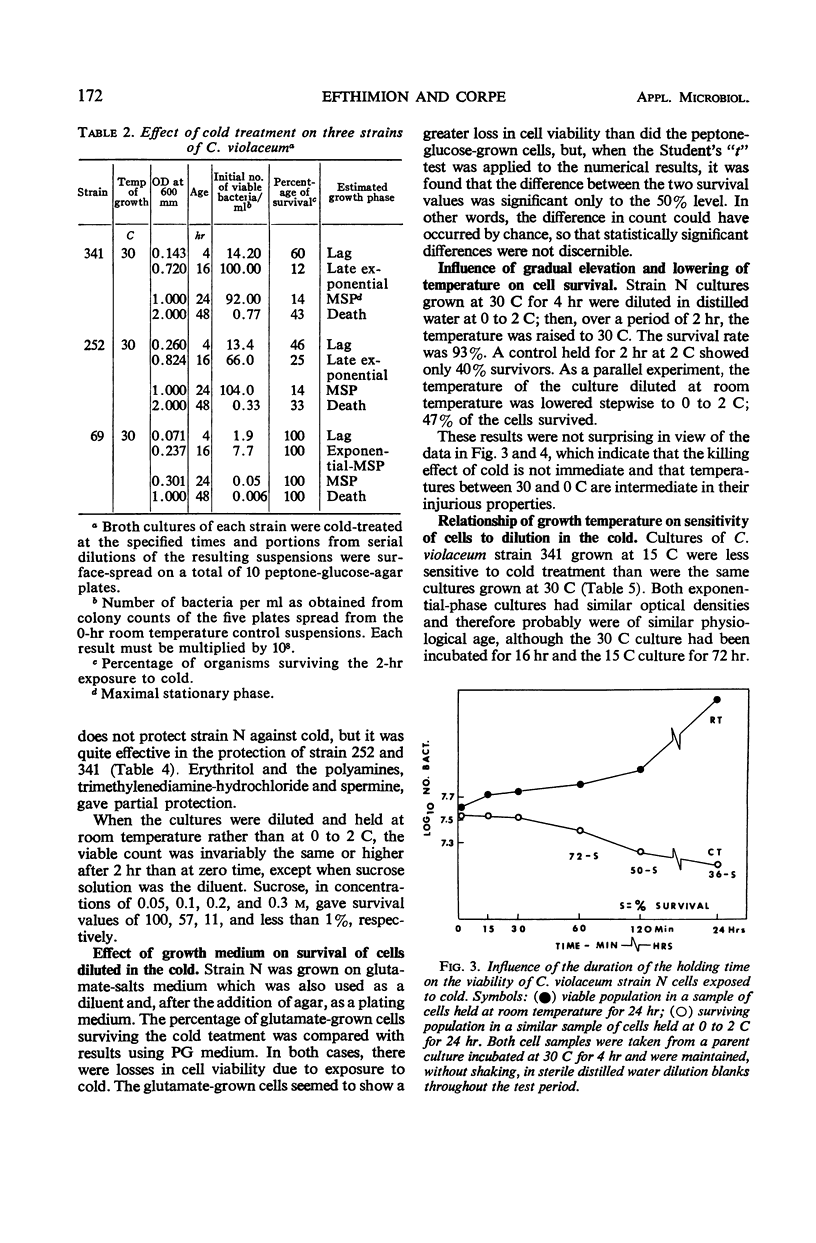
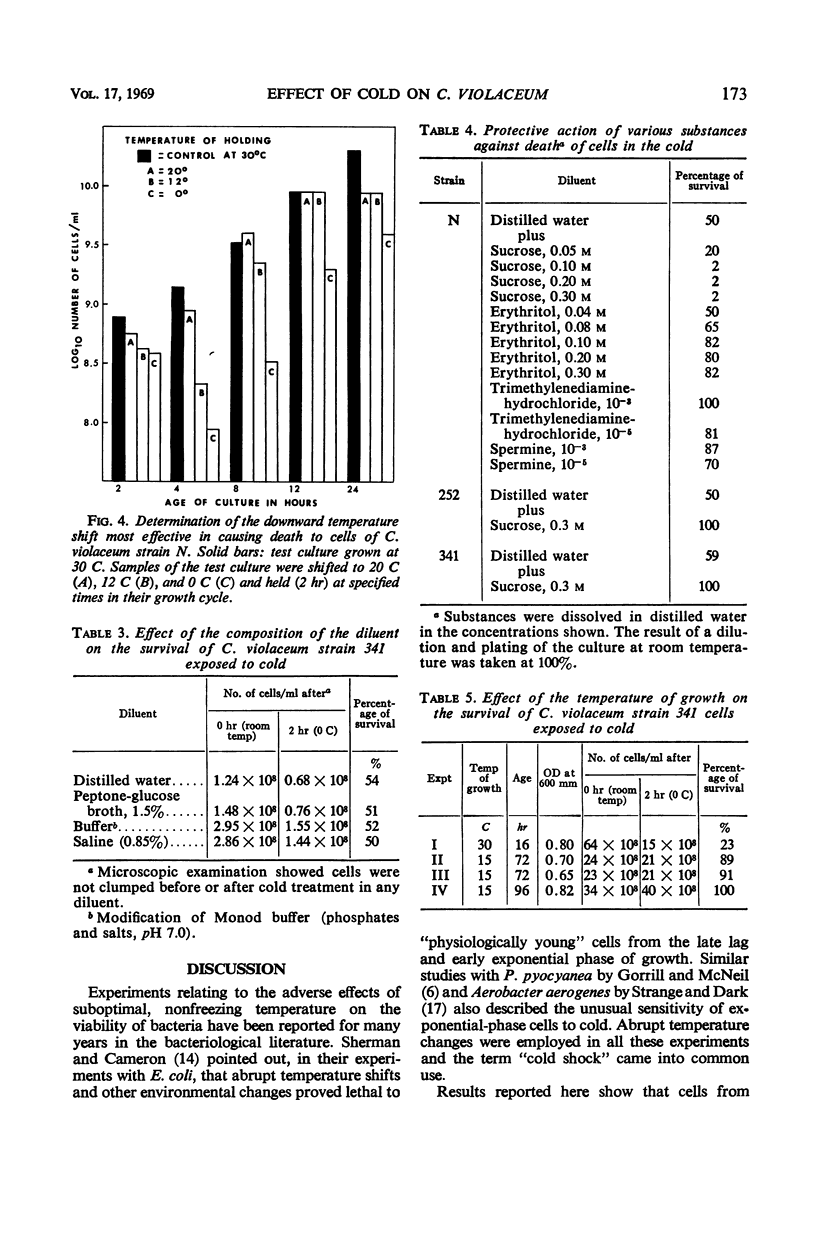
The effect of low, nonfreezing temperatures on the viability of five strains of Chromobacterium violaceum was studied. The viability of cultures grown at 30 C was determined after exposure to various diluents held at 0 to 2 C. A culture diluted at its growth temperature served as the control. Cells of strain N were most sensitive in the early part of the exponential phase of growth. Cells of strains 252 and 341 were most sensitive in the late exponential, early stationary phase of growth. Cells of strain 9 showed greatest loss of viability during the maximal stationary phase. Strain 69 was completely resistant throughout its growth cycle to cold injury. Cell viability was much greater in buffered salts solution than in distilled water, broth, or physiological saline, whether cultures were diluted at room temperature or in the cold. The proportion of cells surviving after exposure to cold, however, was the same regardless of the composition of the diluent. Loss of viability was progressive at 0 to 2 C and reached a maximum after 2 hr. There was no loss of viability of cells exposed to 20 C, but there was some loss at 12 C. Strain 341 cultivated at 15 C was much less sensitive to 0 to 2 C than when it was cultivated at 30 C. The composition of the growth medium seemed to have no effect on the survival of cells exposed to cold. The polyamines, spermine and trimethylenediamine, as well as erythritol and sucrose, exerted some protective action against the effects of cold but not uniformly for all strains studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. G., STILL J. L. FATTY ACID METABOLISM IN SERRATIA MARCESCENS. III. THE CONSTITUENT FATTY ACIDS OF THE CELL. J Lipid Res. 1963 Jan;4:81–86. [PubMed] [Google Scholar]
- BYRNER P., CHAPMAN D. LIQUID CRYSTALLINE NATURE OF PHOSPHOLIPIDS. Nature. 1964 Jun 6;202:987–988. doi: 10.1038/202987a0. [DOI] [PubMed] [Google Scholar]
- Corpe W. A., Salton M. R. Properties of surface materials released from cells of Chromobacterium violaceum. Biochim Biophys Acta. 1966 Jul 27;124(1):125–135. doi: 10.1016/0304-4165(66)90320-5. [DOI] [PubMed] [Google Scholar]
- Farrell J., Rose A. H. Cold shock in a mesophilic and a psychrophilic pseudomonad. J Gen Microbiol. 1968 Mar;50(3):429–439. doi: 10.1099/00221287-50-3-429. [DOI] [PubMed] [Google Scholar]
- GORRILL R. H., McNEIL E. M. The effect of cold diluent on the viable count of Pseudomonas pyocyanea. J Gen Microbiol. 1960 Apr;22:437–442. doi: 10.1099/00221287-22-2-437. [DOI] [PubMed] [Google Scholar]
- Hegarty C. P., Weeks O. B. Sensitivity of Escherichia coli to Cold-Shock during the Logarithmic Growth Phase. J Bacteriol. 1940 May;39(5):475–484. doi: 10.1128/jb.39.5.475-484.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- Sherman J. M., Albus W. R. Physiological Youth in Bacteria. J Bacteriol. 1923 Mar;8(2):127–139. doi: 10.1128/jb.8.2.127-139.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M., Cameron G. M. Lethal Environmental Factors Within the Natural Range of Growth. J Bacteriol. 1934 Apr;27(4):341–348. doi: 10.1128/jb.27.4.341-348.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]



