Abstract
Proper brain wiring during development is pivotal for adult brain function. Neurons display a high degree of polarization both morphologically and functionally, and this polarization requires the segregation of mRNA, proteins, and lipids into the axonal or somatodendritic domains. Recent discoveries have provided insight into many aspects of the cell biology of axonal development including axon specification during neuronal polarization, axon growth, and terminal axon branching during synaptogenesis.
Introduction
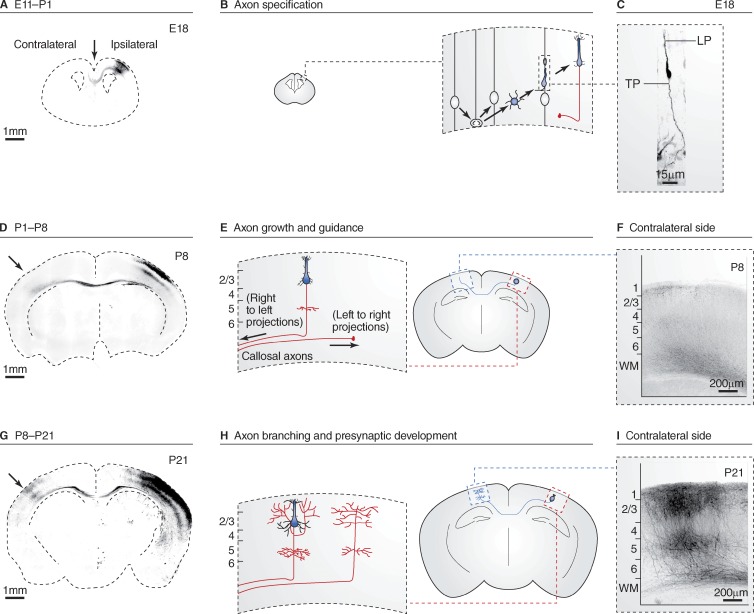
Axon development can be divided into three main steps: (1) axon specification during neuronal polarization, (2) axon growth and guidance, and (3) axon branching and presynaptic differentiation (Fig. 1; Barnes and Polleux, 2009; Donahoo and Richards, 2009). These three steps are exemplified during neocortical development in the mouse: upon neurogenesis, newly born neurons engage long-range migration and polarize (Fig. 1, A and B) by adopting a bipolar morphology with a leading and a trailing process (Fig. 1 C). During migration (approximately from embryonic day [E]11 to E18 in the mouse cortex), the trailing process becomes the axon and extends rapidly while being guided to its final destination (lasts until around postnatal day [P]7 in mouse corticofugal axons with distant targets like the spinal cord; Fig. 1, D–F). Finally, upon reaching its target area, extensive axonal branching occurs during the formation of presynaptic contacts with specific postsynaptic partners (during the second and third postnatal week in the mouse cortex; Fig. 1, G–I). Disruption of any of these steps is thought to lead to various neurodevelopmental disorders ranging from mental retardation and infantile epilepsy to autism spectrum disorders (Zoghbi and Bear, 2012). This review will provide an overview of some of the cellular and molecular mechanisms underlying axon specification, growth, and branching.
Figure 1.
Axon specification, growth, and branching during mouse cortical development. Three stages of the development of callosal axons of cortical pyramidal neurons from the superficial layers 2/3 of the somatosensory cortex in the mouse visualized using long-term in utero cortical electroporation. For this class of model axons, development can be divided in three main stages: (1) neurogenesis and axon specification, occurring mostly at embryonic ages (A–C); (2) axon growth/guidance during the first postnatal week (D–F); and (3) axon branching and synapse formation until approximately the end of the third postnatal week (G–I). A, D, and G show coronal sections of mouse cortex at the indicated ages after in utero cortical electroporation of a GFP-coding plasmid at E15.5 in superficial neuron precursors in one brain hemisphere only (GFP signal in inverted color, dotted line indicates the limits of the brain). B, E, and H are a schematic representation of the main morphological changes observed in callosally projecting axons (red) at the corresponding ages. C shows the typical bipolar morphology of a migrating neuron emitting a trailing process (TP) and a leading process (LP) that will ultimately become the axon and dendrite, respectively. F and I show typical axon projections of layer 2/3 neurons located in the primary somatosensory area at P8 and P21, respectively. Neurons and axons in C, F, and I are visualized by GFP expression (inverted color). Image in C is modified from Barnes et al. (2007) with permission from Elsevier. Images in D, F, G, and I are reprinted from Courchet et al. (2013) with permission from Elsevier.
Neuronal polarization and axon specification
Neuronal polarization is the process of breaking symmetry in the newly born cell to create the asymmetry inherent to the formation of the axonal and somatodendritic compartments (Dotti and Banker, 1987). The mechanisms underlying this process have been studied extensively in vitro and more recently in vivo, but the exact sequence of events has remained elusive (Neukirchen and Bradke, 2011) partly because it is studied in various neuronal cell types that might not use the same extrinsic/intrinsic mechanisms to polarize. It is highly likely that at least three factors underlie neuronal polarization: extracellular cues, intracellular signaling cascades, and subcellular organelle localization. The partition-defective proteins (PARs) are a highly conserved family of proteins including two dyads (Par3/Par6 adaptor proteins and the Par4/Par1 serine/threonine kinases) that are required for polarization and axon formation (Shi et al., 2003, 2004; Barnes et al., 2007; Shelly et al., 2007; Chen et al., 2013), while many other intracellular signaling molecules also support axon formation (Oliva et al., 2006; Rašin et al., 2007; Barnes and Polleux, 2009; Shelly et al., 2010; Cheng et al., 2011; Hand and Polleux, 2011; Cheng and Poo, 2012; Gärtner et al., 2012). These intracellular signaling pathways are influenced by localized extracellular cues that instruct which neurite becomes the axon by either directly promoting axon extension or repressing axon growth in favor of dendritic growth (Adler et al., 2006; Yi et al., 2010; Randlett et al., 2011b; Shelly et al., 2011).
The role of organelle subcellular localization during neuronal polarization is a more controversial issue. Initially, the orientation of organelles, including the Golgi complex, centrosomes, mitochondria, and endosomes, was shown to correlate with the neurite that becomes the axon in vitro (Bradke and Dotti, 1997; de Anda et al., 2005, 2010) and in vivo (de Anda et al., 2010). However, more recent studies suggest that the positioning of the centrosome is not necessary for neuronal polarization (Distel et al., 2010; Nguyen et al., 2011). Centrosome localization is likely constrained by microtubule organization within the cell, and therefore the centrosome position within the cell changes dynamically during different stages of polarization (Sakakibara et al., 2013). The question of how the interplay between extracellular cues, intracellular signaling, and organelle localization lead to polarization has pushed the field to perform more extensive in vivo imaging studies as in vitro systems/models have a difficult time recapitulating the complex environment and rely on neurons that were previously polarized in vivo.
Like other epithelial cells, neural progenitors present a high degree of polarization along the apico-basal axis (Götz and Huttner, 2005). One of the major questions still needing to be addressed is how, or if, newly born mammalian neurons inherit some level of asymmetry from their parent progenitors (Barnes and Polleux, 2009). Recent studies have attempted to answer this question in vivo but have found that the answer might vary in each neuronal subtype. Retinal ganglion cells (RGCs), retinal bipolar neurons, and tegmental hindbrain nuclei neurons seem to inherit the apical/basolateral polarity from their progenitors (Morgan et al., 2006; Zolessi et al., 2006; Distel et al., 2010; Randlett et al., 2011a). In cortical neurons, hippocampal neurons, and cerebellar granule neurons, this relationship is unclear, in part because newly born cortical neurons first exhibit a multipolar morphology with dynamic neurites emerging from the cell body before adopting a bipolar morphology, suggesting they may not retain a predisposed parental polarity (Hand et al., 2005; Barnes et al., 2007). Other factors also suggest that different neuronal subtypes use different mechanisms during polarization. One such factor is the position where neurons specify their axon relative to the original apical/basolateral axis of their progenitors. As an example, cortical neurons in the mouse brain protrude an axon from the membrane facing the original apical surface toward the ventricular zone (Hand et al., 2005; Barnes et al., 2007; Shelly et al., 2007), whereas zebrafish RGCs form their axon from the membrane on the basolateral side (Zolessi et al., 2006; Randlett et al., 2011b). Another significant difference between cortical neurons and RGCs is related to the timing of axogenesis and dendrogenesis. RGCs tend to form their axons and dendrites at the same time during migration (Zolessi et al., 2006; Randlett et al., 2011b). However, cortical neurons form a long axon during migration before significant dendrite arborization takes place. These differences in the regulation of polarization and sequence of axon versus dendrite outgrowth may be linked to the localization of extracellular cues relative to the immature neuron during polarization (Yi et al., 2010).
Neuronal polarization, cytoskeletal dynamics, and polarized transport
What exactly makes the axonal compartment distinct from the somatodendritic domain? This can most easily be illustrated by focusing on the cytoskeleton that forms the framework of the developing axon. The cytoskeleton is composed of microtubules, actin filaments, and intermediate filaments (also called neurofilaments) along with their associated binding partners. Microtubules are composed of α- and β-tubulin subunits that polymerize to form a long filament intrinsically polarized by the addition of tubulin subunits to only one side of the growing filament called the plus end, while on the opposite side depolymerization occurs. It was discovered more than thirty years ago that the axon of a neuron contains a very uniform distribution of microtubules with the plus end facing away from the cell body (Heidemann et al., 1981). Through the years this observation was confirmed in many neuron cell types, and it was determined that dendrites do not have this uniform plus-end out network of microtubules (Fig. 2; Baas et al., 1988). Dendrites appear to have a complex array of microtubule orientations that may vary between species and/or neuronal subtypes. Current research shows that proximal dendrites are composed of mainly minus-end out microtubules, whereas more distal dendrites transition from an equal distribution of minus-end out and plus-end out microtubules to mainly plus-end out microtubules (Stone et al., 2008; Yin et al., 2011; Ori-McKenney et al., 2012). The orientation of microtubules matters greatly because it determines the relative contribution of microtubule-dependent motor proteins (kinesins and dyneins), which are the main motor proteins carrying various cargoes within cells and in particular are responsible for long-range transport in very large cells such as neurons. Dynein (a minus end–directed microtubule motor) is known to be responsible for both the transport of microtubules away from the cell body and for the uniform polarity of microtubules in the axon (Ahmad et al., 1998; Zheng et al., 2008). Recently, it was discovered that kinesin-1 (a plus end–directed microtubule motor) is required for the minus-end out orientation of microtubules in the dendrites of Caenorhabditis elegans DA9 neurons through selective transport of plus-end out microtubule fragments out of the dendrite (Yan et al., 2013). Another hallmark that differentiates the axonal and somatodendritic compartments is the microtubule-associated proteins (MAPs) that decorate microtubules to regulate their bundling and stability (Hirokawa et al., 2010). Microtubules in the axon are mainly decorated by Tau and MAP1B, whereas microtubules in the dendrites are labeled by proteins of the MAP2a-c family. The role of Tau in axon elongation remains controversial because early reports (Harada et al., 1994; Tint et al., 1998; Dawson et al., 2001) of Tau knockout alone suggested that axons were unaffected, but this apparent lack of phenotype might originate from the functional redundancy between MAPs as Tau/MAP1b double knockout mice show clear axon growth defects (Takei et al., 2000).
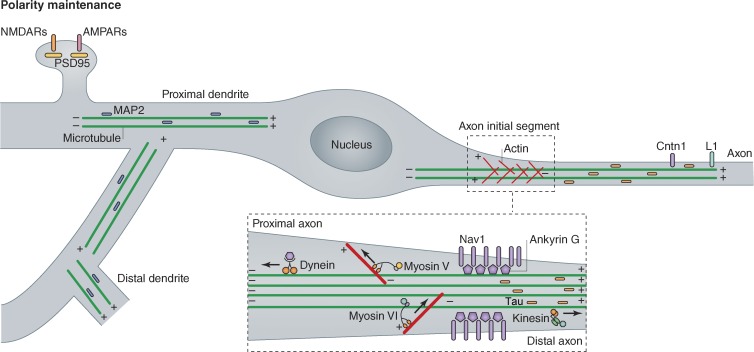
Figure 2.
Polarity maintenance and trafficking of somatodendritic and axonal proteins. Neurons are polarized into two main compartments: the somatodendritic domain and the axon. These domains are characterized by the underlying cytoskeleton and their unique protein signatures. The axonal cytoskeleton is defined by its uniform microtubule orientation where each microtubule is oriented with its plus end away from the cell body, while the dendrites contain a mixture of microtubules oriented in both directions. The proximal axon is characterized by a structure known as the axon initial segment (AIS, see inset). This highly ordered structure creates a diffusion barrier between the axonal compartment and the rest of the cell. F-actin is responsible for the cytoplasmic barrier, while sodium channels anchored by Ankyrin G form the basis of the plasma membrane barrier. Tau is retained in the axon by a microtubule-based filter at the AIS. Molecular motors (including kinesin, dynein, and myosin) then use the underlying cytoskeleton to restrict cargo transport to either the axon (such as Cntn1 and L1) or the dendrites (such as PSD95, AMPARs, and NMDARs).
The dynamics of actin polymerization into actin filaments (F-actin) also play an important role in defining the axonal compartment, and contain an intrinsic polarity based on the polymerization of the free G-actin subunits (Hirokawa et al., 2010). Beyond the well-documented early role of F-actin dynamics in neurite outgrowth, multiple groups have shown that the disruption of actin polymerization allows dendritically localized proteins to incorrectly enter the axonal compartment (Winckler et al., 1999; Lewis et al., 2009; Song et al., 2009). The existence of a “diffusion barrier” in the proximal part of newly formed axons (Song et al., 2009) was long suspected. One of the current hypotheses is that a dense F-actin meshwork creates a cytoplasmic diffusion barrier shortly after polarization, which in part separates the axonal compartment from the neuronal cell body (Fig. 2, inset). Based on functional analysis and electron microscopy analysis, this “F-actin–based filter” is oriented so that the plus ends point toward the cell body while the minus ends point into the axon (Lewis et al., 2009, 2011; Watanabe et al., 2012). Two recent papers show via high resolution imaging techniques that indeed the axon has a unique F-actin network that is not found in dendrites (Watanabe et al., 2012; Xu et al., 2013). The development of this F-actin meshwork appears to directly precede the formation of the axon initial segment (AIS; Song et al., 2009; Galiano et al., 2012). An intra-axonal diffusion barrier, composed of Spectrins and Ankyrin B, defines the eventual position of the AIS. This boundary excludes Ankyrin G, which instead clusters in the most proximal part of the axon close to the cell body, where the AIS will form (Galiano et al., 2012). Ankyrin G is required for AIS formation and maintenance, and its loss causes the axon to start forming protrusions resembling dendritic spines (Hedstrom et al., 2008). Microtubules also play an important role at the AIS, as recent evidence suggests that Tau is retained in the axon through a microtubule-based diffusion barrier independently of the F-actin based filter (X. Li et al., 2011). The AIS is important in the formation of a plasma membrane barrier between the axonal and somatodendritic domains and its disruption affects both neuronal polarity and function because it is critical for clustering of voltage-dependent sodium channels and action potential initiation (Rasband, 2010).
One of the critical cellular mechanisms underlying neuronal polarization is the polarized transport of various cargoes in axons and dendrites. Transport of proteins and various organelles is performed by the microtubule-dependent motor proteins kinesin and dynein (Hirokawa et al., 2010). Studies from many laboratories have demonstrated that kinesin motors can carry cargo to both the axonal and dendritic compartments (Burack et al., 2000; Nakata and Hirokawa, 2003). The mechanism for how selection occurs is not completely understood, but it probably incorporates both the affinity of the kinesin head for microtubules and the cargo bound to the motor protein (Nakata and Hirokawa, 2003; Song et al., 2009; Jenkins et al., 2012). In the axon, dynein works to bring cargo and retrograde signals back to the cell body, whereas in the dendrites it is responsible for much of the transport from the soma into the dendrites (Zheng et al., 2008; Kapitein et al., 2010; Harrington and Ginty, 2013). Additionally, the F-actin–dependent myosin motors can affect the polarized transport of cargos by using the F-actin–based cytoplasmic filter at the AIS to deny or facilitate entry of vesicles into the axon. Loss of the actin filter or myosin Va activity (a plus end–directed motor) allows dendritic cargos into the axon, whereas myosin VI (a minus end–directed motor) both removes axonal proteins from the dendritic surface and funnels vesicles containing axonal proteins through the actin filter at the AIS (Lewis et al., 2009, 2011; Al-Bassam et al., 2012). A current working hypothesis is that vesicles composed of multiple cargoes contain binding sites for each of these motors, and that through unknown mechanisms the activity of the motors can be differentially regulated to control the directionality of transport. An interesting example of how the interplay between different motors and cargo adaptors could lead to polarized transport was recently described for mitochondria (van Spronsen et al., 2013).
Axon growth
Microtubule dynamics regulate axon growth.
After axon specification, axon growth constitutes the second step of axonal development and is tightly linked to axon guidance toward the proper postsynaptic targets. Axon elongation by the growth cone is the product of two opposite forces (Fig. 3): slow axonal transport and the polymerization of microtubules providing a pushing force from the axon shaft, and the retrograde flow of actin providing a pulling force at the front of the growth cone (Letourneau et al., 1987; Suter and Miller, 2011). Although coordinated actin and microtubule dynamics are required for the proper function of the growth cone, it was reported that agents disrupting the actin cytoskeleton have limited consequences on axon elongation and are rather involved in axon guidance in vitro (Marsh and Letourneau, 1984; Ruthel and Hollenbeck, 2000) and in vivo (Bentley and Toroian-Raymond, 1986). Furthermore, local disruption of actin organization in the growth cone of minor neurites allows them to turn into axons (Bradke and Dotti, 1999; Kunda et al., 2001), indicating that the dense actin network present at the periphery of an immature neuronal cell body and in immature neurites may prevent microtubule protrusion and elongation necessary for axon specification.
Figure 3.
Cytoskeletal changes during axon elongation and branching. Representation of axon elongation and collateral branch formation in a cultured neuron. Axon growth is a discontinuous process, and collateral branches often originate from sites where the growth cone paused (gray dotted line), after it has resumed its progression. Other modalities of branch formation can occur through the formation of filopodia and lamellipodia. Red box shows a magnification of the main growth cone. Microtubules from the axon shaft spread into the central (C) zone. Some microtubules pass through the transition (T) zone, containing F-actin arcs, to explore filopodia from the peripheral (P) zone. Upon the proper stimulation by extracellular guidance cues or growth-promoting cues, microtubules are stabilized and invade the P-zone where they provide a pushing force, which, combined with the traction force from the actin treadmilling, provides the force required for growth cone extension. Green box shows the cytoskeletal changes occurring during collateral branch formation in the axon. Filopodia and lamellipodia are primarily F-actin–based protrusions that get invaded by microtubules, then elongate upon microtubule bundling. At later developmental stages, axon branches are stabilized or retracted (blue box) by mechanisms relying on the access to extracellular neurotrophins and/or neuronal activity and synapse formation.
Contrary to actin, microtubule polymerization is required to sustain axon elongation and branching (Letourneau et al., 1987; Baas and Ahmad, 1993). Axonal proteins and cytoskeletal elements are transported along the axon through slow axonal transport by molecular motors (Yabe et al., 1999; Xia et al., 2003). It is still controversial whether tubulin and other cytoskeletal elements are transported in the axon as monomers and/or as polymers (Roy et al., 2000; Terada et al., 2000; Wang et al., 2000; Brown, 2003; Terada, 2003). Nonetheless, disruption of the slow transport of tubulin impairs the pushing force resulting from microtubule polymerization and impairs axon elongation (Suter and Miller, 2011). Therefore, it is not surprising that axon growth is affected in vitro and in vivo by disruption of plus-end microtubule-binding proteins such as APC (Shi et al., 2004; Zhou et al., 2004; Yokota et al., 2009; Chen et al., 2011) or EB1 and EB3 (Zhou et al., 2004; Jiménez-Mateos et al., 2005; Geraldo et al., 2008), microtubule-associated proteins such as MAP1B (Black et al., 1994; Takei et al., 2000; Dajas-Bailador et al., 2012; Tortosa et al., 2013), or proteins regulating microtubule severing and reorganization such as KIF2A (Homma et al., 2003), katanin, and spastin (Karabay et al., 2004; Yu et al., 2005; Wood et al., 2006; Butler et al., 2010).
The contribution of microtubule dynamics to axon growth is not limited to growth cone dynamics but also involves axonal transport and polymerization along the axon shaft. Moreover, changing the balance between microtubule stabilization and depolymerization by local application of microtubule stabilizing agents is sufficient to instruct one neurite to grow and adopt an axon fate (Witte et al., 2008). Many kinase pathways converge on Tau and other axonal MAPs to regulate their function by phosphorylation (Morris et al., 2011). Among them, the MARK kinases regulate microtubule stability and axonal transport through phosphorylation of Tau (Drewes et al., 1997; Mandelkow et al., 2004). Interestingly, MARK-related kinases SAD-A/B control axon specification in part through phosphorylation of Tau (Barnes et al., 2007) and have very recently been linked to the growth and branching of the axons of sensory neurons (Lilley et al., 2013). Our work recently demonstrated that another family member related to MARKs and SAD kinases, called NUAK1, controls axon branching of mouse cortical neurons through the regulation of presynaptic mitochondria capture (Courchet et al., 2013). To what extent the regulation of Tau and other MAPs by the MARKs, SADs, and NUAK1 kinases contributes to axon elongation remains to be explored.
Where does axon elongation take place?
Growth cone progression and guidance constitute the main driver of axonal growth during development, but this process is unlikely to account for the totality of axon elongation. This is especially true after the axon has reached its final target but the axon shaft keeps growing in proportion to the rest of the body. One mechanism that may contribute to this “interstitial” form of axon elongation during brain/body size increase (see Fig. 1 for an example during postnatal cortex growth) is axon stretching, a process that can induce axon elongation in vitro (Smith et al., 2001; Pfister et al., 2004; Loverde et al., 2011) and in vivo (Abe et al., 2004). Aside from extreme stretching performed through the application of external forces, stretching could also contribute to the natural elongation of the axon in response to the tension resulting from growth cone progression (Suter and Miller, 2011).
Axon elongation requires the addition of new lipids, proteins, cytoskeleton elements, and organelles along the axon. Where does the synthesis and incorporation of new components take place? Polysaccharides and cholesterol synthesis mostly occur in the cell body; however, lipid synthesis and/or incorporation can occur along the axon as well (Posse De Chaves et al., 2000; Hayashi et al., 2004). The growth cone is also a site of endocytosis, membrane recycling, and exocytosis (Kamiguchi and Yoshihara, 2001; Winckler and Yap, 2011; Nakazawa et al., 2012). One of the best studied examples of endocytosis and its role in axon growth and neuronal survival is the retrograde trafficking of TrkA receptor by target-derived nerve growth factor (NGF) in the peripheral nervous system (Harrington and Ginty, 2013).
Axon branching and presynaptic differentiation
Where do axon branches form?
The last step of axon development is terminal branching, which allows a single axon to connect to a broad set of postsynaptic targets. Collateral branches are formed along the axon through two distinct mechanisms: the first modality of branching is through splitting or bifurcation of the growth cone, which is linked to axon guidance and to the capacity of one single neuron to reach two targets that are far apart with one single axon. Growth cone splitting is observed in vivo in various neuron types including cortical neurons (Sato et al., 1994; Bastmeyer and O’Leary, 1996; Dent et al., 1999; Tang and Kalil, 2005), sympathetic neurons (Letourneau et al., 1986), motorneurons (Matheson and Levine, 1999), sensory neurons (Ma and Tessier-Lavigne, 2007), or mushroom body neurons in Drosophila (Wang et al., 2002). The second modality, known as interstitial branching, occurs through the formation of collateral branches directly along the axon shaft. Contrary to growth cone splitting, interstitial branching serves the purpose of raising axon coverage locally in order to define their “presynaptic territory”, and may contribute to increased network connectivity (Portera-Cailliau et al., 2005). Although both mechanisms can occur simultaneously in the same neuron, the relative importance of splitting versus interstitial branching is highly divergent from one neuron type to the other (Bastmeyer and O’Leary, 1996; Matheson and Levine, 1999; Portera-Cailliau et al., 2005).
In culture, the axon grows in a non-continuous fashion with frequent growth cone pausing. Time-lapse imaging of sensorimotor neurons revealed that interstitial branching often occurs at the site where the growth cone paused, shortly after it has continued its course (Szebenyi et al., 1998). Accordingly, treatments with neurotrophins that slow the growth cone correlate with increased axon branching (Szebenyi et al., 1998). This suggests that growth cone pausing leaves a “mark” along the axon shaft that might predetermine future sites of branching (Kalil et al., 2000). However, local applications of neurotrophins shows that aside from growth cone pause sites the axon shaft remains competent to form collateral branches upon stimulation by extracellular factors (Gallo and Letourneau, 1998; Szebenyi et al., 2001), through the formation of filopodia or lamellipodia. Similar observations in vivo revealed that cortical axons are highly dynamic during development and form multiple filopodia that are the origin of collateral branches (Bastmeyer and O’Leary, 1996). Lamellipodia can be observed as motile, F-actin–dependent “waves” along the axon in vitro (Ruthel and Banker, 1998) and in vivo (Flynn et al., 2009). Moreover, disruption of microtubule organization impairs lamellipodia formation along the axon and is correlated with decreased axon branching (Dent and Kalil, 2001; Tint et al., 2009).
Cytoskeleton dynamics and axon branch formation.
Regardless of what type of protrusion gives rise to a branch, cytoskeletal reorganization in the nascent branch generally follows a similar sequence (Fig. 3): initially F-actin filament reorganization gives rise to a protrusion (filopodia, lamellipodia), followed by microtubule invasion of this otherwise transient structure to consolidate it, before the mature branch starts elongating through microtubule bundling (Gallo, 2011). Actin filaments accumulate along the axon and form “patches” that serve as nucleators for axon protrusions such as filopodia and lamellipodia (Korobova and Svitkina, 2008; Mingorance-Le Meur and O’Connor, 2009; Ketschek and Gallo, 2010). The mRNA for β-actin and regulators of actin polymerization such as Wave1 or Cortactin accumulate along the axons of sensory neurons and form hot-spots of local translation that are associated to NGF-dependent branching (Spillane et al., 2012; Donnelly et al., 2013). Subsequently, microtubules in the axon shaft undergo fragmentation at branch points as a prelude to branch invasion by microtubules (Yu et al., 1994, 2008; Gallo and Letourneau, 1998; Dent et al., 1999; Hu et al., 2012), a process that may disrupt transport locally to help trap molecules and organelles at branch points. Moreover, severed microtubules are transported into branches, a process required for branch stabilization (Gallo and Letourneau, 1999; Ahmad et al., 2006; Qiang et al., 2010; Hu et al., 2012). Interestingly, it is clear that, just like growth cone–mediated axon elongation, F-actin and microtubule reorganization events are interconnected to sustain axon branching (Dent and Kalil, 2001). As an example, microtubule-severing enzymes can also contribute to actin nucleation and filopodia formation (Hu et al., 2012).
Is axon branching linked to axon elongation?
Like in the growth cone, cytoskeleton reorganization constitutes the backbone of branch formation. It is therefore not surprising that many manipulations of the cytoskeleton affect both axon elongation and branch formation (Homma et al., 2003; Chen et al., 2011). Moreover, conditions that primarily disrupt axon elongation could secondarily disrupt branching by impairing the ability of the nascent branch to grow. However, axon elongation and axon branching can be considered as two separate phenomena and can be operationally separated because conditions disrupting one do not systematically affect the other. As an example, the microtubule-severing proteins katanin and spastin have differential consequences on axon elongation (primarily dependent upon katanin function) and branching (mostly spastin mediated; Qiang et al., 2010), taxol treatment (which stabilizes microtubules) affects axon elongation but not branching (Gallo and Letourneau, 1999), and disruption of TrkA endocytosis by knock-down of Unc51-like kinase (ULK1/2) proteins has opposite effects on axon elongation and branching (Zhou et al., 2007). In vivo, superficial layer cortical neurons initially go through a phase of elongation through the corpus callosum without branching (see Fig. 1), then stop elongating and form collateral branches in the contralateral cortex (Mizuno et al., 2007; Wang et al., 2007). It is conceivable that even before myelination, axons are actively prevented from branching at places and stages when they elongate (for example in the white matter of the neocortex) where they tend to be highly fasciculated. The identities of the molecules that inhibit interstitial branching along the axon shaft are currently unknown.
Regulation of axon branching by activity.
Immature neurons display spontaneous activity in the form of calcium waves (Gu et al., 1994; Gomez and Spitzer, 1999; Gomez et al., 2001) and spontaneous vesicular release long before they have completed axon development, which suggested a role for early neuronal activity in axon development and guidance (Catalano and Shatz, 1998). Cell-autonomous silencing of neurons in vivo by transfection of the hyperpolarizing inward-rectifying potassium channel Kir2.1 in olfactory neurons (Yu et al., 2004), in RGCs (Hua et al., 2005) or in cortical pyramidal neurons (Mizuno et al., 2007; Wang et al., 2007), or in vitro through infusion of tetrodotoxin (which blocks action potentials generation) in co-cultures of thalamo-cortical projecting neurons (Uesaka et al., 2007) results in a decrease in terminal axon branching, indicating that synaptic activity is required for axons to fully develop their branching pattern. Moreover, inhibition of synaptic release by expression of tetanus toxin light chain (TeTN-LC; Wang et al., 2007) also abolished terminal axon branching, suggesting that the formation of functional presynaptic release sites is required cell autonomously to control terminal axon branching. However, one potential limitation of the experiments involving TeTN-LC is that it blocks most VAMP-mediated vesicular release (with the exception of VAMP7, also called tetanus toxin–independent VAMP, or TI-VAMP). Therefore TeTN-LC action may not be limited to blocking synaptic vesicle release, but could also inhibit peptide release through vesicles containing neurotrophins for example, or other important trophic factors required for axon branching. More recent experiments through silencing of postsynaptic targets revealed that branching of callosal or thalamocortical axons is also dependent upon the activity of the postsynaptic targets (Mizuno et al., 2010; Yamada et al., 2010), albeit activity of the presynaptic neuron is required earlier during the branching process than activity of the postsynaptic targets (Mizuno et al., 2010). Activity is also required in some neurons at the phase of axon elongation through a feedback loop involving the activity-dependent up-regulation of guidance molecules (Mire et al., 2012).
How much does spontaneous or evoked neuronal activity contribute to branching? Reduction of neuronal activity through hyperpolarization induced by overexpression of Kir2.1 significantly reduces axon branching without completely eliminating it (Hua et al., 2005; Mizuno et al., 2007; Wang et al., 2007). Activity seems to serve as a competitive regulator of axon branching with regard to its neighbors because silencing of neighboring axons restores normal branching (Hua et al., 2005). Interestingly, neuronal activity induces neurotrophin expression locally, suggesting that activity can contribute to branching partly through activation of activity-independent branching mechanisms (Calinescu et al., 2011).
Neuronal activity can regulate branching through modification of the actin cytoskeleton via RhoA activation (Ohnami et al., 2008), and mRNA accumulates at presynaptic sites, indicating a correlation between local translation and synaptic activity (Lyles et al., 2006; Taylor et al., 2013). Neuronal activity is associated with changes in intracellular Ca2+ signaling, which has been shown to play a deterministic function in axon growth (Gomez and Spitzer, 1999). Calcium signaling activates the Ca2+/calmodulin-dependent kinases (CAMKs) that are known to regulate axon branching in vitro (Wayman et al., 2004; Ageta-Ishihara et al., 2009) and in vivo (Ageta-Ishihara et al., 2009).
Stabilization and refinement of the axonal arborization.
Axon branches are often formed in excess during development, then later refined to select for specific neural circuits (Luo and O’Leary, 2005). Long-range axon branch retraction has long been observed in layer V cortical neurons that initially project to the midbrain, hindbrain, and spinal cord (O’Leary and Terashima, 1988; Bastmeyer and O’Leary, 1996). At later stages, pyramidal neurons from the primary visual cortex will retract their spinal projection through axon pruning, whereas pyramidal neurons from the primary motor cortex will stabilize this projection but retract their axonal branches growing toward visual targets such as the superior colliculus. The molecular mechanisms controlling this area-specific pattern of axon branch pruning are still poorly understood, but seem to involve extracellular cues such as semaphorins and Rac1-dependent signaling (Bagri et al., 2003; Low et al., 2008; Riccomagno et al., 2012). Another example is the well-characterized refinement of retino-geniculate axons during the selective elimination of binocular input of RGC axon synapses onto relay neurons in the dorsal lateral geniculate nucleus (Muir-Robinson et al., 2002). Interestingly, some axons use caspase-dependent pathways locally to induce the selective retraction of axon branches during the process of pruning (Nikolaev et al., 2009; Simon et al., 2012).
Circuit refinement and selective branch retraction can be observed in vivo at the level of the neuromuscular junction where individual branches of motor axons are eliminated asynchronously (Keller-Peck et al., 2001). In the developing CNS, neurotrophin-induced branch retraction can also be observed in a context of competition between neighboring axons (Singh et al., 2008). One other way of stabilizing axon branches is through the formation of synapses with postsynaptic targets. In the visual system, the initial axon arbor is refined to establish ocular dominance through activity-dependent retraction of less active branches (Ruthazer et al., 2003). Time-lapse imaging of RGC axons in zebrafish or in Xenopus tadpole revealed that the formation of presynaptic sites occurs concomitantly to axon branching, and branches that form presynaptic structures are less likely to retract (Meyer and Smith, 2006; Ruthazer et al., 2006). The stabilization of axon branches through formation of synaptic contacts parallels with the stabilization of dendritic branches through synapse formation and stabilization (Niell et al., 2004; J. Li et al., 2011). The role of presynaptic bouton formation goes beyond the stabilization of axonal branches because in vivo, new axon branches can emerge from existing presynaptic terminals (Alsina et al., 2001; Javaherian and Cline, 2005; Panzer et al., 2006).
In conclusion, axon growth and branching can be regulated by both activity-dependent and activity-independent mechanisms during development. However, for mammalian CNS axons, much more work is needed to define (1) the precise molecular mechanisms underlying axon branching; (2) the cellular and molecular mechanisms regulating the key transition between axon growth and branching when axons start forming presynaptic contacts with their postsynaptic partners; and (3) the mechanisms regulating axon pruning during synapse elimination.
Acknowledgments
We thank previous and current members of the Polleux laboratory for helpful discussions, in particular Randal Hand for the original draft of Figure 1.
This work was supported in part by a grant from the National Institutes of Health (AG031524 to F. Polleux and F32NS080464 to T.L. Lewis). Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
Footnotes
Abbreviations used in this paper:
- AIS
- axon initial segment
- E
- embryonic day
- MAP
- microtubule-associated protein
- NGF
- nerve growth factor
- P
- postnatal day
- RGC
- retinal ganglion cell
References
- Abe I., Ochiai N., Ichimura H., Tsujino A., Sun J., Hara Y. 2004. Internodes can nearly double in length with gradual elongation of the adult rat sciatic nerve. J. Orthop. Res. 22:571–577 10.1016/j.orthres.2003.08.019 [DOI] [PubMed] [Google Scholar]
- Adler C.E., Fetter R.D., Bargmann C.I. 2006. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9:511–518 10.1038/nn1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ageta-Ishihara N., Takemoto-Kimura S., Nonaka M., Adachi-Morishima A., Suzuki K., Kamijo S., Fujii H., Mano T., Blaeser F., Chatila T.A., et al. 2009. Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. J. Neurosci. 29:13720–13729 10.1523/JNEUROSCI.3018-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F.J., Echeverri C.J., Vallee R.B., Baas P.W. 1998. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J. Cell Biol. 140:391–401 10.1083/jcb.140.2.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F.J., He Y., Myers K.A., Hasaka T.P., Francis F., Black M.M., Baas P.W. 2006. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 7:524–537 10.1111/j.1600-0854.2006.00403.x [DOI] [PubMed] [Google Scholar]
- Al-Bassam S., Xu M., Wandless T.J., Arnold D.B. 2012. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2:89–100 10.1016/j.celrep.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B., Vu T., Cohen-Cory S. 2001. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat. Neurosci. 4:1093–1101 10.1038/nn735 [DOI] [PubMed] [Google Scholar]
- Baas P.W., Ahmad F.J. 1993. The transport properties of axonal microtubules establish their polarity orientation. J. Cell Biol. 120:1427–1437 10.1083/jcb.120.6.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P.W., Deitch J.S., Black M.M., Banker G.A. 1988. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA. 85:8335–8339 10.1073/pnas.85.21.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A., Cheng H.J., Yaron A., Pleasure S.J., Tessier-Lavigne M. 2003. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 113:285–299 10.1016/S0092-8674(03)00267-8 [DOI] [PubMed] [Google Scholar]
- Barnes A.P., Polleux F. 2009. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32:347–381 10.1146/annurev.neuro.31.060407.125536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A.P., Lilley B.N., Pan Y.A., Plummer L.J., Powell A.W., Raines A.N., Sanes J.R., Polleux F. 2007. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 129:549–563 10.1016/j.cell.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Bastmeyer M., O’Leary D.D.M. 1996. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J. Neurosci. 16:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D., Toroian-Raymond A. 1986. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 323:712–715 10.1038/323712a0 [DOI] [PubMed] [Google Scholar]
- Black M.M., Slaughter T., Fischer I. 1994. Microtubule-associated protein 1b (MAP1b) is concentrated in the distal region of growing axons. J. Neurosci. 14:857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Dotti C.G. 1997. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron. 19:1175–1186 10.1016/S0896-6273(00)80410-9 [DOI] [PubMed] [Google Scholar]
- Bradke F., Dotti C.G. 1999. The role of local actin instability in axon formation. Science. 283:1931–1934 10.1126/science.283.5409.1931 [DOI] [PubMed] [Google Scholar]
- Brown A. 2003. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J. Cell Biol. 160:817–821 10.1083/jcb.200212017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack M.A., Silverman M.A., Banker G. 2000. The role of selective transport in neuronal protein sorting. Neuron. 26:465–472 10.1016/S0896-6273(00)81178-2 [DOI] [PubMed] [Google Scholar]
- Butler R., Wood J.D., Landers J.A., Cunliffe V.T. 2010. Genetic and chemical modulation of spastin-dependent axon outgrowth in zebrafish embryos indicates a role for impaired microtubule dynamics in hereditary spastic paraplegia. Dis. Model. Mech. 3:743–751 10.1242/dmm.004002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calinescu A.A., Liu T., Wang M.M., Borjigin J. 2011. Transsynaptic activity-dependent regulation of axon branching and neurotrophin expression in vivo. J. Neurosci. 31:12708–12715 10.1523/JNEUROSCI.2172-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano S.M., Shatz C.J. 1998. Activity-dependent cortical target selection by thalamic axons. Science. 281:559–562 10.1126/science.281.5376.559 [DOI] [PubMed] [Google Scholar]
- Chen S., Chen J., Shi H., Wei M., Castaneda-Castellanos D.R., Bultje R.S., Pei X., Kriegstein A.R., Zhang M., Shi S.-H. 2013. Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev. Cell. 24:26–40 10.1016/j.devcel.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tian X., Kim W.Y., Snider W.D. 2011. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PLoS ONE. 6:e24335 10.1371/journal.pone.0024335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.L., Poo M.M. 2012. Early events in axon/dendrite polarization. Annu. Rev. Neurosci. 35:181–201 10.1146/annurev-neuro-061010-113618 [DOI] [PubMed] [Google Scholar]
- Cheng P.L., Lu H., Shelly M., Gao H., Poo M.M. 2011. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron. 69:231–243 10.1016/j.neuron.2010.12.021 [DOI] [PubMed] [Google Scholar]
- Courchet J., Lewis T.L., Jr, Lee S., Courchet V., Liou D.Y., Aizawa S., Polleux F. 2013. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 153:1510–1525 10.1016/j.cell.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F., Bonev B., Garcez P., Stanley P., Guillemot F., Papalopulu N. 2012. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci. 15:697–699 10.1038/nn.3082 [DOI] [PubMed] [Google Scholar]
- Dawson H.N., Ferreira A., Eyster M.V., Ghoshal N., Binder L.I., Vitek M.P. 2001. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114:1179–1187 [DOI] [PubMed] [Google Scholar]
- de Anda F.C., Meletis K., Ge X., Rei D., Tsai L.H. 2010. Centrosome motility is essential for initial axon formation in the neocortex. J. Neurosci. 30:10391–10406 10.1523/JNEUROSCI.0381-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda F.C., Pollarolo G., Da Silva J.S., Camoletto P.G., Feiguin F., Dotti C.G. 2005. Centrosome localization determines neuronal polarity. Nature. 436:704–708 10.1038/nature03811 [DOI] [PubMed] [Google Scholar]
- Dent E.W., Kalil K. 2001. Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 21:9757–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E.W., Callaway J.L., Szebenyi G., Baas P.W., Kalil K. 1999. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 19:8894–8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel M., Hocking J.C., Volkmann K., Köster R.W. 2010. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191:875–890 10.1083/jcb.201004154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoo A.L., Richards L.J. 2009. Understanding the mechanisms of callosal development through the use of transgenic mouse models. Semin. Pediatr. Neurol. 16:127–142 10.1016/j.spen.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Donnelly C.J., Park M., Spillane M., Yoo S., Pacheco A., Gomes C., Vuppalanchi D., McDonald M., Kim H.H., Merianda T.T., et al. 2013. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 33:3311–3322 10.1523/JNEUROSCI.1722-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C.G., Banker G.A. 1987. Experimentally induced alteration in the polarity of developing neurons. Nature. 330:254–256 10.1038/330254a0 [DOI] [PubMed] [Google Scholar]
- Drewes G., Ebneth A., Preuss U., Mandelkow E.M., Mandelkow E. 1997. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 89:297–308 10.1016/S0092-8674(00)80208-1 [DOI] [PubMed] [Google Scholar]
- Flynn K.C., Pak C.W., Shaw A.E., Bradke F., Bamburg J.R. 2009. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev. Neurobiol. 69:761–779 10.1002/dneu.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano M.R., Jha S., Ho T.S.-Y., Zhang C., Ogawa Y., Chang K.-J., Stankewich M.C., Mohler P.J., Rasband M.N. 2012. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 149:1125–1139 10.1016/j.cell.2012.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. 2011. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev. Neurobiol. 71:201–220 10.1002/dneu.20852 [DOI] [PubMed] [Google Scholar]
- Gallo G., Letourneau P.C. 1998. Localized sources of neurotrophins initiate axon collateral sprouting. J. Neurosci. 18:5403–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Letourneau P.C. 1999. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J. Neurosci. 19:3860–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner A., Fornasiero E.F., Munck S., Vennekens K., Seuntjens E., Huttner W.B., Valtorta F., Dotti C.G. 2012. N-cadherin specifies first asymmetry in developing neurons. EMBO J. 31:1893–1903 10.1038/emboj.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S., Khanzada U.K., Parsons M., Chilton J.K., Gordon-Weeks P.R. 2008. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat. Cell Biol. 10:1181–1189 10.1038/ncb1778 [DOI] [PubMed] [Google Scholar]
- Gomez T.M., Spitzer N.C. 1999. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 397:350–355 10.1038/16927 [DOI] [PubMed] [Google Scholar]
- Gomez T.M., Robles E., Poo M., Spitzer N.C. 2001. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 291:1983–1987 10.1126/science.1056490 [DOI] [PubMed] [Google Scholar]
- Götz M., Huttner W.B. 2005. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6:777–788 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- Gu X., Olson E.C., Spitzer N.C. 1994. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 14:6325–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Polleux F. 2011. Neurogenin2 regulates the initial axon guidance of cortical pyramidal neurons projecting medially to the corpus callosum. Neural Dev. 6:30 10.1186/1749-8104-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Bortone D., Mattar P., Nguyen L., Heng J.I., Guerrier S., Boutt E., Peters E., Barnes A.P., Parras C., et al. 2005. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 48:45–62 10.1016/j.neuron.2005.08.032 [DOI] [PubMed] [Google Scholar]
- Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. 1994. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 369:488–491 10.1038/369488a0 [DOI] [PubMed] [Google Scholar]
- Harrington A.W., Ginty D.D. 2013. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 14:177–187 10.1038/nrn3253 [DOI] [PubMed] [Google Scholar]
- Hayashi H., Campenot R.B., Vance D.E., Vance J.E. 2004. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 279:14009–14015 10.1074/jbc.M313828200 [DOI] [PubMed] [Google Scholar]
- Hedstrom K.L., Ogawa Y., Rasband M.N. 2008. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 183:635–640 10.1083/jcb.200806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S.R., Landers J.M., Hamborg M.A. 1981. Polarity orientation of axonal microtubules. J. Cell Biol. 91:661–665 10.1083/jcb.91.3.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Niwa S., Tanaka Y. 2010. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 68:610–638 10.1016/j.neuron.2010.09.039 [DOI] [PubMed] [Google Scholar]
- Homma N., Takei Y., Tanaka Y., Nakata T., Terada S., Kikkawa M., Noda Y., Hirokawa N. 2003. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 114:229–239 10.1016/S0092-8674(03)00522-1 [DOI] [PubMed] [Google Scholar]
- Hu J., Bai X., Bowen J.R., Dolat L., Korobova F., Yu W., Baas P.W., Svitkina T., Gallo G., Spiliotis E.T. 2012. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr. Biol. 22:1109–1115 10.1016/j.cub.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J.Y., Smear M.C., Baier H., Smith S.J. 2005. Regulation of axon growth in vivo by activity-based competition. Nature. 434:1022–1026 10.1038/nature03409 [DOI] [PubMed] [Google Scholar]
- Javaherian A., Cline H.T. 2005. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron. 45:505–512 10.1016/j.neuron.2004.12.051 [DOI] [PubMed] [Google Scholar]
- Jenkins B., Decker H., Bentley M., Luisi J., Banker G. 2012. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J. Cell Biol. 198:749–761 10.1083/jcb.201205070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Mateos E.M., Paglini G., González-Billault C., Cáceres A., Avila J. 2005. End binding protein-1 (EB1) complements microtubule-associated protein-1B during axonogenesis. J. Neurosci. Res. 80:350–359 10.1002/jnr.20453 [DOI] [PubMed] [Google Scholar]
- Kalil K., Szebenyi G., Dent E.W. 2000. Common mechanisms underlying growth cone guidance and axon branching. J. Neurobiol. 44:145–158 [DOI] [PubMed] [Google Scholar]
- Kamiguchi H., Yoshihara F. 2001. The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J. Neurosci. 21:9194–9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein L.C., Schlager M.A., Kuijpers M., Wulf P.S., van Spronsen M., MacKintosh F.C., Hoogenraad C.C. 2010. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20:290–299 10.1016/j.cub.2009.12.052 [DOI] [PubMed] [Google Scholar]
- Karabay A., Yu W., Solowska J.M., Baird D.H., Baas P.W. 2004. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 24:5778–5788 10.1523/JNEUROSCI.1382-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Peck C.R., Walsh M.K., Gan W.B., Feng G., Sanes J.R., Lichtman J.W. 2001. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron. 31:381–394 10.1016/S0896-6273(01)00383-X [DOI] [PubMed] [Google Scholar]
- Ketschek A., Gallo G. 2010. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J. Neurosci. 30:12185–12197 10.1523/JNEUROSCI.1740-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F., Svitkina T. 2008. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol. Biol. Cell. 19:1561–1574 10.1091/mbc.E07-09-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda P., Paglini G., Quiroga S., Kosik K., Caceres A. 2001. Evidence for the involvement of Tiam1 in axon formation. J. Neurosci. 21:2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P.C., Shattuck T.A., Ressler A.H. 1986. Branching of sensory and sympathetic neurites in vitro is inhibited by treatment with taxol. J. Neurosci. 6:1912–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P.C., Shattuck T.A., Ressler A.H. 1987. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil. Cytoskeleton. 8:193–209 10.1002/cm.970080302 [DOI] [PubMed] [Google Scholar]
- Lewis T.L., Jr, Mao T., Svoboda K., Arnold D.B. 2009. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat. Neurosci. 12:568–576 10.1038/nn.2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.L., Jr, Mao T., Arnold D.B. 2011. A role for myosin VI in the localization of axonal proteins. PLoS Biol. 9:e1001021 10.1371/journal.pbio.1001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Erisir A., Cline H. 2011. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 69:273–286 10.1016/j.neuron.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kumar Y., Zempel H., Mandelkow E.M., Biernat J., Mandelkow E. 2011. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO J. 30:4825–4837 10.1038/emboj.2011.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley B.N., Pan Y.A., Sanes J.R. 2013. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron. 79:39–53 10.1016/j.neuron.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loverde J.R., Ozoka V.C., Aquino R., Lin L., Pfister B.J. 2011. Live imaging of axon stretch growth in embryonic and adult neurons. J. Neurotrauma. 28:2389–2403 10.1089/neu.2010.1598 [DOI] [PubMed] [Google Scholar]
- Low L.K., Liu X.B., Faulkner R.L., Coble J., Cheng H.J. 2008. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc. Natl. Acad. Sci. USA. 105:8136–8141 10.1073/pnas.0803849105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., O’Leary D.D. 2005. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28:127–156 10.1146/annurev.neuro.28.061604.135632 [DOI] [PubMed] [Google Scholar]
- Lyles V., Zhao Y., Martin K.C. 2006. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 49:349–356 10.1016/j.neuron.2005.12.029 [DOI] [PubMed] [Google Scholar]
- Ma L., Tessier-Lavigne M. 2007. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J. Neurosci. 27:6843–6851 10.1523/JNEUROSCI.1479-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E.M., Thies E., Trinczek B., Biernat J., Mandelkow E. 2004. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J. Cell Biol. 167:99–110 10.1083/jcb.200401085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Letourneau P.C. 1984. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J. Cell Biol. 99:2041–2047 10.1083/jcb.99.6.2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson S.F., Levine R.B. 1999. Steroid hormone enhancement of neurite outgrowth in identified insect motor neurons involves specific effects on growth cone form and function. J. Neurobiol. 38:27–45 [DOI] [PubMed] [Google Scholar]
- Meyer M.P., Smith S.J. 2006. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J. Neurosci. 26:3604–3614 10.1523/JNEUROSCI.0223-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance-Le Meur A., O’Connor T.P. 2009. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 28:248–260 10.1038/emboj.2008.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire E., Mezzera C., Leyva-Díaz E., Paternain A.V., Squarzoni P., Bluy L., Castillo-Paterna M., López M.J., Peregrín S., Tessier-Lavigne M., et al. 2012. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat. Neurosci. 15:1134–1143 10.1038/nn.3160 [DOI] [PubMed] [Google Scholar]
- Mizuno H., Hirano T., Tagawa Y. 2007. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J. Neurosci. 27:6760–6770 10.1523/JNEUROSCI.1215-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H., Hirano T., Tagawa Y. 2010. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. Eur. J. Neurosci. 31:410–424 10.1111/j.1460-9568.2009.07070.x [DOI] [PubMed] [Google Scholar]
- Morgan J.L., Dhingra A., Vardi N., Wong R.O.L. 2006. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat. Neurosci. 9:85–92 10.1038/nn1615 [DOI] [PubMed] [Google Scholar]
- Morris M., Maeda S., Vossel K., Mucke L. 2011. The many faces of tau. Neuron. 70:410–426 10.1016/j.neuron.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Robinson G., Hwang B.J., Feller M.B. 2002. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 22:5259–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T., Hirokawa N. 2003. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162:1045–1055 10.1083/jcb.200302175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa H., Sada T., Toriyama M., Tago K., Sugiura T., Fukuda M., Inagaki N. 2012. Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J. Neurosci. 32:12712–12725 10.1523/JNEUROSCI.0989-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirchen D., Bradke F. 2011. Neuronal polarization and the cytoskeleton. Semin. Cell Dev. Biol. 22:825–833 10.1016/j.semcdb.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Nguyen M.M., Stone M.C., Rolls M.M. 2011. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 6:38 10.1186/1749-8104-6-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell C.M., Meyer M.P., Smith S.J. 2004. In vivo imaging of synapse formation on a growing dendritic arbor. Nat. Neurosci. 7:254–260 10.1038/nn1191 [DOI] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O’Leary D.D.M., Tessier-Lavigne M. 2009. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 457:981–989 10.1038/nature07767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- O’Leary D.D., Terashima T. 1988. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and “waiting periods”. Neuron. 1:901–910 10.1016/0896-6273(88)90147-X [DOI] [PubMed] [Google Scholar]
- Ohnami S., Endo M., Hirai S., Uesaka N., Hatanaka Y., Yamashita T., Yamamoto N. 2008. Role of RhoA in activity-dependent cortical axon branching. J. Neurosci. 28:9117–9121 10.1523/JNEUROSCI.1731-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A.A., Jr, Atkins C.M., Copenagle L., Banker G.A. 2006. Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26:9462–9470 10.1523/JNEUROSCI.2625-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K.M., Jan L.Y., Jan Y.-N. 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 76:921–930 10.1016/j.neuron.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzer J.A., Song Y., Balice-Gordon R.J. 2006. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J. Neurosci. 26:934–947 10.1523/JNEUROSCI.3656-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister B.J., Iwata A., Meaney D.F., Smith D.H. 2004. Extreme stretch growth of integrated axons. J. Neurosci. 24:7978–7983 10.1523/JNEUROSCI.1974-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C., Weimer R.M., De Paola V., Caroni P., Svoboda K. 2005. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 3:e272 10.1371/journal.pbio.0030272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse De Chaves E.I., Vance D.E., Campenot R.B., Kiss R.S., Vance J.E. 2000. Uptake of lipoproteins for axonal growth of sympathetic neurons. J. Biol. Chem. 275:19883–19890 10.1074/jbc.275.26.19883 [DOI] [PubMed] [Google Scholar]
- Qiang L., Yu W., Liu M., Solowska J.M., Baas P.W. 2010. Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol. Biol. Cell. 21:334–344 10.1091/mbc.E09-09-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O., Norden C., Harris W.A. 2011a. The vertebrate retina: a model for neuronal polarization in vivo. Dev. Neurobiol. 71:567–583 10.1002/dneu.20841 [DOI] [PubMed] [Google Scholar]
- Randlett O., Poggi L., Zolessi F.R., Harris W.A. 2011b. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 70:266–280 10.1016/j.neuron.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband M.N. 2010. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11:552–562 10.1038/nrn2852 [DOI] [PubMed] [Google Scholar]
- Rašin M.-R., Gazula V.-R., Breunig J.J., Kwan K.Y., Johnson M.B., Liu-Chen S., Li H.-S., Jan L.Y., Jan Y.-N., Rakic P., Šestan N. 2007. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat. Neurosci. 10:819–827 10.1038/nn1924 [DOI] [PubMed] [Google Scholar]
- Riccomagno M.M., Hurtado A., Wang H., Macopson J.G., Griner E.M., Betz A., Brose N., Kazanietz M.G., Kolodkin A.L. 2012. The RacGAP β2-Chimaerin selectively mediates axonal pruning in the hippocampus. Cell. 149:1594–1606 10.1016/j.cell.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Coffee P., Smith G., Liem R.K., Brady S.T., Black M.M. 2000. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J. Neurosci. 20:6849–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer E.S., Akerman C.J., Cline H.T. 2003. Control of axon branch dynamics by correlated activity in vivo. Science. 301:66–70 10.1126/science.1082545 [DOI] [PubMed] [Google Scholar]
- Ruthazer E.S., Li J., Cline H.T. 2006. Stabilization of axon branch dynamics by synaptic maturation. J. Neurosci. 26:3594–3603 10.1523/JNEUROSCI.0069-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G., Banker G. 1998. Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil. Cytoskeleton. 40:160–173 [DOI] [PubMed] [Google Scholar]
- Ruthel G., Hollenbeck P.J. 2000. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J. Neurosci. 20:2266–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A., Sato T., Ando R., Noguchi N., Masaoka M., Miyata T. 2013. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb. Cortex. [DOI] [PubMed] [Google Scholar]
- Sato M., Lopez-Mascaraque L., Heffner C.D., O’Leary D.D. 1994. Action of a diffusible target-derived chemoattractant on cortical axon branch induction and directed growth. Neuron. 13:791–803 10.1016/0896-6273(94)90246-1 [DOI] [PubMed] [Google Scholar]
- Shelly M., Cancedda L., Heilshorn S., Sumbre G., Poo M.M. 2007. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 129:565–577 10.1016/j.cell.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Shelly M., Lim B.K., Cancedda L., Heilshorn S.C., Gao H., Poo M.M. 2010. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 327:547–552 10.1126/science.1179735 [DOI] [PubMed] [Google Scholar]
- Shelly M., Cancedda L., Lim B.K., Popescu A.T., Cheng P.L., Gao H., Poo M.M. 2011. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 71:433–446 10.1016/j.neuron.2011.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.H., Jan L.Y., Jan Y.N. 2003. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 112:63–75 10.1016/S0092-8674(02)01249-7 [DOI] [PubMed] [Google Scholar]
- Shi S.-H., Cheng T., Jan L.Y., Jan Y.-N. 2004. APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr. Biol. 14:2025–2032 10.1016/j.cub.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Simon D.J., Weimer R.M., McLaughlin T., Kallop D., Stanger K., Yang J., O’Leary D.D.M., Hannoush R.N., Tessier-Lavigne M. 2012. A caspase cascade regulating developmental axon degeneration. J. Neurosci. 32:17540–17553 10.1523/JNEUROSCI.3012-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.K., Park K.J., Hong E.J., Kramer B.M., Greenberg M.E., Kaplan D.R., Miller F.D. 2008. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat. Neurosci. 11:649–658 10.1038/nn.2114 [DOI] [PubMed] [Google Scholar]
- Smith D.H., Wolf J.A., Meaney D.F. 2001. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 7:131–139 10.1089/107632701300062714 [DOI] [PubMed] [Google Scholar]
- Song A.H., Wang D., Chen G., Li Y., Luo J., Duan S., Poo M.M. 2009. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 136:1148–1160 10.1016/j.cell.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Spillane M., Ketschek A., Donnelly C.J., Pacheco A., Twiss J.L., Gallo G. 2012. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J. Neurosci. 32:17671–17689 10.1523/JNEUROSCI.1079-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.C., Roegiers F., Rolls M.M. 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell. 19:4122–4129 10.1091/mbc.E07-10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter D.M., Miller K.E. 2011. The emerging role of forces in axonal elongation. Prog. Neurobiol. 94:91–101 10.1016/j.pneurobio.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G., Callaway J.L., Dent E.W., Kalil K. 1998. Interstitial branches develop from active regions of the axon demarcated by the primary growth cone during pausing behaviors. J. Neurosci. 18:7930–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G., Dent E.W., Callaway J.L., Seys C., Lueth H., Kalil K. 2001. Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J. Neurosci. 21:3932–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y., Teng J., Harada A., Hirokawa N. 2000. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 150:989–1000 10.1083/jcb.150.5.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Kalil K. 2005. Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J. Neurosci. 25:6702–6715 10.1523/JNEUROSCI.0871-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.M., Wu J., Tai H.C., Schuman E.M. 2013. Axonal translation of β-catenin regulates synaptic vesicle dynamics. J. Neurosci. 33:5584–5589 10.1523/JNEUROSCI.2944-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S. 2003. Where does slow axonal transport go? Neurosci. Res. 47:367–372 10.1016/j.neures.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Terada S., Kinjo M., Hirokawa N. 2000. Oligomeric tubulin in large transporting complex is transported via kinesin in squid giant axons. Cell. 103:141–155 10.1016/S0092-8674(00)00094-5 [DOI] [PubMed] [Google Scholar]
- Tint I., Slaughter T., Fischer I., Black M.M. 1998. Acute inactivation of tau has no effect on dynamics of microtubules in growing axons of cultured sympathetic neurons. J. Neurosci. 18:8660–8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tint I., Jean D., Baas P.W., Black M.M. 2009. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J. Neurosci. 29:10995–11010 10.1523/JNEUROSCI.3399-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa E., Galjart N., Avila J., Sayas C.L. 2013. MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. EMBO J. 32:1293–1306 10.1038/emboj.2013.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N., Hayano Y., Yamada A., Yamamoto N. 2007. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J. Neurosci. 27:5215–5223 10.1523/JNEUROSCI.4685-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M., Mikhaylova M., Lipka J., Schlager M.A., van den Heuvel D.J., Kuijpers M., Wulf P.S., Keijzer N., Demmers J., Kapitein L.C., et al. 2013. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 77:485–502 10.1016/j.neuron.2012.11.027 [DOI] [PubMed] [Google Scholar]
- Wang C.L., Zhang L., Zhou Y., Zhou J., Yang X.J., Duan S.M., Xiong Z.Q., Ding Y.Q. 2007. Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci. 27:11334–11342 10.1523/JNEUROSCI.3380-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zugates C.T., Liang I.H., Lee C.H., Lee T. 2002. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 33:559–571 10.1016/S0896-6273(02)00570-6 [DOI] [PubMed] [Google Scholar]
- Wang L., Ho C.L., Sun D., Liem R.K., Brown A. 2000. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2:137–141 10.1038/35004008 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Al-Bassam S., Miyazaki Y., Wandless T.J., Webster P., Arnold D.B. 2012. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2:1546–1553 10.1016/j.celrep.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman G.A., Kaech S., Grant W.F., Davare M., Impey S., Tokumitsu H., Nozaki N., Banker G., Soderling T.R. 2004. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 24:3786–3794 10.1523/JNEUROSCI.3294-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B., Yap C.C. 2011. Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors. Traffic. 12:1099–1108 10.1111/j.1600-0854.2011.01213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B., Forscher P., Mellman I. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 397:698–701 10.1038/17806 [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. 2008. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180:619–632 10.1083/jcb.200707042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.D., Landers J.A., Bingley M., McDermott C.J., Thomas-McArthur V., Gleadall L.J., Shaw P.J., Cunliffe V.T. 2006. The microtubule-severing protein Spastin is essential for axon outgrowth in the zebrafish embryo. Hum. Mol. Genet. 15:2763–2771 10.1093/hmg/ddl212 [DOI] [PubMed] [Google Scholar]
- Xia C.H., Roberts E.A., Her L.S., Liu X., Williams D.S., Cleveland D.W., Goldstein L.S. 2003. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161:55–66 10.1083/jcb.200301026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Zhong G., Zhuang X. 2013. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 339:452–456 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe J.T., Pimenta A., Shea T.B. 1999. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J. Cell Sci. 112:3799–3814 [DOI] [PubMed] [Google Scholar]
- Yamada A., Uesaka N., Hayano Y., Tabata T., Kano M., Yamamoto N. 2010. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc. Natl. Acad. Sci. USA. 107:7562–7567 10.1073/pnas.0900613107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Chao D.L., Toba S., Koyasako K., Yasunaga T., Hirotsune S., Shen K. 2013. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. eLife. 2:e00133 10.7554/eLife.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.J., Barnes A.P., Hand R., Polleux F., Ehlers M.D. 2010. TGF-β signaling specifies axons during brain development. Cell. 142:144–157 10.1016/j.cell.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Takei Y., Kido M.A., Hirokawa N. 2011. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 70:310–325 10.1016/j.neuron.2011.02.049 [DOI] [PubMed] [Google Scholar]
- Yokota Y., Kim W.-Y., Chen Y., Wang X., Stanco A., Komuro Y., Snider W., Anton E.S. 2009. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 61:42–56 10.1016/j.neuron.2008.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.R., Power J., Barnea G., O’Donnell S., Brown H.E., Osborne J., Axel R., Gogos J.A. 2004. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 42:553–566 10.1016/S0896-6273(04)00224-7 [DOI] [PubMed] [Google Scholar]
- Yu W., Ahmad F.J., Baas P.W. 1994. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J. Neurosci. 14:5872–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Solowska J.M., Qiang L., Karabay A., Baird D., Baas P.W. 2005. Regulation of microtubule severing by katanin subunits during neuronal development. J. Neurosci. 25:5573–5583 10.1523/JNEUROSCI.0834-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Qiang L., Solowska J.M., Karabay A., Korulu S., Baas P.W. 2008. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell. 19:1485–1498 10.1091/mbc.E07-09-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wildonger J., Ye B., Zhang Y., Kita A., Younger S.H., Zimmerman S., Jan L.Y., Jan Y.N. 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10:1172–1180 10.1038/ncb1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.-Q., Zhou J., Dedhar S., Wu Y.-H., Snider W.D. 2004. NGF-induced axon growth is mediated by localized inactivation of GSK-3β and functions of the microtubule plus end binding protein APC. Neuron. 42:897–912 10.1016/j.neuron.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Zhou X., Babu J.R., da Silva S., Shu Q., Graef I.A., Oliver T., Tomoda T., Tani T., Wooten M.W., Wang F. 2007. Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc. Natl. Acad. Sci. USA. 104:5842–5847 10.1073/pnas.0701402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi H.Y., Bear M.F. 2012. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4:a009886–a009886 10.1101/cshperspect.a009886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi F.R., Poggi L., Wilkinson C.J., Chien C.B., Harris W.A. 2006. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 1:2 10.1186/1749-8104-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]