Abstract
Objective. To analyze serum interleukin-6 (IL-6) expression level and its clinical significance in patients with dermatomyositis. Methods. Blood samples from 23 adult patients with dermatomyositis (DM), 22 with systemic lupus erythematosus (SLE), 22 with rheumatoid arthritis (RA), 16 with Sjögren's syndrome (SS), and 20 healthy controls were collected. The IL-6 concentration was detected by chemiluminescence immunoassay. Correlations between IL-6 expression levels and clinical features or laboratory findings in patients with DM were investigated. Results. IL-6 expression level of DM patients was significantly higher than that of normal controls, significantly lower than that of RA patients, and slightly lower than that of SLE or SS patients with no significant differences. The incidence of fever was significantly higher in the IL-6 elevated group. Serum ferritin (SF) and C-reactive protein (CRP) were positively correlated with IL-6. Conclusions. IL-6 plays a less important role in DM than in RA. IL-6 monoclonal antibodies may have poor effect in patients with DM.
1. Introduction
Idiopathic inflammatory myopathy (IIM) is a series of disorders characterized by chronic muscle inflammation of unknown cause. Dermatomyositis (DM) is one of the most common forms of IIM with muscle, skin, and internal organs involved [1, 2]. Interleukin-6 (IL-6) is a potent, pleiotropic Th2 cytokine that regulates the immune defense response. IL-6 acts as both a proinflammatory and an anti-inflammatory cytokine and plays a central role in the transition from the acute to the chronic phase of the inflammatory process [3, 4]. Elevated levels of IL-6 have been documented in a variety of autoimmune diseases, such as rheumatoid arthritis, inflammatory bowel disease, glomerular nephritis, and so forth [5–7]. In this study, we tried to determine whether IL-6 could contribute to the inflammation associated with DM. We compared serum IL-6 level in DM patients with other three connective tissue diseases: systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome (SS). Clinical significance of IL-6 was evaluated.
2. Materials and Methods
2.1. Patients and Controls
All patients were enrolled in West China Hospital from August 2012 to March 2013. Patients with DM satisfied the criteria of Bohan/Peter [8, 9]. The disease controls were patients with SLE, RA, and SS, based on the Criteria of ACR for SLE [10], the Criteria of ACR for RA [11], and the revised American-European Criteria for SS [12], respectively. Patients with an overlapping syndrome were excluded. Healthy controls were matched to DM patients by age and gender. This study was approved by the Ethical Committee of West China Hospital of Sichuan University, and informed consent was obtained from all patients.
2.2. Methods
Serum IL-6 levels were measured by chemiluminescence immunoassay (ROCHE Modular Analytics E170) in DM patients and controls. Blood parameters including creatine phosphokinase (CPK), CRP, erythrocyte sedimentation rate (ESR), SF, antinuclear antibody (ANA) and anti-Jo-1 antibody were measured by standard methods. Clinical data were obtained from medical records on admission.
2.3. Statistics
Statistical analysis was performed by using Fisher's exact test for comparison of frequencies and Mann-Whitney U test for comparison of median levels. Correlation coefficients were established by using Spearman's correlation coefficients. The data were analyzed by SPSS 17.0 software. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Serum IL-6 Levels in DM Patients and Controls
Twenty-Three patients with DM, 22 with SLE, 22 with RA, 16 with SS, and 20 healthy controls were enrolled in this study. The demography and the IL-6 levels in DM patients and controls were shown in Table 1 and Figure 1. The age at onset and sex were not significantly different between DM patients and controls. The serum level of IL-6 was 21.5 ± 30.3, 43.3 ± 55.6, 102.6 ± 82.8, 26.7 ± 29.9, and 1.2 ± 2.6 pg/mL in DM, SLE, RA, SS patients, and healthy controls, respectively. The level of IL-6 was significantly higher in DM patients than that in healthy controls (P < 0.01), and was significantly lower than that in RA patients (P < 0.001). Compared with SLE and SS patients, IL-6 level was slightly lower in DM patients, but no significant difference was found.
Table 1.
The demography and the IL-6 levels in DM patients and controls.
| Variables | DM | SLE | RA | SS | Healthy controls |
|---|---|---|---|---|---|
| n = 23 | n = 22 | n = 22 | n = 16 | n = 20 | |
| Age, years | 49 ± 11 | 41 ± 16 | 53 ± 14 | 44 ± 14 | 45 ± 12 |
| Male/female | 9/14 | 1/21 | 8/14 | 1/15 | 10/10 |
| IL-6, pg/mL | 21.5 ± 30.3 | 43.3 ± 55.6 | 102.6 ± 82.8 | 26.7 ± 29.9 | 1.2 ± 2.6 |
| P value | 0.100 | <0.001 | 0.662 | <0.01 |
P value was obtained from the statistical comparisons of serum IL-6 between the DM group and the SLE, RA, SS, and healthy groups. DM: dermatomyositis, SLE: systemic lupus erythematosus, RA: rheumatoid arthritis, SS: Sjögren's syndrome, and IL-6: interleukin-6.
Figure 1.
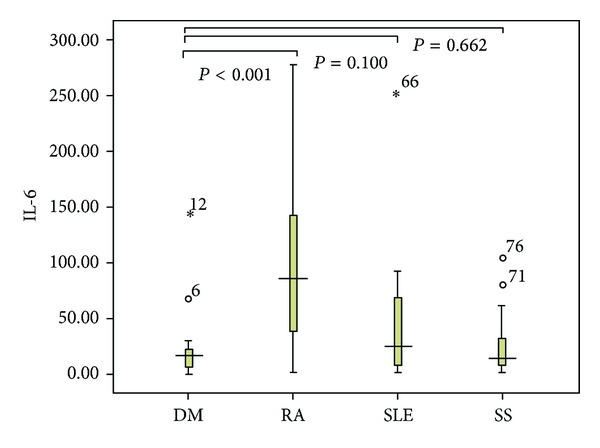
Serum IL-6 levels in patients with DM, RA, SLE, and SS.
3.2. Correlation between IL-6 and Clinical Features or Laboratory Markers
Correlation coefficients were established by using Spearman's correlation coefficients. Considering the clinical characteristics, significantly positive correlation was found between IL-6 and fever (r s = 0.569; P = 0.005, Table 2). As shown in Figure 2, the receiver-operator characteristic curve (ROC curve) analysis was used to determine the level of IL-6 when fever occurred. The area under the curve was 0.831, and the cutpoint value was 22.35 pg/mL. All DM patients had fever when IL-6 level was above the cutpoint. The sensitivity and specificity for IL-6 cutoff point value predicting fever in DM patients were 60% and 100%, respectively. In addition, no correlation was found between IL-6 and sex, age, course of disease, muscle weakness, interstitial lung disease, Gottron's sign, heliotrope eruption, or arthritis. As for laboratory markers, IL-6 was found positively correlated with CRP (r s = 0.595; P = 0.004) and SF (r s = 0.789; P = 0.004, Table 3). No correlation was found between IL-6 and abnormal electromyogram, CPK, positive muscle biopsy findings, ESR, ANA, or anti-Jo-1.
Table 2.
Correlation between IL-6 and clinical features in patients with DM.
| Variables | r s | P value |
|---|---|---|
| Sex | −0.013 | 0.952 |
| Age (years) | 0.009 | 0.968 |
| Course of DM (months) | −0.322 | 0.134 |
| Muscle weakness | −0.011 | 0.961 |
| ILD | 0.100 | 0.651 |
| Gottron's sign | 0.215 | 0.325 |
| Heliotrope eruption | 0.121 | 0.582 |
| Arthritis | 0.079 | 0.721 |
| Fever | 0.569 | 0.005 |
ILD: interstitial lung disease.
Figure 2.
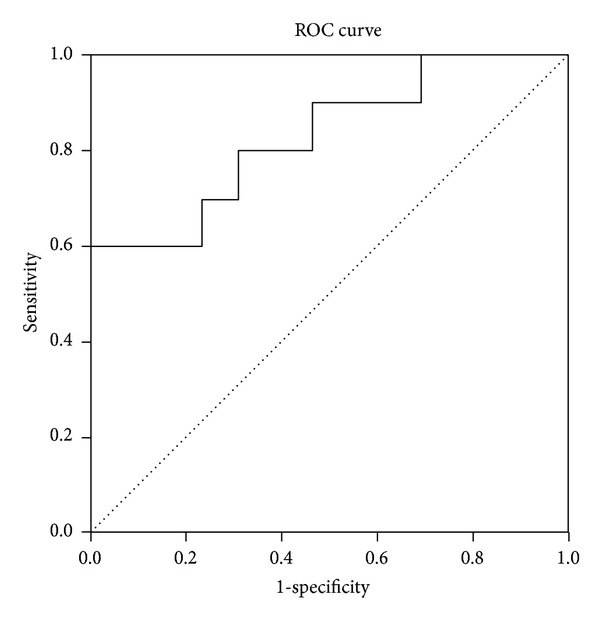
The ROC curve of DM patients with fever.
Table 3.
Correlation between IL-6 and laboratory markers in patients with DM.
| Variables | r s | P value |
|---|---|---|
| EMG | 0.081 | 0.715 |
| CPK | 0.405 | 0.055 |
| Biopsy | 0.228 | 0.295 |
| ESR | −0.019 | 0.930 |
| CRP | 0.595 | 0.004 |
| SF | 0.789 | 0.004 |
| ANA | −0.079 | 0.721 |
| Anti-Jo-1 | −0.111 | 0.613 |
EMG: electromyogram, CPK: creatine phosphokinase, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, SF: serum ferritin, and ANA: antinuclear antibody.
4. Discussion
It is widely accepted that DM arises from CD4 + T cell- and B cell-mediated muscle inflammation, in which the complement system is activated, resulting in membrane deposition of attack complex within muscle capillaries [13]. It is unclear which pathway mediates the inflammation and perivasculitis [14]. As far as we know, IL-6 is an important proinflammatory cytokine and contributes to the inflammatory process. Elevated levels of IL-6 have been documented in a variety of autoimmune diseases, including rheumatoid arthritis, colitis, Crohn's disease, glomerular nephritis, and so forth [15]. Besides, IL-6 plays central roles in the regulation of both innate and adaptive inflammatory immune responses, as well as both humoral and cell-mediated autoimmune reactions, such as B cell differentiation activity and the T cell growth and differentiation [16]. In literature, evidence concerning the pathogenic role of IL-6 in DM is sparse. Our study demonstrated that the level of serum IL-6 was significantly higher in DM patients than in healthy controls, and IL-6 had significant correlation with the inflammatory marker CRP or serum ferritin (SF). In pathophysiology, tissue injury of skin, muscle, or other internal organs activates monocytes and macrophages to release cytokines, inducing in turn hepatic synthesis of acute phase reactants (APRs) [17]. CRP and SF are classic APRs, and IL-6 is a mainly stimulator of APRs [18]. These findings showed that IL-6 played a role in the inflammatory process of DM. Interestingly, this conclusion was verified in an experimental mouse model of myosin-induced myositis, indicating that the deficiency of IL-6 led to marked amelioration of the clinical signs and pathologic findings of muscle injury [19].
Furthermore, we found that the level of IL-6 in DM patients was significantly lower than that in RA patients, which suggesting a less important role of IL-6 in DM than in RA [20]. The level of IL-6 in DM was even slightly lower than those in SLE and SS. In DM patients, the degree of inflammatory reaction is not always consistent with clinical severity, and inflammation decrescence may be accompanied with aggravated systemic injuries. The effect of anti-inflammatory drugs as glucocorticoid on DM is limited to some degree. These phenomena provided clinical evidence to our deduction that inflammation is only a small chapter in the pathogenesis of DM, and IL-6 plays a minor role in it. Therefore, we can conclude that although blockade of IL-6 and IL-6 signaling have been shown to be effective in treating several inflammatory diseases (e.g., tocilizumab for rheumatoid arthritis and inflammatory bowel disease [21, 22]), its effect in patients with DM should be limited.
RA, SLE, and SS are known to have immune activation, producing a lot of antibodies. However, few DM patients have myositis-specific antibody (MSA) or myositis-associated antibody (MAA) [23]. Autoantibody production relies on B cell differentiation and activation. As mentioned above, IL-6 plays central roles in B cell differentiation activity. Thus, lower serum IL-6 level in DM than in RA, SLE, and SS may be an explanation to the differences in autoantibody production in these rheumatic diseases. In this study, we found that the level of IL-6 was closely related to fever. DM patients whose IL-6 level was above the cutoff point almost had fever. In view of pathology and physiology, IL-6 plays an important role in the mechanism of fever by changing the thermoregulation set-point [24]. It is interesting that IL-6 is significantly higher in RA patients, but fever is seldom seen. The mechanism for this needs further research.
In conclusion, we believe that IL-6 plays a minor role in DM. Large samples and longitudinal studies are required to elucidate the exact relationship between IL-6 and DM.
5. Conclusions
IL-6 plays a less important role in DM than in RA. The effect of the therapy targeting IL-6 on DM may be limited.
Conflict of Interests
There is no conflict of interests to disclose.
Acknowledgment
This paper was supported by the National Natural Science Foundation of China (Grants Nos. 81172869 and 30901339).
References
- 1.Wu H, Geng D, Xu J. An approach to the development of interstitial lung disease in dermatomyositis: a study of 230 cases in China. Journal of International Medical Research. 2013;41(2):493–501. doi: 10.1177/0300060513476435. [DOI] [PubMed] [Google Scholar]
- 2.Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Annals of the Rheumatic Diseases. 2004;63(3):297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka T, Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory disease. International Journal of Biological Sciences. 2012;8(9):1226–1227. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst SM, Wilkinson TS, McLoughlin RM, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 5.Atreya R, Mudter J, Finotto S, et al. Erratum: blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature Medicine. 2000;6(5):583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annual Review of Immunology. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 7.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Research and Therapy. 2006;8(supplement 2, article S3) doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis anddermatomyositis (first of two parts) The New England Journal of Medicine. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 9.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) The New England Journal of Medicine. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 12.Vatajli C, Bombardiere S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the Rheumatic Diseases. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalakas MC. Immunopathogenesis of inflammatory myopathies. Annals of Neurology. 1995;37(supplement 1):S74–S86. doi: 10.1002/ana.410370709. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. The Lancet. 2003;362(9388):971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 15.Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Annals of the Rheumatic Diseases. 2000;59(1):i21–i27. doi: 10.1136/ard.59.suppl_1.i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Hirano T. The pathological and physiological roles of IL-6 amplifier activation. International Journal of Biological Sciences. 2012;8(9):1267–1280. doi: 10.7150/ijbs.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caravaca FS, Martín MV, Barroso S, Ruiz B, Hernández-Gallego R. Do inflammatory markers add predictive information of death beyond that provided by age and comorbidity in chronic renal failure patients? Nephrology Dialysis Transplantation. 2006;21(6):1575–1581. doi: 10.1093/ndt/gfl033. [DOI] [PubMed] [Google Scholar]
- 18.Arlet JB, Boutin-Le Thi Huong D, Pouchot J, Piette JC. Current concepts on the pathogenesis of adult-onset Still’s disease. Revue de Medecine Interne. 2005;26(7):549–556. doi: 10.1016/j.revmed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Scuderi F, Mannella F, Marino M, Provenzano C, Bartoccioni E. IL-6-deficient mice show impaired inflammatory response in a model of myosin-induced experimental myositis. Journal of Neuroimmunology. 2006;176(1-2):9–15. doi: 10.1016/j.jneuroim.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Cronstein BN. Interleukin-6-a key mediator of systemic and local symptoms in rheumatoid arthritis. Bulletin of the NYU Hospital for Joint Diseases. 2007;65(supplement 1):S11–S15. [PubMed] [Google Scholar]
- 21.Ash A, Emery P. The role of Tocilizumab in the management of rheumatoid arthritis. Expert Opinion on Biological Therapy. 2012;12(9):1277–1289. doi: 10.1517/14712598.2012.707178. [DOI] [PubMed] [Google Scholar]
- 22.Ahluwalia JP. Immunotherapy in inflammatory bowel disease. Medical Clinics of North America. 2012;96(3):525–544. doi: 10.1016/j.mcna.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nature Reviews Neurology. 2011;7(6):343–354. doi: 10.1038/nrneurol.2011.63. [DOI] [PubMed] [Google Scholar]
- 24.Yokota S. Interleukin-6 as a pathogenic factor of systemic-onset juvenile idiopathic arthritis. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31(2):99–103. doi: 10.2177/jsci.31.99. [DOI] [PubMed] [Google Scholar]


