Abstract
Endocrine resistance is a significant problem in breast cancer treatment. Thus identification and validation of novel resistance determinants is important to improve treatment efficacy and patient outcome. In our work, AGR2 expression was determined by qRT-PCR in Tru-Cut needle biopsies from tamoxifen-treated postmenopausal breast cancer patients. Our results showed inversed association of AGR2 mRNA levels with primary treatment response (P = 0.0011) and progression-free survival (P = 0.0366) in 61 ER-positive breast carcinomas. As shown by our experimental and clinical evaluations, elevated AGR2 expression predicts decreased efficacy of tamoxifen treatment. From this perspective, AGR2 is a potential predictive biomarker enabling selection of an optimal algorithm for adjuvant hormonal therapy in postmenopausal ER-positive breast cancer patients.
1. Introduction
Breast cancer is the most common women's malignancy, with growing incidence primarily in advanced countries. Despite improvements in treatment, 30–40% of women are diagnosed with metastatic cancer or develop metastases and die from their disease [1]. The most important group of breast cancers is hormone sensitive tumors, characterized by expression of estrogen and progesterone receptors (ER and PgR). These tumors encompass approximately 70% of all breast cancers and are significantly clinicopathologically different from ER-negative tumors. Thus, determination of ER status is an essential part of the diagnostic procedure in breast cancer patients. The presence of ER and PgR indicates response to endocrine therapy and improved disease-free survival [2, 3]. The treatment of choice represents tamoxifen, which has been used for systemic treatment for all stages of ER-positive breast cancer during the past 30 years. Despite the undeniable benefit, approximately one third of patients with ER-positive breast cancer either do not respond to tamoxifen or develop resistance, which constitutes a serious clinical problem. Thus, identification of novel, reliable, and easily identifiable biomarkers indicating resistance to this drug is of general interest.
Recent findings suggest that AGR2 plays a prominent role in mediating pro-oncogenic signals of ER, and there is a correlation between increased AGR2 expression and poor outcome of therapy in patients with ERα-positive breast cancers [4, 5]. Historically, AGR2 mRNA was discovered as selectively expressed in ER-positive breast cancer cell lines [6]. AGR2 has been functionally characterized and shown to act as an inhibitor of the tumor suppressor p53 [7] and a mediator of metastatic spread in rodent models [8]. Data from Wang et al. have shown that AGR2 can also transform cells and mediate cell migration [9]. In our previous work, we found that AGR2 mediates a prosurvival pathway in human breast cancer cells and is involved in pro-oncogenic signals of ER. More strikingly, AGR2 expression was elevated both in vitro and in vivo in response to tamoxifen adjuvant therapy, indicating that AGR2 mediates an agonist effect of this drug [4, 10]. Although the mode of action of AGR2 after tamoxifen treatment remains to be defined, we hypothesized that AGR2 may significantly affect the development and progression of hormone sensitive breast tumors and response to anti-hormonal treatment.
2. Material and Methods
2.1. Clinical Samples and Processing
Our retrospective study includes 61 Tru-Cut needle biopsies from ER-positive invasive breast carcinomas of postmenopausal patients who received tamoxifen as primary treatment at the Masaryk Memorial Cancer Institute (MMCI) during the period 2000–2004. These patients due to advanced age (median 79 years) or comorbidities could not take any other primary treatment for their disease. More than half of the patients had locally advanced disease, which on one hand facilitated the assessment of treatment response; on the other hand, complications associated with advanced tumor resulted in the addition of local radiotherapy in 15 patients and a surgical solution in 1 patient. Detailed characteristics of the patients are given in Table 1. Biopsies were fixed in 10% formalin, embedded in paraffin wax, and stained with hematoxylin/eosin for histological examination. Clinical data including response to therapy were evaluated by oncologist from the hospital's patient records. The study was approved by the local Ethical Commission and informed consent was obtained from each patient.
Table 1.
Characteristics of patients.
| Total (n = 61) | % | |
|---|---|---|
| Age (years) | ||
| Median (range) | 79 (62–93) | |
| Age >70 | 54 | 89 |
| Performance status | ||
| Karnofsky index ≤70% | 32 | 52 |
| Histology | ||
| Invasive ductal | 52 | 85 |
| Invasive lobular | 9 | 15 |
| Clinical stage | ||
| I | 2 | 3 |
| II | 22 | 36 |
| III | 27 | 44 |
| IV | 10 | 17 |
| T4 tumors | 33 | 54 |
| N+ | 48 | 79 |
| Primary treatment | ||
| Hormonal therapy | 61 | 100 |
| Tamoxifen only | 54 | 89 |
| Tamoxifen switched to Al* | 7 | 11 |
| Radiation therapy | 15 | 25 |
| Surgery | 1 | 2 |
| Initial response to primary treatment | ||
| Responders** | 48 | 79 |
| Complete response (CR) | 6 | 10 |
| Partial response (PR) | 39 | 64 |
| Stable disease (SD) | 3 | 5 |
| Nonresponders*** | 13 | 21 |
| Progressive disease (PD) | 9 | 15 |
| Stable disease (SD) | 4 | 6 |
Annotations. *Switch of the tamoxifen to an aromatase inhibitor was carried out due to tamoxifen's side effects (mostly endometrial hyperplasia). All seven patients achieved partial remission in the course of tamoxifen therapy.
**Patients who achieved complete or partial (reduction of disease by 30% or more) remission or had long-lasting disease stabilization (stable disease for at least 33 months; median of PFS).
***Patients who never responded to primary treatment or achieved stable disease for less than 12 months during the period of primary treatment.
2.2. Treatment Evaluation
The best response recorded during the primary treatment was used for response analyses. Patients who achieved complete or partial response (reduction of disease by 30% or more) or had long-lasting disease stabilization (stable disease for at least 33 months; median of PFS) were classified as responders. Patients who never responded to primary treatment or achieved stable disease for less than 12 months were classified as nonresponders. Tumor response to tamoxifen treatment was evaluated using mammography or ultrasound. Patients whose general health status or disease state (e.g., extensive T4 tumors) did not allow these tests were examined using caliper and palpation of regional lymph nodes. Progression-free survival (PFS) was measured from the first day of tamoxifen therapy until progression or death from any cause occurred. Patients who were alive and who had not experienced disease progression, or who were lost to follow up, were censored at the date that they were last known to be alive and progression-free. Overall survival (OS) was measured from the date of diagnosis until death from any cause. Patients who have not died or who were lost to follow-up were censored when they were last known to be alive.
2.3. Reverse Transcription and Quantitative PCR
Under the supervision of an experienced pathologist, corresponding samples of tumor tissue were collected and used for extraction of total cellular RNA by TRI Reagent (MRC, Cincinnati, OH, USA). cDNA synthesis was carried out using the M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Triplicate samples were subjected to quantitative PCR analysis using TaqMan for 18S rRNA (Applied Biosystems, Foster City, CA, USA) and SYBR Green (Sigma-Aldrich, St Louis, MO, USA) for AGR2 and GAPDH. The primer pairs used for AGR2 were as follows: forward: 5′-GGAGCTCTATATAAATCCAAGACAAGCA-3′ and reverse: 5′-GCCAATTTCTGGATTTCTTTATTTTC-3′, and the primer pairs for GAPDH were as follows: forward: 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse: 5′-GAAGATGGTGATGGGATTTC-3′. PCR reaction was performed using default conditions: initial denaturation 95°C, then 40 cycles 95°C 15 sec and 60°C 1 min. To obtain absolute quantitation, dilution series of plasmids pDEST12.2 with cloned respective sequences were used in range from 2 to 20 million of copies to receive standard curves. Two different housekeeping genes 18S rRNA and GAPDH were used, to confirm experimental setup and homogeneity of biopsy samples. The results obtained in both cases were fully comparable.
2.4. Immunohistochemical Staining
Immunohistochemical (IHC) staining was performed on 4 μm thick freshly cut tissue sections. Sections were deparaffinized in xylene and rehydrated into PBS through a graded ethanol series. Endogenous peroxidase activity was quenched in 3% hydrogen peroxide in PBS for 15 minutes. Antigen retrieval was performed in citrate buffer pH 6 in 94°C for 20 minutes. The sections were incubated overnight at 4°C with either anti-AGR2 antibody (HPA007912, Sigma-Aldrich, St. Louis, MO, USA) or SP1 antibody against ER or SP2 antibody against progesterone receptor (both Lab Vision and NeoMarkers, Fremont, CA, USA). A streptavidin-biotin peroxidase detection system was used according to the manufacturer's instructions (Vectastain Ellite ABC Kit, Vector Laboratories, Burlingame, CA, USA). Signal was visualized by 3,3′-diaminobenzidine (Liquid DAB+ Substrate Chromogen System, Dako, Glostrup, Denmark). Tissue sections of small intestine and lymph node served as external positive and negative controls for AGR2.
2.5. Statistical Analysis
Fisher's exact test was used to derive P values from the 2 × 2 contingency tables. Survival analyses were performed by Kaplan-Meier method, and the differences between the survival curves were evaluated by the log-rank test to determine statistical significance levels. In all cases, a P value less than 0.05 was considered significant. The MedCalc Version 9.3.9.0 (MedCalc Software, Ostend, Belgium) was used for all calculations.
3. Results
The study included 61 postmenopausal ER-positive breast cancer patients receiving tamoxifen in first line of therapy. In particular, there were patients who could not undergo surgery due to their advanced age on date of diagnosis ranging from 62 to 93 years with median age 79 and average age 78.2 years or comorbidities (more than 52% of patients had the Karnofsky index 70% or less). More than half of the cases showed significantly locally advanced disease (54% T4 tumors, 79% had proven involvement of regional lymph nodes), which facilitated the evaluation of response to treatment. Systemic hormonal treatment by tamoxifen was accompanied by radiotherapy in 15 patients and mastectomy in 1 patient due to assessment of local tumor extent. Breast cancer was diagnosed in a fourth clinical stage in 10 patients; however, seven of them showed bone involvement only. In total, 48 patients (79%) were classified as “responders” who benefited from tamoxifen; most frequently partial regression of disease was detected. The remaining 13 patients (21%) did not respond to treatment and were classified as “nonresponders”. Detailed characteristics of the patients are given in Table 1.
AGR2 mRNA expression showed an exponential distribution (P < 0.01; Kolmogorov-Smirnov test) within the studied group of patients. Thus AGR2 mRNA expression was defined according to the median, where tumors with levels below the median were classified as the low expression group, and tumors with expression above the median were classified as high expression. There is an inverse relationship between AGR2 mRNA expression and response to tamoxifen (P = 0.0011; Fisher's exact test) (Figure 1). In parallel, immunohistochemical staining was used to determine AGR2 expression on protein level (Figure 2). IHC staining confirmed AGR2 expression in all tested samples. IHC score was calculated as the sum of the percentage of cells with weak, moderate, and strong staining with a range from 0 to 300. This scoring system has been used as it takes into account both extent of reactivity and intensity [11, 12]. Due to IHC score data distribution, ROC analysis was used to determine cut-off level for “low” and “high” AGR2 levels. Nevertheless, no significant association with treatment response was found (P = 0.3183; Fisher's exact test).
Figure 1.
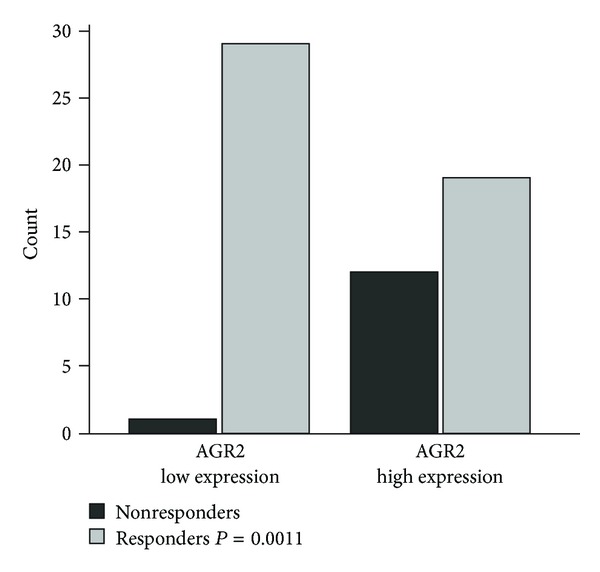
Numbers of postmenopausal breast cancer patients who responded or did not respond to primary tamoxifen treatment in relation to AGR2 expression.
Figure 2.
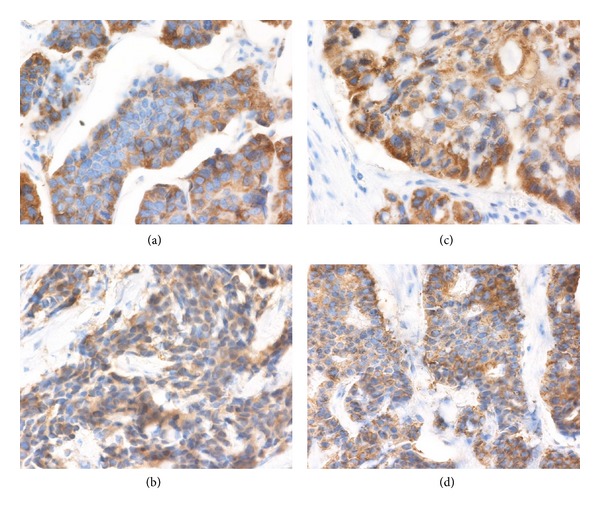
Illustration of immunohistochemical detection of AGR2 protein in Tru-Cut needle biopsies. (a) IHC score 80, (b) IHC score 100, (c) IHC score 180, and (d) IHC score 220. As cut-off level determined by ROC analysis was 110, samples (a) and (b) were classified in group denoted as “low” and AGR2 expression and samples (c) and (d) were included in group denoted as “high” AGR2 expression.
Disease progression was observed in 36 patients during or after primary treatment. Second line of therapy with aromatase inhibitors was applied in 33 of these patients. Median progression-free survival (PFS) reached 33.5 months and overall survival (OS) 52.3 months in the whole cohort of patients. Patients with low AGR2 mRNA expression showed significantly longer PFS compared to cases with elevated AGR2 expression (HR 0.57; 95% CI: 0.31 to 0.96; P = 0.0366; Figure 3(a)). Although there was a similar trend between AGR2 mRNA expression and OS, the data were not statistically significant (HR 0.69; 95% CI: 0.38–1.18; P = 0.1655). As expected, neither PFS (HR 1.34; 95% CI: 0.69 to 2.75; P = 0.3569; Figure 3(b)) nor OS (HR 1.31; 95% CI: 0.68 to 2.66; P = 0.3985) did correlate significantly with the level of AGR2 determined by IHC.
Figure 3.
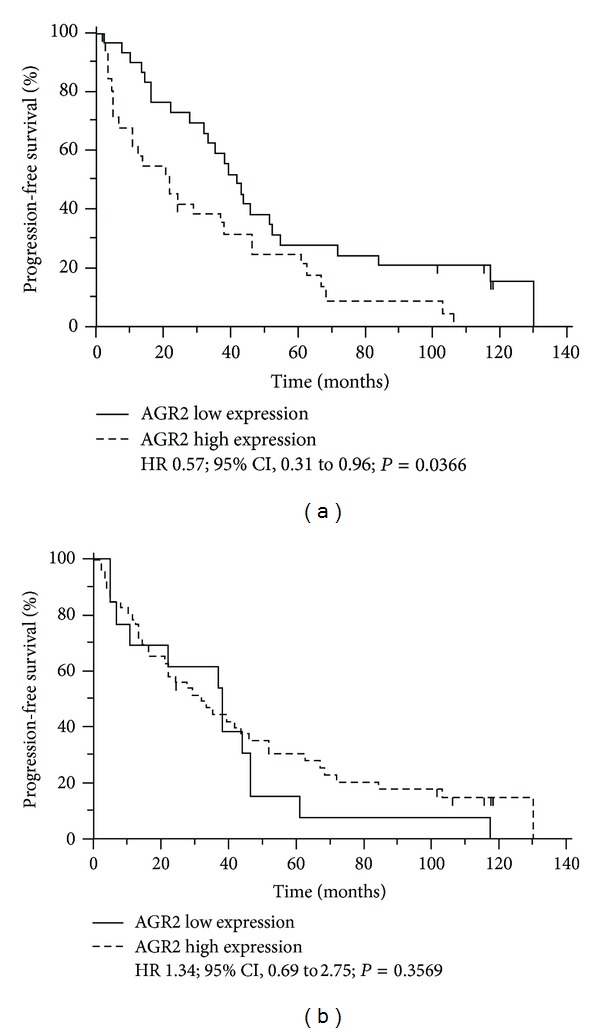
Progression-free survival of patients with low or high AGR2 tumor expression is determined using Kaplan-Meier curves. (a) Determination of PFS with respect to AGR2 mRNA levels. There were 4 censored observations in survival curve for low AGR2 mRNA expression and 2 censored observations in survival curve for high AGR2 mRNA level. (b) Determination of PFS with respect to AGR2 IHC staining. There were no censored observations in survival curve for low AGR2 IHC level and 6 censored observations in survival curve for high AGR2 IHC level. Hazard ratio (HR) with 95% CI as well as P value calculated using log-rank test is provided for both curves.
4. Discussion
Tamoxifen resistance in breast cancer patients represents the main problem limiting treatment efficacy. We previously showed in consecutive group of ER-positive breast carcinomas that elevated levels of AGR2 mRNA predict significantly shorter disease free survival [4]. Nevertheless, the direct effect of AGR2 overexpression on the sensitivity to treatment with tamoxifen needs more precise characterization. Thus, in the present work, we have investigated the expression of AGR2 in a cohort of inoperable postmenopausal breast cancer patients with respect to response to tamoxifen treatment.
We confirmed that breast cancer patients with low AGR2 mRNA expression more readily respond to primary treatment by tamoxifen compared with tumors exhibiting AGR2 overexpression (Figure 1). These data are supported by determination of PFS showing more favorable outcome in patients with decreased AGR2 mRNA levels, which underscores the importance of AGR2 as a predictive biomarker. On the other hand, statistical analysis of OS proved to be insignificant, although survival curves showed similar tendencies as PFS, which may be due to (i) old age associated with increased mortality not only due to cancer and/or (ii) the effect of second or next line of endocrine therapy using aromatase inhibitors in progressing patients.
Although the correlation between AGR2 mRNA and protein levels has been demonstrated by previous studies [4, 5], IHC staining in our cohort of patients did not show statistical association between AGR2 protein level and response to tamoxifen treatment due to semiquantitative character of the IHC staining. This is also supported by the fact that in comparison with AGR2 mRNA levels, where cut-off levels determined by median and ROC analysis were more and less the same, ROC analysis for IHC staining calculated significantly different cut-off compared to median. It is important to take into account that our cohort of sample biopsies consists of ER-positive breast carcinomas only, which show elevated AGR2 levels. IHC staining sensitivity seems to be insufficient to distinguish modest differences in AGR2 levels in our group of tumor samples. This is in fact supported by examples of IHC staining in Figure 2 showing very similar levels of AGR2 in more than half of all samples (IHC score: 80–220) within both groups divided according to AGR2 at “low” and “high”. On the other hand, we positively confirmed association of ER with AGR2 expression on both mRNA and protein levels in vivo since AGR2 was detected in all analyzed samples. In particular, we have shown that the increased expression of AGR2 detectable on mRNA level may reflect increased transcriptional activity of ER linked to tamoxifen agonistic effect predicting worse response to tamoxifen treatment [13].
Although ER expression itself is the main predictor of response to endocrine therapy, crosstalk between ER and other signaling pathways involved in regulation of cellular growth, survival, stress, and cytokine levels has been mechanistically described in resistance to endocrine agents. The clinical relevance of ER crosstalk with growth factor signaling pathways was confirmed by prospective trials in patients with metastatic disease, showing that tamoxifen resistance is associated with high expression of receptor tyrosine kinases HER2 and EGFR [14]. Interestingly, recent reports indicate that AGR2 is involved in the crosstalk between ER and EGFR [15] or PI3K/AKT [16] resulting in endocrine resistance, providing a potential mechanistic basis for our observations.
The identification of novel predictive biomarkers is essential for personalized endocrine therapies. Our data indicate that AGR2 may serve as one such biomarker with decreased AGR2 mRNA levels identifying a subset of postmenopausal breast cancer patients who respond and have clearly benefit from tamoxifen-based therapy. On the other hand, our data suggest that high AGR2 mRNA levels may predict a subset of postmenopausal breast cancer patients that are less likely to show adequate tumor growth control following tamoxifen therapy and for whom other options may therefore be more appropriate.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This work was supported by RECAMO CZ.1.05/2.1.00/03.0101, GACR P301/13/00956S, MH CZ-DRO (MMCI, 00209805), and IGA NT13794-4/2012.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Ca: A Cancer Journal for Clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Katzenellenbogen BS, Frasor J. Therapeutic targeting in the estrogen receptor hormonal pathway. Seminars in Oncology. 2004;31(1):28–38. doi: 10.1053/j.seminoncol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JFR, Cannon PM, Nicholson RI, Blamey RW. Oestrogen and progesterone receptors as prognostic variables in hormonally treated breast cancer. International Journal of Biological Markers. 1996;11(1):29–35. doi: 10.1177/172460089601100106. [DOI] [PubMed] [Google Scholar]
- 4.Hrstka R, Nenutil R, Fourtouna A, et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010;29(34):4838–4847. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- 5.Innes HE, Liu D, Barraclough R, et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. British Journal of Cancer. 2006;94(7):1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochemical and Biophysical Research Communications. 1998;251(1):111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 7.Pohler E, Craig AL, Cotton J, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Molecular and Cellular Proteomics. 2004;3(6):534–547. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Research. 2005;65(9):3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Research. 2008;68(2):492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 10.Hengel SM, Murray E, Langdon S, et al. Data-independent proteomic screen identifies novel tamoxifen agonist that mediates drug resistance. Journal of Proteome Research. 2011;10(10):4567–4578. doi: 10.1021/pr2004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abd El-Rehim DM, Ball G, Finder SE, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. International Journal of Cancer. 2005;116(3):340–350. doi: 10.1002/ijc.21004. [DOI] [PubMed] [Google Scholar]
- 12.McCarty KS, Jr., Miller LS, Cox EB, Konrath J, McCarty KS., Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Archives of Pathology and Laboratory Medicine. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 13.Hodges LC, Cook JD, Lobenhofer EK, et al. Tamoxifen functions as a molecular agonist inducing cell cycle-associated genes in breast cancer cells. Molecular Cancer Research. 2003;1(4):300–311. [PubMed] [Google Scholar]
- 14.Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clinical Cancer Research. 2004;10(17):5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 15.Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of Amphiregulin through hippo pathway co-activator YAP1 activation. The Journal of Biological Chemistry. 2011;286(20):18301–18310. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrstka R, Murray E, Brychtova V, Fabian P, Hupp TR, Vojtesek B. Identification of an AKT-dependent signalling pathway that mediates tamoxifen-dependent induction of the pro-metastatic protein anterior gradient-2. Cancer Letters. 2013;333:187–193. doi: 10.1016/j.canlet.2013.01.034. [DOI] [PubMed] [Google Scholar]


