Abstract
Interleukin-23 is a key cytokine involved in the generation of Th17 effector cells. Clinical efficacy of an anti-p40 mAb blocking both IL-12 and IL-23 and disease association with single nucleotide polymorphisms in the IL23R gene raise the question of a functional role of IL-23 in psoriasis. In this study, we provide a comprehensive analysis of IL-23 and its receptor in psoriasis and demonstrate its functional importance in a disease-relevant model system. The expression of IL-23 and its receptor was increased in the tissues of patients with psoriasis. Injection of a mAb specifically neutralizing human IL-23 showed IL-23–dependent inhibition of psoriasis development comparable to the use of anti-TNF blockers in a clinically relevant xenotransplant mouse model of psoriasis. Together, our results identify a critical functional role for IL-23 in psoriasis and provide the rationale for new treatment strategies in chronic epithelial inflammatory disorders.
Psoriasis is one of the most common epithelial inflammatory disorders and has a significant inherited component (1-3). Recent interest in psoriasis research has focused on the functional and potential therapeutic role of IL-23 (4). IL-23 binds to a heterodimeric receptor consisting of the IL-12Rβ1 and IL-23R chain (5). IL-23 is a key cytokine for the generation of peripheral effector Th17 cells (6). Expression data indicate the presence of IL-23 in psoriasis lesions (7). Treatment of patients with anti-p40 mAb targeting both IL-12 and IL-23 has demonstrated clinical efficacy in patients with psoriasis (8). Accumulating data including mouse models (9), human expression analyses (7), and genetic association studies (10, 11) provide the first indications that IL-23 plays a key role in chronic epithelial inflammation of the gut and skin. However, it is currently unclear if IL-23 plays a functional role or represents a potential therapeutic target in psoriasis.
In this study, we investigate the expression and function of IL-23 and its receptor in human psoriasis tissue samples, as well as in an in vivo animal xenograft model of human psoriasis. We provide functional evidence for a key role of IL-23 in psoriasis with significant implications for future IL-23–targeted therapy.
Materials and Methods
Xenotransplantation model
Xenotransplantation experiments and assessment of samples were performed as published (12). Dosage and schedule of Ab administration were based on previous publications (13) and administered as follows: 1 mg anti-human anti–IL-23 (mouse IgG1, clone 7G10, kindly provided by Schering-Plough Biopharma/Merck Research Laboratories, Palo Alto, CA) and 1 mg murine isotype-matched Ab (mouse IgG1, clone 27F11, kindly provided by Schering-Plough Biopharma, Palo Alto, CA) were both administered s.c. every second week starting at day 7; 1 mg infliximab (anti-human TNF-α mAb, Remicade, Centocor Ortho Biotech, Horsham, PA) was administered i.v. every second week starting at day 7. All patients gave their informed consent. Animal studies were approved by the Kantonale Veterinäramt of Zurich, Switzerland, and human studies by the Ethical Committee of the Kanton Zurich and the Guy’s Hospital Research Ethics Committee.
Flow cytometry analysis and immunohistochemistry
Cell isolation was performed as previously described (13). Cells were stained using anti-human CD8 (BD Pharmingen, San Jose, CA), CD4 (BD Pharmingen), CD3 (BD Pharmingen), HLA-DR (BD Pharmingen), CD11c (BD Pharmingen), IL-23R (R&D Systems, Minneapolis, MN), pan cytokeratin (Gene-Tex, Irvine, CA), IL-23 (kindly provided by Schering-Plough Biopharma/Merck Research Laboratories), or isotype-matched control mAbs.
Quantitative RT-PCR
RNA extraction and PCR were performed as previously described (14). Primer sequences used were designed for human IL-23 (5′-CAGCAACCCTGAGTCCCTAA-3′, 5′-TCAACATATGCAGGTCCCACT-3′) and for human GAPDH (5′-ATTGCCCTCAACGACCACTTTG-3′, 5′-TTGATGGTACATGACAAGGTGCGG-3′).
Statistical analysis
Values are expressed as the mean ± SD. Comparisons were calculated by unpaired t test or ANOVA followed by Bonferroni-corrected p value for multiple comparisons.
Results and Discussion
Analysis of IL-23 and IL-23R in psoriatic tissue
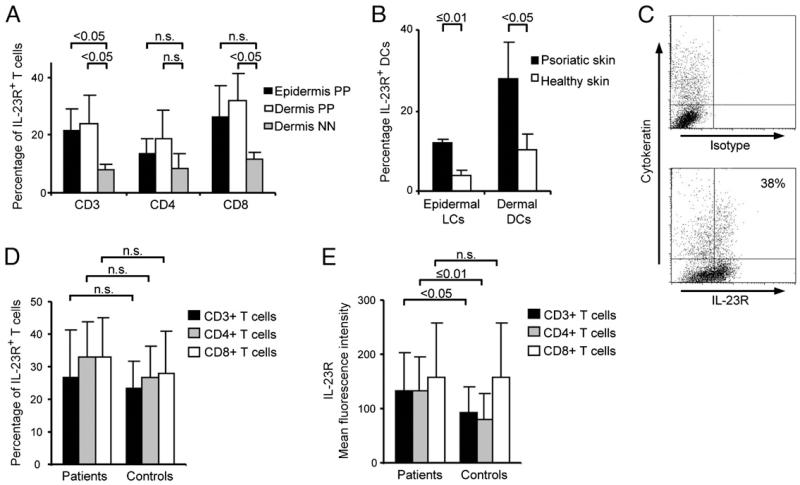
To explore IL-23 expression in psoriatic tissues, we first isolated RNA from lesional and symptomless noninflamed (PN) tissue samples from patients with psoriasis comparing expression of IL-23p19, IL-12p40, and IL12-p35 transcripts. We found expression of all three transcripts with high expression in lesional compared with PN skin, in line with previous data (7, 15, 16) (data not shown). Immunohistochemical analysis confirmed expression of IL-23 in psoriatic skin (Supplemental Fig. 1). The major cell type expressing IL-23 appeared to be cells with dendritic shape in the upper dermis. Although expression of human IL-23 has been reasonably well studied in normal and diseased skin, less is known about the expression of IL-23R. We therefore studied the expression of the IL-23R in different cell types in psoriatic skin and blood. IL-23R was expressed on both psoriatic immune cells and keratinocytes (KCs) as assessed by flow cytometry analysis of freshly isolated cell suspensions. Expression of IL-23R was significantly increased on epidermal (p < 0.05) and dermal (p < 0.05) psoriatic CD3+ and dermal CD8+ (p < 0.05) T cells when compared with dermal CD3+ and dermal CD8+ T cells of healthy individuals (Fig. 1A). There was also an increased expression of IL-23R on psoriatic dermal dendritic cells (DCs) (p < 0.05) and psoriatic epidermal Langerhans cells (p ≤ 0.01) compared with respective DC populations isolated from skin of healthy donors (Fig. 1B). Representative flow cytometry analysis of IL-23R expression in epidermal cell suspension isolated from psoriatic plaque lesions demonstrated expression of IL-23R on psoriatic and normal KCs (Fig. 1C). KCs isolated from the skin of psoriatic patients did not show a statistically significant difference of IL-23R expression (mean 37 ± 11.2; n = 3) compared with KCs of healthy individuals (mean 23 ± 9.47; n = 3) (Supplemental Fig. 2).
FIGURE 1.
Expression of IL-23R in psoriatic patients and healthy donors. Significantly increased levels of IL-23R expression were detected on epidermal (n = 3) and dermal (n = 3) psoriatic CD3+ and dermal CD8+ T cells when compared with dermal CD3+ and CD8+ lymphocytes of healthy individuals (n = 3) (A). Dermal DCs (n = 4) and epidermal Langerhans cells (LCs; n = 3) isolated from the skin of psoriatic patients expressed significantly higher IL-23R levels than DCs isolated from skin of healthy donors (B). Representative flow cytometry analysis of IL-23R expression in epidermal cell suspension isolated from psoriatic plaque lesions (n = 3; see Supplemental Fig. 2) (C). Expression of IL-23R on peripheral blood T cells of psoriasis patients and healthy controls. Percentage and MFI of IL-23R–expressing CD3+, CD4+ and CD8+ T cells was analyzed in peripheral blood of patients with psoriasis (n = 17) and healthy controls (n = 33). Percentage of IL-23R–expressing cells did not show statistically significant differences between patients with psoriasis and healthy controls (D). MFI of IL-23R expression was significantly higher on CD3+ and CD4+ T cells of patients with psoriasis than on those of healthy controls (E). NN, skin from healthy individuals; PP, lesional psoriatic skin.
In peripheral blood, there was no significant difference in numbers of IL-23R–expressing CD3+, CD4+, or CD8+ T cells between psoriasis patients and healthy controls (Fig. 1D). Recently, Kagami and colleagues (17) described an increase in IL-23R expression on CD4+ T cells in peripheral blood of psoriasis patients as compared with healthy controls. The evaluated psoriasis population in the study by Kagami and colleagues (17) had a mean Psoriasis Area and Severity Index of 22; our patient group had a mean Psoriasis Area and Severity Index of 10. This difference in disease severity might explain the discrepancy in the results obtained. However, we found significantly higher median fluorescence intensity (MFI) of IL-23R expression levels on CD3+ (p < 0.05) and CD4+ T cells (p ≤ 0.01) of patients with psoriasis (Fig. 1E).
Kinetics of IL-23 expression in developing psoriasis lesions
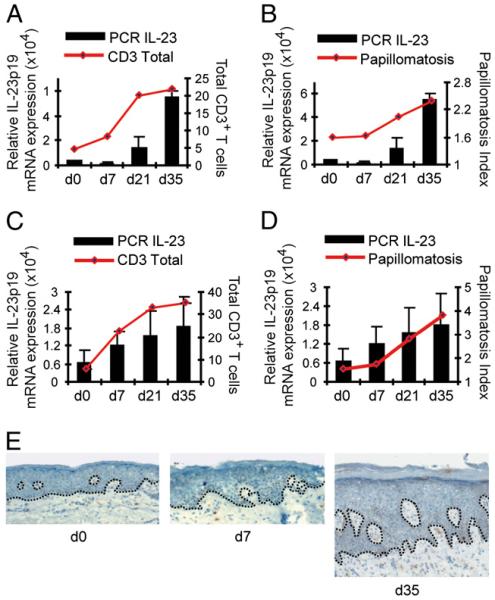
We next sought to put our tissue expression data into a clinically relevant functional context. We have recently established a model system using immunosuppressed AGR129 (RAG−/−, IFNRI−/−, IFNRII−/−) mice transplanted with clinically symptomless grafts from patients with psoriasis (12). These human skin grafts spontaneously develop typical features of psoriasis postgrafting over the course of 4–6 wk, including thickening of the epidermis (acanthosis), elongation of the rete ridges (papillomatosis), and increased numbers of dermal and epidermal T cells, closely reflecting the pathology of patient samples. Our model has been shown to be dependent on CD3+ T cells and TNF (12) and has been used to functionally assess relevant immunological pathways in psoriasis (13, 14). We first assessed the temporal regulation of IL-23 production during psoriasis graft development and found a kinetic course compatible with low initial IL-23 production followed by a steady increase that was correlated to the development of epithelial pathology (as indicated by the Papillomatosis Index) and expansion of CD3+ T cells (Fig. 2). Thus, IL-23 seems to have a unique expression profile distinct from the initial burst of IFN-α production in the early phases of psoriasis (13). IL-23 production seems to be occurring later and in a more sustained fashion, coinciding with the development of typical epithelial and immune features of psoriasis.
FIGURE 2.
IL-23 expression correlates with onset of psoriatic phenotype and expansion of T cells. Kinetics of human IL-23p19 mRNA expression (normalized to human GAPDH) during psoriasis development were compared with the number of total CD3+ T cells (A, C) and papillomatosis indices representing psoriatic epithelial pathology (B, D). Grafts were analyzed on the day of transplantation and after 7, 21, and 35 d. RNA was extracted from skin grafts, and quantitative RT-PCR was performed using human specific primers for IL-23p19. Two independent experiments were performed with skin from two different patients (first patient: A, B; second patient: C, D). Immunohistochemistry staining of xenotransplants for IL-23 at days 0, 7, and 35 (E). Original magnification ×100.
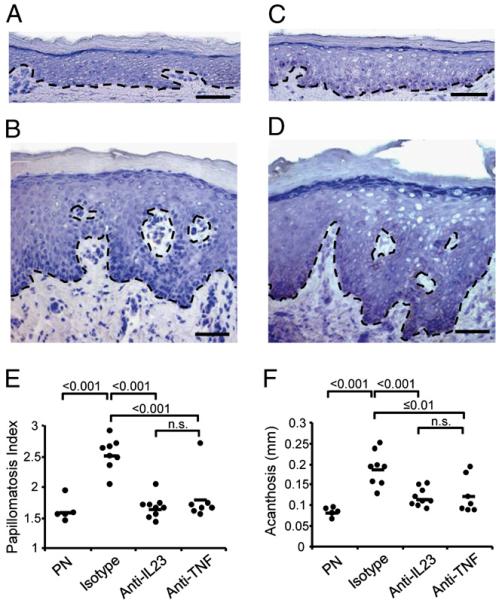
Anti–IL-23–specific mAb blocks psoriasis development in a clinically relevant psoriasis model
To further elucidate the role of the IL-23 pathway in our model, we took advantage of a human IL-23–specific mouse mAb that does not cross-react with mouse IL-23. The mAb was injected s.c. at days 7 and 21 and its effect compared with isotype control treatment and our current benchmark anti-TNF mAb (infliximab). Fig. 3 shows a microscopic view of PN skin on the day of transplantation (day 0, Fig. 3A) and lesional psoriatic skin of the same patient (Fig. 3B). Isotype control-treated PN grafts developed psoriasis over the course of 35 d (Fig. 3D). Injection of anti-human IL-23 mAb significantly blocked the development of psoriasis, producing a microscopic picture (Fig. 3C) that was nearly indistinguishable from symptomless skin at day 0 (Fig. 3A). Papillomatosis index (Fig. 3E) and acanthosis (Fig. 3F) were quantified in skin grafts prior to transplantation onto AGR129 mice and posttreatment with either anti-human IL-23 mAb or isotype-matched control Ab. There was a statistically significant reduction of psoriasis acanthosis (p < 0.001) and papillomatosis index (p < 0.001) in grafts of mice treated with anti-human IL-23 mAb compared with isotype control-treated mice. The efficacy of the treatment with anti-human IL-23 mAb in blocking psoriasis development was comparable to anti-TNF mAb (infliximab) treatment (Fig. 3E, 3F). To assess the impact of blocking the IL-23 pathway on the immune compartment, we quantified total human CD3+ T cells, including dermal versus epidermal CD3+ T cells in PN skin grafts pretransplantation, 35 d posttransplantation, and treatment with either anti-human IL-23 mAb, isotype-matched control, or anti-TNF mAb (infliximab). We observed a statistically significant reduction of CD3+ total T cells per high-power field (p < 0.001) in grafts treated with anti-human IL-23 mAb (mean 2.23 ± 1.4) compared with isotype control-treated mice (mean 9.4 ± 2.5) (Supplemental Fig. 3A). Treatment with anti-human IL-23 mAb led to a significant reduction of both CD3+ epidermal and dermal T cells in grafted skin, which was especially pronounced in the epidermal compartment (Supplemental Fig. 3B). It is noteworthy that the effect of anti-human IL-23 mAb was as pronounced as anti-TNF mAb, one of the current benchmark treatments of psoriasis (18). Intriguingly, after both treatments with either anti–IL-23 or anti-TNF mAb, not only T cell numbers but also IL-23R expressing T cells in the skin decreased significantly (Supplemental Fig. 3C). These findings indicate a profound effect of blocking the IL-23 pathway on both the epithelial as well as the immunological component of psoriasis in a clinically relevant humanized model system. With IFN-γ as one of the major cytokines in psoriasis, IL-12 might also play an important pathogenic role by inducing Th1 cell response (19, 20). However, recent evidence suggests that it is rather IL-23 that represents a key cytokine in psoriasis pathogenesis by driving Th17 cell-mediated skin inflammation. Interestingly, neither IFN-γ nor IL-17 gene expression showed any variation after anti–IL-23 treatment in our mouse model, whereas the Th17 cytokine IL-22 was expressed at very low levels but showed a downward trend with anti–IL-23 treatment (Supplemental Fig. 4). Although not significant, these findings were in line with recent studies showing that IL-22 is particularly important for IL-23–induced skin inflammation and acanthosis (21, 22).
FIGURE 3.
Blockade of IL-23 prevents development of psoriasis. Microscopic view of PN skin on the day of transplantation (A) and lesional psoriatic skin of the same patient (B). Uninvolved skin 5 wk posttransplantation onto AGR129 mice treated with anti-human IL-23 mAb (C) or isotype control Ab (D). Original magnification ×100. Papillomatosis indices (E) and acanthosis (F) in skin grafts pretransplantation onto AGR129 mice and after 35 d treatment with either anti-human IL-23 or isotype-matched control Ab. There was a statistically significant reduction of psoriasis papillomatosis indices (p < 0.001) and acanthosis (p < 0.001) in grafts of mice treated with anti-human IL-23 as compared with isotype control-treated mice. The efficacy of the treatment with anti-human IL-23 in inhibiting psoriasis development was comparable to anti-TNF (infliximab) (E, F). Dots represent independently grafted mice samples. Scale bars in A–D, 20 μm.
Encouraging results have been obtained using anti-p40 mAb targeting both IL-12 and IL-23 in psoriasis (8); more selective targeting of the IL-23 pathway might be considered advantageous in future risk/benefit evaluations of biological treatment approaches in chronic inflammation.
Taken together, our findings provide functional evidence for a major role of the IL-23 pathway in the pathogenesis of psoriasis and support therapeutic approaches specifically targeting IL-23 in cutaneous inflammation and potentially other chronic epithelial inflammatory disorders.
Supplementary Material
Acknowledgments
We thank C. Dudli for excellent technical assistance and D. Mihic for help with immunohistochemistry staining in Supplemental Fig. 1A and Fig. 3E.
This work was supported by Wellcome Trust Programme GR078173MA, National Institutes of Health Grant RO1AR040065, Medical Research Council UK Programme G0601387, Dunhill Medical Trust, Bonizzi-Theler Foundation, Hermann Klaus Foundation, Hartmann Müller Foundation, and Schering-Plough Biopharma/Merck Research Laboratories. This work was also supported by the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St. Thomas’ National Health Service Foundation Trust in partnership with King’s College London and King’s College Hospital National Health Service Foundation Trust.
Abbreviations used in this paper
- DC
dendritic cell
- KC
keratinocyte
- LC
Langerhans cell
- MFI
median fluorescence intensity
- NN
skin from healthy individuals
- PN
symptomless noninflamed
- PP
lesional psoriatic skin
Footnotes
Disclosures
T.K.M., W.M.B., E.O., and R.K. are employees of Merck Research Laboratories (formerly Schering-Plough Research).
The online version of this article contains supplemental material.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, Voorhees JJ. Psoriasis, T cells and autoimmunity. J. R. Soc. Med. 1996;89:315–319. doi: 10.1177/014107689608900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Invest. Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 5.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB, PHOENIX 1 study investigators Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 9.Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J. Immunol. 2003;170:5438–5444. doi: 10.4049/jimmunol.170.11.5438. [DOI] [PubMed] [Google Scholar]
- 10.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, Timms K, Gutin A, Abkevic V, Burden AD, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 11.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, Nestle FO. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 15.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 16.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J. Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 17.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE, EXPRESS study investigators Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 19.Nestle FO, Conrad C. The IL-12 family member p40 chain as a master switch and novel therapeutic target in psoriasis. J. Invest. Dermatol. 2004;123:xiv–xv. doi: 10.1111/j.0022-202X.2004.23488.x. [DOI] [PubMed] [Google Scholar]
- 20.Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J. Dermatol. Sci. 2009;54:99–105. doi: 10.1016/j.jdermsci.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 22.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.