In recent years the group of arboviruses has been attracting considerable interest as causative agent of emerging and reemerging infectious diseases in humans. The importance of arboviruses for transfusion medicine has been discussed in an earlier publication of this working group [1]. Especially West Nile virus (WNV), a member of the family of Flaviviridae, has been gaining particular attention during the last years. Since its first description in 1940 this pathogen had been assumed to have a broad geographical distribution (Africa, Asia, Middle East, Southern Europe and Australia (with subtype Kunjin virus)), but usually its pathogenicity has been considered to be low or moderate. This perception changed in 1999 when WNV was first detected in the USA, and infections with this virus induced severe human central nervous system disorders, some of them with fatal outcome. This led to a reassessment of previous WNV outbreaks in Europe and an intensification of the epidemiological surveillance. Since then thorough investigations have been addressing the epidemiology and pathogenesis as well as the biological properties of the virus.
1. Current Knowledge about the Pathogen
WNV is grouped to the arboviruses (arthropod-borne viruses) that are able to propagate both in arthropods (e.g. mosquitoes, ticks) and in vertebrates (e.g. birds and mammals). Such viruses can be transmitted by infected arthropods, especially mosquitoes, during their blood meal on vertebrates. WNV was first isolated in 1937 from the blood of a febrile female patient who was examined in the context of a study of sleeping sickness in the West Nile District of Uganda [2]. After the region where it had first been observed this virus was named West Nile virus. WNV is neurotropic in mice and is a member of the genus Flavivirus within the family of Flaviviridae. The type species of this virus family is the yellow fever virus (YFV; Latin flavus = yellow). There is a close antigenic relationship of WNV to other members of the genus Flavivirus, and WNV is grouped to the Japanese encephalitis virus (JEV) antigen complex because of a pronounced cross-reactivity in serological assays (table 1). This group includes a number of important human and animal pathogens such as the eponym JEV, the St. Louis encephalitis virus (SLEV), the Murray Valley encephalitis virus (MVEV), the Australian WNV variant Kunjin virus (KUNV), and the Usutu virus (USUV) [3]. Additional viruses are assigned to the JEV antigen complex; however, little is known about the relevance of these pathogens for humans and animals (table 1).
Table 1.
Japanese Encephalitis Virus (JEV) Antigen Complex (Serogroup)
| Virus | Main areas of circulation | Human diseases | Animals infected | Reservoirs | Vector (WNV isolation) |
|---|---|---|---|---|---|
| Japanese Encephalitis Virus (JEV) | South East Asia, India, Japan, Korea, Philippines | fever, encephalitis, meningitis | pigs, horses | pigs, birds | mosquitoes |
| West Nile Virus (WNV) | worldwide | fever, encephalitis, meningoencephalitis | birds, mammals, reptiles | birds | mosquitoes (ticks) |
| Murray Valley Encephalitis Virus (MVEV) | Australia | fever, encephalitis, meningoencephalitis | sheep, birds | water fowl | mosquitoes |
| St. Louis Encephalitis Virus (SLEV) | USA, Central and South America | fever, encephalitis | none known | birds | mosquitoes |
| Usutu Virus (USUV) | Africa, Austria, Hungary, Southern Europe, Germany | fever, encephalitis in immunosuppressed individuals | birds | rodents, birds | mosquitoes |
| Rabensburg Virus (RABV) | Austria/Czech Republic | none known | none known | unknown | mosquitoes |
| Koutanga Virus (KOUV) | Senegal, Somalia, Central African Republic | none known | none known | (rodents) | mosquitoes, ticks |
| Alfuy Virus (ALFV) | Australia | fever | none known | birds | mosquitoes |
| Yaounde Virus (YAOUV) | Cameroon, Senegal, Central African Republic, DR Congo | none known | none known | (rodents) | mosquitoes |
| Cacipacore Virus (CPCV) | Brazil | (fever) | (horses) | birds | unknown |
At present, WNV is geographically the most prevalent mosquito-transmitted virus, and WNV infections are being observed on all five continents. The natural transmission cycle runs between ornithophilic mosquitoes (vector) and birds that serve as reservoir or amplifying hosts. Mosquitoes become infected during blood meals on viremic birds and can transmit the virus to other birds during subsequent blood meals. Humans and mammals that are infected by mosquito bites can develop encephalitis or meningitis but are considered as dead-end hosts. Until about 1999 it had been assumed that the different WNV circulating in Africa, Europe, Asia, and Australia cause generally either asymptomatic infections or induce only mild courses of disease in humans and horses. Only occasionally severe courses of disease had been observed. This had been assumed for both WNV lineages. WNV lineage 1 circulated in Europe, the Mediterranean basin, Asia and Australia, and lineage 2 was first described in South Africa in 1974 in an outbreak of febrile illness in humans and horses [4, 5, 6, 7]. After the introduction of WNV into the USA in 1999 severe courses of disease in humans were frequently observed.
1.1 Characteristics of WNV
WNV is characterized by its property to replicate in arthropods (mosquitoes, ticks), reptiles (including alligators), birds, and mammals (including humans and horses). This requires an adaptation to the temperature of the respective infected host. In mosquitoes as poikilothermic hosts WNV is able to replicate at an ambient temperature as low as 14 °C, whereas in febrile birds WNV grows at temperatures as high as 45 °C [8, 9].
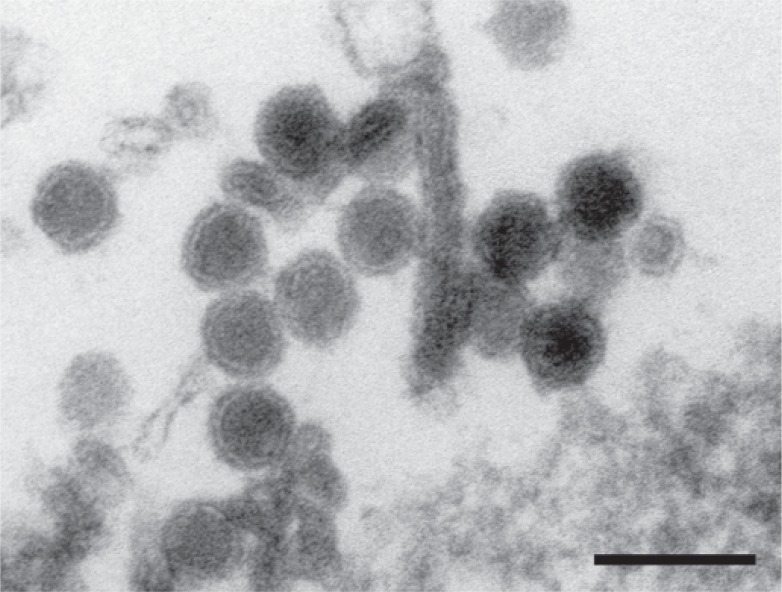
WNV is an enveloped virus; its lipid membrane is derived from membranes of the endoplasmic reticulum (ER). The icosahedral virus particle has a diameter of about 50 nm and contains the capsid with a diameter of about 30 nm [10] (fig. 1). Like other flaviviruses, the capsid encloses the positive-strand RNA genome with a size of about 11 kb.
Fig. 1.
Ultra-thin section of a West Nile Virus-infected cell culture. Enveloped virus particles with the viral capsid are visible (negative contrast with uranyl acetate). The bar represents 100 nm. EM micrograph by Dr. H. R. Gelderblom, Robert Koch-Institut.
In the infected cell the viral genome serves as mRNA for the synthesis of viral structural and non-structural (NS) proteins and is infectious like all other positive-strand RNA genomes of viruses (except for retroviruses). Like all eukaryotic mRNA, the 5′-end of the genome has a cap structure which is important for the stability of the mRNA and for the translation onto the ribosome. Unlike cellular mRNA, the viral genome is not polyadenylated at the 3′-end.
The genome encodes a polyprotein with a size of about 3,300 amino acids (fig. 2). The first three genes encode the structural proteins: the capsid protein (C), the precursor membrane protein/membrane protein (prM/M) and the envelope protein (E). The structural proteins are necessary for the formation of virus particles, while the seven non-structural proteins are involved in viral replication and assembly of the virus particles. The 5′- and the 3′-ends of the genome are flanked by untranslated regulatory sequences (UTR, untranslated region). Host-cell furin-like proteases are responsible for the cleavage of the polyprotein precursor at the junctions of C/prM, prM/E as well as E/NS1, while the viral NS3 serine protease processes the other viral proteins. NS2B serves as a cofactor for the activity of NS3. The full-length NS3 protein has further enzymatic activities, a 5′-RNA triphosphatase (RTPase), a nucleoside triphosphatase (NTPase), and an ATP-dependent RNA helicase. NS5 encodes the RNA-dependent RNA polymerase as well as an N-7- and a 2′O-methyltransferase (MTase) which are necessary for the methylation of the 5′-RNA cap structure. The multifunctional NS3 protein as well as the NS5 protein represent suitable targets for the development of antiviral drugs [11]. NS1 is secreted in large quantities from infected cells and induces an immune response in infected individuals [12]. The NS1 protein inhibits signalling of the innate immunity, facilitating the spread of the virus in the organism [13]. NS2A is involved in virus assembly. Furthermore NS2A inhibits activation of the IFN-β promoter, while NS4B blocks the IFN response. NS4A modifies the structure of the ER and enables efficient replication of the virus [14, 15]. All structural and NS proteins are required for efficient replication of WNV.
Fig. 2.

Genome structure of WNV. C = Capsid protein, prM = precursor of the membrane protein, E = envelope protein, NS = non-structural protein, UTR = untranslated region.
The infectious virus particle contains three structural proteins, the core or capsid protein C, the envelope protein E and the membrane protein M. The outer layer of the virus consists of E proteins which form homodimers. Crystal structure analysis showed that the E protein has three structural domains, like in other flaviviruses. The domain III is involved in cell receptor binding and induces the majority of neutralizing antibodies [16].
The virus particle attaches to the cell with the viral envelope protein E; the receptors on the cell that are essential for attachment and entry into the cell have not yet been characterized in detail. Depending on the target cell, WNV can use several different cell surface receptors.
After uptake into the cell by receptor-mediated endocytosis, the particles are transported to the endosomes. The acidic environment of the endosome induces a conformation change of the E protein, enabling the fusion of the viral envelope with the membrane of the endosome and the release of the capsid into the cytoplasm [17]. The viral genome serves as mRNA for the synthesis of the viral precursor protein and as template for the synthesis of the negative-strand RNA intermediate that is used by the viral RNA-dependent RNA polymerase for the synthesis of the genomic RNA. After replication of the genome and synthesis of viral proteins, virus particles are assembled, and the maturation of the virions takes place at the membranes of the ER. Mature virus particles are released from the cell by exocytosis [18, 19]. During virus maturation prM is cleaved by a cellular furin-like protease in the ER, resulting in an M protein that is approximately 70 amino acids long and the pr peptide that remains associated with the virus particles in the slightly acidic environment of the cell. After release of the virus particles into the neutral environment the pr peptide dissociates from the virus particles, and a conformational change of the E protein takes place. The mature virus particle contains 90 E homodimers and 180 M protein molecules.
Analyses of the mature virus particles by cryo-electron microscopy showed a relatively smooth surface of the virus particles, which is formed by the 90 anti-parallel E homodimers [10]. Only mature flavivirus particles are infectious [20, 21]. However, it could be shown that cleavage of the prM protein during virus maturation is incomplete and that the virus particles partly express prM determinants on their surface. Thus the released virus particles form a heterogeneous population containing both mature and immature virus particles as well as particles containing both cleaved and uncleaved prM [22]. In infected individuals antibodies against the prM protein are formed which can bind to immature virus particles. Such virus-antibody complexes can enter the cell by Fc receptor-mediated uptake and infect cells [23]. In further studies it was shown that antibodies against prM enable infection not only in vitro but also in vivo, as antibody-opsonized immature virus particles were infectious to mice, causing disease and death of the animals [24]. If and to what extent the immune response against prM determinants influences the course of disease in humans is unclear. Furthermore, it is discussed that formation of immature virus particles could be a strategy of flaviviruses to evade the immune response of the infected host [25]. For the development of efficient prophylactic vaccines it is necessary to understand the role of the different virus-specific proteins in the immune response and in pathogenesis.
Phylogenetic analysis of WNV isolates obtained from different species shows that variants of WNV with different pathogenic potential circulate which can be differentiated at the genome level (table 2).
Table 2.
WNV lineages: differences in genome sequence and pathogenicity
| WNV | Area of circulation | Diseases in humans | Infection in animals | Isolated from | |
|---|---|---|---|---|---|
| Lineage 1 (clade 1a) [27] | worldwide | fever, meningitis, encephalitis, acute flaccid paralysis (AFP) | birds, reptiles, mammals | humans, mosquitoes, birds, mammals, reptiles | |
| Lineage 1 (clade 1b) [28] | Australia (Kunjin) | fever, meningitis, encephalitis | birds | mosquitoes, humans | |
| Lineage 2 [7] | South and Central Africa, Madagascar, Europe | fever, meningitis, encephalitis | birds, horses | mosquitoes, birds, horses, humans | |
| Potentially novel lineages | |||||
| Lineage 3 (RABV) [29] | Austria/Czech Republic | unknown | unknown | Culex pipiens | |
| Lineage 4 [30] | Russia | unknown | unknown | Dermacentor marginatus | |
| Lineage 5 (lineage 1 clade 1c) [31] | India | fever, meningitis, encephalitis | fruit bats | mosquitoes, humans, bats | |
| Lineage 6 (KUNV) [28] | Malaysia | fever, meningitis, encephalitis | birds | mosquitoes, humans | |
| Lineage 7 [32] | Spain | unknown | unknown | Culex pipiens | |
Of particular interest is the finding that individual WNV isolates differ in their growth properties in cell cultures at higher temperatures (44 °C) [26]. Therefore, it is possible that such changes in growth behavior have an impact on the spread of the pathogen in the arthropod vectors as well as in the reservoir hosts.
Previously it had been assumed that all WNV highly pathogenic for humans and animals could be grouped in the phylogenetic lineage 1. However, in recent years it was reported that WNV lineage 2 isolates from South Africa and from Europe could also induce severe courses of disease in humans and in horses [33, 34, 35, 36].
An extension of the original classification of WNV into lineages 1 and 2 is being discussed. It was proposed that an isolate (Rabensburg virus, RABV) detected only in mosquitoes (Culex pipiens) at the border of Austria and the Czech Republic [29] should be classified as WNV lineage 3. Furthermore, a WNV isolate from ticks in the Caucasus differs considerably from lineage 1 and 2 viruses and was therefore tentatively classified as lineage 4 [30]. Phylogenetic analysis of complete genome sequences of human and mosquito isolates from India suggests that these isolates, originally classified as lineage 1 clade 1c, might represent WNV lineage 5 [31]. Originally, the Malaysian WNV isolate was grouped as KUNV. However, phylogenetic analysis shows that there are significant sequence differences between the Malaysian isolate and KUNV that justify a classification as WNV lineage 6 [28]. From a pool of Culex pipiens in Spain, a WNV was isolated that can be phylogenetically differentiated from all WNV sequenced previously and could therefore be assigned to a new lineage 7 [32]. Further studies on the molecular epidemiology of various viruses isolated from humans, animals and arthropods are necessary to obtain information about the pathogenic potential of WNV.
1.1.1 Stability
WNV is thermolabile and, like other flaviviruses, is inactivated rapidly by heat. In serum treated for 30 min at 56 °C, infectious virus was no longer detectable [37]. At low temperatures (4 °C) WNV was stable for more than 96 h, and at 28 °C the titer of the virus decreased by a factor of 103 in the same period [38]. In erythrocyte concentrates experimentally contaminated with WNV, infectious virus could be detected after storage for 42 days at low temperature (1–6 °C) [39]. Treatment with detergent or low pH, similar to that used in the production of plasma products, inactivated WNV below the limit of detection [40].
1.2 Infection and Infectious Diseases
Humans are infected by mosquitoes that take their blood meal on both birds and humans. Serological studies have shown that the majority of infections (approximately 80%) remain asymptomatic [41, 42]. After an incubation period of 2–14 days, about 20% of infected individuals develop a self-limiting febrile illness (West Nile fever; WNF), which lasts about 3–6 days. The following clinical symptoms are observed: malaise, headache, eye and muscle pain, nausea, vomiting, diarrhea, anorexia, rash on legs, arms and body, swollen lymph nodes, and fatigue. Predominant are the sudden onset of fever and symptoms of a flu-like infection. Only about 1% of infected persons fall seriously ill with neurological symptoms (meningitis, encephalitis, paresis or paralysis with poliomyelitis-like symptoms (acute flaccid paralysis, AFP), for review articles see [43, 44, 45]).
The onset of WNV-caused meningitis (WNM) and encephalitis (WNE) is similar to other virus-induced neurological diseases, beginning with fever, headache, neck stiffness, and photophobia. Altered mental status is observed frequently, such as stupor and disorientation right up to coma. Furthermore, poliomyelitis-like symptoms like AFP (West Nile poliomyelitis, WNP) and other neurological symptoms (ataxia, optic neuritis, extrapyramidal symptoms, cranial nerve damage, polyradiculitis, spinal cord inflammation, or seizures) are diagnosed [46, 47]. In previous outbreaks in the USA, Romania, Israel, and Russia (Volgograd) the proportion of fatal outcomes of WNV infections in hospitalized persons with neurological symptoms was between 4 and 14% [48]. Risk factors for a severe course of disease with neurological symptoms are, in addition to age (>50 years), diabetes mellitus, hypertension, liver disease, and immunosuppression as well as genetic host factors [49].
Long-term sequelae can occur as a result of WNV infection [50]. Even months after clinical recovery, about half of the patients suffering from WNM, WNE, WNP, or WNF complained about health problems such as fatigue and muscle pain, concentration and memory are impaired [50, 51].
Experimental infection of hamsters showed that WNV can persist in tissues of these animals and that infectious WNV is excreted in the urine. It was also reported that WNV could be detected in the urine of humans during the acute phase [52]. It is discussed controversially whether WNV persists in convalescent patients and whether virus can be excreted in the urine [53, 54]. WNV could be detected in the brain of patients who died during the acute phase of WNV infection, but not in persons who died several months after onset of symptoms. However, persistence of WNV for months could be determined in a patient with meningoencephalitis suffering from B-cell lymphoma as well as in patients with therapy-related immunosuppression [55].
Immunocompromised patients have a higher risk of a more severe course of disease. Approximately 40–60% of immunosuppressed transplant patients developed a severe neurological disease as a result of WNV infection, regardless of whether the infection had been acquired with the organ, by transfusion, or by mosquito bite [56, 57, 58, 59]. For these patients, the risk of developing a severe course of disease is about 40–60 times higher than for the general population.
Both viral and host factors play an important role for the course of the disease. The host innate immunity is of great importance for the control of pathogens. In the infected organism various chemokines and chemokine receptors are involved in controlling the pathogen [49]. Persons who have a deletion in the gene encoding the chemokine receptor 5 (CCR5δ32) and thus have a loss of the CCR5 function, develop severe symptoms after WNV infection [60, 61]. Furthermore, mouse experiments emphasize that CCR5 is necessary for an effective immune response against WNV and other flaviviruses [62]. This is in contrast to infection with HIV, where CCR5 serves as a major coreceptor for HIV, and persons with CCR5δ32 are protected against infection with M-tropic HIV-1 [63]. It is yet unknown whether CCR5 antagonists, used as therapeutic drugs against HIV replication, influence an infection with WNV [64].
The European Centre for Disease Prevention and Control (ECDC) developed case definitions that define general criteria for the diagnosis of infectious diseases and their pathogens including WNV (http://ec.europa.eu/health/ph_threats/com/docs/1589_2008_en.pdf).
1.3 Epidemiology
After its first description in 1940, it had long been believed that WNV circulating in Africa, Asia, and Australia generally cause asymptomatic infections or diseases with mild symptoms [5]. However, since the mid-1990s researchers have been reporting outbreaks associated with severe neurological diseases in Europe (Romania and Russia) and Israel [27, 65]. WNV achieved particular importance when it was isolated in New York in 1999 for the first time from diseased birds and humans. Starting in New York, WNV has spread across the USA and southern Canada within 3 years [53]. In subsequent years, WNV was then demonstrated in the Caribbean as well as in Central and South America [53]. The epidemic in the USA was characterized by severe courses of disease in humans and horses and a high mortality in birds, especially in crows (for maps showing the dynamic of virus spread see: www.cdc.gov/ncidod/dvbid/westnile/index.htm). Today WNV is endemic in the Americas from Canada to Argentina [48, 66].
The WNV epidemic in the USA and other American countries is caused by a phylogenetically homogenous virus that could be grouped to clade 1 of WNV lineage 1a. So far, the virus has been detected in the USA in more than 320 bird species, in humans and many other mammals (including horses, dogs, cats), but also in reptiles like alligators (www.cdc.gov/ncidod/dvbid/westnile/birds and mammals. htm; http://www.aphis.usda.gov/vs/nahss/equine/wnv/). Sequence comparison of the isolates from the different species collected in different regions and at different time points showed only minor sequence differences [67].
1.3.1 Human WNV Infections in Europe and the Mediterranean Basin
Eastern and Western Mediterranean Region: In the early 1950s, cases of meningitis and encephalitis in humans as a consequence of a WNV infection were first reported from Israel [68]. In the following years individual infections and occasionally major outbreaks were confirmed by laboratory tests. During an outbreak in 1957, deaths as a result of WNE were reported for the first time in Israel [69]. A major outbreak occurred at the end of the 1970s / at the beginning of the 1980s [69]. With virological and serological methods the disease-causing viruses were classified as WNV lineage 1.
Starting from the mid-1990s, an increase of cases of WNV disease in humans and horses was being observed in Israel. In 1998, outbreaks of WNV in goose farms were reported in Israel, and at the same time WNV was isolated from storks migrating to the overwintering areas in Africa [68, 69, 70]. Increased surveillance of WNV infections in humans using serological, virological and molecular methods showed neutralizing antibodies against WNV in a high percentage of persons (about 86%) with close contact to diseased geese as well as in persons (about 28%) living in areas preferably visited by migratory birds [68]. In the summer of 2000, more than 250 cases of WNV disease were detected in humans in Israel. The phylogenetic analysis showed that two different WNV variants were responsible for the outbreak, which were either closely related to isolates from Israeli and American birds from 1999 or with Romanian isolates from 1997 [69]. Seroepidemiological studies revealed that a seroprevalence of about 50% was determined in humans in the same region where cases of WNV disease occurred, showing that a large number of asymptomatic infections in humans have been taking place [69]. Ongoing studies of patients with neuro-invasive disease and serological investigations of persons of different age groups imply that WNV is endemic in Israel [69, 71, 72].
A high prevalence was also observed in Egypt in the 1950s. Recent studies showed a seroprevalence in humans of 24% [73]. The detection of seroconversion in humans and sentinel chickens provides evidence that WNV is endemic in Egypt.
Severe courses of disease in humans and horses have been reported from the western Mediterranean region in the 1990s [74, 75]: Algeria (1994, about 50 patients with encephalitis), Morocco (1996, 100 ill horses) and Tunisia (approximately 170 patients with encephalitis or meningoencephalitis). All isolates obtained from humans and horses in the western Mediterranean region since 1996 are closely related and regarded as a cluster of WNV lineage 1a. The close relationship of the isolates suggests that WNV was probably introduced into this region only once and became endemic [27, 75].
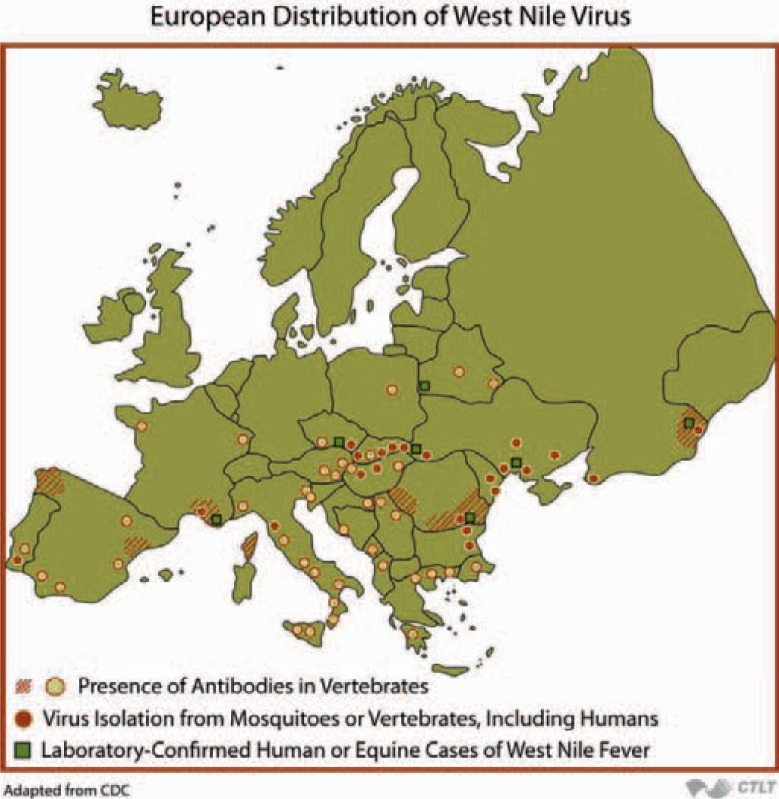
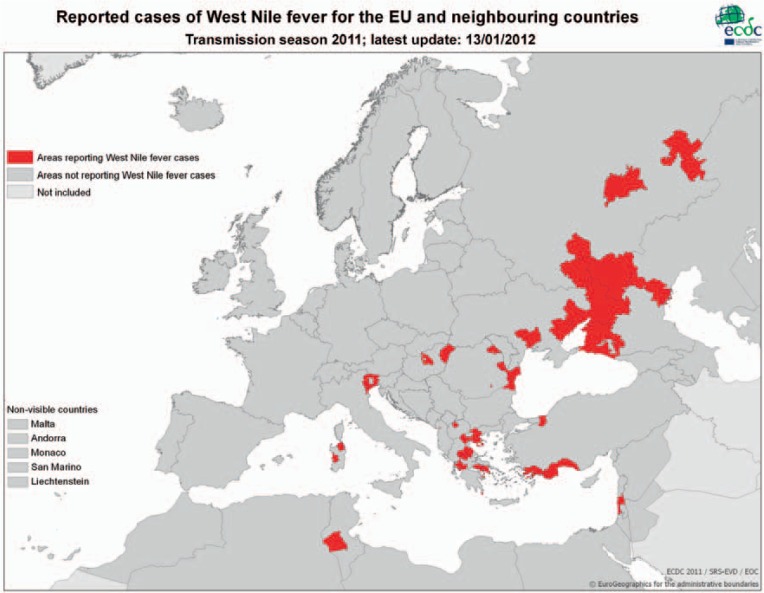
In Europe, sporadic cases of disease in horses and humans were observed occasionally in France, Portugal, Spain, Italy, Czech Republic, Romania, and Hungary (fig. 3) [71]. In general, cases were diagnosed in the period from late July to late October. Since about 2005 the prevalence of notified WNV has been changing considerably in Europe, and since 2008 WNV-related cases of disease have been reported regularly from various European countries. At an expert meeting at the ECDC in 2009 the state of knowledge on the spread of WNV in Europe was summarized [71]. In addition, a web site was created and the numbers of WNV infections in various European countries are being published regularly (fig. 4; http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/index.aspx).
Fig. 3.
European Distribution of West Nile Virus’ from Epidemiology of Infectious Diseases. Available at: http://ocw.jhsph.edu. Copyright © Johns Hopkins Bloomberg School of Public Health. Creative Commons BY-NC-SA.
Fig. 4.
Detection of human WNV infections in Europe and neighboring regions in the year 2011. Source: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/index.aspx.
Horses were diagnosed with WNV in southern France (Camargue) as early as 1962, but no human infections were reported. Sporadic cases of WNV infection in humans and horses in this region were then diagnosed in the following years [6].
The first major WNV outbreak in Europe with 17 deaths was observed in Romania in 1996. Laboratory tests confirmed WNV infection in 393 of 835 hospitalized patients with neurological symptoms [76]. In the following years, evidence has been growing that WNV has become endemic in Romania and was responsible for diseases of humans and animals [71, 77].
The first cases of WNV infections in Italy (Tuscany) were reported in horses in 1998 [78], but no further WNV activity was observed over a period of 10 years. In August of 2008, a large number of WNV infections in horses were diagnosed in Northern Italy [79]. At the same time WNV was detected in diseased birds (magpies, crows, and pigeons). Again in 2009 infections in horses and in humans were diagnosed in the same northern Italian regions [80, 81]. Further investigations suggested that WNV was also responsible for cases of disease in other regions of Italy [82, 83]. Retrospective studies of patients with neurological symptoms that are compatible with WNV infection indicate that WNV had been circulating in northern Italy before 2008, causing infections in humans [84]. Recently, evidence was published that WNV is also present in blood donors in endemic regions of Italy [83, 85, 86]. The repeated detection of WNV infections in Italy in recent years and the isolation of the virus from mosquitoes suggest that WNV has become endemic in Italy. WNV isolates are closely related to those of other WNV circulating in the western Mediterranean region [27, 65, 83].
In August of 2010, Greece reported the first cases of WNV disease in humans [36, 87, 88]. For the year 2010 a total of 262 infections were reported [88], and 69 additional cases were registered by mid-October of 2011 [89]. Seroepidemiological studies conducted in previous years had already indicated that WNV or a closely related virus was circulating in Greece although no WNV cases had been observed [90]. Molecular epidemiological studies suggest that WNV circulating in Greece in 2010/2011 was closely related to WNV lineage 2 found in Hungary, Austria, and Russia as well as in South Africa [36].
1.3.2 WNV in Russia
Between 1963 and 1993 different WNV were isolated in the European part of Russia and western Siberia from birds, mosquitoes, and ticks. Serological tests in the same regions showed that 0.4–8% of the population were antibody-positive [91, 92]. This suggests that WNV has been endemic in these regions for some time. Although sporadic clinical cases had occurred in humans especially in the Volga delta, WNV infections had not been considered a health problem. In the summer of 1999 increasing numbers of WNV cases were observed in residents of the Volgograd Region (a total of about 1,000 cases, including more than 400 with WNE or WNM) [92]. Sequence analysis showed that the epidemic was caused by a virus closely related to an isolate from Romania detected in 1996 and Israeli isolates from 1999. As one Israeli virus was isolated from a stork migrating to the wintering grounds in Africa, it might be hypothesized that the stork had been infected on its flight from the breeding grounds in Eastern Europe and that this WNV variant was endemic in these areas [27, 93]. In recent years southern Russia has been reporting diseases caused by WNV more frequently (Volgograd region, Rostov, Astrakhan, Voronezh, and the Republic of Kalmykia) ([71]; http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/index.aspx). Summarizing the epidemiological data, it can be assumed that WNV is circulating in southern Russia and is especially endemic in the Volga delta. The different, genetically distinct WNV isolates from this region can be grouped to lineage 1a [27, 93]. To what extent this region is also the starting point for the spread of WNV by migratory birds has not been studied in detail so far.
1.3.3 WNV Lineage 2 in Europe
Previously it had been assumed that WNV lineage 2 viruses circulated only in central and southern Africa and Madagascar, causing only mild diseases in vertebrates. Thus it was unexpected when WNV lineage 2 was isolated in South Africa not only from birds but also from horses with neurological symptoms as well as from birds in Hungary and Austria [29, 33, 79, 94]. According to the present state of knowledge, diseases in humans and horses in Europe are not only caused by WNV lineage 1 (clade 1a), but also by WNV lineage 2. Phylogenetic investigations showed that outbreaks observed in Russia, Romania, and Greece in 2007 and 2010 and in Italy in 2011 were caused by lineage 2 WNV [36, 88, 95, 96, 97]. Patients showing neurological symptoms were reported in South Africa during the same period [34].
The invasion of WNV lineage 2 into Europe and the change of the pathogenic potential for birds, horses, and humans show that WNV is subject to continuing evolution and selection. Further investigations are needed to answer the question to what extent the increase in prevalence of WNV in the Mediterranean region, and also in Romania, Russia, Austria, Hungary and the Czech Republic, can be explained by genetic changes in the viral genome, resulting in a preferential reproduction of the pathogen in local vectors or in birds.
1.3.4 Imported WNV Infections and Knowledge about Infections in Germany
Isolated cases of disease caused by imported WNV infections were diagnosed in several European countries during the years 1998–2005. The majority of cases have been observed in France (one case from Senegal in 1998 and one from the USA in 2002 and three cases in 2003, one from Tunisia in 2003, and four cases from Djibouti in 2005). The Czech Republic (2002), Denmark (2002), the Netherlands (three cases in 2002), and Germany (two cases in 2002 one in 2004) also reported cases imported from the USA. Two cases in Ireland acquired their WNV infection in Portugal [98, 99]. In Switzerland, a WNV infection imported from Egypt was diagnosed, and most recently a WNV infection was reported of a Dutch citizen returning from Israel [100]. These observations show that travelling in areas where WNV is endemic poses a risk for acquiring a WNV infection. Nothing is known about the estimated number of unreported, asymptomatic infections of persons returning from WNV-endemic areas.
Sera from acutely ill humans or horses were investigated by WNV-specific PCR in Germany in order to get information about potentially unrecognized WNV infections of humans or horses with neurological diseases that are compatible with the case definition for WNV infections [101]. No viral RNA could be detected in any of the investigated cases, indicating that at the time of sampling no unrecognized WNV infections had occurred in Germany.
1.3.5 Further Transmission Routes (Iatrogenic, Mother-Child, Transplantation, Occupational Transmission)
In the USA, WNV infections transmitted by blood components were reported for the first time in 2002 [102]. Transmission of WNV by mothers infected with WNV during pregnancy was investigated in the USA. A mother acutely infected with WNV in the 27th week of pregnancy transmitted WNV to the child [103]. To what extent the birth defects in this child were due to the infection is not clear, as in the following years other children born by mothers infected during pregnancy had no apparent defects. Out of 72 infants studied, only 3 children were infected congenitally [104]. Transmission seems to be possible also by breastfeeding, as viral RNA was detected in breast milk and WNV-specific IgM was found in an infant although the child did not develop symptoms [103].
Also in 2002 the first transmission of WNV by transplanted organs occurred in the USA [105]. Transmission of WNV to recipients of organ transplants was reported from Italy in 2009, while the organ donor showed no sign of infection and no WNV genome could be detected by PCR in the blood of the donor [106, 107, 108]. Donor screening by PCR is performed due to the epidemiological situation in Italy.
Transmission of WNV lineage 2 during autopsy of a horse and a needlestick injury were reported from South Africa. Both persons developed neurological symptoms [34]. In the USA, two WNV lineage 1 infections through needlestick injuries were reported; both individuals developed only mild symptoms. In addition, an infection obtained during handling of infected tissues has been observed in the USA [109].
1.3.6 Usutu Virus (USUV) Infections
In 2009 the diagnosis of a USUV infection of an immunosuppressed patient who developed encephalitis was unexpected in northern Italy [110]. Like WNV, USUV belongs to the JEV antigen complex and its first occurrence in Europe was reported in dead birds in Austria [111]. In recent years Hungary, Switzerland, Spain, and Italy have been reporting increasing numbers of infections in birds and for the first time also Germany in 2011. Until its first detection in birds in Austria in 2002, USUV had only been known to occur in Africa. In the following years USUV became endemic in Austria and in other regions of Europe [3, 112, 113, 114, 115]. Epidemiologically the spread of USUV in Europe parallels the dissemination of WNV after its introduction into the USA. So far it is unclear to what extent nucleic acid testing (NAT) systems for the detection of WNV react with USUV sequences, causing false-positive results in the NAT [116].
1.3.7 Birds
Birds are considered to be the reservoir or amplifying host for WNV. Up to now, lethal outcomes of WNV infections have been observed in 326 bird species in the USA (www.cdc.gov/ncidod/dvbid/westnile/birdspecies.htm). It can be assumed that enzootic infection cycles between wild birds and ornithophilic mosquitoes occur in wetlands in temperate zones in Europe. Examples are the Camargue in France, the Po valley in Italy, the Danube plain in Romania, and the Volga delta in Russia. Mosquitoes multiply particularly in warm weather when suitable breeding grounds are available. Therefore, it is not unexpected that there is a relatively high prevalence of WNV-infected birds in these areas.
Infected birds usually develop a viremia which is high enough to infect blood-sucking mosquitoes (≥105/ml blood). It is assumed that WNV is being introduced into Europe by migrating birds which are being infected during migration through WNV-endemic areas of Africa. The sporadic WNV outbreaks in the Mediterranean region can therefore be explained by bird migration and lack of immunity of the native birds [75]. There is increasing evidence that in the past decade WNV have become endemic in various regions of Europe, such as Romania, Italy, Greece, and Russia [27, 75].
When WNV first started to be spread across the USA, it was observed that a large number of birds died of an acute WNV infection, especially species that are grouped into the family of crows (order of Passeriformes). In contrast, no deaths of birds have been reported in Europe, Africa, and Asia [5, 117], and the first sporadic cases of death of birds or outbreaks in poultry farms were reported during the mid-1990s [33, 36, 69, 118]. The differences in the course of WNV infections in birds in Europe, Asia, and Africa compared to those in the USA could be explained by the fact that WNV had long been endemic in Africa and Asia, and birds living in these areas had to cope permanently with the pathogen. This may have selected birds with reduced susceptibility to WNV infection. The co-circulation of WNV with varying pathogenicity in endemic areas in Africa could have supported this selection. In contrast to the epidemiological situation in the old WNV-endemic areas, one single highly pathogenic WNV strain was imported to the USA, which is grouped to the lineage 1a and is closely related to highly pathogenic isolates from birds in Israel [93].
In the USA, the virulence of different WNV isolates and the susceptibility of different bird species were investigated by experimental infection of American birds. It was shown that some bird species such as crows were highly susceptible to WNV infection with the American NY99 isolate and died, while other species were susceptible to infection and developed viremia, but did not die [119]. In further studies it was shown that crows did not develop disease when they were infected with the Australian Kunjin isolate [120]. However, a decline in virulence of WNV circulating in the USA could be observed [121].
There are no comprehensive investigations of the course of infection in European sedentary or migratory birds with WNV isolates circulating in Europe. Unlike in the USA, no particular mortality in bird populations has been observed in Europe in parallel with WNV cases of horses and humans. The high seroprevalence in birds in some European regions indicates that WNV is either endemic or migratory birds were infected in their wintering grounds in Africa [3, 33, 65].
WNV infections were diagnosed in deceased geese and wild birds in Hungary in 2003 [33]. The isolate from geese was closely related to the Israeli and American WNV lineage 1a isolates. WNV lineage 2, which had been found only in South Africa before, was isolated from a hawk in Hungary [33]. In 2008 WNV was detected for the first time in Austria in dead raptors (hawk, gyrfalcon) [122]. Whether these birds had been infected by their prey or by mosquitoes is unclear.
To clarify whether there was a measurable risk that migratory birds introduce WNV into Germany, two comprehensive serological studies of birds in Germany have been performed so far [123, 124]. In both studies neutralizing antibodies against WNV were detected in a low percentage of migratory birds, but there was no evidence that the infections had been acquired in Germany. These results are consistent with studies reporting antibodies against WNV in free-living sedentary and migratory birds in the Czech Republic (Moravia) and Poland [125, 126].
The detection of WNV in birds as well as horses in Austria and Hungary since about 2003 implies that WNV might also become established in Germany. Therefore, systematic surveillance of dead birds as well as horses and humans with neurological symptoms appears worthwhile. Sentinel birds like chickens or ducks are used in different countries to monitor the circulation of arboviruses that use birds as their reservoir. The animals are maintained under conditions, which enable the transmission of the pathogens by arthropods. Sentinel birds are screened on a regular basis for the development of pathogen-specific antibodies. Chickens can be infected with WNV but show no clinical symptoms. Using such monitoring systems, the spread of WNV could be traced in North America, Italy, Romania, and Egypt [65, 73, 77, 127]. Comparable studies using sentinel birds for the monitoring of virus infection in poultry and other birds in Germany showed no evidence of transmission of WNV in Germany [128].
1.3.8 Importance of Other Vertebrates
Most mammals are regarded as dead-end hosts for WNV. The number of infectious viral particles present during the viremic phase of infection in species such as humans or horses is in general too low to enable the infection of mosquitoes. However, it was shown by experimental infection that some mammalian species like fox squirrels (Sciurus niger) [129] or reptiles such as alligators [130] develop a viremia high enough to infect mosquitoes.
In several European countries like France and Italy, WNV infections in horses were considered as the first signs of invasion of the pathogen into these regions [74, 78]. Horses seem to be especially prone to infections with WNV [131]. Approximately 10% of infected animals develop an encephalomyelitis with symptoms such as fever (>40 °C), depression, anorexia, ataxic movements, and a hind leg weakness, which in many cases progress to recumbency with mortality rates of about 50% [131]. But serological studies have shown that the majority of infections are asymptomatic, and in Europe high antibody prevalences are observed in humans in areas where WNV diseases had been detected in horses [77, 132, 133]. Until recently all WNV isolates from horses in Europe could be grouped to lineage 1a; therefore, it was unexpected that a WNV lineage 2 was isolated from horses with neurological symptoms in several regions of Hungary where no WNV disease in horses had been observed previously [35].
Investigation by PCR of over 100 horses with neurological symptoms in Germany revealed no evidence of infection with WNV [101].
In the European Union encephalomyelitis in horses is notifiable (nationally to the Friedrich-Loeffler-Institute and to the Animal Disease Notification System (ADNS) of the EU); furthermore, these diseases have to be reported to the World Organization for Animal Health (OIE). The detection of WNV infection in horses should be considered as early warning system and should increase the attention when analyzing neurological diseases in humans.
1.3.9 Importance of Mosquitoes as WNV Vectors
WNV has a natural cycle in ornithophilic mosquitoes (vector) and birds (reservoir or amplifying host). Field studies have shown that WNV can be isolated from a variety of different mosquito species and from ticks [134, 135, 136, 137, 138]. More than 60 mosquito species have been identified that are naturally, or can be experimentally, infected with WNV and must therefore be regarded as potential vectors [139]. Investigations of different mosquito populations suggest that the vector competence of a species may differ depending on time and region [140]. The role of ticks in maintaining the transmission cycle of WNV is discussed controversially. Experimentally infected ticks of the genus Ixodes were unable to transmit WNV to susceptible birds or lizards [141].
In general, viremic birds are the reservoir, infecting mosquitoes with WNV during blood meals. In this way birds serve as amplifying host and as source for the regional and longdistance dissemination of the infection. Female mosquitoes become infected during blood meals, which they take after having been fertilized by a male, since mosquitoes need vertebrate proteins for egg maturation. One can distinguish ornithophilic mosquito species and those who take their blood meal on birds as well as mammals, and reptiles. These mosquito species are therefore referred to as bridge vectors. Representatives of the genus Culex play a major role worldwide as vector for the infection of vertebrates [139].
After ingestion of the blood meal, the first virus reproduction takes place in the gut of the mosquito. From there the pathogen spreads throughout the organism and ultimately reaches the salivary glands. Under experimental conditions it was shown that during a blood meal mosquitoes could transmit viruses to animals only when the virus was present in the saliva of mosquitoes. Mosquitoes remain persistently infected for life without showing discernible symptoms. However, ultrastructural studies of mosquitoes that were infected some time ago showed organ and cell changes that could result in a decrease of virus titer and thus reduce the probability of transmission [142].
Several studies have shown that the development of viremia in the mosquitoes and thus the competence to transmit the pathogen to vertebrates is temperature-dependent [143]. The higher the average ambient temperature after the blood meal, the faster the spread of the pathogen takes place in the mosquito and the more mosquitoes develop the competence to transmit the virus during a subsequent blood meal [8, 144]. After experimentally infecting mosquitoes with WNV and maintaining them at a constant ambient temperature of 30 °C, they transmitted WNV with a higher efficiency to test animals than those maintained at 26 °C [145]. Orally infected mosquitoes, which were kept at an ambient temperature of 26 °C and 30 °C, developed virus titers sufficiently high to enable virus transmission (about 107/mosquito) after 16 and 11 days after infection, respectively. At an average ambient temperature of 18 °C and 14 °C, comparable titers were measured after 22 or 58 days, respectively. In addition, it was demonstrated that the survival of mosquitoes was prolonged at low ambient temperatures. Therefore, there was also an increased likelihood that WNV can be transmitted by hibernating mosquitoes. This hypothesis has been supported by the analysis of overwintering mosquitoes, which were infected either experimentally or naturally [146, 147, 148].
For different flaviviruses it was shown that, in addition to infecting mosquitoes during the blood meal, virus could also be transmitted vertically to the egg [147, 149]. This was demonstrated both by examining the progeny of experimentally infected mosquitoes as well as by virus isolation from male mosquitoes which must have been infected vertically because males do not take blood meals but rather feed on plant juice [130, 150]. The transovarial transmission of the virus may be important for the maintenance of the infection cycle between mosquitoes and birds, as it has been shown that mosquitoes were infected with WNV during the winter diapause without having taken a blood meal [148]. The transmission rate to the egg depends on the mosquito species and the virus strain and is in the range of 1:20 to 1:1,000 [151, 152, 153].
In various infection experiments it was shown that about 105 infectious virus particles/ml of blood are needed to infect a mosquito via a blood meal [119]. The interdependency between various mosquito species and different WNV variants (isolates) appears to be complex and is only partially understood. Experimental infection revealed differences in the susceptibility of various mosquito species as well as in the dose of infectious virus particles necessary to establish a WNV infection in different mosquito species. In other words, after exposure of different mosquito species to the same virus dose the percentage of infected mosquitoes able to transmit can be different [137, 154]. Therefore, it is important to understand the correlation of WNV variants, distribution of susceptible mosquito species as well as landscape and climate factors (temperature, humidity etc.) to assess the risk of WNV becoming endemic in a particular region such as Germany [155].
1.3.10 Evidence of WNV in European Mosquitoes
In several investigations in Europe it was shown that native mosquito species are infected with WNV or potentially able to transmit virus. WNV belonging to lineages 1 or 2 were isolated from different mosquito species. Furthermore, additional WNV variants have exclusively been found in mosquitoes so far, such as the Rabensburg virus (isolated from Culex pipiens and Aedes rossicus) (table 2). In Europe WNV lineage 1a has been isolated from Culex spp. (pipiens, univittatus, and modestus), Coquillettidia richiardii, Aedes spp. (cantans, caspius, ecrucians, rossicus, and vexans) and Anopheles maculipennis [3, 65]. In Europe, WNV lineage 2 was isolated in Hungary for the first time, later on WNV lineage 2 was detected in Culex pipiens in Greece. Studies of mosquito populations in northern Italy showed that the sequences of the isolates were closely related to those of isolates from birds and humans, which had circulated in the region in previous years [156]. This close relationship between viruses isolated from birds and humans as well as from mosquitoes in consecutive years suggests that WNV has become endemic in this region and is hibernating in the mosquito population [157].
Since 1991 the German Mosquito Control Association (Kommunale Aktionsgemeinschaft zur Bekämpfung der Schnakenplage Phillipsburg; KAPS) has been investigating the prevalence of different mosquito species in the upper Rhine valley. These studies have shown the prevalence of mosquito species in this region, which are potentially able to transmit WNV between birds, but also from birds to humans or other mammals like horses [158]. So far no WNV-positive mosquitoes were detected [159]. Comparative studies on the distribution of mosquitoes were carried out in other European regions [160]. These studies show that various factors such as climate and temperature changes as well as travel and transportation of goods, especially from southern Europe, have an impact on the spread of mosquito species previously unknown in Central Europe. A close surveillance of the spread of such mosquito species in Germany and in neighboring countries will enable a better risk assessment regarding the transmission of arboviruses.
1.3.11 Notification Requirements in Europe
Reports on the detection of human WNV infections in Greece have prompted the ECDC to alert the European countries again to intensify their WNV monitoring program. On the basis of the European Commission Decision 2009/312/ EC [161], an epidemiological surveillance within the European Community network is to be performed for WNV infections. Presently single cases of WNV disease are not notifiable in Germany according to the Protection against Infection Act (Infektionsschutzgesetz;IfSG). Currently, it is mandatory to report WNV infections in humans as a disease with a severe course in accordance with article 6 (1) clause 5a, as well as an increased incidence of WNV cases in accordance with article 6 (1) clause 5b of the IfSG. In France and Italy further precautionary measures for the control of the spread of WNV have been implemented [162, 163]. Among other things, this includes the detection of WNV in dead birds (grade 1) as well as WNV infections in diseased horses (grade 2) and in diseased humans (grade 3). In the case of WNV infections in humans, appropriate measures to prevent transmission by blood components have to be taken in these countries. In Italy, blood and organ donors are screened for viral genome by PCR in those areas where WNV is circulating [83]. Also in Switzerland WNF cases, the detection of WNV as well as WNV antibodies in humans have to be notified to the authorities. Starting on July 1, 2011, WNV was included into the Swiss Epizootic Disease Act as disease to be under surveillance (www.admin.ch/ch/d/sr/916_401/index.html).
The ECDC has set up web sites for information on the actual WNV distribution in Europe (http://ecdc.europa.eu/en/activities/diseaseprogrammes/emerging_and_vector_borne_diseases/Pages/West_Niles_fever_Risk_Maps.aspx; http://ecdc.europa.eu/en/healthtopics/west_nile_fever/epidemiological_data/Pages/annual_epidemiological_report.aspx).
1.4 Detection Methods and Their Significance
WNV infections can be diagnosed using a variety of different serological methods, genome detection with NAT techniques or direct detection by virus isolation. Commercial tests are available for the serological detection and the analysis of the virus genome; the Food and Drug Administration (FDA) has approved some of the tests in the USA. Commercial WNV-NATs with CE-IVD marking are available in Europe, but it should be mentioned that these tests have mainly been developed for use in the USA. The determination of WNV-specific IgM antibodies in serum or in cerebrospinal fluid gives serological evidence of an acute WNV infection. About 8 days after onset of symptoms more than 90% of patients have developed WNV-specific IgM antibodies. It was shown that in some patients WNV-specific IgM antibodies were detectable even 1 year later [164]. Therefore, the detection of WNV-specific IgM antibodies alone is not sufficient for the diagnosis of a fresh WNV infection [165, 166].
When sera from the early phase of infection are compared with later sera (for example convalescent sera), an at least fourfold increase of the antibody titer confirms a WNV infection. To rule out the presence of cross-reacting flavivirus-specific antibodies or that false-positive results were obtained, reactive (positive) antibody findings have to be confirmed by other WNV-specific detection methods, especially when they are the results of an enzyme immunoassay, immunofluorescence, or hemagglutination inhibition test. The gold standard for confirmation of WNV-specific antibodies is the plaque-reduction neutralization test (PRNT). The evaluation of laboratory tests for IgM antibodies in sera reactive in antibody screening tests showed that about 72% of the reactive sera in the USA could not be confirmed by PRNT and should therefore be considered as false-positive [167]. It is expected that in regions such as Germany, where WNV is not endemic, antibody screening tests will give a similarly high or even higher number of false-reactive results [168, 169].
Studies have shown that cross-reactions can occur with other flaviviruses, for example after vaccination against yellow fever, Japanese encephalitis and tick-borne encephalitis, or after infection with other flaviviruses such as DENV, TBEV, JEV or SLEV which was acquired during stays in endemic countries [36, 168, 169]. The differentiation of antibodies against flaviviruses is generally done by PRNT.
A serological differentiation of whether a virus belonging to lineage 1 or lineage 2 has infected humans or animals is not yet possible. Monoclonal antibodies, which were prepared against the Australian WNV isolate KUNV or against a standard WNV were able to differentiate different subgroups of lineage 1 and lineage 2 viruses by ELISA [170]. This indicates that isolates differ in distinct antigenic determinants (epitopes). To what extent such differences might affect the development of vaccines must be investigated.
In general, WNV can only be isolated in cell cultures from blood or cerebrospinal fluid of patients in the early phase of infection. From deceased humans virus can be successfully isolated from a variety of organs. According to the European Directive 2000/54/EC of the European Parliament and the Council of 18 September 2000 on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work (http://europa.eu/legislation_summaries/employment_and_social_policy/health_hygiene_safety_at_work/em0039_en.htm), virus isolation or work with infectious WNV requires laboratories of biosafety level 3 (BSL3) and can therefore be performed only in specialized laboratories. NAT methods such as polymerase chain reaction (RT-PCR and real-time PCR) or transcription-mediated amplification (TMA) have been shown to be sensitive and specific WNV detection methods. The tests approved in the USA were designed for the detection of isolates circulating in the USA, which are all closely related. However, WNV circulating in Europe, Asia, and Africa are phylogenetically different lineage 1 as well as lineage 2 viruses. Therefore, it has to be shown that tests used for diagnostics contain primers and probes that detect all known isolates with high sensitivity and specificity. The establishment of appropriate primers and probes also enables the differentiation of lineage 1 and 2 viruses [171]. For further characterization of the genome, sequencing of the viral RNA followed by phylogenetic analysis allows the molecular epidemiological classification of isolates and circulating viruses.
2 Blood and Plasma Donors
2.1 Prevalence and Incidence in Donor Populations
Screening of about 24,000 blood donations in Hesse [169] and Austria [172] revealed that antibodies against WNV were detectable only in a few donations. Exploratory studies yielded that 5.9% of the sera were reactive in antibody screening tests, but only four of the reactive sera could be confirmed by PRNT, which corresponds to a rate of 0.03% of the donations examined. No WNV genome sequences could be detected by PCR in any of approximately 10,000 donations [169].
According to these findings, it can be assumed that in Germany the prevalence of antibody-positive donations is very low and that most probably WNV infections were acquired during travelling in endemic areas.
Immunoglobulin preparations manufactured from plasma donated from German, Austrian or Czech donors in the years 2006–2010 showed a significant rise of antibody titers in 2009 and 2010 [173], implying an increase in the number of WNV antibody-positive donors. Whether this increase is caused by seroconversion due to infection in the respective countries or whether the donors acquired the infection while travelling in endemic areas cannot be concluded yet. Tests performed by PCR at the Paul-Ehrlich-Institut [169] showed that no WNV genome could be detected in any of the plasma pools (n = 96) prepared from European donors, whereas 32 out of 174 plasma pools produced in 2004 and 2005 from donors in the USA were PCR-positive [169].
Immunoglobulin preparations produced in the USA were examined for their content of WNV-specific antibodies. A significant increase in neutralizing antibodies was demonstrated in immunoglobulins produced from donations collected in 2002, 3 years after the onset of the WNV epidemic in 1999. As expected, the antibody titers directed against WNV in immunoglobulins increased in the USA in the following years due to the progressive dissemination of the virus [174].
2.2 Definition of Exclusion Criteria
When it became known that WNV could be transmitted through blood products, exclusion criteria for travellers returning from the USA were developed (deferral from donation for 4 weeks after returning from North America in the period from July 1 to November 30; bulletin in the German Federal Gazette (Bundesanzeiger No. 180, September 25, 2003, page 21665)). Taking into account the epidemiological situation in Europe and Asia as well as in Africa, the measures were extended. It was suggested to defer blood donors for 4 weeks after returning from areas with ongoing transmission of WNV or to test donations for the presence of WNV genomes. This measure is in accordance with the Commission Directive 2004/33/EC of March 22, 2004 which determines deferral of the donor for a period of 28 days (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:091:0025:0039:EN:PDF).
2.3 Donor Testing and Significance
Testing of donations with antibody screening tests is not performed and appears not to be justified in Germany because antibody-positive donations give only evidence of a previous infection with WNV. Blood and plasma donations can be tested by PCR for the presence of viral RNA. In contrast to the USA and Canada, various WNV grouped to lineages 1 and 2 are circulating in Europe and are inducing diseases in humans. The selection of suitable tests should ascertain that all known WNV variants are detected with comparable sensitivity and specificity [175, 176]. To what extent viruses which are closely related to WNV but not yet clearly classified have to be included in the development of genome detection systems remains an open question as long as it is not proven that such viruses infect vertebrates or humans.
2.4 Donor Interviews
Donors are asked whether they have travelled to tropical regions or had general symptoms of an infection/disease. A stay in North America from July to November and in other areas where WNV is endemic represents a risk of transmission of WNV and causes a deferral from blood donation for at least 4 weeks (see Commission Directive 2004/33/EC).
2.5 Donor Information and Counselling
Information and specific advice on WNV infections and prophylaxis can be obtained from infectiological centers or institutes for tropical medicine.
3 Recipients
3.1 Prevalence and Incidence of Blood-Associated Infections and Infectious Diseases in Recipient Populations
Currently there is no evidence that WNV infections can be acquired in Germany. In recent years, however, WNV infections in humans have repeatedly been observed in the Mediterranean region (Israel, Italy, Greece, and North Africa), in Romania, Hungary, Russia, and some Central Asian states. These infections were induced by WNV lineage 1 as well as lineage 2. NAT should therefore be performed with test systems detecting all known WNV sequences.
3.2 Immune Status (Resistance, Existing Immunity, Immune Response, Age, Exogenous Factors)
In recent years, outbreaks with highly pathogenic WNV variants have been described in the USA and Canada as well as in Israel, Romania, Italy, Greece, and several Russian regions. This is in contrast to WNV infection caused by viruses with low pathogenicity which had circulated until about the mid-1990s in Europe, Africa, and Australia. The majority of WNV infections is asymptomatic or causes only mild symptoms such as fever and malaise. Age and immune status, e.g. iatrogenic immunosuppression, may influence the course of a WNV infection. Severe courses of disease are more frequently observed in the elderly. Patients who were immunosuppressed during chemotherapy or after transplantation frequently developed central nervous symptoms. Furthermore, it was also shown that the course of the disease in such patients was protracted. In one case, no immune response against WNV was detectable, and the patient was persistently infected for months [55].
In Germany, there are reports of sporadic infections and diseases caused by WNV. Single travellers returning from the USA had been infected during their stay [98]. Bird ringers were investigated as it was assumed that they had a risk of infection when ringing migratory birds returning from areas potentially endemic for WNV in the spring. None of the ringers showed evidence of having been infected during banding activities in Germany [168]. However, it was shown that bird ringers who had also banded birds in Africa had developed WNV-neutralizing antibodies. But it is noteworthy that ringers working in TBE- or yellow fever-endemic areas are usually vaccinated against these viral diseases. To what extent vaccination against TBEV and YFV also induces protection against WNV infections or disease is unclear. Studies on the seroprevalence of blood donors suggest that only a few blood donors have antibodies against WNV, and none of the donors tested were WNV-RNA positive [169, 172].
3.3 Severity and Course of the Disease
Approximately 80% of human infections are asymptomatic. After an incubation period of 2–14 days, about 20% of infected persons develop a self-limiting febrile disease (WWNF) with flu-like symptoms such as malaise, headache, eye and muscle pain, nausea, vomiting, diarrhea, anorexia, and fatigue [41, 42]. One out of 150 infected individuals develops neurological symptoms (meningitis, encephalitis, paralysis or paresis with AFP symptoms; reviewed in [44, 45]). Approximately 4–10% of hospitalized patients with neurological symptoms died [48].
Immunosuppressed patients and transplant recipients have an approximately 40-fold higher risk to contract an infection of the brain induced by WNV. Treatment of patients with neurological symptoms with immunoglobulin preparations with high antibody titers against WNV improved the prognosis and may prevent neuroinvasive disease [59, 106].
3.4 Therapy and Prophylaxis
In the absence of a pathogen-specific therapy, the treatment of patients with WNV infection is generally symptomatic using antipyretics and fluid substitution.
3.4.1 Antiviral Drugs
At present there is no WNV-specific antiviral therapy. Because ribavirin inhibits WNV replication in neuron cell cultures [177], patients in Israel were treated with ribavirin, but unsuccessfully and even possibly worsening the course of the disease [178]. Comparable negative results were observed in the hamster infection model, which showed that ribavirin led to an increased death rate of infected animals [179]. WNV-specific antiviral substances or substances that are effective against other flaviviruses are thoroughly investigated in different working groups [reviewed in 18, 180]. For these studies WNV-infected cell cultures or WNV replicon models are used. The demands on the antiviral compounds are high, as on the one hand they should not interfere with the immune system and on the other hand should cross the blood-brain barrier.
3.4.2 Use of Immunoglobulins
Until now, there were just case reports on the treatment of patients with immunoglobulins. These show that the course of the disease in patients with neurological symptoms improved and that treated patients survived [59, 106, 181]. At present, limited amounts of specific immunoglobulin preparations are available that were produced in Israel from WNV antibody-positive plasma. Depending on the batch, high neutralizing antibody titers were also determined in immunoglobulin preparations produced from US American plasma [174]. The potency of immunoglobulin preparations containing neutralizing antibodies was proven in animal experiments as well as by treatment of WNV-infected patients [182]. It has to be further investigated to what extent humanized monoclonal antibodies with high neutralizing activity are suitable for the treatment of WNV infections. It is conceivable to administer mixtures of monoclonal antibodies with neutralizing capacity to prevent the selection of neutralization-resistant strains during therapy. A humanized monoclonal antibody directed against the viral surface protein E is in the phase I of clinical trial [183].
3.4.3 Vaccines and Vaccine Development
Up to now, no vaccines are approved for prophylaxis in humans [reviewed in 184]. A chimeric live vaccine (containing prM and E of the US American isolate WN02) on the basis of a yellow fever vaccine virus (YFV-17D) has been investigated in clinical trial phases I and II and induced neutralizing antibodies as well as a T-cell response in vaccinees [185, 186, 187]. A DNA vaccine encoding prM and E induced a T-cell response and neutralizing antibodies and has successfully passed phase I [188]. Boosting of mice with purified DIII-domain protein fragments induced a high level of protection in mice against challenge with or exposure to infectious WNV [189]. In addition, formalin-inactivated virus particles are under development as vaccines [190].
For the protection of horses against diseases caused by WNV lineage 1, formalin-inactivated virus particles (cell culture virus) and genetically engineered live vaccines based on Canary pox virus were approved in the USA by the Department of Agriculture in 2003 (USDA; www.aaep.org/pdfs/AAEP_WNV_Guidelines_2005.pdf). In the same year an inactivated cell culture virus was licensed for the vaccination of geese in Israel. A chimeric live vaccine based on the YFV was approved in 2006 for vaccination of horses in the USA. An inactivated WNV vaccine for use in horses has received European approval by the European Medicines Agency (EMA).
Experimental studies on the protection of horses against disease caused by WNV lineage 2 have shown that the chimeric vaccine based on the Canary pox virus protects efficiently against a challenge infection with WNV lineage 2 [191].
3.4.4 Prophylaxis
As currently no human vaccines are available, the only effective prophylaxis is to take preventive measures against mosquito bites in areas where WNV is endemic or where transmissions of WNV are being reported.
3.5 Transmissibility
WNV is usually transmitted by infected mosquitoes. In 2002 more than 23 WNV infections by blood components from donors who were asymptomatic at the time of blood donation were reported in the USA [102]. Therefore, testing of blood donations for viral nucleic acid in minipools was introduced, starting at the end of June of 2003 [192]. Despite the introduction of NAT testing, a low number of WNV infections through donations was still observed. These donations had a viremia below the detection limit of NAT, especially if minipool testing was performed [193, 194, 195]. Therefore, the test algorithms were aligned in the following years to the particular actual local or regional epidemiological situation [196, 197]. In principle, minipools prepared from up to 16 donations were tested, but when the number of positive results increased, each donation was tested individually until the epidemiological situation permitted to return to minipool testing. A good correlation was shown between the analysis of epidemiological data on the incidence of WNV infection in the population as well as the results of the donation screening by PCR and the detection of virus-positive donations [198].
Also in 2003, the screening of blood donations for WNV-RNA was introduced in Canada. Similar to the USA, the NAT is performed in minipools of six donations and in individual donations in regions with high WNV incidence [199, 200].
Since September of 2010, Italy has implemented, in the framework of the National WNV Surveillance Plan, screening of all blood and plasma donations by NAT in areas where WNV is circulating, in the period from June 15 to November 15. Furthermore, all tissue and organ donations are tested by PCR in Italy [83].
3.6 Frequency of Administration, Type, and Amount of Blood Products
In the USA, transmissions of WNV were reported through not inactivated blood products (red blood cells and platelet concentrates, fresh frozen plasma) but not through virus-inactivated blood components or plasma products [194, 195]. Up to now, no transmission of WNV through blood or blood products has been observed in Germany. Deferral of donors for 4 weeks after a febrile infection or for 28 days after returning from a region where WNV is endemic reduces the risk of virus transmission through transfusion.
4 Blood Products
4.1 Infectious Load of the Starting Material and Test Methods
There are several commercial NAT tests available for the detection of WNV-RNA. Studies on the virus load in blood and plasma donations were primarily achieved in the USA or performed on plasma of American origin. In viremic donations virus titers of about 6 × 105 genome equivalents/ml
were determined using quantitative PCR [192, 201]. Quantitative analyses of plasma pools prepared in the USA between 2003 and 2004 showed virus loads of up to approximately 103 genome equivalents/ml of plasma in individual pools [169]. After the introduction of WNV-NAT, plasma pools of blood donations from USA showed no measurable virus load [202].
4.2 Methods for Removal and Inactivation of the Infectious Agent
To ensure the viral safety of plasma products, the production processes have been validated in numerous studies using relevant as well as model viruses. After the finding that WNV can be transmitted through blood and blood components, the production processes of plasma derivatives were investigated using WNV. It was demonstrated that in the validation studies WNV behaved comparable to other enveloped viruses belonging to the Flaviviridae used as model viruses for the validation of manufacturing processes (bovine viral diarrhea virus (BVDV) and TBEV) [40, 203, 204]. In inactivation studies, a reduction of the infectious virus titers by a factor of ≥104 was shown for WNV as well as for the model viruses. Due to the epidemiological situation in Germany, plasma from individual donations as well as pooled plasma preparations produced by the solvent/detergent (S/D) process are safe since WNV and other enveloped viruses are efficiently inactivated by S/D treatment [reviewed in 205]. Like many other viruses, WNV is rapidly inactivated in plasma by methylene blue photoinactivation [206]. The treatment of plasma or platelet preparations with amotosalen hydrochloride (psoralen, S-59) and UV light (intercept method) also inactivates a variety of pathogens including WNV [207, 208, 209]. Furthermore, it was shown that addition of riboflavin and UV light treatment (Mirasol method) leads to an effective reduction of a variety of pathogens in plasma and platelet concentrates [210]. Solheim [211] and Rock [212] reviewed the assessment of the safety and the therapeutic application of blood components manufactured by using various pathogen inactivation methods.
4.3 Feasibility and Validation of Procedures for Removal/Inactivation of the Infectious Agent
WNV can be grown in cell cultures to sufficiently high titers. The determination of infectious virus particles is done using the plaque assay (determination of plaque-forming units) or the end-point titration (determination of the tissue culture infectious dose 50%, TCID50). Blood components and plasma products can be experimentally contaminated with WNV from culture supernatant of infected cells. Validation of the elimination/inactivation capacity of the different production steps of blood components or plasma products is done by determining infectious WNV by virus titration. In general, model viruses (viruses belonging to the same virus family) are used, as these viruses share comparable properties with the relevant viruses.
5 Assessment
So far only a few imported WNV infections have been reported in Germany. However, the epidemiological situation has changed since the mid-1990s in the Mediterranean basin, Russia and Romania, and especially in Hungary and Austria during the last 2 years. Therefore, increased attention must be paid to the spreading of WNV, especially as some WNV isolates seem to be highly pathogenic for humans and animals. Patients with etiologically unclear meningitis or encephalitis should be tested for the presence of a WNV infection. Encephalitis induced by other arboviruses as well as herpes encephalitis, Guillain-Barré syndrome and bacterial meningoencephalitis must be excluded by differential diagnostics.
To estimate the risk of WNV spreading in Germany, a multidisciplinary collaboration of entomologists, biologists (ornithologists), climatologists, veterinarians, and physicians in concerted long-term projects is required. Such projects can also generate additional information on the risk of introduction of other arthropod-borne pathogens.
The determination and population analysis of mosquito species and their distribution in Germany will allow an estimation of the risk of being bitten by those mosquitoes that can efficiently transmit WNV. As was shown in several studies, the propagation of WNV in mosquitoes is temperature-dependent. The higher the average daily temperature, the faster and the more efficiently the pathogen multiplies in the mosquitoes. As a consequence of appropriate climate changes, WNV can potentially become endemic in Germany if the pathogen is introduced for example by migratory birds. Central nervous system diseases in birds or horses may be indicators for WNV infections in animals. Investigation of suspected cases in animals and humans is possible with molecular, virological and serological methods. Human diseases that are compatible with the case definitions of a WNV infection (ECDC) should be clarified by appropriate detection methods.
Blood and plasma products that are manufactured using validated viral inactivation procedures are safe and do not transmit WNV. As demonstrated in the USA, WNV can be transmitted through non-inactivated blood components. Fever and neurological disorders after transfusion of non-inactivated blood components may be an indication of a transfusion-transmitted WNV infection. On the strength of past experiences, the use of NAT detection methods is able to reduce the risk of transmission by these products but cannot eliminate it completely. The optimization and validation of NAT methods for the detection of all WNV lineages circulating in Europe or other endemic regions are necessary regarding their specificity and sensitivity.
At present, deferral from blood donation after returning from travels to North America or other (tropical) endemic regions is implemented in Germany. In case there is a significant extension in the area of circulation, especially if WNV should spread to Germany, it might become necessary to screen donations with sensitive NAT comparable to the test strategies in the USA and Canada.
This paper was completed on February 16, 2012, and approved by the German Advisory Committee Blood (Arbeitskreis Blut) on March 30, 2012. It was compiled by the members of the subgroup ‘Assessment of Pathogens Transmissible by Blood’ of the German Advisory Committee Blood (Arbeitskreis Blut):
References
- 1.Blümel J, Burger R, Gerlich W, Gürtler L, Heiden M, Hitzler W, Jansen B, Klamm H, Lefèvre H, Löwer J, Ludwig WD, Montag-Lessing T, Offergeld R, Paessens A, Pauli G, Seitz R, Schlenkrich U, Willkommen H. Arboviren – durch Arthropoden übertragbare Viren. Stellungnahmen des Arbeitskreises Blut des Bundesministeriums für Gesundheit und Soziale Sicherung (Arboviruses–viruses transmitted by arthropods. Opinion of the Task Force on Blood of the Federal Ministry for Health and Social Security) Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2004;47:910–918. doi: 10.1007/s00103-004-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20:471–492. [Google Scholar]
- 3.Weissenböck H, Hubálek Z, Bakonyi T, Nowotny N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet Microbiol. 2010;140:271–280. doi: 10.1016/j.vetmic.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh BM, Jupp PG, Dos Santos I, Meenehan GM. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. S Afr J Sci. 1976;72:295–300. [Google Scholar]
- 5.Hayes CG. West Nile Fever. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton: CRC Press; 1988. pp. 59–88. [Google Scholar]
- 6.Murgue B, Murri S, Triki H, Deubel V, Zeller HG. West Nile in the Mediterranean basin: 1950–2000. Ann N Y Acad Sci. 2001;951:117–126. doi: 10.1111/j.1749-6632.2001.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 7.Burt FJ, Grobbelaar AA, Leman PA, Anthony FS, Gibson GV, Swanepoel R. Phylogenetic relationships of southern African West Nile virus isolates. Emerg Infect Dis. 2002;8:820–826. doi: 10.3201/eid0808.020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Kinney RM, Huang CY, Whiteman MC, Bowen RA, Langevin SA, Miller BR, Brault AC. Avian virulence and thermostable replication of the North American strain of West Nile virus. J Gen Virol. 2006;87:3611–3622. doi: 10.1099/vir.0.82299-0. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248–248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 11.Lescar J, Luo D, Xu T, Sampath A, Lim SP, Canard B, Vasudevan SG. Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from Dengue virus as a target. Antiviral Res. 2008;80:94–101. doi: 10.1016/j.antiviral.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald J, Tonry J, Hall RA, Williams B, Palacios G, Ashok MS, Jabado O, Clark D, Tesh RB, Briese T, Lipkin WI. NS1 protein secretion during the acute phase of West Nile virus infection. J Virol. 2005;79:13924–13933. doi: 10.1128/JVI.79.22.13924-13933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82:8262–8271. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie JM, Khromykh AA, Jones MK, Westa-way EG. Subcellular localization and some biochemical properties of the flavivirus Kunjin non-structural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 15.Evans JD, Seeger C. Differential effects of mutations in NS4B on West Nile virus replication and inhibition of interferon signaling. J Virol. 2007;81:11809–11816. doi: 10.1128/JVI.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai R, Kar K, Anthony K, Gould LH, Ledizet M, Fikrig E, Marasco WA, Koski RA, Modis Y. Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80:11000–11008. doi: 10.1128/JVI.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 18.Diamond MS. Progress on the development of therapeutics against West Nile virus. Antiviral Res. 2009;83:214–227. doi: 10.1016/j.antiviral.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol. 2010;84:10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moesker B, Rodenhuis-Zybert IA, Meijerhof T, Wilschut J, Smit JM. Characterization of the functional requirements of West Nile virus membrane fusion. J Gen Virol. 2010;91:389–393. doi: 10.1099/vir.0.015255-0. [DOI] [PubMed] [Google Scholar]
- 22.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, Malasit P, Kuhn RJ, Sittisombut N. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol. 2010;84:8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colpitts TM, Rodenhuis-Zybert I, Moesker B, Wang P, Fikrig E, Smit J. prM-antibody renders immature West Nile virus infectious in vivo. J Gen Virol. 2011;92:2281–2285. doi: 10.1099/vir.0.031427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Partial maturation: an immune-evasion strategy of dengue virus? Trends Microbiol. 2011;19:248–254. doi: 10.1016/j.tim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Andrade CC, Maharaj PD, Reisen WK, Brault AC. North American West Nile viral genotype isolates demonstrate differential replicative capacity in response to temperature. J Gen Virol. 2011;92:2523–2533. doi: 10.1099/vir.0.032318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehender G, Ebranati E, Bernini F, Lo Presti A, Rezza G, Delogu M, Galli M, Ciccozzi M. Phylogeography and epidemiological history of West Nile virus genotype 1a in Europe and the Mediterranean basin. Infect Genet Evol. 2011;11:646–653. doi: 10.1016/j.meegid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56:338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 29.Bakonyi T, Hubálek Z, Rudolf I, Nowotny N. Novel flavivirus or new lineage of West Nile virus, central Europe. Emerg Infect Dis. 2005;11:225–231. doi: 10.3201/eid1102.041028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lvov DK, Butenko AM, Gromashevsky VL, Kovtunov AI, Prilipov AG, Kinney R, Aristova VA, Dzharkenov AF, Samokhvalov EI, Savage HM, Shchelkanov MY, Galkina IV, Deryabin PG, Gubler DJ, Kulikova LN, Alkhovsky SK, Moskvina TM, Zlobina LV, Sadykova GK, Shatalov AG, Lvov DN, Usachev VE, Voronina AG. West Nile virus and other zoonotic viruses in Russia: examples of emerging-reemerging situations. Arch Virol Suppl. 2004;18:85–96. doi: 10.1007/978-3-7091-0572-6_7. [DOI] [PubMed] [Google Scholar]
- 31.Bondre VP, Jadi RS, Mishra AC, Yergolkar PN, Arankalle VA. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol. 2007;88:875–884. doi: 10.1099/vir.0.82403-0. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez A, Sanchez-Seco MP, Ruiz S, Molero F, Hernandez L, Moreno J, Magallanes A, Tejedor CG, Tenorio A. Putative new lineage of West Nile virus, Spain. Emerg Infect Dis. 2010;16:549–552. doi: 10.3201/eid1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venter M, Swanepoel R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector Borne Zoonotic Dis. 2010;10:659–664. doi: 10.1089/vbz.2009.0230. [DOI] [PubMed] [Google Scholar]
- 35.Kutasi O, Bakonyi T, Lecollinet S, Biksi I, Ferenczi E, Bahuon C, Sardi S, Zientara S, Szenci O. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J Vet Intern Med. 2011;25:586–591. doi: 10.1111/j.1939-1676.2011.0715.x. [DOI] [PubMed] [Google Scholar]
- 36.Papa A, Xanthopoulou K, Gewehr S, Mourelatos S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin Microbiol Infect. 2011;17:1176–1180. doi: 10.1111/j.1469-0691.2010.03438.x. [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, Brault AC, Reisen WK. Comparative thermostability of West Nile, St. Louis encephalitis, and western equine encephalomyelitis viruses during heat inactivation for serologic diagnostics. Am J Trop Med Hyg. 2009;80:862–863. [PubMed] [Google Scholar]
- 38.Mayo DR, Beckwith WH., 3rd Inactivation of West Nile virus during serologic testing and transport. J Clin Microbiol. 2002;40:3044–3046. doi: 10.1128/JCM.40.8.3044-3046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mather T, Takeda T, Tassello J, Ohagen A, Serebryanik D, Kramer E, Brown F, Tesh R, Alford B, Chapman J, Lazo A. West Nile virus in blood: stability, distribution, and susceptibility to PEN110 inactivation. Transfusion. 2003;43:1029–1037. doi: 10.1046/j.1537-2995.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 40.Kreil TR. West Nile virus: recent experience with the model virus approach. Dev Biol (Basel) 2004;118:101–105. [PubMed] [Google Scholar]
- 41.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 42.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 43.DeBiasi RL, Tyler KL. West Nile virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2:264–275. doi: 10.1038/ncpneuro0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer DL, Li J, Shi PJ. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 45.DeBiasi RL. West Nile virus neuroinvasive disease. Curr Infect Dis Rep. 2011;13:350–359. doi: 10.1007/s11908-011-0193-9. [DOI] [PubMed] [Google Scholar]
- 46.Sejvar JJ, Leis AA, Stokic DS, Van Gerpen JA, Marfin AA, Webb R, Haddad MB, Tierney BC, Slavinski SA, Polk JL, Dostrow V, Winkelmann M, Petersen LR. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis. 2003;9:788–793. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sejvar JJ, Marfin AA. Manifestations of West Nile neuroinvasive disease. Rev Med Virol. 2006;16:209–224. doi: 10.1002/rmv.501. [DOI] [PubMed] [Google Scholar]
- 48.Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease – United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 49.Lim JK, Murphy PM. Chemokine control of West Nile virus infection. Exp Cell Res. 2011;317:569–574. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook RL, Xu X, Yablonsky EJ, Sakata N, Tripp JH, Hess R, Piazza P, Rinaldo CR. Demographic and clinical factors associated with persistent symptoms after West Nile virus infection. Am J Trop Med Hyg. 2010;83:1133–1136. doi: 10.4269/ajtmh.2010.09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 52.Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;72:320–324. [PubMed] [Google Scholar]
- 53.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fisher-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibney KB, Lanciotti RS, Sejvar JJ, Nugent CT, Linnen JM, Delorey MJ, Lehman JA, Boswell EN, Staples JE, Fischer M. West Nile virus RNA not detected in urine of 40 people tested 6 years after acute West Nile virus disease. J Infect Dis. 2011;203:344–347. doi: 10.1093/infdis/jiq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn RG, Guarner J, Sejvar JJ, Hartman H, Mc-Comb RD, Nevins DL, Bhatnagar J, Zaki SR. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42:680–683. doi: 10.1086/500216. [DOI] [PubMed] [Google Scholar]
- 56.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 57.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation. 2004;77:399–402. doi: 10.1097/01.TP.0000101435.91619.31. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention West Nile virus transmission via organ transplantation and blood transfusion – Louisiana, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1263–1267. [PubMed] [Google Scholar]
- 59.Rhee C, Eaton EF, Concepcion W, Blackburn BG. West Nile virus encephalitis acquired via liver transplantation and clinical response to intravenous immunoglobulin: case report and review of the literature. Transpl Infect Dis. 2011;13:312–317. doi: 10.1111/j.1399-3062.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 60.Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection, but not for viral transmission. J Infect Dis. 2010;201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kindberg E, Mickiene A, Ax C, Akerlind B, Vene S, Lindquist L, Lundkvist A, Svensson L. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008;197:266–269. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
- 63.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 64.Ahuja SK, He W. Double-edged genetic swords and immunity: Lesson from CCR5 and beyond. J Infect Dis. 2010;201:171–174. doi: 10.1086/649427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calistri P, Giovannini A, Hubálek Z, Ionescu A, Monaco F, Savini G, Lelli R. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol J. 2010;4:29–37. doi: 10.2174/1874357901004010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen LR, Hayes EB. West Nile virus in the Americas. Med Clin North Am. 2008;92:1307–1322. doi: 10.1016/j.mcna.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Armstrong PM, Vossbrinck CR, Andreadis TG, Anderson JF, Pesko KN, Newman RM, Lennon NJ, Birren BW, Ebel GD, Henn MR. Molecular evolution of West Nile virus in a northern temperate region: Connecticut, USA 1999–2008. Virology. 2011;417:203–210. doi: 10.1016/j.virol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinberger M, Pitlik SD, Gandacu D, Lang R, Nassar F, Ben David D, Rubinstein E, Izthaki A, Mishal J, Kitzes R, Siegman-Igra Y, Giladi M, Pick N, Mendelson E, Bin H, Shohat T. West Nile fever outbreak, Israel, 2000: epidemiologic aspects. Emerg Infect Dis. 2001;7:686–691. doi: 10.3201/eid0704.010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bin H, Grossman Z, Pokamunski S, Malkinson M, Weiss L, Duvdevani P, Banet C, Weisman Y, Annis E, Gandaku D, Yahalom V, Hindyieh M, Shulman L, Mendelson E. West Nile fever in Israel 1999–2000: from geese to humans. Ann NY Acad Sci. 2001;951:127–142. doi: 10.1111/j.1749-6632.2001.tb02691.x. [DOI] [PubMed] [Google Scholar]
- 70.Malkinson M, Banet C, Weisman Y, Pokamunski S, King R, Drouet MT, Deubel V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis. 2002;8:392–397. doi: 10.3201/eid0804.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ECDC: Meeting Report. Expert consultation on West Nile virus infection. Stockholm, 21–22 April 2009. http://ecdc.europa.eu/en/publications/Publications/0909_MER_Expert_consultation_on_WNV.pdf
- 72.Kopel E, Amitai Z, Bin H, Shulman LM, Mendelson E, Sheffer R: Surveillance of West Nile virus disease, Tel Aviv District, Israel, 2005 to 2010. Euro Surveill 2011;16:pii = 19894. [PubMed]
- 73.Soliman A, Mohareb E, Salman D, Saad M, Salama S, Fayez C, Hanafi H, Medhat I, Labib E, Rakha M, El-Sayed N, Yingst S, Tjaden J, Earhart K. Studies on West Nile virus infection in Egypt. J Infect Public Health. 2010;3:54–59. doi: 10.1016/j.jiph.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Murgue B, Murri S, Zientara S, Durand B, Durand JP, Zeller H. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis. 2001b;7:692–696. doi: 10.3201/eid0704.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sotelo E, Fernández-Pinero J, Llorente F, Agüero M, Hoefle U, Blanco JM, Jiménez-Clavero MA. Phylogenetic relationships of Western Mediterranean West Nile virus strains (1996–2010) using full-length genome sequences: single or multiple introductions? J Gen Virol. 2011;92:2512–2522. doi: 10.1099/vir.0.033829-0. [DOI] [PubMed] [Google Scholar]
- 76.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 77.Neghina AM, Neghina R. Reemergence of human infections with West Nile virus in Romania, 2010: an epidemiological study and brief review of the past situation. Vector Borne Zoonotic Dis. 2011;11:1289–1292. doi: 10.1089/vbz.2010.0206. [DOI] [PubMed] [Google Scholar]
- 78.Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, Lelli R, Murri S, Scicluna MT. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002;8:1372–1378. doi: 10.3201/eid0812.020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calistri P, Giovannini A, Savini G, Monaco F, Bonfanti L, Ceolin C, Terregino C, Tamba M, Cordioli P, Lelli R. West Nile virus transmission in 2008 in north-eastern Italy. Zoonoses Public Health. 2010;57:211–219. doi: 10.1111/j.1863-2378.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 80.Rizzo C, Vescio F, Declich S, Finarelli AC, Macini P, Mattivi A, Rossini G, Piovesan C, Barzon L, Palù G, Gobbi F, Macchi L, Pavan A, Magurano F, Ciufolini MG, Nicoletti L, Salmaso S, Rezza G. West Nile virus transmission with human cases in Italy, August–September 2009. Euro Surveill. 2009;14 pii = 19353. [PubMed] [Google Scholar]
- 81.Barzon L, Squarzon L, Cattai M, Franchin E, Pagni S, Cusinato R, Palu G. West Nile virus infection in Veneto region, Italy, 2008–2009. EuroSurveill. 2009;14 doi: 10.2807/ese.14.31.19289-en. pii = 19289. [DOI] [PubMed] [Google Scholar]
- 82.Calistri P, Monaco F, Savini G, Guercio A, Purpari G, Vicari D, Cascio S, Lelli R. Further spread of West Nile virus in Italy. Vet Ital. 2010;46:467–474. [PubMed] [Google Scholar]
- 83.Barzon L, Pacenti M, Cusinato R, Cattai M, Franchin E, Pagni S, Martello T, Bressan S, Squarzon L, Cattelan A, Pellizzer G, Scotton P, Beltrame A, Gobbi F, Bisoffi Z, Russo F, Palu G. Human cases of West Nile Virus Infection in north-eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011;16(33) pii = 19949. [PubMed] [Google Scholar]
- 84.Cusi MG, Roggi A, Terrosi C, Gori Savellini G, Toti M. Retrospective diagnosis of West Nile virus infection in a patient with meningoencephalitis in Tuscany, Italy. Vector Borne Zoonotic Dis. 2011;11:1511–1512. doi: 10.1089/vbz.2010.0248. [DOI] [PubMed] [Google Scholar]
- 85.Pezzotti P, Piovesan C, Barzon L, Cusinato R, Cattai M, Pacenti M, Piazza A, Franchin E, Pagni S, Bressan S, Martello T, Potenza R, Scipioni C, Ammendola R, Breda A, Palu G, Russo F, Rezza G. Prevalence of IgM and IgG antibodies to West Nile virus among blood donors in an affected area of north-eastern Italy, summer 2009. Euro Surveill. 2011;16 doi: 10.2807/ese.16.10.19814-en. pii = 19814. [DOI] [PubMed] [Google Scholar]
- 86.Pierro A, Gaibani P, Manisera C, Dirani G, Rossini G, Cavrini F, Ghinelli F, Ghinelli P, Finarelli AC, Mattivi A, Macini PL, Castellani G, Landini MP, Sambri V. Seroprevalence of West Nile virus-specific antibodies in a cohort of blood donors in northeastern Italy. Vector Borne Zoonotic Dis. 2011;11:1605–1607. doi: 10.1089/vbz.2011.0616. [DOI] [PubMed] [Google Scholar]
- 87.Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, Kamaria F, Liona A, Mellou K, Saroglou G, Panagiotopoulos T. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro Surveill. 2010b;15 doi: 10.2807/ese.15.34.19644-en. pii = 19644. [DOI] [PubMed] [Google Scholar]
- 88.ECDC: Meeting Report. Expert Consultation on West Nile VIRUS Infection Thessaloniki, 25–26 January 2011.
- 89.Danis K, Papa A, Papanikolaou E, Dougas G, Terzaki I, Baka A, Vrioni G, Kapsimali V, Tsakris A, Kansouzidou A, Tsiodras S, Vakalis N, Bonovas S, Kremastinou J. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Euro Surveill. 2011;16 pii = 19951. [PubMed] [Google Scholar]
- 90.Papa A, Perperidou P, Tzouli A, Castilletti C. West Nile virus – neutralizing antibodies in humans in Greece. Vector Borne Zoonotic Dis. 2010;10:655–658. doi: 10.1089/vbz.2010.0042. [DOI] [PubMed] [Google Scholar]
- 91.Platonov AE. West Nile encephalitis in Russia 1999–2001: were we ready? Are we ready? Ann N Y Acad Sci. 2001;951:102–116. doi: 10.1111/j.1749-6632.2001.tb02689.x. [DOI] [PubMed] [Google Scholar]
- 92.Platonov AE, Fedorova MV, Karan LS, Shopenskaya TA, Platonova OV, Zhuravlev VI. Epidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (Diptera: Culicidae) bionomics. Parasitol Res. 2008;103(suppl 1):S45–53. doi: 10.1007/s00436-008-1050-0. [DOI] [PubMed] [Google Scholar]
- 93.May FJ, Davis CT, Tesh RB, Barrett AD. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85:2964–2974. doi: 10.1128/JVI.01963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Venter M, Human S, Zaayman D, Gerdes GH, Williams J, Steyl J, Leman PA, Paweska JT, Setzkorn H, Rous G, Murray S, Parker R, Donnellan C, Swanepoel R. Lineage 2 West Nile virus as cause of fatal neurologic disease in horses, South Africa. Emerg Infect Dis. 2009;15:877–884. doi: 10.3201/eid1506.081515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sirbu A, Ceianu CS, Panculescu-Gatej RI, Vázquez A, Tenorio A, Rebreanu R, Niedrig M, Nicolescu G, Pistol A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16 pii = 19762. [PubMed] [Google Scholar]
- 96.Chaskopoulou A, Dovas CI, Chaintoutis SC, Bouzalas I, Ara G, Papanastassopoulou M. Evidence of enzootic circulation of West Nile virus (Nea Santa-Greece-2010, lineage 2), Greece, May to July 2011. Euro Surveill. 2011;16 pii = 19933. [PubMed] [Google Scholar]
- 97.Bagnarelli P, Marinelli K, Trotta D, Monachetti A, Tavio M, Del Gobbo R, Capobianchi M, Menzo S, Nicoletti L, Magurano F, Varaldo P. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro Surveill. 2011;16 pii = 20002. [PubMed] [Google Scholar]
- 98.Pauli G. West-Nil-Virus: Prävalenz und Bedeutung als Zoonoseerreger. Bundesgesundhbl Gesundheitsforsch Gesundheitsschutz. 2004;47:653–660. doi: 10.1007/s00103-004-0864-x. [DOI] [PubMed] [Google Scholar]
- 99.ECDC: Annual Epidemiological Report on Communicable Diseases in Europe. p 250. http://ecdc.europa.eu/en/publications/Publications/0706_SUR_Annual_Epidemiological_Report_2007.pdf, 2007.
- 100.Aboutaleb N, Beersma MF, Wunderink HF, Vossen AC, Visser LG. Case report: West-Nile virus infection in two Dutch travellers returning from Israel. Euro Surveill. 2010;15 doi: 10.2807/ese.15.34.19649-en. pii = 19508. [DOI] [PubMed] [Google Scholar]
- 101.Linke S: Die Prävalenz und Inzidenz von West-Nil-Virus in Deutschland. Dissertation, Fachbereich der Biologie, Chemie und Pharmazie der Freien Universität Berlin, 2007.
- 102.Tobler LH, Bianco C, Glynn SA, Schreiber GB, Dille BJ, Prince HE, Lanciotti RS, Linnen JM, Gallarda J, Shyamala V, Smith D, Kleinman SH, Busch MP, NHLBI Retrovirus Epidemiology Study (REDS) Detection of West Nile virus RNA and antibody in frozen plasma components from a voluntary market withdrawal during the 2002 peak epidemic. Transfusion. 2005;45:480–486. doi: 10.1111/j.0041-1132.2005.04266.x. [DOI] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention Possible West Nile virus transmission to an infant through breast-feeding – Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002a;51:877–878. [PubMed] [Google Scholar]
- 104.O'Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ, Kightlinger LK, Beecham BD, Miller TK, Neitzel DF, Michaels SR, Campbell GL, Lanciotti RS, Hayes EB. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003–2004. Pediatrics. 2006;117:e537–545. doi: 10.1542/peds.2005-2024. [DOI] [PubMed] [Google Scholar]
- 105.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE, DiazGranados CA, Winquist AG, Perlino CA, Wiersma S, Hillyer KL, Goodman JL, Marfin AA, Chamberland ME, Petersen LR, West Nile Virus in Transplant Recipients Investigation Team Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 106.Morelli MC, Sambri V, Grazi GL, Gaibani P, Pierro A, Cescon M, Ercolani G, Cavrini F, Rossini G, Capobianchi MR, Di Caro A, Menzo S, Pagliaro PP, Ghinelli F, Lazzarotto T, Landini MP, Pinna AD. Absence of neuroinvasive disease in a liver transplant recipient who acquired West Nile virus (WNV) infection from the organ donor and who received WNV antibodies prophylactically. Clin Infect Dis. 2010;51:e34–37. doi: 10.1086/655146. [DOI] [PubMed] [Google Scholar]
- 107.Capobianchi MR, Sambri V, Castilletti C, Pierro AM, Rossini G, Gaibani P, Cavrini F, Selleri M, Meschi S, Lapa D, Di Caro A, Grossi P, De Cillia C, Venettoni S, Landini MP, Ippolito G, Nanni Costa A, on behalf of the Italian Transplant Network Retrospective screening of solid organ donors in Italy, 2009, reveals unpredicted circulation of West Nile virus. Euro Surveill. 2011;15 doi: 10.2807/ese.15.34.19648-en. pii = 19648. [DOI] [PubMed] [Google Scholar]
- 108.Nanni Costa A, Capobianchi MR, Ippolito G, Palù G, Barzon L, Piccolo G, Andreetta B, Filippetti M, Fehily D, Lombardini L, Grossi P. West Nile virus: the Italian National Transplant Network reaction to an alert in the north-eastern region, Italy 2011. Euro Surveill. 2011;16 pii = 19991. [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention Laboratory-acquired West Nile virus infections – United States, 2002. MMWR Morb Mortal Wkly Rep. 2002b;51:1133–1135. [PubMed] [Google Scholar]
- 110.Tamba M, Bonilauri P, Bellini R, Calzolari M, Albieri A, Sambri V, Dottori M, Angelini P. Detection of Usutu virus within a West Nile virus surveillance program in Northern Italy. Vector Borne Zoonotic Dis. 2011;11:551–557. doi: 10.1089/vbz.2010.0055. [DOI] [PubMed] [Google Scholar]
- 111.Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nikolay B, Diallo M, Boye CS, Sall AA. Usutu Virus in Africa. Vector Borne Zoonotic Dis. 2011;11:1417–1423. doi: 10.1089/vbz.2011.0631. [DOI] [PubMed] [Google Scholar]
- 113.Savini G, Monaco F, Terregino C, Di Gennaro A, Bano L, Pinoni C, De Nardi R, Bonilauri P, Pecorari M, Di Gialleonardo L, Bonfanti L, Polci A, Calistri P, Lelli R. Usutu virus in Italy: an emergence or a silent infection? Vet Microbiol. 2011;151:264–274. doi: 10.1016/j.vetmic.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 114.Steinmetz HW, Bakonyi T, Weissenböck H, Hatt JM, Eulenberger U, Robert N, Hoop R, Nowotny N. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland – genomic and pathologic comparison to other central European outbreaks. Vet Microbiol. 2011;148:207–212. doi: 10.1016/j.vetmic.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 115.Jöst H, Bialonski A, Maus D, Sambri V, Eiden M, Groschup MH, Günther S, Becker N, Schmidt-Chanasit J. Isolation of Usutu Virus in Germany. Am J Trop Med Hyg. 2011;85:551–553. doi: 10.4269/ajtmh.2011.11-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaibani P, Pierro AM, Cavrini F, Rossini G, Landini MP, Sambri V. False-positive transcription-mediated amplification assay detection of West Nile virus in blood from a patient with viremia caused by an Usutu virus infection. J Clin Microbiol. 2010;48:3338–3339. doi: 10.1128/JCM.02501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet Res. 2009;40:43. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jourdain E, Toussaint Y, Leblond A, Bicout DJ, Sabatier P, Gauthier-Clerc M. Bird species potentially involved in introduction, amplification, and spread of West Nile virus in a Mediterranean wetland, the Camargue (Southern France) Vector Borne Zoonotic Dis. 2007;7:15–33. doi: 10.1089/vbz.2006.0543. [DOI] [PubMed] [Google Scholar]
- 119.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koenig WD, Hochachka WM, Zuckerberg B, Dickinson JL. Ecological determinants of American crow mortality due to West Nile virus during its North American sweep. Oecologia. 2010;163:903–909. doi: 10.1007/s00442-010-1627-z. [DOI] [PubMed] [Google Scholar]
- 122.Wodak E, Richter S, Bagó Z, Revilla-Fernández S, Weissenböck H, Nowotny N, Winter P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. 2011;149:358–366. doi: 10.1016/j.vetmic.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 123.Linke S, Niedrig M, Kaiser A, Ellerbrok H, Müller K, Müller T, Conraths FJ, Mühle RU, Schmidt D, Köppen U, Bairlein F, Berthold P, Pauli G. Serologic evidence of West Nile virus infections in wild birds captured in Germany. Am J Trop Med Hyg. 2007a;77:358–364. [PubMed] [Google Scholar]
- 124.Seidowski D, Ziegler U, von Rönn JA, Müller K, Hüppop K, Müller T, Freuling C, Mühle RU, Nowotny N, Ulrich RG, Niedrig M, Groschup MH. West Nile virus monitoring of migratory and resident birds in Germany. Vector Borne Zoonotic Dis. 2010;10:639–647. doi: 10.1089/vbz.2009.0236. [DOI] [PubMed] [Google Scholar]
- 125.Hubálek Z, Halouzka J, Juricová Z, Sikutová S, Rudolf I, Honza M, Janková J, Chytil J, Marec F, Sitko J. Serologic survey of birds for West Nile flavivirus in southern Moravia (Czech Republic) Vector Borne Zoonotic Dis. 2008;8:659–666. doi: 10.1089/vbz.2007.0283. [DOI] [PubMed] [Google Scholar]
- 126.Hubálek Z, Wegner E, Halouzka J, Tryjanowski P, Jerzak L, Sikutová S, Rudolf I, Kruszewicz AG, Jaworski Z, Wlodarczyk R. Serologic survey of potential vertebrate hosts for West Nile virus in Poland. Viral Immunol. 2008b;21:247–253. doi: 10.1089/vim.2007.0111. [DOI] [PubMed] [Google Scholar]
- 127.Komar N. West Nile virus surveillance using sentinel birds. Ann N Y Acad Sci. 2001;951:58–73. doi: 10.1111/j.1749-6632.2001.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 128.Ziegler U, Seidowski D, Globig A, Fereidouni SR, Ulrich RG, Groschup MH. Sentinel birds in wild-bird resting sites as potential indicators for West Nile virus infections in Germany. Arch Virol. 2010;155:965–969. doi: 10.1007/s00705-010-0618-z. [DOI] [PubMed] [Google Scholar]
- 129.Tiawsirisup S, Blitvich BJ, Tucker BJ, Halbur PG, Bartholomay LC, Rowley WA, Platt KB. Susceptibility of fox squirrels (Sciurus niger) to West Nile virus by oral exposure. Vector Borne Zoonotic Dis. 2010;10:207–209. doi: 10.1089/vbz.2008.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Unlu I, Kramer WL, Roy AF, Foil LD. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. J Med Entomol. 2010;47:625–633. doi: 10.1603/me09087. [DOI] [PubMed] [Google Scholar]
- 131.Castillo-Olivares J, Wood J. West Nile virus infection of horses. Vet Res. 2004;35:467–483. doi: 10.1051/vetres:2004022. [DOI] [PubMed] [Google Scholar]
- 132.Leblond A, Hendrikx P, Sabatier P. West Nile virus outbreak detection using syndromic monitoring in horses. Vector Borne Zoonotic Dis. 2007;7:403–410. doi: 10.1089/vbz.2006.0593. [DOI] [PubMed] [Google Scholar]
- 133.Savini G, Monaco F, Calistri P, Lelli R. Phylogenetic analysis of West Nile virus isolated in Italy in 2008. Euro Surveill. 2008;13 pii = 19048. [PubMed] [Google Scholar]
- 134.Hubálek Z, Halouzka J. West Nile fever – a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. Vol. 267. Berlin, Springer: Curr Topics Microbiol Immunol; 2002. pp. 241–252. [DOI] [PubMed] [Google Scholar]
- 136.Mumcuoglu KY, Banet-Noach C, Malkinson M, Shalom U, Galun R. Argasid ticks as possible vectors of West Nile virus in Israel. Vector Borne Zoonotic Dis. 2005;5:65–71. doi: 10.1089/vbz.2005.5.65. [DOI] [PubMed] [Google Scholar]
- 137.Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile Virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 138.Moskvitina NS, Romanenko VN, Ternovoi VA, Ivanova NV, Protopopova EV, Kravchenko LB, Kononova IuV, Kuranova VN, Chausov EV, Moskvitin SS, Pershikova NL, Gashkov SI, Konovalova SN, Bol'shakova NP, Loktev VB. Detection of the West Nile Virus and its genetic typing in ixodid ticks (Parasitiformes: Ixodidae) in Tomsk City and its suburbs. Parazitologiia. 2008;42:210–225. [PubMed] [Google Scholar]
- 139.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;20:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaidyanathan R, Scott TW. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis. 2007;7:193–198. doi: 10.1089/vbz.2006.0589. [DOI] [PubMed] [Google Scholar]
- 141.Reisen WK, Brault AC, Martinez VM, Fang Y, Simmons K, Garcia S, Omi-Olsen E, Lane RS. Ability of transstadially infected Ixodes pacificus (Acari: Ixodidae) to transmit West Nile virus to song sparrows or western fence lizards. J Med Entomol. 2007;44:320–327. doi: 10.1603/0022-2585(2007)44[320:aotiip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 142.Girard YA, Popov V, Wen J, Han V, Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2005;42:429–444. doi: 10.1093/jmedent/42.3.429. [DOI] [PubMed] [Google Scholar]
- 143.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cornel AJ, Jupp PG, Blackburn NK. Environmental temperature on the vector competence of Culex univittatus (Diptera: Culicidae) for West Nile virus. J Med Entomol. 1993;30:449–456. doi: 10.1093/jmedent/30.2.449. [DOI] [PubMed] [Google Scholar]
- 146.Rosen L. Overwintering mechanisms of mosquito-borne arboviruses in temperate climates. Am J Trop Med Hyg. 1987;37(suppl):69S–76S. doi: 10.4269/ajtmh.1987.37.69s. [DOI] [PubMed] [Google Scholar]
- 147.Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- 148.Andreadis TG, Armstrong PM, Bajwa WI. Studies on hibernating populations of Culex pipiens from a West Nile virus endemic focus in New York City: parity rates and isolation of West Nile virus. J Am Mosq Control Ass. 2010;26:257–264. doi: 10.2987/10-6004.1. [DOI] [PubMed] [Google Scholar]
- 149.Baqar S, Hayes CG, Murphy JR, Watts DM. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am J Trop Med Hyg. 1993;48:757–762. doi: 10.4269/ajtmh.1993.48.757. [DOI] [PubMed] [Google Scholar]
- 150.Miller BR, Nasci RS, Godsey MS, Savage HM, Lutwama JJ, Lanciotti RS, Peters CJ. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley province, Kenya. Am J Trop Med Hyg. 2000;62:240–246. doi: 10.4269/ajtmh.2000.62.240. [DOI] [PubMed] [Google Scholar]
- 151.Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- 152.Phillips RA, Christensen K. Field-caught Culex erythrothorax larvae found naturally infected with West Nile virus in Grand County, Utah. J Am Mosq Control Assoc. 2006;22:561–562. doi: 10.2987/8756-971X(2006)22[561:FCELFN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 153.Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae) J Med Entomol. 2008;45:445–451. doi: 10.1603/0022-2585(2008)45[445:eipfha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 154.Jiang SF, Zhang YM, Guo XX, Dong YD, Xing D, Xue RD, Zhao TY. Experimental studies on comparison of the potential vector competence of four species of Culex mosquitoes in China to transmit West Nile virus. J Med Entomol. 2010;47:788–790. doi: 10.1603/me08292. [DOI] [PubMed] [Google Scholar]
- 155.Brugger K: Globale Erwärmung und neu auftretende Infektionskrankheiten am Beispiel der Usutu Virus Epidemie in Österreich. Dissertation Universität Wien, 2008. www.univie.ac.at/img-wien/dipldiss/diss/Diss_Brugger.htm
- 156.Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, Galletti G, Gelati A, Barbieri I, Tamba M, Lelli D, Carra E, Cordioli P, Angelini P, Dottori M. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS ONE. 2010;5:e14324. doi: 10.1371/journal.pone.0014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monaco F, Savini G, Calistri P, Polci A, Pinoni C, Bruno R, Lelli R. 2009 West Nile disease epidemic in Italy: first evidence of overwintering in Western Europe? Res Vet Sci. 2011;91:321–326. doi: 10.1016/j.rvsc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 158.Becker N, Huber K, Pluskota B, Kaiser A. Ochlerotatus japonicus japonicus – a newly established neozoan in Germany and a revised list of the German mosquito fauna. Eur Mosq Bull. 2011;29:88–102. [Google Scholar]
- 159.Timmermann U, Becker N. Mosquito-borne West Nile virus (WNV) surveillance in the Upper Rhine Valley, Germany. J Vector Ecol. 2010;35:140–143. doi: 10.1111/j.1948-7134.2010.00039.x. [DOI] [PubMed] [Google Scholar]
- 160.Votýpka J, Šeblová V, Rádrová J. Spread of the West Nile virus vector Culex modestus and the potential malaria vector Anopheles hyrcanus in central Europe. J Vector Ecol. 2008;33:269–277. doi: 10.3376/1081-1710-33.2.269. [DOI] [PubMed] [Google Scholar]
- 161.European Commission: European Commission Decision 2009/312/EC. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:091:0027:0030:EN:PDF
- 162.Grazzini G, Liumbruno GM, Pupella S, Silvestri AR, Randi V, Pascarelli N, Zucchelli P, Di Caro A, Spataro N, D'Angelo E, Sambri V. West Nile virus in Italy: a further threat to blood safety, a further challenge to the blood system. Blood Transfus. 2008;6:235–237. doi: 10.2450/2008.0065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ministère du travail, des relations sociales, de la famille et de la solidarité, Ministère de la santé, de la jeunesse, des sports et de la vie associative: Guide de procédures de lutte contre la circulation du virus West Nile en France métropolitaine. www.sante.gouv.fr/fichiers/bo/2009/09-08/ste_20090008_0100_0125.pdf
- 164.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levett PN, Sonnenberg K, Sidaway F, Shead S, Niedrig M, Steinhagen K, Horsman GB, Drebot MA. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol. 2005;43:5873–5875. doi: 10.1128/JCM.43.12.5873-5875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fox JL, Hazell SL, Tobler LH, Busch MP. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol. 2006;13:33–36. doi: 10.1128/CVI.13.1.33-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Janusz KB, Lehman JA, Panella AJ, Fischer M, Staples E. Laboratory testing practices for West Nile virus in the United States. Vector Borne Zoonotic Dis. 2011;11:597–599. doi: 10.1089/vbz.2010.0058. [DOI] [PubMed] [Google Scholar]
- 168.Linke S, Muehlen M, Niedrig M, Ellerbrok H, Kaiser A, Fiedler W, Sonnenberg K, Alpers K, Stark K, Pauli G. Assessing the exposure of German and Austrian bird ringers to West Nile virus (Flavivirus) and evaluating their potential risk of infection. J Ornithol. 2008;149:271–275. [Google Scholar]
- 169.Pfleiderer C, Blümel J, Schmidt M, Roth WK, Houfar MK, Eckert J, Chudy M, Menichetti E, Lechner S, Nübling CM. West Nile virus and blood product safety in Germany. J Med Virol. 2008;80:557–563. doi: 10.1002/jmv.21110. [DOI] [PubMed] [Google Scholar]
- 170.Hall RA, Scherret JH, Mackenzie JS. Kunjin virus: an Australian variant of West Nile? Ann N Y Acad Sci. 2001;951:153–160. [PubMed] [Google Scholar]
- 171.Linke S, Ellerbrok H, Niedrig M, Nitsche A, Pauli G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. J Virol Methods. 2007b;146:355–358. doi: 10.1016/j.jviromet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 172.Pfleiderer C, König C, Chudy M, Schmidt M, Roth WK, Nübling CM. Molecular epidemiology of West Nile Virus in humans. Dev Biol (Basel) 2006;126:197–201. discussion 326–327. [PubMed] [Google Scholar]
- 173.Rabel PO, Planitzer CB, Farcet MR, Orlinger KK, Ilk R, Barrett PN, Kreil TR. Increasing West Nile virus antibody titres in central European plasma donors from 2006 to 2010. Euro Surveill. 2011;16 doi: 10.2807/ese.16.10.19812-en. pii = 19812. [DOI] [PubMed] [Google Scholar]
- 174.Planitzer CB, Modrof J, Yu MY, Kreil TR. West Nile virus infection in plasma of blood and plasma donors, United States. Emerg Infect Dis. 2009;15:1668–1670. doi: 10.3201/eid1510.080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Niedrig M, Linke S, Zeller H, Drosten C. First international proficiency study on West Nile virus molecular detection. Clin Chem. 2006;52:1851–1854. doi: 10.1373/clinchem.2005.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Linke S, Mackay WG, Scott C, Wallace P, Niedrig M. Second external quality assessment of the molecular diagnostic of West Nile virus: are there improvements towards the detection of WNV? J Clin Virol. 2011;52:257–260. doi: 10.1016/j.jcv.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 177.Jordan I, Briese T, Fischer N, Lau JY, Lipkin WI. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J Infect Dis. 2000;182:1214–1217. doi: 10.1086/315847. [DOI] [PubMed] [Google Scholar]
- 178.Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, Rubinshtein E, Itzhaki A, Mishal J, Siegman-Igra Y, Kitzes R, Pick N, Landau Z, Wolf D, Bin H, Mendelson E, Pitlik SD, Weinberger M. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675–678. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- 180.Malet H, Massé N, Selisko B, Romette JL, Alvarez K, Guillemot JC, Tolou H, Yap TL, Vasudevan S, Lescar J, Canard B. The flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008;80:23–35. doi: 10.1016/j.antiviral.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 181.Walid MS, Mahmoud FA. Successful treatment with intravenous immunoglobulin of acute flaccid paralysis caused by West Nile virus. Perm J. 2009;13:43–46. doi: 10.7812/tpp/09-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ben-Nathan D, Gershoni-Yahalom O, Samina I, Khinich Y, Nur I, Laub O, Gottreich A, Simanov M, Porgador A, Rager-Zisman B, Orr N. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect Dis. 2009;9:18. doi: 10.1186/1471-2334-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob Agents Chemother. 2010;54:2431–2436. doi: 10.1128/AAC.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011;3:269–285. doi: 10.2217/imt.10.93. [DOI] [PubMed] [Google Scholar]
- 185.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 186.Biedenbender R, Bevilacqua J, Gregg AM, Watson M, Dayan G. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infect Dis. 2011;203:75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith HL, Monath TP, Pazoles P, Rothman AL, Casey DM, Terajima M, Ennis FA, Guirakhoo F, Green S. Development of antigen-specific memory CD8+ T cells following live-attenuated chimeric West Nile virus vaccination. J Infect Dis. 2011;203:513–522. doi: 10.1093/infdis/jiq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, Enama ME, Nelson S, Nason M, Gu W, Bundrant N, Koup RA, Bailer RT, Mascola JR, Nabel GJ, Graham BS, VRC 303 Study Team a West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schneeweiss A, Chabierski S, Salomo M, Delaroque N, Al-Robaiy S, Grunwald T, Bürki K, Liebert UG, Ulbert S. A DNA vaccine encoding the E protein of West Nile Virus is protective and can be boosted by recombinant domain DIII. Vaccine. 2011;29:6352–6357. doi: 10.1016/j.vaccine.2011.04.116. [DOI] [PubMed] [Google Scholar]
- 190.Posadas-Herrera G, Inoue S, Fuke I, Muraki Y, Mapua CA, Khan AH, Parquet Mdel C, Manabe S, Tanishita O, Ishikawa T, Natividad FF, Okuno Y, Hasebe F, Morita K. Development and evaluation of a formalin-inactivated West Nile Virus vaccine (WN-VAX) for a human vaccine candidate. Vaccine. 2010;28:7939–7946. doi: 10.1016/j.vaccine.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 191.Minke JM, Siger L, Cupillard L, Powers B, Bakonyi T, Boyum S, Nowotny N, Bowen R. Protection provided by a recombinant ALVAC®-WNV vaccine expressing the prM/E genes of a lineage 1 strain of WNV against a virulent challenge with a lineage 2 strain. Vaccine. 2011;29:4608–4612. doi: 10.1016/j.vaccine.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 192.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 193.Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, Linnen JM, Shyamala V, Tomasulo P, Kleinman SH. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460–467. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 194.Montgomery SP, Brown JA, Kuehnert M, Smith TL, Crall N, Lanciotti RS, Macedo de Oliveira A, Boo T, Marfin AA, 2003 West Nile Virus Transfusion-Associated Transmission Investigation Team Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion. 2006;46:2038–2046. doi: 10.1111/j.1537-2995.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 195.Centers for Disease Control and Prevention West Nile virus transmission through blood transfusion–South Dakota, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:76–79. [PubMed] [Google Scholar]
- 196.Kleinman SH, Williams JD, Robertson G, Caglioti S, Williams RC, Spizman R, Morgan L, Tomasulo P, Busch MP. West Nile virus testing experience in 2007: evaluation of different criteria for triggering individual-donation nucleic acid testing. Transfusion. 2009;49:1160–1170. doi: 10.1111/j.1537-2995.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 197.Biggerstaff BJ, Petersen LR. A modeling framework for evaluation and comparison of trigger strategies for switching from minipool to individual-donation testing for West Nile virus. Transfusion. 2009;49:1151–1159. doi: 10.1111/j.1537-2995.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 198.O'Brien SF, Scalia V, Zuber E, Hawes G, Alport EC, Goldman M, Fearon MA. West Nile virus in 2006 and 2007: the Canadian Blood Services’ experience. Transfusion. 2010;50:1118–1125. doi: 10.1111/j.1537-2995.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 199.Cameron C, Reeves J, Antonishyn N, Tilley P, Alport T, Eurich B, Towns D, Lane D, Saldanha J. West Nile virus in Canadian blood donors. Transfusion. 2005;45:487–491. doi: 10.1111/j.0041-1132.2005.04384.x. [DOI] [PubMed] [Google Scholar]
- 200.Vamvakas EC, Kleinman S, Hume H, Sher GD. The development of West Nile virus safety policies by Canadian blood services: guiding principles and a comparison between Canada and the United States. Transfus Med Rev. 2006;20:97–109. doi: 10.1016/j.tmrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 201.Zou S, Foster GA, Dodd RY, Petersen LR, Stramer SL. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354–1361. doi: 10.1086/656602. [DOI] [PubMed] [Google Scholar]
- 202.Food and Drug Administration . Guidance for Industry: Use of Nucleic Acid Tests to Reduce the Risk of Transmission of West Nile Virus from Donors of Whole Blood and Blood Components Intended for Transfusion. Rockville: US Department of Health and Human Services, Food and Drug Administration; 2008. [Google Scholar]
- 203.Kreil TR, Berting A, Kistner O, Kindermann J. West Nile virus and the safety of plasma derivatives: verification of high safety margins, and the validity of predictions based on model virus data. Transfusion. 2003;43:1023–1028. doi: 10.1046/j.1537-2995.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 204.Jakubik JJ, Vicik SM, Tannatt MM, Kelley BD. West Nile Virus inactivation by the solvent/detergent steps of the second and third generation manufacturing processes for B-domain deleted recombinant factor VIII. Haemophilia. 2004;10:69–74. doi: 10.1046/j.1365-2516.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 205.Hellstern P, Solheim BG. The use of solvent/detergent treatment in pathogen reduction of plasma. Transfus Med Hemother. 2011;38:65–70. doi: 10.1159/000323552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mohr H, Knüver-Hopf J, Gravemann U, Redecker-Klein A, Müller TH. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion. 2004;44:886–890. doi: 10.1111/j.1537-2995.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- 207.Lin L, Hanson CV, Alter HJ, Jauvin V, Bernard KA, Murthy KK, Metzel P, Corash L. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion. 2005;45:580–590. doi: 10.1111/j.0041-1132.2005.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gallian P, Vignoli C, Dombey AM, Mayaudon V, Lin L, Galichet V, Cantaloube JF, De Micco P. Inactivation of a European strain of West Nile virus in single-donor platelet concentrate using the INTERCEPT blood system. Vox Sang. 2006;91:345–347. doi: 10.1111/j.1423-0410.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 209.Irsch J, Lin L. Pathogen inactivation of platelet and plasma blood components for transfusion using the INTERCEPT Blood System™. Transfus Med Hemother. 2011;38:19–31. doi: 10.1159/000323937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Marschner S, Goodrich R. Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus Med Hemother. 2011;38:8–18. doi: 10.1159/000324160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 212.Rock G. A comparison of methods of pathogen inactivation of FFP. Vox Sang. 2011;100:169–178. doi: 10.1111/j.1423-0410.2010.01374.x. [DOI] [PubMed] [Google Scholar]