Abstract
Exposure to addictive drugs can result in maladaptive alterations in neural circuit function. This review highlights recent progress made in identifying the organization, function, and cellular plasticity of the ventral tegmental area (VTA) and nucleus accumbens (NAc), two brain regions strongly implicated in substance use disorders. Emphasis is given to advances made with new research methodologies, particularly optogenetics, which have provided scientists with an unprecedented ability to map neural circuitry and pinpoint drug-induced synaptic modifications. A better understanding of these adaptive events will aid the development of pharmacological treatments for drug addiction and, more generally, further our understanding of motivated behaviors.
Introduction
The main pathological features of substance use disorders — compulsive drug seeking, loss of self-control, and propensity to relapse — are shared between all classes of addictive drugs. This is suggestive of a common neural mechanism and, indeed, many drug-induced changes in the brain are common to the addicted state.
One of the most prominent effects observed in the brain immediately following drug exposure is an elevation of dopamine (DA) levels, particularly in the NAc [1]. Accordingly, much attention has been devoted to the triggers and consequences of dopamine release, especially in relationship to drug-dependent behaviors [2,3,4•,5]. At the synaptic level, a DA-dependent strengthening of glutamatergic synapses has been observed in both the NAc and VTA after a single in vivo exposure to cocaine, as well as other abused drugs [6,7,8•,9]. While it is clear that activity in these two structures has a profound influence on animals’ propensity to seek out and work for rewards, research has been hindered by the heterogeneity of cell types and signaling molecules in these regions [10•,11]. In both the NAc and VTA, intermingled populations of cells appear to participate in completely segregated, opposing circuits [12••,13]. Fortunately, advances in experimental techniques, and particularly the development of optogenetics, have now made it possible to stimulate specific cell types and neural pathways with a exceptional degree of spatial, temporal, and neurochemical precision [14]. This review highlights recent studies that have employed these techniques to better understand the circuitry and synaptic modifications underlying substance use disorders (Figure 1) [15–19].
Figure 1.
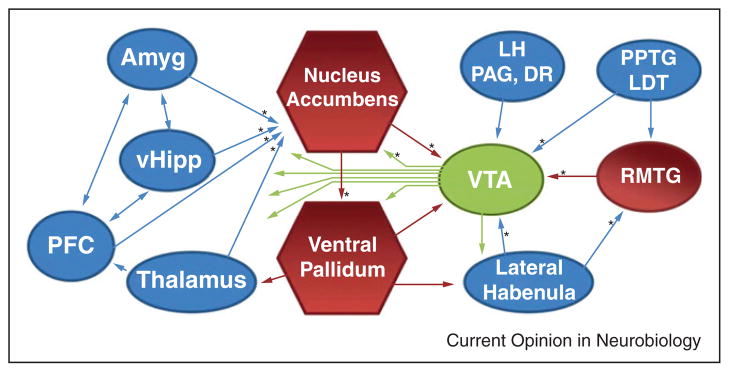
Schematic of the principal brain regions that innervate the VTA and NAc. Red indicates inhibitory structures and blue indicates excitatory structures. Pathways that have been examined with optogenetic techniques are indicated with an *. Amyg, amygdala; vHipp, ventral hippocampus; LH, lateral hypothalamus; PAG, periaqueductal gray; DR, dorsal raphe; PPTG/LDT, pedunculopontine and laterodorsal tegmentum; RMTG, rostromedial tegmental nucleus.
Optogenetic approaches
Optogenetic techniques capitalize on light-sensitive proteins to control neuronal activity. A wide variety of these proteins have now been optimized for use in neurons, including ion channels and pumps as well as G protein coupled receptors [20,21•,22]. To get selective expression of these proteins, DNA encoding the opsin is introduced into neurons in an anatomically restricted manner, commonly by viral delivery or in vivo electroporation [23,24]. Expression of the protein within neurons can be further restricted with the use of selective promoters or DNA recombination systems [25,26]. Once the protein is expressed, it will diffuse within the membrane of cells and over time can be found in distal processes. This allows for the direct manipulation of spiking activity in axons. Thus, there are multiple levels by which photo-stimulation can be restricted: location of DNA delivery, cell-type specific expression, and localized light delivery. When these approaches are used together, anatomically localized, genetically defined neural pathways can be repeatedly stimulated or inhibited, in vitro and in vivo.
Neural circuitry of the ventral tegmental area
The VTA has a central and complicated role in motivated behaviors [10•]. It is a heterogeneous structure, where DA, GABA, and glutamate projection neurons are all intermingled [27,28]. In vivo electrophysiological recordings have been employed in this region to gain insight into when and why these neurons fire, but it has been difficult to unequivocally identify the type of neuron that is, being recorded. Optical tagging refers to the use of optogenetics to determine the identity of neurons during in vivo recordings [29,30]. By targeting optical stimulation or inhibition to a specific subset of cells, electrophysiological responses can indicate if the active cell is a neuron that expresses the opsin [31,32•]. This type of approach was recently used to unambiguously identify cell types in the VTA and confirm that it is indeed just DA neurons that signal discrepancies between expected and actual rewards [33••]. Neighboring GABA neurons similarly respond to reward-predicting cues, but their firing pattern is not further modulated in real-time by receipt or omission of the expected reward [33••].
It has long been appreciated that DA plays a major role in reinforcement learning, and now selective photostimulation of VTA DA neurons has confirmed that enhanced activity in these cells is sufficient to reinforce instrumental behavior [34–37]. The firing rate of DA neurons is an important factor, as only high frequency activity patterns can condition a place preference [38]. Selective photo-stimulation of DA neurons is also sufficient to strengthen glutamatergic synapses in the VTA, a consequence common to all addictive drugs [9]. This demonstrates that there is a direct link between DA neuron activity and an increase in the strength of their excitatory synaptic inputs. Whether this plasticity is restricted to specific glutamatergic synapses in the VTA or is a more general phenomenon is still an open question.
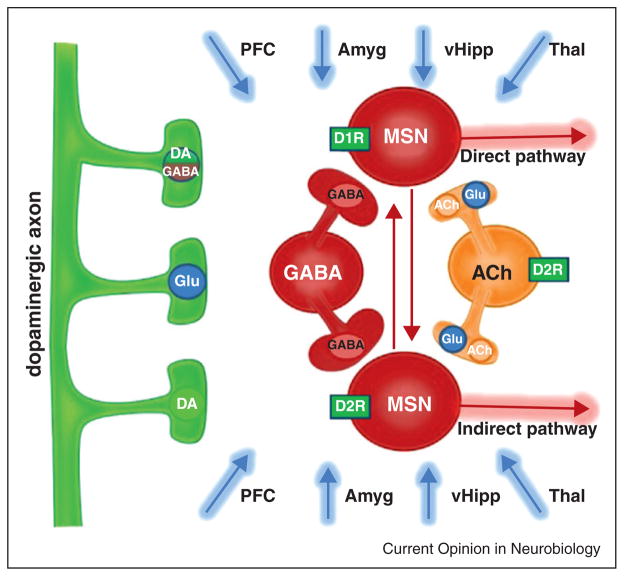
An important consideration is that while photostimulation can be highly selective for cell types or pathways, many types of neurons, including DA neurons, release more than one neurotransmitter. Recent optogenetic studies have shown that DA neurons can release glutamate and GABA to varying extents, in addition to DA (Figure 2). While it is presently unclear if these neurotransmitters share the same synaptic vesicles, glutamate release has been observed from dopaminergic axons in the NAc [39–42]. In the dorsal striatum it has been demonstrated that dopaminergic axons scavenge and release GABA [43••]. How widespread these phenomena are among other dopaminergic projections is not yet known. This complexity in the signaling molecules released by DA neurons is part of the reason it has been so difficult to characterize how the activity of these neurons influences downstream structures [44].
Figure 2.
Schematic of the organization of the nucleus accumbens. Both dopaminergic and glutamatergic fibers innervate each local cell type. Multiple neurotransmitters are released from dopaminergic and cholinergic axons. The two types of MSNs can be differentiated by the dopamine receptors they express and their access to the midbrain, which is either direct or indirect.
Whether aversive stimuli activate or inhibit different VTA neurons has been another ongoing question, and recent work demonstrates that indeed both effects can occur, just in different, neighboring dopaminergic circuits [33••,45]. One circuit that is, activated by aversive stimuli involves an excitatory projection from the lateral habenula to DA neurons that preferentially innervate the prefrontal cortex (PFC) [12••]. Most of the lateral habenula input, however, inhibits midbrain DA neurons through a GABAergic relay in the rostromedial tegmental nucleus [46•,47,48]. These DA cells, which are inhibited in response to aversive stimuli, preferentially innervate the NAc [48,49]. This is consistent with voltammetry data showing aversive stimuli acutely reduces DA levels there [50,51]. It is unclear if these NAc-projecting DA cells are distinct from those that receive excitatory input from the pedunculopontine tegmentum, which is an input that can generate a conditioned place preference when activated [12••]. These data highlight how neighboring cells can participate in completely segregated circuits. Since inputs to VTA come from all over the brain and outputs go to a similarly wide variety of structures, much more circuit mapping remains to be done here [52••].
Neural circuitry of the nucleus accumbens
The NAc integrates reward-related information from several areas throughout the brain, including the VTA [53]. The principal cell type in the NAc is a GABAergic projection neuron known as a medium spiny neuron (MSN). There are two main types of MSNs and the primary feature that separates them is the direct or indirect route by which they influence neural activity in the midbrain [54]. Optogenetic experiments examining outputs from both the NAc and dorsal striatum have recently provided functional evidence that both direct and indirect output neurons innervate downstream GABA neurons in their respective targeted structures [55,56]. While these studies did not find any evidence for direct pathway innervation of DA neurons, there is strong anatomical evidence that this connection exists [52••,57]. Optogenetic studies have confirmed that MSNs form inhibitory synapses onto each other as well as onto local cholinergic interneurons, but these cells do not synapse on some of the other interneuron populations, such as fast-spiking interneurons [55].
Selective photostimulation of MSN subtypes has confirmed that activity in these cells has opposing influences on behavior, with activity in direct and indirect pathway neurons often encouraging and discouraging behavior, respectively [58]. For example, photostimulation of direct and indirect pathway MSNs in the NAc can bidirectionally alter the ability of cocaine to induce a conditioned place preference [59]. In the dorsal striatum, selective stimulation of MSN subtypes can increase or decrease locomotion, depending on the targeted cell type [31]. These stimulations are also capable of reinforcing or discouraging exploration behavior and can introduce opposing biases in goal-directed action selection [32•,60••]. Likewise, the inhibition of these cell types differentially alters the behavioral plasticity associated with repeated drug treatment [61]. When all MSNs are photostimulated, without regard for subtype, behavioral effects similar to direct pathway stimulation are typically observed. Specifically, photostimulation of all NAc shell MSNs supports intracranial self-stimulation and enhances the rewarding effects of cocaine [59,62••]. Similarly, a place preference is elicited when spiking activity is increased in both MSN populations by photostimulation of Gq-coupled α1A-adrenergic receptors [63].
The influence of cholinergic interneuron populations on behavior has also been explored with selective optogenetic manipulations, and neither activating nor inhibiting these neurons produced gross behavioral effects or elicited a place preference [64]. Inhibiting these neurons did however increase the firing rate of neighboring cells, and when this was done during cocaine exposure it decreased conditioned place preference [64]. This observation is inconsistent with the finding that indiscriminate activation of all NAc neurons augments cocaine reward, but cholinergic interneuron inhibition may preferentially activate indirect pathway MSNs. These cholinergic inter-neurons can be inhibited in a physiological manner by GABAergic projections from the VTA, which send a remarkably specific input to just these striatal neurons [65•]. Overall the cholinergic interneurons have a very intricate role in modulating NAc physiology, since their photostimulation triggers both DA release and feed-forward inhibition onto MSNs [66–68]. Moreover, these interneurons can also corelease glutamate [69].
Drug-induced synaptic plasticity in the nucleus accumbens
Excitatory input to the NAc primarily comes from the ventral hippocampus, basolateral amygdala, medial pre-frontal cortex, and midline thalamus, and recent optogenetic studies have identified several idiosyncrasies in pathway-specific synaptic properties (Figure 1) [62••]. Innervation patterns are noteworthy as the afferents are heterogeneously distributed and connection strength varies throughout the NAc. Basolateral amygdala inputs are fairly robust throughout the entire ventral striatum, while ventral hippocampal inputs are uniquely localized to and predominate in the medial NAc shell [62••]. Each excitatory input innervates both MSN subtypes but, at least in the dorsomedial NAc core, hippocampal inputs to indirect pathway MSNs are relatively weak [70]. These fibers preferentially innervate small distal spines on indirect pathway MSNs, which makes the hippocampal input to these cells relatively ineffective at driving postsynaptic action potentials. PFC and thalamus inputs, in contrast, similarly innervate both MSN subtypes in the NAc core [70]. The data overall suggest that differences between NAc inputs might be more a matter of degree than categorical. This raises the possibility that the various excitatory inputs have similar ways of engaging striatal microcircuitry. Indeed, photostimulation of different sets of glutamate axons in the NAc, whether they are from the hippocampus, amygdala, or PFC, can similarly reinforce instrumental behavior [62••,71•]. Of course, particular environmental stimuli differentially activate these pathways, but photo-stimulation of each group of axons is sufficient to drive intracranial self-stimulation and induce a place preference. This indicates the information encoded in these inputs has incentive properties and can contribute to reinforcement learning. Determining what exactly is encoded in these specific inputs is an area of ongoing investigation.
Repeated exposure to cocaine initiates a cascade of events in the NAc [72,73]. One particularly intriguing effect that appears a week or two after cocaine use starts is a potentiation of excitatory inputs to MSNs [74•,75]. When cocaine is self-administered under extended access paradigms, this synaptic potentiation involves the insertion of GluR2-lacking AMPA receptors, and blocking these specific receptors can attenuate compulsive drug seeking [76–78]. It is presently unclear if this particular type of synaptic plasticity is localized to specific inputs or cell types in the NAc. However, several pathway-specific synaptic modifications have recently been identified following various drug-administration paradigms. For example, following withdrawal from self-administered cocaine, there is an increase in transmitter release probability in PFC, but not amygdala, inputs to the NAc [79].
Presently, drug-induced, pathway-specific postsynaptic modifications have only been reported after experimenter-administered, intraperitoneal injections of cocaine. With this paradigm, ventral hippocampus inputs in the medial NAc shell are selectively potentiated when compared with basolateral amygdala and prefrontal cortex inputs [62••]. A separate study which distinguished MSN subtypes and focused on PFC infralimbic inputs to the NAc found a selective potentiation of these synapses on direct pathway MSNs [8•]. Intriguingly, depotentiation of these synapses was effective at reversing cocaine-induced locomotor sensitization [8•]. This parallels what happens with direct optical inhibition of the infralimbic cortex, which is effective at stopping habitual behavior on a T-maze [80•]. Other PFC regions have also been implicated in drug-related behaviors, and optical inhibition of the prelimbic cortex, as well as a more selective inhibition of the prelimbic inputs to the NAc core, blocks cocaine-primed cocaine seeking [81]. Together, these studies raise the possibility that attenuating PFC input to the NAc may be an effective treatment for drug abuse disorders.
Another avenue of research explores if small subsets or ensembles of MSNs are responsible for specific drug-related behaviors. Indeed, only a small minority of MSNs show c-fos activation in response to cocaine exposure [82]. Inactivating these specific cells, which exhibit their own unique drug-induced synaptic modifications, is sufficient in disrupting cocaine-induced psychomotor sensitization [83•,84]. These data highlight the complexity of drug-induced synaptic plasticity and stress the importance of identifying what modifications underlie the pathological features of drug abuse disorders.
Conclusions
Because of the complex assortment of cell types, neurotransmitters systems, and subcircuits within the VTA and NAc, optogenetic techniques have been a boon for research on these structures. Many well-established hypotheses have been confirmed, such as the fact that some DA neurons are activated by aversive stimuli and corelease glutamate [33••,39,41]. Likewise, the opposing forces of the two MSN subtypes in the striatum have been verified, and now we have detailed information on what this means for specific behaviors [31,32•,59]. There have also been many surprises from the use of optogenetic techniques, such as the fact that dopamine neurons can release GABA and that glutamatergic input to the NAc from any number of sources can carry a rewarding signal [43••,62••]. Future research will further benefit from the use of temporally precise optical inhibition to uncover the specific physiological role of different neural circuits. This type of work has already revealed circuits that can attenuate habitual behavior, which is particularly important for the goal of overcoming drug addiction [8•,80•].
Acknowledgments
Authors are supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse. We thank Dr. Ross McDevitt for helpful editorial comments and the National Institute on Drug Abuse Visual Media Department for help with the figures.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. This review discusses the complex mechanisms by which DA regulates electrical and biochemical aspects of neuronal function in the striatum and PFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci U S A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 7.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 8•.Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. These authors identify a pathway-specific, cocaine-induced synaptic potentiation in infralimbic inputs to direct pathway MSNs in the NAc shell. Depotentiation of these cortical inputs abolished cocaine-induced locomoter sensitization. [DOI] [PubMed] [Google Scholar]
- 9.Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. This review discusses the complex roles of dopamine in behavioral functions related to motivation. It distinguishes between aspects of motivation that are differentially affected by dopaminergic manipulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales M, Pickel VM. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Ann N Y Acad Sci. 2012;1248:71–88. doi: 10.1111/j.1749-6632.2011.06299.x. [DOI] [PubMed] [Google Scholar]
- 12••.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012 doi: 10.1038/nature11527. This paper characterizes two distinct circuits involving VTA DA neurons, which when activated can elicit either reward or aversion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology (Bethesda) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao ZF, Burdakov D, Sarnyai Z. Optogenetics: potentials for addiction research. Addict Biol. 2011;16:519–531. doi: 10.1111/j.1369-1600.2011.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez R, Lobo MK, Zhang F, de Lecea L. Neural integration of reward, arousal, and feeding: recruitment of VTA, lateral hypothalamus, and ventral striatal neurons. IUBMB Life. 2011;63:824–830. doi: 10.1002/iub.539. [DOI] [PubMed] [Google Scholar]
- 17.Stamatakis AM, Stuber GD. Optogenetic strategies to dissect the neural circuits that underlie reward and addiction. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. 2012 doi: 10.1016/j.brainres.2012.11.001. in press [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei Y, Zhang F. Molecular tools and approaches for optogenetics. Biol Psychiatry. 2012;71:1033–1038. doi: 10.1016/j.biopsych.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9:159–172. doi: 10.1038/nmeth.1808. This paper systematically compares opsins under matched experimental conditions and identifies key parameters that should be considered when designing, conducting, and interpreting optogenetic experiments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prigge M, Schneider F, Tsunoda SP, Shilyansky C, Wietek J, Deisseroth K, Hegemann P. Color-tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem. 2012;287:31804–31812. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britt JP, McDevitt RA, Bonci A. Use of channelrhodopsin for activation of CNS neurons. Curr Protoc Neurosci. 2012;Chapter 2(Unit 2):16. doi: 10.1002/0471142301.ns0216s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders A, Johnson CA, Sabatini BL. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits. 2012;6:47. doi: 10.3389/fncir.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0452-z. in press [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kravitz AV, Kreitzer AC. Optogenetic manipulation of neural circuitry in vivo. Curr Opin Neurobiol. 2011;21:433–439. doi: 10.1016/j.conb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 2012 doi: 10.1016/j.brainres.2012.11.018. in press [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. By directly photostimulating MSNs in the striatum, these authors show that reward and aversion can be elicited by direct and indirect pathway neurons, respectively, in a dopamine receptor-independent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. This is the first paper to use optogenetics to identify DA and GABA neurons during in vivo electrophysiological recordings. It confirms that DA neurons both encode a reward-prediction error and often respond to aversive stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg EE, Janak PH. Establishing causality for dopamine in neural function and behavior with optogenetics. Brain Res. 2012 doi: 10.1016/j.brainres.2012.09.036. in press [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KM, Baratta MV, Yang A, Lee D, Boyden ES, Fiorillo CD. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE. 2012;7:e33612. doi: 10.1371/journal.pone.0033612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur J Neurosci. 2011;33:1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- 43••.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. This paper is the first to show that GABA can transport through the vesicular monoamine transporter VMAT2 and be released from dopaminergic axons in the striatum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulzer D, Rayport S. Dale’s principle and glutamate corelease from ventral midbrain dopamine neurons. Amino Acids. 2000;19:45–52. doi: 10.1007/s007260070032. [DOI] [PubMed] [Google Scholar]
- 45.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. These authors show that aversive stimuli activates lateral habenula inputs to the midbrain. This excitatory projection inhibits VTA DA neurons via a GABAergic relay in the rostromedial tegmental nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. Using a modified rabies virus to comprehensively identify monosynaptic inputs to midbrain DA neurons from throughout the brain, these authors shows that some MSNs directly innervate midbrain DA neurons. [DOI] [PubMed] [Google Scholar]
- 53.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 58.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 59.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. These authors found that transient optogenetic stimulation of dorsal striatal MSNs during decision-making could introduce opposing biases in the distribution of choices made by mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. These authors explore the synaptic properties and behavioral relevance of three different excitatory inputs to the NAc. They demonstrate that activation of glutamate axons in the NAc is sufficient to reinforce instrumental behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 64.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Brown MT, Tan KR, O‘Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. These authors demonstrate that GABAergic projection neurons in the VTA selectively innervate cholinergic interneurons in the NAc. Activation of this pathway in behaving mice enhances discrimination of a motivationally important stimulus. [DOI] [PubMed] [Google Scholar]
- 66.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 69.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS ONE. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macaskill AF, Little JP, Cassel JM, Carter AG. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat Neurosci. 2012;15:1624–1626. doi: 10.1038/nn.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. These authors demonstrate that brief optical inhibition of fibers from the amygdala to the NAc can reduce cue-evoked intake of sucrose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. This review describes in depth the current literature regarding cocaine-induced changes in AMPA receptors in the NAc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suska A, Lee BR, Huang YH, Dong Y, Schluter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci U S A. 2012;110:713–718. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. This paper describes how optogenetic inhibition of infralimbic cortex can stop habitual responding in a T-maze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2012;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koya E, Hope BT. Cocaine and synaptic alterations in the nucleus accumbens. Biol Psychiatry. 2011;69:1013–1014. doi: 10.1016/j.biopsych.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. These authors identify synaptic modifications that are unique to the minority of NAc neurons that exhibit robust c-fos expression following repeated cocaine injections. These neurons are central to cocaine-induced psychomotor sensitization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]