Abstract
Objective
The Sequential Organ Failure Assessment (SOFA) score is validated to measure severity of organ dysfunction in critically ill patients. However, in some practice settings, daily arterial blood gas (ABG) data required to calculate the respiratory component of the SOFA score are often unavailable. The objectives of this study were to derive SpO2/FiO2 (SF) ratio correlations with the PaO2/ FiO2 (PF) ratio to calculate the respiratory parameter of the SOFA score, and to validate the respiratory SOFA obtained using SF ratios against clinical outcomes.
Patients and measurements
We obtained matched measurements of SpO2 and PaO2 from two populations: Group 1- patients undergoing general anesthesia and Group 2- patients from the ARDS network -low versus high tidal volume for the Acute Respiratory Management of ARDS (ARMA) database. Using a linear regression model, we first determined SF ratios corresponding to PF ratios of 100, 200, 300 and 400. Second, we evaluated the contribution of positive end expiratory pressure (PEEP) on the relationship between SF and PF, for patients on PEEP in centimeters of water (cm H2O) of <8, 8–12 and >12. Third, we calculated the SOFA scores in a separate cohort of ICU patients using the derived SF ratios and validated them against clinical outcomes.
Results
The total SOFA scores calculated using SF ratios and PF ratios were highly correlated (Spearman's rho 0.85, p<0.001) in all patients and in the 3 stratified PEEP categories (<8 cm H20, Spearman's rho 0.87, p<0.001; PEEP 8–12 cm H20, Spearman's rho 0.85, p<0.001; PEEP>12 cm H20, Spearman's rho 0.85, p<0.001). The respiratory SOFA scores based on SF ratios and PF ratios correlated similarly with ICU length of stay and ventilator-free days, when validated in a cohort of critically ill patients.
Conclusion
The total and respiratory SOFA scores obtained with imputed SF values correlate with the corresponding SOFA score using PF ratios. Both the derived and original respiratory SOFA scores similarly predict outcomes.
Introduction
Advances in modern medicine have resulted in marked improvements in patients' survival of diseases that were once considered to be fatal. Concurrently, there has also been a tremendous growth in the elderly population with many surviving into their 8th and 9th decades.(1) These changing demographics, along with the sheer increase in the volume and acuity of patients admitted to hospitals, necessitate the need for scoring systems to describe evolving morbidity, treatment endpoints, organ dysfunction, and predictors of mortality.(2)
The Sequential Organ Failure Assessment (SOFA)(3) score is one such scoring system, which allows daily evaluation of organ dysfunction in critically ill patients. The SOFA score was validated as a measure of severity of illness over time within patients, and thus can be used to follow the course of organ dysfunction and response to treatment.(4) The SOFA score has become the premier scoring system of patients in multi-organ failure, given its high specificity and sensitivity as a predictor of morbidity and mortality in critically ill patients.(2–6)
The SOFA score is based on six organ categories comprised of the respiratory, cardiovascular, nervous, hepatic, renal and coagulation systems.(3) Each of these categories is graded from a score of 1 (least organ dysfunction) to 4 (severe organ dysfunction), based on predefined cutoffs that are easily measured in the Intensive Care Unit (ICU) (Table 1). Of these, the severity of respiratory dysfunction is measured in the SOFA score by PaO2/FiO2 (PF) ratios, which require a daily arterial blood gas measurement if the SOFA score is to be followed during the course of an ICU admission. (3) Often these blood gas data are not available on a daily basis; hence the clinical and research utility of the SOFA and other scoring systems are reduced dramatically. Recently, Rice et. al.(7) determined the SF ratios corresponding to PF ratios of 200 and 300 to assist in the diagnosis of acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) respectively; however the corresponding SF values for PF ratios of 100 and 400 needed for the SOFA score are presently unavailable. Furthermore, it is not known if the level of positive end expiratory pressure (PEEP) required adds predictive value to the PF or SF ratios. While PEEP does not affect the oxyhemoglobin curve and hence the relationship between SpO2 and PaO2, PEEP may impact the PF ratio by improving ventilation perfusion matching. The aims of this study, therefore, were to expand on the work done by Rice et al.(7) and derive and validate a reliable system to utilize SF ratios to impute for the PF ratios in assessing the respiratory parameters of the SOFA score, providing clinicians and researchers the ability to monitor daily SOFA scores even when arterial blood gas data are not available.
Table 1.
The Sequential Organ Failure Score (SOFA)*
| SOFA score |
Respiratory PaO2/ FiO2 ratio |
Cardiovascular Mean Arterial Pressure / vasopressors* |
Nervous Glasgow coma score |
Hepatic Bilirubin, mg/dl |
Renal Creatinine, mg/dl (or urine output) |
Coagulation Platelets (×103/mm3) |
|---|---|---|---|---|---|---|
| 1 | <400 | < 70 mm/Hg | 13–14 | 1.2–1.9 | 1.2 – 1.9 | <150 |
| 2 | <300 | dopamine ≤ 5 or dobutamine (any dose) | 10–12 | 2–5.9 | 2.0 – 3.4 | <100 |
| 3 | <200 | dopamine > 5, epinephrine ≤0.1 or norepinephrine ≤ 0.1 | 6–9 | 6–11.9 | 3.5 – 4.9 (or < 500 ml/d) | <50 |
| 4 | <100 | dopamine > 15, epinephrine > 0.1 or norepinephrine > 0.1 | <6 | >12 | > 5.0 (or < 200 ml/d) | <20 |
Vasopressor drug doses are in mcg/kg/min
Methods
The study was approved by Institutional Review Board at Vanderbilt University Medical Center and involved two phases; Phase 1-derivation phase, followed by Phase 2-validation phase. In Phase 1, matched measurements of oxygen saturation by pulse oximetry (SpO2) and partial pressure of oxygen in arterial blood (PaO2) were obtained from 2 groups of patients: Group 1: those undergoing general anesthesia at Vanderbilt University Medical Center from 2002 to 2007 and Group 2: patients from the ARDS network -low versus high tidal volume for the Acute Respiratory Management of ARDS (ARMA) database.(8) We limited data points to those with SpO2 ≤ 98% to maximize matched data in the linear range of the sigmoidal association between SpO2 and PaO2 in the oxyhemoglobin curve, and at the same time maintain clinical relevance and adequate sample size, given that it is unlikely that patients with higher SpO2 would have PF ratios of less than 400 and thus impact the SOFA score. SF ratios corresponding to PF ratios of 100, 200, 300 and 400 were then derived. In Phase 2, the SOFA scores calculated by using these SF ratios were validated against outcomes in a 3rd group of surgical and trauma ICU patients.
Patients
In group 1, the intraoperative data points were obtained from a computerized query of the Microsoft SQL2003 database providing storage for data from Vanderbilt University's electronic anesthesiology documentation system, the Vanderbilt Perioperative Informatics Medical System (VPIMS)©. Data queried from the database included intraoperative pulse oximetry (SpO2) values (Nellcor pulse oximeters via a Philips Intellivue MP 90 monitor) and time correlated intraoperative partial pressure of oxygen (PaO2) determinations made by arterial blood sampling (Laboratory Gem Premier 3000, Instrumentation Technology) in all mechanically ventilated (MV) adults, undergoing general anesthesia. We excluded patients who were scheduled for cardiovascular surgeries, due to the potential effect of cardiopulmonary bypass and hypothermia on blood gas data, and those having thoracotomies with resultant lung resection or hypo-inflation for surgical purposes. In group 2, PaO2 and corresponding SpO2 values were obtained form the ARMA study database of patients with acute respiratory distress syndrome (ARDS).(8)
In our discussions with experts in the field - both clinicians and researchers - the unmet need in this area was to develop an easy to use and reliable imputation for PF ratios by using SF threshold values, without having the clinicians and research personnel perform difficult calculations, incorporating the covariates that may have played a role in this relationship. Hence a decision was made first to develop a model without any covariates, enabling the calculation of the SOFA score when only SpO2 was available in a broad range of patients. To study the possible contribution of PEEP to the predictive value of SF and PF, we evaluated a second model to determine the relationship between SF and PF ratios within 3 categories of PEEP support: <8 centimeters of water (cm H2O), 8–12 cm H2O and > 12 cm H2O.
Statistical analysis
The best fitting association between SF and PF ratios was described by a linear association between log (10) transformed SF and PF. Using the equation derived from the linear regression model, SF ratios corresponding to PF ratios of 100, 200, 300 and 400 were determined in the entire study population consisting of the anesthesia and ARMA databases. The possible contribution of PEEP to the predictive value of SF and PF was examined by stratifying for <8 cm H20 PEEP, 8–12 cm H20 PEEP and >12 cm H20 PEEP, in only the patients in the ARMA database with data on levels of PEEP. Linear regression was then fitted between log (10) transformed SF and PF separately within each stratum. SF threshold values correlating with PF ratios of 100, 200, 300 and 400 were determined separately within each of the 3 PEEP categories, using the equation derived from the models.
To validate the use of SF ratios, the respiratory component of the SOFA score (SOFA respiratory -SF) and the total SOFA (SOFA-SF) were calculated in a cohort of 100 trauma and surgical ICU patients using the derived SF ratio thresholds and were correlated with the respiratory component of the SOFA score (SOFA respiratory-PF) and the total SOFA (SOFA-PF) based on PF values. The SOFA respiratory-SF and SOFA respiratory-PF were then correlated with important outcomes, such as ICU length of stay and ventilator free days, in the same trauma and surgical ICU cohort.
Results
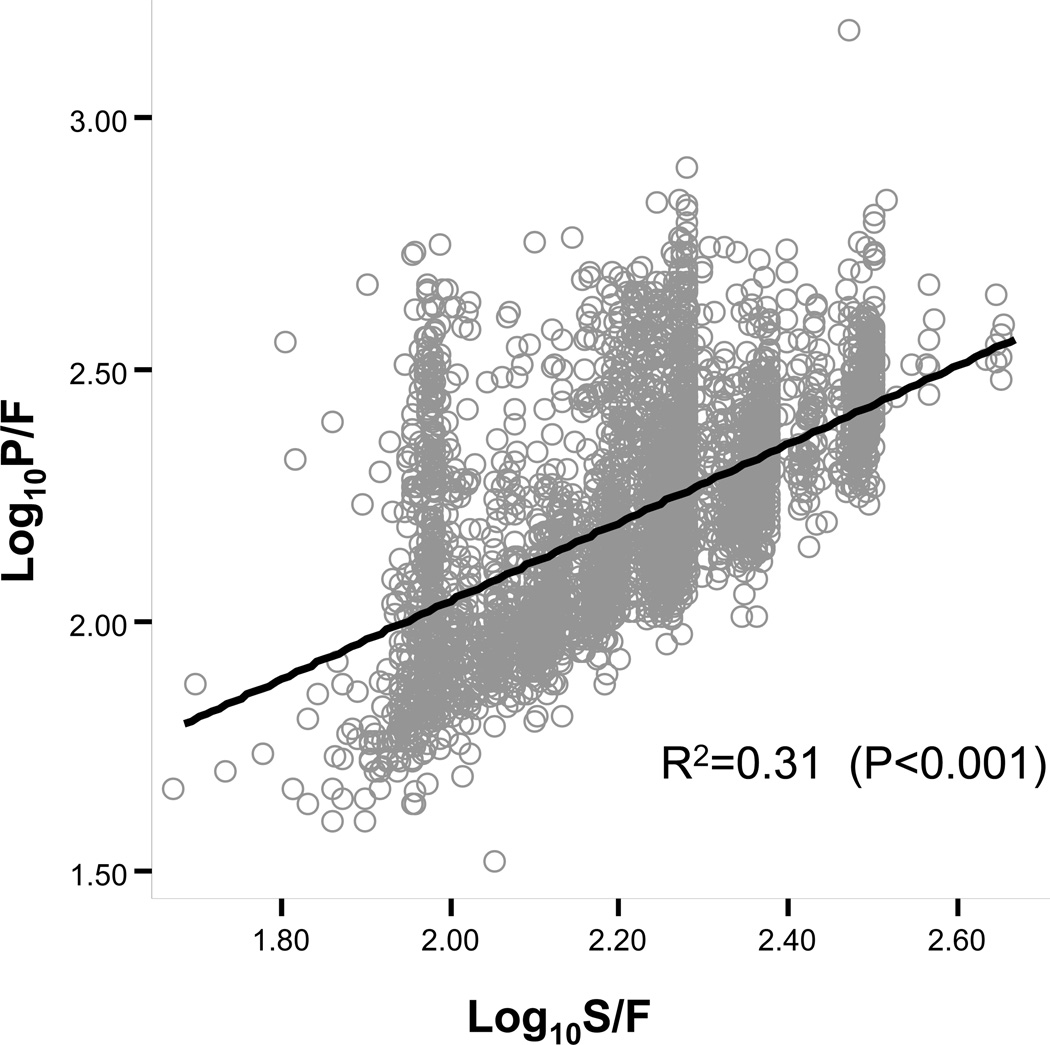
One thousand seven hundred and forty two matched measurements of SpO2 and PaO2 were obtained from patients undergoing anesthesia at Vanderbilt University Medical Center and 2986 measurements from patients in the ARMA database. The best fitting association between SF and PF ratios in the total sample of 4728 matched measurements was described by a linear relationship between the transformed logarithmic value of the SF and PF ratios, with the regression equation Log(PF)=0.48+0.78 × Log(SF) and R-square of 0.31(Figure 1). SF threshold values correlating with PF ratios of 100, 200, 300 and 400 were determined, using the equation derived from the model (Table 2).
Figure 1.
Linear regression describing association between SpO2/FiO2 and PaO2/FiO2 in the combined anesthesia and ARMA database.
The association between SpO2/FiO2 and PaO2/FiO2 was best described by a linear association between log10 transformed PaO2/FiO2values SpO2/FiO2 and. R-square for the model was 0.31 indicating that 31% variability in PaO2/FiO2 was due to SpO2/FiO2.
Table 2.
Derivation of SpO2/FiO2 values corresponding to PaO2/FiO2 ratios in the combined anesthesia and ARMA database*
| SOFA Respiratory score | PaO2/FiO2 | SpO2/FiO2 |
|---|---|---|
| 1 | <400 | <512 |
| 2 | <300 | <357 |
| 3 | <200 | <214 |
| 4 | <100 | <89 |
Data derived from 4728 matched SpO2/FiO2 and PaO2/FiO2 measurements from the combined anesthesia and ARMA database
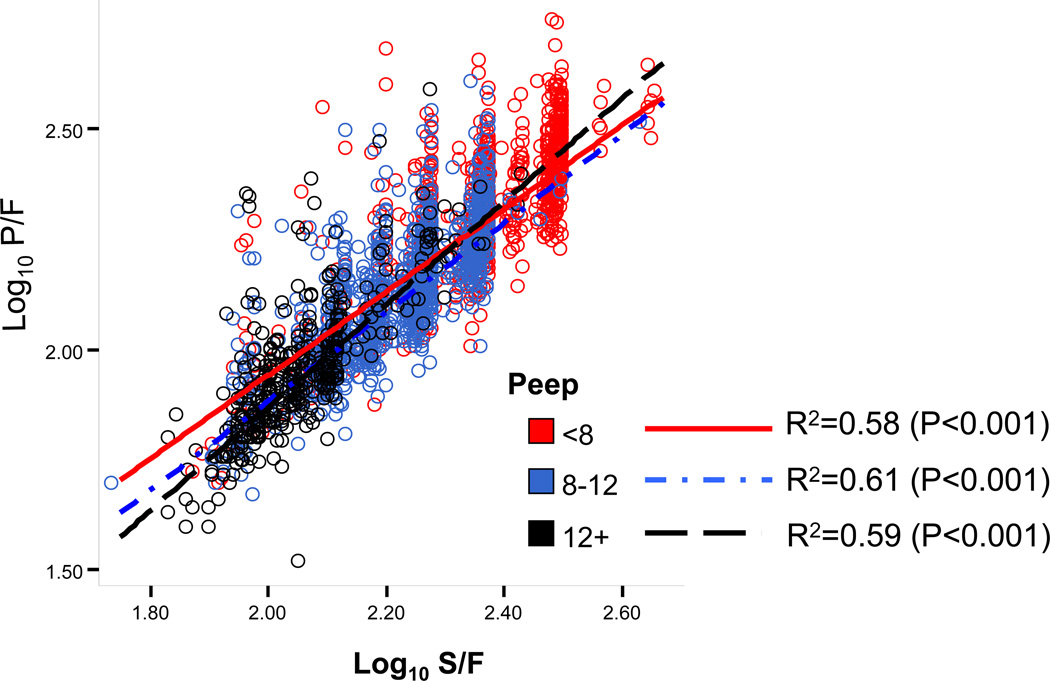
Of the 2986 patients in the ARMA database, 2916 patients had documented PEEP support and were included in the stratified analysis by PEEP. The associations between SF and PF for the 3 models incorporating levels of PEEP (PEEP <8 cm H20, n=1107; PEEP 8–12 cm H20, n=1404 and PEEP >12 cm H20, n= 405), were best described by the following 3 equations: Log(PF)= 0.06 + 0.94 × Log(SF), Log(PF)= −0.13 + 1.01 × Log(SF) and Log(PF)= −0.47 + 1.17 × Log(SF), respectively, with corresponding R-square of 0.58, 0.61 and 0.59 (Figure 2). SF threshold values correlating with PF ratios of 100, 200, 300 and 400, according to level of PEEP are shown in Table 3.
Figure 2.
Linear regression describing association between SpO2/FiO2 and PaO2/FiO2, stratified by level of positive end expiatory pressure in the ARMA database. The association between SpO2/FiO2 and PaO2/FiO2, stratified by the level of PEEP, was best described by a linear association between the log transformed values of SpO2/FiO2 and PaO2/FiO2. R-square for the 3 models (PEEP <8 cm H20, n=1107; PEEP 8–12cm H20, n=1404 and PEEP >12 cm H20, n= 405) were 0.58, 0.61 and 0.59, respectively.
Table 3.
Derivation of SpO2/FiO2 values corresponding to PaO2/FiO2 stratified by PEEP in the ARMA database*
| PaO2/FiO2 ratio | SpO2/FiO2 ratio | |||
|---|---|---|---|---|
| SOFA Respiratory score |
PEEP <8 | PEEP 8–12 | PEEP>12 | |
| 1 | <400 | <502 | <515 | <425 |
| 2 | <300 | <370 | <387 | <332 |
| 3 | <200 | <240 | <259 | <234 |
| 4 | <100 | <115 | <130 | <129 |
Data derived from 2916 matched SpO2/FiO2 and PaO2/FiO2 measurements from the ARMA database of the ARDS network's NIH study(8)
The SOFA respiratory-SF and PF and total SOFA-SF and PF were well correlated (Table 4). Both the SOFA respiratory-SF and PF scores correlated with outcomes when validated in a cohort of 100 surgical and trauma intensive care unit patients (Table 4). Table 5 shows that the SOFA respiratory-SF and PF and total SOFA-SF and PF are correlated in the different PEEP categories and both predict outcomes similarly.
Table 4.
Validation of SOFA scores derived from SpO2/FiO2 from the combined anesthesia and ARMA database *
| Spearman’s rho | p-value | |
|---|---|---|
| SOFA-PF vs. SOFA-SF | 0.85 | <0.001 |
| SOFA respiratory-PF vs. SOFA respiratory-SF | 0.50 | <0.001 |
| ICU LOS vs. SOFA respiratory-PF | 0.26 | 0.005 |
| ICU LOS vs. SOFA respiratory-SF | 0.36 | 0.013 |
| Ventilator free days vs. SOFA respiratory-PF | −0.28 | 0.005 |
| Ventilator free days vs. SOFA respiratory-SF | −0.33 | 0.025 |
Validation performed by calculating SF based SOFA scores and correlating against PF based SOFA scores and clinical outcomes in a separate cohort of surgical and trauma ICU patients.
Table 5.
Validation of SOFA scores derived from SpO2/FiO2, stratified by the PEEP level, from the ARMA database
| Spearman’s rho, p-value | |||
|---|---|---|---|
| PEEP <8 | PEEP 8–12 | PEEP>12 | |
| SOFA-PF vs. SOFA-SF | 0.87, <<0.001 | 0.85, <0.001 | 0.85, <0.001 |
| SOFA respiratory-PF vs. SOFA respiratory-SF | 0.45, <0.001 | 0.41, <0.001 | 0.47, <0.001 |
| ICU LOS vs. SOFA respiratory-PF | 0.28, <0.006 | ||
| ICU LOS vs. SOFA respiratory-SF | 0.36, <0.001 | 0.27, 0.007 | 0.35, <0.001 |
| Ventilator free days vs. SOFA respiratory-PF | −0.28, 0.005 | ||
| Ventilator free days vs. SOFA respiratory-SF | −0.31, 0.002 | −0.25, 0.011 | −0.32, 0.001 |
Validation performed by calculating PEEP stratified SF based SOFA scores and correlating against PF based SOFA scores and clinical outcomes in a cohort of surgical and trauma ICU patients.
Discussion
In this study, we have taken multiple approaches for both clinicians and critical care researchers, to ensure confidence in using pulse oximetry data to calculate a reliable and valid severity of illness score over a wide range of patients in both the operating room and ICU. Our data show that the total SOFA scores calculated by imputing the derived SF ratios (for PF values of 100, 200, 300 and 400) are highly correlated with the total SOFA scores calculated with the PF ratio, and that the respiratory component of the SOFA scores are moderately correlated. In addition, the SF based respiratory SOFA score predicted outcomes when validated in a cohort of trauma and surgical critically ill patients. These findings provide support for using SF ratios to calculate SOFA scores when arterial blood gas data are not available. A second aim of our study was to determine if stratifying for PEEP improved the predictive value of the SF ratio for the PF ratio. Though the correlations between SF and PF for the 3 stratums of PEEP were similar (r-squares of 0.58, 0.61 and 0.59, respectively), subtle differences existed in the SF threshold values for corresponding PF ratios depending on the level of PEEP. Thus level of PEEP may not significantly alter the relationship between SF and PF, and the differences in SF thresholds may not be clinically relevant. However the additional PEEP stratified analysis may provide more precise SF ratios for researchers, incorporating SOFA scores in their studies.
The utility of having a validated model for substituting SF ratios for PF ratios could go beyond the confines of the objective of this study. Extension of this work would allow the correlation we derived for SF vs. PF ratios to be applied to other severity of illness scores such as the Acute Physiology and Chronic Health Evaluation II score (APACHE II),(9) that require a PaO2 or PF ratios for their calculation.
Our study has several strengths and limitations that should be mentioned. First, we studied a heterogeneous group of patients, who were either undergoing general anesthesia or were part of the ARMA study. This strength, along with the large number of paired observations, increases the generalizability of the study given that both surgical and medical patients with varying degrees of pulmonary dysfunction were studied. However, this strength in generalizability is limited by poorer correlation between SF and PF ratios (a lower R-square value), when evaluated in the entire population. This is primarily driven by the group of patients undergoing general anesthesia, who are often oxygenated to maintain saturations above 95%, thus limiting the number of SpO2 measurements in the linear part of the oxyhemoglobin curve. Second, we did not have the levels of PEEP in the patients undergoing general anesthesia, and so the models incorporating the categories of PEEP were limited to the ARMA database of the ARDS network's NIH cohort.(8) Third, in the effort to develop an easy to apply method of completing the SOFA score, we designed a model that did not incorporate covariates such as hemoglobin, age, comorbid illnesses, smoking history, body mass index and positioning of patient (supine versus prone). While it is possible that incorporation of these covariates would have strengthened the model, it would have made the regression equation impractical for daily use.
Conclusion
The respiratory and total SOFA scores obtained with imputed SF values correlate with the respiratory and total SOFA score using PF ratios. Both the derived and original respiratory SOFA score predicted outcomes similarly. SF ratios therefore provide an alternative method for calculating the respiratory component of the SOFA score when the PF ratios are unavailable due to the lack of arterial blood gas data.
Acknowledgements
The authors appreciate the ARDSnet for making its ARMA dataset available for this study.
Funding/Support: Dr. Pandharipande is the recipient of the ASCCA-FAER-Abbot Physician Scientist Award and the Vanderbilt Physician Scientist Development Award. Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award) and the National Institutes of Health (AG0727201)
Abbreviations
- SpO2
Oxygen saturation by pulse oximetry
- PaO2
Partial pressure of oxygen in arterial blood
- FiO2
Fraction of inspired oxygen
- SF
SpO2/FiO2 ratio
- PF
PaO2/FiO2 ratio
- SOFA
Sequential Organ Failure Assessment
- PEEP
Positive End Expiratory Pressure
- ICU
Intensive Care Unit
- ARDS
Acute Respiratory Distress Syndrome
- ARMA
Acute Respiratory Management of ARDS
- MV
Mechanically Ventilated
- SOFA-SF
SOFA score calculated using SpO2/FiO2 ratio
- SOFA-PF
SOFA score calculated using PaO2/FiO2 ratio
- SOFA respiratory-SF
The respiratory component of the SOFA score calculated using SpO2/FiO2 ratio
- SOFA respiratory-PF
The respiratory component of the SOFA score calculated using PaO2/FiO2 ratio
Reference List
- 1.Marik PE. Management of the critically ill geriatric patient. Crit Care Med. 2006;34:S176–S182. doi: 10.1097/01.CCM.0000232624.14883.9A. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Critical Care Clinics. 2000;16:353–366. doi: 10.1016/s0749-0704(05)70114-7. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Mendonca Ad, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Ferreira F, Morena R. Scoring systems for assessing organ dysfunction. Critical Care Clinics. 2000;16:353–366. doi: 10.1016/s0749-0704(05)70114-7. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]