Abstract
We have previously demonstrated that biomarkers of inflammation and immune activity detected within intraoperative renal transplant allograft biopsies are linked to adverse short-term post-transplantation clinical outcomes. Now we provide a post hoc analysis of our earlier data in the light of longer clinical follow-up. A total of 75 consecutively performed renal allografts were analyzed for gene expression of proinflammatory molecules, inflammation-induced adhesion molecules, and antiapoptotic genes expressed 15 minutes after vascular reperfusion to determine whether this analysis can aid in predicting long-term quality of renal function, proteinuria, graft loss, and death-censored graft. We have built predictive models for proteinuria (area under the curve = 0.859, p = 0.0001) and graft loss (area under the curve = 0.724, p = 0.027) 2 years post-transplantation using clinical variables in combination with intragraft gene expression data of tumor necrosis factor–α, interleukin-6, CD40, CD3, and tumor necrosis factor–α, Bcl-2, and interferon-γ, respectively. This post hoc analysis demonstrates that hypothesis-driven, targeted polymerase chain reaction profiling of gene expression in the donor kidney at the time of engraftment can predict 2-year post-transplantation clinical outcomes.
Keywords: Renal transplantation, PCR, Prediction, Proteinuria, Graft loss
1. Introduction
Immunosuppression in clinical solid organ transplantation often follows the “one size fits all” approach with very limited initial use of individualized care. While some institutions use a single regimen for all transplant recipients, others depend upon traditional risk factors to choose a therapeutic regimen. Unfortunately, traditional risk factor assessments allow only imprecise categorization of individual patient's risk for adverse outcomes. These traditional risk factors include several recipient and donor characteristics, such as donor brain death, prolonged cold ischemia, human leukocyte antigen (HLA) mismatching, age, living versus deceased donation, and race [1–5]. We have proposed that hypothesis-driven, targeted polymerase chain reaction (PCR) profile of the donor kidney at the time of transplantation will complement assessment of traditional risk factors in predicting clinical outcomes.
In our initial study, 75 allograft renal biopsy samples were obtained 15 minutes after the creation of vascular anastomosis [6]. In this study, gene expression profiling of proinflammatory, T-cell activation and cytoprotective genes predicted short-term clinical outcomes such as delayed graft function (DGF), acute rejection (AR), and quality of renal function as assessed by the measurement of serum creatinine 6 months after renal transplantation. Elevated expression of certain immune system genes, including tumor necrosis factor (TNF)–α, transforming growth factor (TGF)–β, CD25, intercellular adhesion molecule-1 (ICAM-1), A20, and interleukin (IL)–10 were associated with DGF. The expression of an overlapping but slightly different set of genes was associated with AR (TNF-α, CD25, TGF-β, IL-6, ICAM-1, hemoxygenase-1 (HO-1), and CD3). Decreased intragraft gene expression of the cytoprotective Bcl-xl and amplified intragraft expression of T-cell activation marker CD25 predicted better renal function 6 months after transplantation. Although the cytoprotective gene Bcl-xl was informative in predicting short-term post-transplantation outcomes, the related Bcl-2 gene and other markers like platelet endothelial cell adhesion molecule (PECAM), nuclear factor (NF)–κB, CD40, and interferon (IFN)–γ did not prove useful for prediction of renal function or any of the other short-term outcomes.
Based on these initial results, we asked whether a PCR-based transcriptional profiling strategy focusing on the expression of select proinflammatory, T-cell activation, and cytoprotective genes aids in prediction of not only short-term but also long-term clinical outcomes. We have now assessed clinical outcomes, such as the quality of renal function, proteinuria, graft loss, and death-censored graft loss, over a 4-year follow-up period.
2. Subjects and methods
Study subjects, immunosuppressive regimen, specimen collection, and laboratory methods are as published elsewhere [6].
2.1. Clinical variables
The clinical data included recipient age, race, prior transplantation, type of induction therapy, warm ischemic time (WIT), cold ischemic time (CIT; for cadaveric donor transplants only), donor type (living or deceased), donor age, and donor race, HLA matching, DGF, AR, diabetes in the recipient, sirolimus use, normalized and time-averaged area under the curve (AUC) immunosuppressive drug levels, and triglyceride levels, systolic blood pressure, and diastolic blood pressure values around the 1-year anniversary.
2.2. Criteria for categorizing clinical outcomes
Clinical data were retrieved from computerized medical records and chart reviews. DGF was defined as a requirement for dialysis during the first week post-transplantation in the absence of AR, vascular complications, or urinary tract obstruction. The diagnosis of AR was confirmed by pathologic examination of the graft biopsy. Serum creatinine values closest to the yearly post- transplantation anniversary were selected. If serum creatinine was not obtained within a 3-month period before or after the anniversary, serum creatinine was listed as not available. MDRD glomerular filtration rate (GFR) was calculated using four variables: creatinine, age, race, and gender. Proteinuria was assessed based on dipstick readings from none to 4+. Death-censored graft loss was defined as graft loss for any reason but death.
2.3. Quantification of gene expression and statistical analyses
The comparative Ct method was used to quantify gene expression. The expression of target genes were normalized to 18-second ribosomal RNA and to the calibrator. For PCR quantification, the reader was scaled on a median scale to normalize the data. This approach is widely accepted, as this reduces mean based noise from the data and brings all sample reports onto the same median scale.
This study examined the association between gene expression and several endpoints. Gene expression data were standardized to have a mean of 0 and a standard deviation of 1. Standardizing the variables is important for multivariate analyses because it allows variables measured at different scales to contribute equally to the analyses. We used a logistic regression for the analysis of our dichotomous outcomes and multiple linear regressions for the continuous outcomes.
The selection of relevant predictors is an important step in constructing a predictive model. Because the main focus of this analysis was to obtain a highly predictive model of several outcomes, we performed subset selections to determine our working model. For outcomes that were continuous, the Mallow's Cp statistic for multiple linear regressions was used as the model selection criterion. Mallow's Cp is a consistent measure of the goodness of fit of a model as well as finding the best subset that includes only the most important predictors of the outcome of interest. For outcomes that were binary, we used the branch and bound algorithm with the likelihood score in logistic regression to select the best models. The Branch and Bound search algorithm is a widely used schema for solving combinatorial optimization problems. Upon choosing the models with the highest likelihood scores, we determined the working model using the Akaike Information Criterion (AIC). The AIC statistic is often used in model selection, because it uses the log-likelihood and penalizes it with the number of predictors in a model. This penalty ensures that the increase in log-likelihood will not be due to the inclusion of irrelevant predictors. The AIC helps identify the model that can account for most of the variability with the least amount of variables. Another reason for using Mallow's Cp and the AIC is that it allows us to do model selection without encountering the problem of multiple comparison, as we are not doing any formal tests in constructing our working model.
We performed the model selection on the predictors set of: genes alone, clinical variables alone, and genes plus clinical variables combined. To obtain predicted values under the working model, we performed 10-fold cross-validation. The process of 10-fold cross-validation included partitioning the sample into 10 parts and using nine-tenths of the patients for constructing a model, then validating it on the remaining one-tenth of the patients and repeating the same process 10 times using a different partition. The advantage of this method is that all observations are used for both constructing a model and validating one (each observation is used for validation exactly once). Using the predicted values from the cross-validation, we were then able to construct receiver operating characteristic (ROC) curves from the working regression models. To assess how well the prediction model performs, we used the AUC for the ROC curves. A model with no predictive power would have an AUC of 0.5, whereas a perfectly predictive model would have an AUC of 1.0. We also obtained p values to determine whether the AUC was significantly different from 0.5.
SAS version 9.1 for Windows was used to conduct the model selection and cross-validation, whereas R was used to obtain the ROC curves and AUC values.
3. Results
3.1. Clinical outcomes
Among the 75 consecutive renal transplant recipients who were enrolled in the study, gene expression data were available in 73 patients. These 73 patients were followed for 4 years post-transplantation. After 4 years of follow-up 21, of 73 (28.77%) renal allografts failed (Table 1). Eleven of the 21 (52.4%) graft losses were caused by death, and 10 patients had death-censored graft loss by year 4 year post-transplantation (Table 1). Of the patients with death-censored graft loss, three were because of interstitial fibrosis/tubular atrophy (IF/TA) (14.3%), three were lost because of AR (14.3%), and three were lost because of a combination of IF/TA and AR (14.3%). One allograft was lost to fungal pyelonephritis. Twelve of the 73 (16.4%) patients died during the follow-up period (1 patient died after graft loss and therefore counted as a death-censored graft loss) (Table 1). The cause of death was not known to our transplant center in six cases, Two patients each died of of the following causes: sepsis, cardiovascular disease, and malignancy.
Table 1. Patient characteristics.
| Cumulative incidence of graft loss, death, and death-censored graft loss over 4-year follow-up | |||
|---|---|---|---|
|
| |||
| Clinical outcome | No. of events | % | |
| 1-Year graft loss | 8 | 10.96 | |
| 2-Year graft loss | 15 | 20.55 | |
| 3-Year graft loss | 17 | 23.29 | |
| 4-Year graft loss | 21 | 28.77 | |
| 1-Year death | 8 | 10.96 | |
| 2-Year death | 11 | 15.07 | |
| 3-Year death | 11 | 15.07 | |
| 4-Year death | 12 | 16.44 | |
| 1-Year death-censored graft loss | 1 | 1.37 | |
| 2-Year death-censored graft loss | 5 | 6.85 | |
| 3-Year death-censored graft loss | 7 | 9.59 | |
| 4-Year death-censored graft loss | 10 | 13.7 | |
|
| |||
| Serum creatinine and MDRD GFR over 4-year follow-up among the 73 patients | |||
|
| |||
| No. of events | Creatinine level (mg/dl) (mean ± SD) | MDRD GFR (ml/min/1.73 m2) (mean ± SD) | |
|
| |||
| 1-Year creatinine | 64 | 1.62 ± 0.80 | 51.14 ± 17.93 |
| 2-Year creatinine | 54 | 1.80 ± 0.90 | 49.24 ± 22.46 |
| 3-Year creatinine | 51 | 1.93 ± 1.47 | 49.24 ± 21.94 |
| 4-Year creatinine | 41 | 1.79 ± 0.95 | 49.24 ± 21.52 |
|
| |||
| Dipstick proteinuria over 4-year follow-up among the 73 patients | |||
|
| |||
| No. of events | Proteinuria (g/day) (mean ± SD) | ||
|
| |||
| 1-Year proteinuria | 64 | 1.04 ± 1.30 | |
| 2-Year proteinuria | 46 | 1.23 ± 1.45 | |
| 3-Year proteinuria | 38 | 0.89 ± 1.31 | |
| 4-Year proteinuria | 40 | 1.15 ± 1.41 | |
3.2. Outcomes
We examined the relationship of gene expression data with the following outcomes: serum creatinine, MDRD GFR, proteinuria, graft loss, and death-censored graft loss. These end points were analyzed for the second, third, and fourth years after renal transplantation. The effect of gene expression data on year 3 and 4 outcomes did not remain significant after adjustment for clinical variables (data not shown), so we present only the year2 outcomes. We also performed a prediction analysis with variables selected in our regression models for each of the outcomes (serum creatinine, MDRD GFR, proteinuria, graft loss and death-censored graft loss).
3.2.1. Two-year serum creatinine
Serum creatinine 2 years after renal transplantation was assessed in our linear regression model (Table 2). The gene expression profiles were not significantly associated with higher serum creatinine values. Among the clinical variables, older donor age and an episode of AR showed significant correlation with serum creatinine 2 years after transplantation. Using clinical variables such as donor age, AR, and diabetes in the recipient, we were able to create a predictive model for serum creatinine with an AUC value of 0.755 (p = 0.0001).
Table 2. Association of clinical outcomes with gene expression and select clinical variables.
| Variables | Serum creatinine | MDRD GFR | Proteinuria | Graft loss | Death-censored graft loss |
|---|---|---|---|---|---|
| TNF-α | NS | NS | NS | Positive, p = 0.0524 | Positive, p = 0.0315 |
| CD25 | NS | NS | NS | NS | NS |
| TGF-β | NS | NS | NS | NS | NS |
| A20 | NS | NS | NS | NS | NS |
| Bcl-xl | NS | NS | NS | NS | NS |
| Bcl-2 | NS | NS | NS | Negative, p = 0.0128 | NS |
| IL-10 | NS | NS | NS | NS | NS |
| IL-6 | NS | NS | NS | NS | NS |
| PECAM | NS | Positive, p = 0.023 | NS | NS | NS |
| ICAM-1 | NS | NS | NS | NS | NS |
| Ho-1 | NS | NS | NS | NS | NS |
| CD40 | NS | NS | Negative, p = 0.0067 | NS | NS |
| CD3 | NS | Negative, p = 0.0264 | NS | NS | NS |
| IFN-γ | NS | NS | NS | NS | NS |
| NF-κB | NS | NS | NS | NS | NS |
| Recipient race | NS | NS | NS | NS | NS |
| Donor race | NS | NS | NS | NS | NS |
| Transplant number (first, second, third) | NS | NS | NS | NS | NS |
| Donor type (living or deceased) | NS | NS | NS | NS | NS |
| Recipient age | NS | NS | NS | NS | NS |
| Donor age | Positive, p = 0.0412 | Negative, p = 0.0499 | NS | NS | NS |
| Warm ischemia time | NS | NS | NS | NS | NS |
| Cold ischemia time | NS | NS | NS | NS | NS |
| HLA match | NS | NS | NS | NS | NS |
| Delayed graft function | NS | NS | NS | NS | NS |
| Acute rejection | Positive, p = 0.0279 | NS | Positive, p = 0.0074 | NS | NS |
| Induction therapy with depleting agent | NS | NS | NS | NS | NS |
| Diabetes in the recipient | NS | NS | NS | NS | NS |
| Triglyceride level | NS | NS | NS | NS | NS |
| Systolic blood pressure | NS | NS | NS | NS | NS |
| Diastolic blood pressure | NS | NS | NS | NS | NS |
| Immunosuppressive drug level | NS | NS | NS | NS | NS |
| Sirolimus use | NS | NS | NS | NS | NS |
NS, not significant.
3.2.2. MDRD GFR at 2 years post-transplantation
In the unadjusted regression model TGF-β, Bcl-xl, IL-10, PECAM, CD3 and IFN-γ showed significant association with estimated MDRD GFR 2 years post-transplantation (Table 2). In the adjusted linear regression model, higher donor age was associated with lower MDRD GFR at 2 years, and high PECAM and low CD3 intragraft gene expression at the time of transplantation predicted higher MDRD GFR at 2 years. Using clinical variables, such as recipient race, donor age, degree of HLA matching, but not gene expression data, we were able to create predictive model with an AUC value of 0.652 (p = 0.043).
3.2.3. Two-year proteinuria
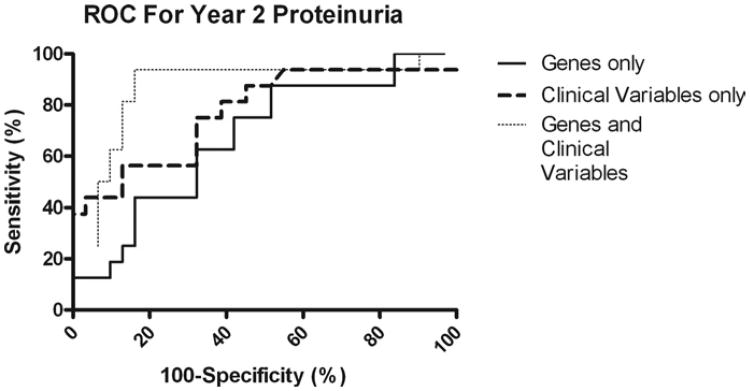
CD40 intragraft expression showed a negative association with proteinuria 2 years after transplantation both in the unadjusted and adjusted logistic regression models. The presence of AR was associated with the presence of proteinuria (Table 2). Using the expression of TNF-α, IL-6, CD40, and CD3, we created a prediction model with an AUC value of 0.673 (p = 0.027). Using clinical variables, such as donor age, AR, and diabetes in the recipient, we built a predictive model with an AUC value of 0.773 (p = 0.001). Combining the gene expression data and the clinical variables our prediction model achieved an AUC value of 0.859 (p = 0.0001) (Fig. 1).
Fig. 1.
Prognostic accuracy of clinical variables and gene expression profiling of the donor kidney at the time of transplantation for proteinuria 2 years after transplantation. The ROC curves show sensitivity (i.e., true positive rates) for all possible 100-specificity (i.e., false-positive rates) values. Increasing AUC indicates better prognostic accuracy.
3.2.4. Two-year overall graft survival
In the unadjusted logistic regression analysis, only high TNF-α intragraft gene expression showed significant association with graft loss 2 years post-transplantation. After adjustment for clinical variables, although no clinical variable became significant in addition to high intragraft TNF-α gene expression, low intragraft Bcl-2 gene expression became significantly associated with graft loss at 2 year post-transplantation follow-up (Table 2).
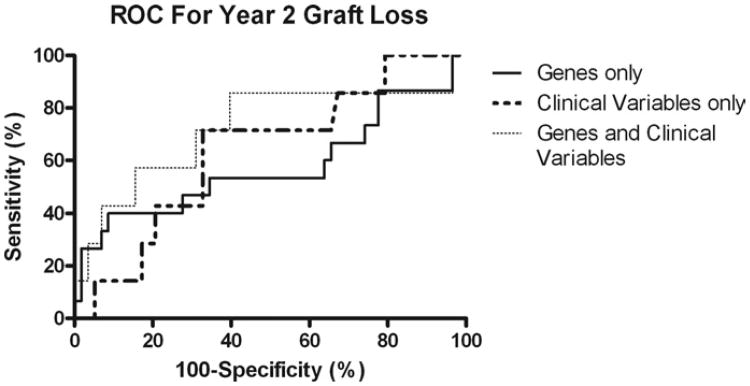
Using the intragraft gene expression of TNF-α, Bcl-2, and IFN-γ, we created a predictive model with an AUC value of 0.577 (p = 0.182). Using clinical variables, DGF, AR, and systolic blood pressure we built a predictive model with an AUC value of 0.637 (p = 0.122). Combining the gene expression data and the clinical variables our prediction model had an AUC value of 0.724 (p = 0.027) (Fig. 2).
Fig. 2.
Prognostic accuracy of clinical variables and gene expression profiling of the donor kidney at the time of transplantation for graft loss 2 years after renal transplantation. The ROC curves show sensitivity (i.e., true-positive rates) for all possible 100-specificity (i.e., false-positive rates) values. Increasing AUC indicates better prognostic accuracy.
3.2.5. Two-year death-censored graft survival
Of all the targeted genes tested in the unadjusted logistic regression model, only high intragraft expression of TNF-α predicted death-censored graft loss at 2-year follow-up. Adjustment for clinical variables available at the time of transplantation did not result in the addition of any other significant variables (Table 2). We were unable to predict death-censored graft loss even by combining the gene expression data with clinical variables.
In summary, after performing multivariate regression modeling for the best predictors of long-term outcomes, the following associations with intraoperative intragraft gene expression were found: (1) high CD3 and low PECAM were associated with worse 2-year allograft function; (2) low CD40 expression was associated with proteinuria; (3) low Bcl-2 predicted worse overall (but not death-censored) 2-year graft survival; (4) high TNF-α predicted worse death-censored and overall 2-year graft survival; (5) using the expression of several genes and clinical variables, a predictive model allowing good prediction could be built for graft loss and proteinuria; and (6) after adjustment for clinical variables, none of the gene expression profiles was associated with distinctive clinical outcomes at 3 or 4 years post-transplantation.
4. Discussion
The harvesting, preservation, and subsequent implantation of a donor kidney necessarily results in ischemia-reperfusion injury. The consequent endothelial activation and injury leads to enhanced endothelial cell–leukocyte adhesion, activation of leukocytes and release of inflammatory mediators, such as cytokines and reactive oxygen species. Donor death and the necessity of cold preservation further complicate injury in deceased donor transplants. This inflammatory response is known to occur in all other solid organ transplants, such as in lung transplantation [7].
Proinflammatory forces promote apoptosis, whereas antiapoptotic processes limit the extent of apoptotic damage occurring in a transplanted kidney. The balance of these two forces may very well determine the long-term fate of a graft. It stands to reason that intragraft expression of proinflammatory genes, such as TNF-α and T-cell markers for graft infiltration such as intragraft CD3 gene expression likely promote an inflammatory response, antiapoptotic genes, such as Bcl-2 and inhibitors of humoral immune responses, such as PECAM-1 should serve to limit the extent of inflammatory responses. We have selected molecular targets based on a hypothesis related to their putative role in promoting or deterring ischemia-reperfusion injury. The ability of this hypothesis-driven, targeted molecular approach to predict short-term outcomes such as DGF, AR, and serum creatinine values 6 months post-transplantation confirmed the validity of our molecular marker selection [6]. Furthermore, as we demonstrate here, genes from the same panel show significant associations with long-term outcomes, such as quality of renal function, proteinuria, graft loss, and death-censored graft loss 2 years after renal transplantation. Specifically, high intragraft gene expression of the proinflammatory molecule TNF-α in the renal allograft at the time of transplantation is associated with higher rates of DGF and AR in the early post-transplant period and an increased likelihood of graft loss and death-censored graft loss years after renal transplantation.
High intragraft gene expression of the T-cell marker CD3 in the renal allograft at the time of transplantation is associated with AR in the early post-transplantation period and lower quality renal function 2 years after renal transplantation [6]. Our data support the hypothesis that evidence of T-cell immunity, as shown by the presence of CD3, a T-cell lineage marker, at the time of transplantation is closely linked to poor renal function 2 years after transplantation.
Low expression of the antiapoptotic Bcl-xl gene is associated with lower-quality renal function 6 months after renal transplantation and the hypo-expression of antiapoptotic Bcl-2 gene is associated with graft loss. Our finding of low Bcl-2 expression predicting graft loss 2 years after transplantation is consistent with a report of low Bcl-xl expression in association with chronic rejection [8]. PECAM-deficient mice show an autoimmune disease phenotype with hyperreactive B-cells and autoantibodies [9]. Although low PECAM expression was not associated with worse 6-month renal function, it is associated with diminished renal function 2 years after renal transplantation.
Acute rejection and low CD40 intragraft gene expression predicted proteinuria 2 years after renal transplantation. Although the prior occurrence of AR predicting proteinuria fits our paradigm of more inflamed organs being associated with worse outcomes, the association between low intragraft expression of the potent co-stimulatory CD40 molecule and proteinuria does not. Our study cannot provide insights into the potential cellular mechanisms behind this observation, but the association observed is strongly significant.
Although several studies have used molecular methods to predict graft survival, they have focused on the role of the recipients in long-term transplant outcomes [10, 11]. Instead, we have concentrated on the donor kidney's role in determining long-term renal allograft outcomes.
Brown et al. have also focused upon donor characteristics. They demonstrated that a specific donor complement-3 (C3) allotype was associated with better long-term renal allograft survival [12]. Although it has yet not been elucidated how this donor C3 allotype results in graft protection, it is known that, in mice, allografts that do not produce C3 survive much longer than allografts that produce C3, which results in a proinflammatory milieu [13].
Current clinical practice for prediction of long-term posttransplantation outcomes is to use easily assessable recipient socioeconomic and clinical characteristics [14–16]. Acute cellular rejection now rarely results in graft loss, but it is still a strong predictor of poor long-term graft survival [17, 18]. The T cells mediating acute cellular rejection do not work in isolation, but their behavior is influenced by their microenvironment. A proinflammatory milieu such as that observed in DGF may determine the behavior of T-cells and, consequently, the fate of the graft [19, 20]. Because clinical risk factors for poor long-term transplant outcomes such as DGF and AR themselves are often correlates of intragraft inflammation, markers of intragraft inflammation detected by real-time PCR may provide important complementary information for predicting clinical outcomes.
The combination of clinical variables and gene expression data can provide prognostic information. Clinical variables predicted proteinuria with an AUC value of 0.773, gene expression data predicted proteinuria with an AUC value of 0.673, but combining both we increased the AUC value of our predictive model to 0.859. The comparative Ct method that we used to quantify PCR results allows the relative quantification of expression compared with a calibrator. To have an absolute cut-off value useful when analyzing a biopsy sample in clinical practice, the standard curve PCR quantification method would be useful. Although the high intragraft expression of a proinflammatory cytokine (TNF-α) predicts graft loss, the high intragraft expression of a T-cell marker (CD3) predicts poor long-term renal function. It is reasonable to assume that the more inflamed the transplanted organ, the poorer the outcome; but, the question arises as to why we are not seeing the exact same genes predicting both graft loss and the quality of renal function, However, many nonimmunologic factors can cause changes in allograft function, such as the institution of angiotensin converting enzyme inhibitors for congestive heart failure, but may not directly negatively influence graft survival.
The outcome of renal transplantation must be dependent on both donor and recipient factors. Intuitively, with the passage of time, recipient factors should become more important in determining the fate of the allograft. Although we could demonstrate that gene expression profiles in the donor kidney at the time of transplantation predict clinical outcomes 2 years after renal transplantation, we were unable to extend this observation to clinical outcomes taking place 3 or 4 years after renal transplantation. Our formal prediction analysis madeitpossible to focus on the interplay between several genes and clinical variables. We have successfully built predictive models for graft loss and proteinuria allowing for improved prediction of these conditions. In the future, molecular analysis of the graft at the time of transplantation may facilitate individualized, optimized care.
Acknowledgments
This work was supported by an Amgen Research Fellowship Grant (to G.B-K.).
References
- 1.Pratschke J, Tullius SG, Neuhaus P. Brain death associated ischemia/reperfusion injury. Ann Transplant. 2004;9:78. [PubMed] [Google Scholar]
- 2.Opelz G, Dohler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83:247. doi: 10.1097/01.tp.0000251781.36117.27. [DOI] [PubMed] [Google Scholar]
- 3.Opelz G. The benefit of exchanging donor kidneys among transplant centers. N Engl J Med. 1988;318:1289. doi: 10.1056/NEJM198805193182001. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 5.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 6.Avihingsanon Y, Ma N, Pavlakis M, Chon WJ, Uknis ME, Monaco AP, et al. On the intraoperative molecular status of renal allografts after vascular reperfusion and clinical outcomes. J Am Soc Nephrol. 2005;16:1542. doi: 10.1681/ASN.2005020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade CF, Kaneda H, Der S, Tsang M, Lodyga M, Chimisso Dos Santos C, et al. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J Heart Lung Transplant. 2006;25:1317. doi: 10.1016/j.healun.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Avihingsanon Y, Ma N, Csizmadia E, Wang C, Pavlakis M, Giraldo M, et al. Expression of protective genes in human renal allografts: A regulatory response to injury associated with graft rejection. Transplantation. 2002;73:1079. doi: 10.1097/00007890-200204150-00011. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- 10.Lazzeri E, Rotondi M, Mazzinghi B, Lasagni L, Buonamano A, Rosati A, et al. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation. 2005;79:1215. doi: 10.1097/01.tp.0000160759.85080.2e. [DOI] [PubMed] [Google Scholar]
- 11.Matz M, Beyer J, Wunsch D, Mashreghi MF, Seiler M, Pratschke J, et al. Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function. Kidney Int. 2006;69:1683. doi: 10.1038/sj.ki.5000343. [DOI] [PubMed] [Google Scholar]
- 12.Brown KM, Kondeatis E, Vaughan RW, Kon SP, Farmer CK, Taylor JD, et al. Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med. 2006;354:2014. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 13.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 14.Krikov S, Khan A, Baird BC, Barenbaum LL, Leviatov A, Koford JK, Goldfarb-Rumyantzev AS. Predicting kidney transplant survival using tree-based modeling. ASAIO J. 2007;53:592. doi: 10.1097/MAT.0b013e318145b9f7. [DOI] [PubMed] [Google Scholar]
- 15.Issa N, Stephany B, Fatica R, Nurko S, Krishnamurthi V, Goldfarb DA, et al. Donor factors influencing graft outcomes in live donor kidney transplantation. Transplantation. 2007;83:593. doi: 10.1097/01.tp.0000256284.78721.ba. [DOI] [PubMed] [Google Scholar]
- 16.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, Chelamcharla M, Habib AN, Wang BJ, et al. Role ofsocioeconomic statusin kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1:313. doi: 10.2215/CJN.00630805. [DOI] [PubMed] [Google Scholar]
- 17.Pallardo Mateu LM, Sancho Calabuig A, Capdevila Plaza L, Franco Esteve A. Acute rejection and late renal transplant failure: Risk factors and prognosis. Nephrol Dial Transplant. 2004;19(Suppl 3):iii38. doi: 10.1093/ndt/gfh1013. [DOI] [PubMed] [Google Scholar]
- 18.Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, Kaplan B. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 2000;70:1098. doi: 10.1097/00007890-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 19.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 20.Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, et al. The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant. 2008;8:78. doi: 10.1111/j.1600-6143.2007.02032.x. [DOI] [PubMed] [Google Scholar]