Abstract
The objective of this investigation is to review existing research pertaining to cognitive impairment and decline following critical illness and describe a case involving a 49-year-old female with sepsis and acute respiratory distress syndrome (ARDS) with no prior neurologic history who, compared to baseline neuropsychological test data, experienced dramatic cognitive decline and brain atrophy following treatment in the medical intensive care unit (ICU) at Vanderbilt University Medical Center. The patient participated in detailed clinical interviews and underwent comprehensive neuropsychological testing and neurological magnetic resonance imaging (MRI) at approximately 8 months and 3.5 years after ICU discharge. Compared to pre-ICU baseline test data, her intellectual function declined approximately 2 standard deviations from 139 to 106 (from the 99th to the 61st percentile) on a standardized intelligence test 8 months post-discharge, with little subsequent improvement. Initial diffusion tensor brain magnetic resonance imaging (DT-MRI) at the end of ICU hospitalization showed diffuse abnormal hyperintense areas involving predominately white matter in both hemispheres and the left cerebellum. A brain MRI nearly 4 years after ICU discharge demonstrated interval development of profound and generalized atrophy with sulcal widening and ventricular enlargement. The magnitude of cognitive decline experienced by ICU survivors is difficult to quantify due to the unavailability of pre-morbid neuropsychological data. The current case, conducted on a patient with baseline neuropsychological data, illustrates the trajectory of decline occurring after critical illness and ICU-associated brain injury with marked atrophy and concomitant cognitive impairments.
Keywords: ARDS, brain injury, critical care, executive dysfunction, sepsis
Review of Cognitive Functioning in Survivors of Critical Illness
The primary outcomes of interest following critical illness, defined as illness requiring treatment in the intensive care unit (ICU), have traditionally been mortality and morbidity. However, recent advances in technology and medicine have significantly reduced mortality rates1 and extended the lives of the critically ill, thus shifting concerns from survival to preservation of quality of life and emotional and cognitive functioning–domains threatened and potentially comprised by the effects of critical illness. Such concerns are well-founded, particularly as they relate to cognitive abilities which are highly vulnerable due to ICU-related insults2,3 and are vital to the well-being and functional outcomes of ICU survivors.
While research is, in many respects, in its infancy, evidence from over a dozen epidemiologic investigations (totaling approximately 500 subjects) suggests that over 50% of ICU survivors may experience significant and persistent cognitive impairment, widely referred to as long-term cognitive impairment (LTCI).4–9 The impairment occurs in wide-ranging domains including executive functioning, memory, attention, visual-spatial construction, language, and other areas.7,10,11 In general, impairment ranges in severity from mild to very pronounced and is often sufficiently disabling enough to effect quality of life and the ability to return to work.5–7 Patients appear to be susceptible to the development of cognitive impairment regardless of age, level of education, or severity of illness.2,7 However, virtually all investigations conducted to date have been done with relatively young subjects (mean age 54 years), so the degree to which advanced age is a risk factor for the development of impairment remains largely unknown. As is true following most kinds of brain injury, partial recovery of functioning occurs over time, with patients rarely returning to premorbid baselines.3 Among those with pre-existing early forms of neurodegenerative disease such as mild cognitive impairment (MCI), critical illness may amplify and accelerate the descent into frank dementia, as has been shown in animal models.12
Potential contributors to cognitive decline in ICU survivors have not been widely studied and, in light of the highly heterogeneous nature of critical illness, there is almost certainly no single explanation for the neuropsychological impairment experienced by these individuals.11 Biologically plausible mechanisms which are increasingly the focus of attention include delirium, inflammation, and others.13,14 Delirium occurs in up to 80% of ICU patients15,16 and appears to be associated with hypoperfusion in frontal, temporal, and subcortical brain regions (susceptible to even slight alterations in blood flood), thus leading to the development of neuropsychological deficits.17–19 Inflammation is associated with a wide array of conditions resulting in critical illness; inflammation occurs in all patients with septic shock, sepsis, and acute respiratory distress syndrome (ARDS) and has clear negative implications for the brain.20,21 Inflammatory responses are mediated by cytokines that penetrate the blood-brain barrier and directly or indirectly modulate brain activity, potentially altering neurotransmission22 and presumably worsening cognitive impairment or contributing to new cognitive impairment.
Although a consensus is emerging among clinicians and researchers regarding the long-term effects of critical illness on cognitive functioning, little if anything is known regarding the precise magnitude of cognitive decline in ICU survivors. In the case of individuals undergoing a transplant or an elective surgical procedure (e.g., cardiac surgery), neuropsychological testing is often done prior to hospital admission in order to accurately quantify baseline cognitive functioning and to determine the degree of change following surgery.23–26 Medical ICU admissions are not elective as they typically occur due to rapidly developing conditions such as pneumonia, sepsis, or ARDS; as such, it is nearly impossible to precisely determine baseline levels of cognitive functioning in patients with such conditions. No such baseline data exist among medical ICU cohorts, as comprehensive baseline neuropsychological test data is nonexistent among all of the roughly 500 participants in the aforementioned studies, as it is not possible to determine who will develop a critical illness.
We present a case that is both novel and unique relative to available literature involving a 49-year-old survivor of sepsis and ARDS in whom we were able to quantify the degree of intellectual and cognitive decline due to the presence of previous neuropsychological test data. She underwent IQ testing at 32 years of age and, following her critical illness, she was retested with a revised version of the same IQ instrument [Wechsler Adult Intelligence Scale-III (WAIS-III)] while undergoing comprehensive neuropsychological testing and structural brain MRI. Further, we documented neuropathologic changes both initially and longitudinally on brain MRI scans.
Materials and Methods
The patient participated in detailed clinical interviews and underwent comprehensive neuropsychological testing and neuroimaging (MRI) at approximately 8 months and 3.5 years after ICU discharge. Her inpatient hospital medical records were reviewed. Institutional Review Board (IRB) approval was obtained from the Vanderbilt University IRB, and written consent was obtained from the patient to publish the findings presented herein.
Results
Clinical Course
History of illness
Following a 9-day history of sore throat, fevers, chills, nausea, vomiting, and productive cough with presyncope, this 49-year-old female with a history of mild asthma and hypertension, but no neurologic history, presented to a local community hospital with community-acquired pneumonia. Despite 5 days of intravenous antibiotics, her condition progressed to severe sepsis that necessitated transfer to the Vanderbilt University Medical Center ICU. Upon arrival at the ICU, the subject was profoundly hypoxic and hypotensive and required immediate intubation, mechanical ventilation, and vasopressor support (Table 1 shows her medical data). Bilateral lung lower lobe infiltrates without pleural effusions were found on chest radiographs, consistent with the diagnosis of ARDS. She became septic and progressed to septic shock. After approximately 3 weeks of aggressive medical care in the ICU, her clinical course began to improve slowly. She was successfully extubated on day 36 and was transferred to the general medical unit on day 37. Thereafter, she was discharged to local rehabilitation, with a total hospital length of stay of 43 days.
Table 1.
Clinical data for ARDS patient with subsequent cognitive impairment
| Length of stay (d) | |
| Hospital | 43 |
| ICU | 39 |
| Rehabilitation unit | 21 |
| Vanderbilt medical ICU admission data | |
| APACHE II Score | 22 |
| PaO2/FiO2 | 93 |
| Other notable findings | |
| Lowest PaO2 (mm Hg) | 70 |
| Highest FiO2 (%) | 1.0 |
| Highest positive end-expiratory pressure (cm H2O) | 18 |
| Duration of mechanical ventilation (d) | 31 |
| Duration of FiO2 >60% (d) | 8 |
| Duration of Glasgow Coma Scale score of 3 (d) | 16 |
| Duration of comaa (d) | 24 |
| Duration of deliriumb (d) | 11 |
| Duration of vasopressors (d) | 13 |
| Average daily dose of fentanyl while comatose (µg) | ~3525 |
| Average daily dose of midazolam while comatose (mg) | ~171 |
| Duration of continuous veno-venous hemodialysis (d) | 11 |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; APACHE II, acute physiology and chronic health evaluation II
Coma was defined as a Richmond Agitation Sedation Scale score of −4 or −5.
Delirium was defined as either present or absent using the Confusion Assessment Method for the ICU.
Neurologic Events/Neuroimaging
At the time of her Vanderbilt hospital admission, the patient remained sedated on midazolam and fentanyl. On ICU admission, the Glasgow Coma Scale (GCS) score was 3 (GSC scores of 3 to 8 are typically thought to indicate coma), and the Richmond Agitation and Sedation Scale (RASS)27 score was −5 (RASS scores range from +4 “Combative” to 0 “Alert and Calm” to −5 “Unarousable to Verbal or Physical Stimulation”). Throughout her hospitalization, she had no focal neurologic deficits, but was sedated in order to maintain her oxygen saturation (>89%) and avoid patient-ventilator asynchrony. For the ensuing 7 days, she experienced hypoactive (ie “quiet”) delirium [measured using the Confusion Assessment Method-ICU15], with several episodes of agitation (hyperactive delirium).
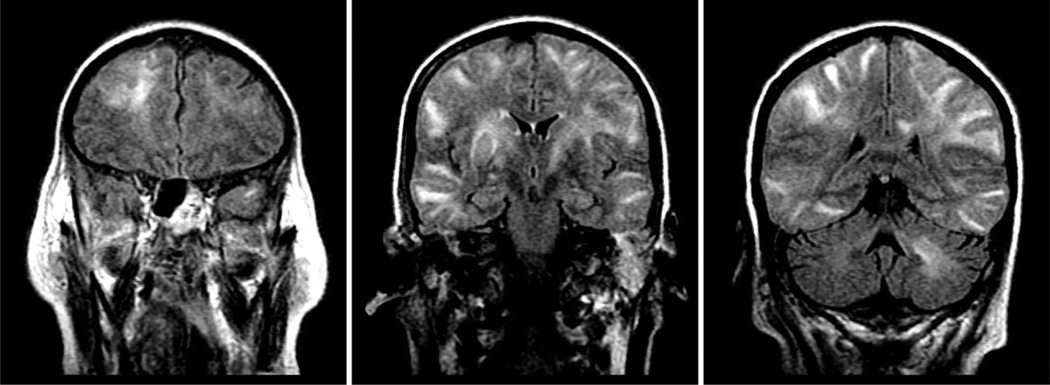
On ICU day 28, the patient developed a new decline in mental status. She received no sedatives or analgesics for three days in an effort to wean her from mechanical ventilation. The neurologic exam remained nonfocal, but the patient developed hypertension likely due to midazolam and/or fentanyl withdrawal. She had a single tonic-clonic seizure that resolved after treatment. After consultation with neurology and neurosurgery, it was thought that her seizure was secondary to medication withdrawal. Postseizure electroencephalogram indicated nonspecific, but severe, generalized cerebral dysfunction. Brain computed tomography (CT) demonstrated normal ventricle volumes but showed areas of low attenuation in subcortical white matter in the posterolateral left frontal lobe and in the posteromedial right hippocampus. Brain diffusion-weighted MRI on ICU day 31 revealed marked, diffuse abnormal white matter and hyperintense areas involving both cerebral hemispheres and the left cerebellum; there was no atrophy or ventricular enlargement (Fig. 1). There was no evidence of a stroke or intracranial hemorrhage.
Fig. 1.
Coronal diffusion weighted brain imaging in a patient with acute respiratory distress syndrome during intensive care unit hospitalization. The scan on the left shows a hyperintense area in the right frontal lobe white matter. The middle scan shows hyperintense areas in bilateral posterior frontal lobes, temporal lobes, and deep white matter tracts. The right scan shows hyperintense areas in the bilateral posterior temporal and parietal lobes and in the left cerebellum.
Following a 48-hour propofol-induced coma, during which antiepileptic therapy was titrated, her level of consciousness began to improve on ICU day 32. She was successfully extubated on ICU day 36, with wide fluctuations in her mental status until 36 hours prior to ICU discharge (GCS range: 3–15; RASS range: −5 to +2). Altogether, she had a drug-induced coma for 24 days and hypoactive or mixed hypoactive and hyperactive delirium for 11 days.
At the time of admission to a rehabilitation facility, she was alert and oriented to person, place, and time, and, according to medical records, grossly appeared to be “cognitively intact.” She received 3 weeks of inpatient physical rehabilitation for general weakness and physical deconditioning and was then discharged home. She returned to work on a part-time basis 4 weeks after completing rehabilitation and transitioned to a full-time schedule one month later but failed to return to her previous level of functioning. Her physical functioning steadily improved, although she acknowledged decreased energy and fatigue when running errands or engaging in light housework. Persistent problems with stamina and decreased energy had largely resolved at the time of her second evaluation almost 4 years later. She described a history of good health since her hospital discharge and denied new medical problems or new hospital admissions.
Neuropsychological Course
Premorbid function
The subject was a 49-year-old woman who lived alone and who had worked for over 20 years at the same large corporation. At the time of her critical illness, she was a mid-level manager. She was a university graduate with a degree in mathematics and a 3.7 grade point average. She displayed superior premorbid intellectual ability as evidenced by her 1986 WAIS-R scores (mean = 100, SD =15) with a Verbal Intelligence Quotient (VIQ) of 142, Performance Intelligence Quotient (PIQ) of 128, and Full Scale Intelligence Quotient (FSIQ) of 139. She had no significant past medical history of neurological trauma, stroke, intracranial hemorrhage, or cognitive deficits and had no significant psychiatric disorders.
Neuropsychological assessment—Initial post-ICU evaluation
Approximately 8 months after ICU discharge, the subject was referred by her intensivist for an initial evaluation after complaining of mood and cognitive changes. Table 2 shows the neuropsychological tests administered at initial (8 month) follow up. Her Beck Depression Inventory II (BDI-II)28 score was 10, suggesting the absence of significant depressive symptoms. Her score on the Impact of Events Scale – Revised (IES-R)29 was 27, indicating the presence of mild symptoms of post-traumatic stress disorder (PTSD), including recurrent and intrusive thoughts of her ICU stay. A structured psychological interview determined that she did not meet the diagnostic criteria for PTSD.
Table 2.
Neuropsychological test scores
| Test | Initial follow-up examination results |
3.5 yr follow-up examination results |
|---|---|---|
| Attention/concentration | ||
| Trails A (46) | 37 S;T score = 37 | 21 S; T = 58 |
| Stroop test (47) | ||
| Word score | 128; T = 60 | 136; T = 68 |
| Color score | 87; T = 58 | 87; T = 54 |
| Color-word score | 52; T = 62 | 46; T = 56 |
| Interference | −50 | −5.0; T = 46 |
| Executive functioning | ||
| Booklet categories (48) | 88 errors; T = 26 | 77 errors, T = 34 |
| Trails B (46). | 67 S; T = 42 | 46 S; T = 58 |
| Global cognitive functioning |
||
| MMSE (49) | 30; T = 56 | 30; T = 54 |
| Intellectual functioning | ||
| WAIS-III (FS) (50)a | 106 | 118 |
| Verbal IQ | 110 | 113 |
| Performance IQ | 100 | 122 |
| Language | ||
| Verbal fluency (S and C) (46) |
63; T = 48 | 63; T = 49 |
| Memory | ||
| RAVLT (51) | ||
| Trial 1 | 8; T = 59 | 5; T = 43 |
| Trial 2 | 11; T = 60 | 10; T = 56 |
| Trial 3 | 12; T = 56 | 12; T = 57 |
| Trial 4 | 12; T = 52 | 12; T = 54 |
| Trial 5 | 13; T = 54 | 13; T = 56 |
| Interference | 4; T = 40 | 7; T = 60 |
| Trial 6 | 15; T = 67 | 12; T = 59 |
| Rey-Osterreith (51) | ||
| 30 minute delay | 13 | 27 |
| WMS-III (52)b | ||
| Primary indexes | ||
| Auditory memory | 114 | 117 |
| Visual immediate memory |
112 | 109 |
| Immediate memory | 112 | 116 |
| Auditory delayed memory |
136 | 124 |
| Visual delayed memory |
112 | 115 |
| Auditory recognition delayed |
125 | 115 |
| General memory | 130 | 124 |
| Visuoconstruction | ||
| Rey-Osterreith (51) | ||
| Copy | 34; T = 56 | 36; T = 63 |
MMSE, Mini Mental State Exam; RAVLT, Rey Auditory Verbal Learning Test; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition.
Both the WAIS-III and the WMS-III have a mean score of 100 and a SD of 15. All other test scores are presented using T-scores (mean of 50 and SD of 10).
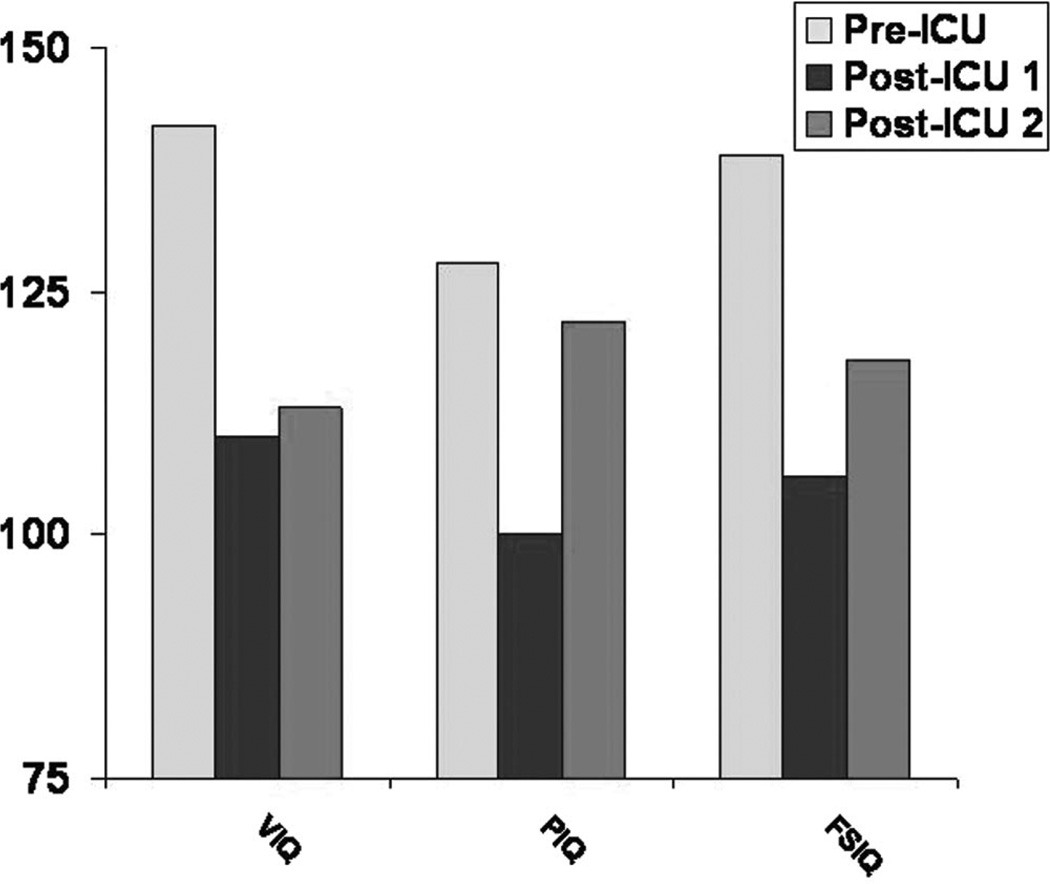
Her composite scores on the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) were in the average range. When compared to her precritical illness scores, her obtained IQ scores (WAIS-R, 1986) showed substantial decline (VIQ =110 vs. 142; PIQ = 100 vs. 128; FSIQ = 106 vs. 139) (Fig. 2). The largest decrease occurred on the Arithmetic subtest (6 points), with a 5-point decrease on picture arrangement, a 4-point decrease on information, and a 3-point decrease on picture completion and comprehension (Fig. 3). There were no subtests in which improvement occurred.
Fig. 2.
Comparison of IQ scores. A comparison of scores on verbal, performance, and full-scale intelligence quotients before ICU hospitalization, at post-ICU time 1 (8 months), and at post-ICU time 2 (3.5 years) in a 52-year-old survivor of ARDS. VIQ, PIQ, and FSIQ scores have a mean of 100 and a SD of 15.
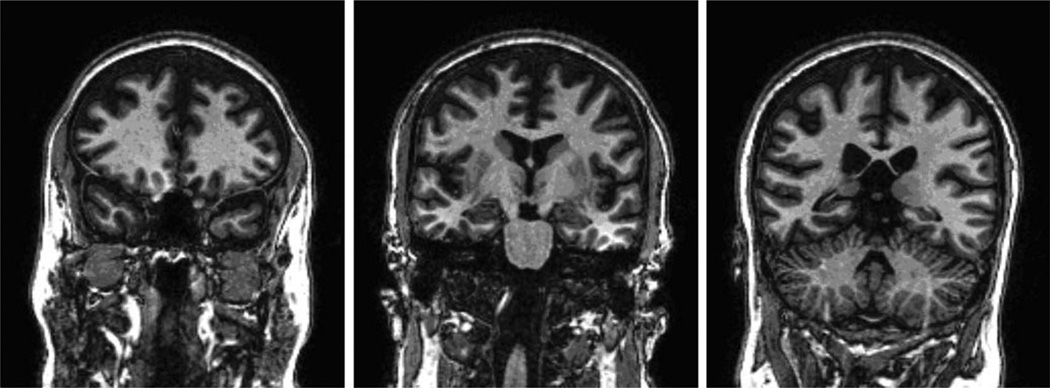
Fig. 3.
Coronal T2 weighted brain imaging (radiologic view: right is left and left is right) at 3.5-year follow-up. The scan on the left shows a view through the frontal lobes showing marked brain atrophy with sulcal widening. The middle scan shows a view through the hippocampus, showing enlargement of the temporal horns of the lateral ventricles, mild hippocampal atrophy, and sulcal widening. The right scan shows a view through the posterior temporal and parietal lobes and cerebellum with marked brain atrophy with sulcal widening and ventricular enlargement.
Neuropsychological scores (Table 2) reflected marked variability, although the patient generally performed within or above normal limits on most measures. She was alert and fully oriented and her global cognition was intact [Mini-Mental State Examination (MMSE) = 30]. While her overall memory was superior (Weschler Memory Scale-Third Edition (WMS-III) General Memory Index), she demonstrated relative weaknesses in visual memory (Faces I and II).30 She evidenced mild deficits in attention and concentration (Trail-making Test A).31 Her executive functioning abilities were also variable. She scored within normal limits on Trailmaking Test B31 but committed 88 errors on the Booklet Categories test, indicating significant impairment on this measure.
Neuropsychological assessment—3.5-Year post-ICU evaluation
The patient, now 52 years old, participated in a second follow-up evaluation 3.5 years after ICU discharge (Table 2) which included neuroimaging. She reported experiencing better overall cognitive functioning but described ongoing difficulties with organization, planning, and multitasking (abilities essential for success at her job). She indicated her inefficiency in the workplace was due to an inability to sustain focus, deficits in prioritizing, and a tendency to misplace things. Although she was meeting minimal standards, her performance was far below the superior level that had previously characterized her work. She ultimately decided to retire approximately 3 years after her ICU discharge. Her emotional functioning had improved steadily. As before, she had no significant symptoms of depression via the BDI-II. Symptoms of PTSD had largely resolved, with scores in the subclinical range on the Impact of Events Scale – Revised (IES-R).29
Her intellectual functioning was again assessed via the WAIS-III. Her composite IQ scores remained lower than her premorbid scores (VIQ =113 vs. 142; PIQ = 122 vs. 128; FIQ = 118 vs. 139) although her scores were uniformly higher than her 8 month follow-up evaluation. An analysis of performance on individual WAIS-III subtests showed improvement from her 8-month evaluation, with the largest improvement occurring on Picture Completion (7 points) and Digit Symbol Coding (3 points) subtests. Neuropsychological scores (Table 2) showed a general pattern of improvement since her initial evaluation, with scores in the average or above average range on all measures (although likely below her premorbid level), with the exception of the Booklet Categories Test, where she continued to have significant impairment (74 errors). Her overall memory abilities were variable and lower than those predicted given her IQ, although her lowest scores were within the normal range.
Brain imaging—Follow-up
Brain T1-weighted MRI showed marked atrophy (all lobes) with sulcal widening and ventricular enlargement (Fig. 3) that were not present on her MRI obtained during her acute illness. Quantitative MRI analysis comparing the patient’s brain volumes to age- and gender-matched controls32 showed decreased total brain volume (>8 standard deviations [SD] below controls) and increased ventricular size (1.3 SD larger than controls). Table 3 shows the quantitative MRI results for this patient compared to matched controls (females ages 46–55).
Table 3.
Quantitative brain imaging analysis—ARDS patient versus controls
| Brain regions (cm3) |
Total brain volumes |
Lateral ventricles |
Left temporal horn |
Right temporal horn |
III ventricle |
IV ventricle |
|---|---|---|---|---|---|---|
| Patient | 1075.13 | 19.01 | 0.11 | 0.2 | 1.04 | 2.01 |
| Controls | 1321.12 | 14.2 | 0.15 | 0.18 | 0.81 | 1.36 |
| Controls SD | 29.23 | 3.61 | 0.11 | 0.11 | 0.24 | 0.5 |
| SD difference | −8.8 | 1.3 | 0.38 | 0.2 | 0.96 | 1.3 |
Control data are from a sample of 24 females between the ages of 46 and 55. ARDS, acute respiratory distress syndrome; SD, standard deviation.
Discussion
The present case involves a critically ill 49-year-old female (49 years old at the time of ICU admission) with a minimal prior medical history and no pre-existing cognitive impairment who developed sepsis and ARDS but who did not experience frank neurologic insults during the course of her critical illness. Her case demonstrates the profound impact of critical illness on long-term neurocognitive functioning and brain integrity and the magnitude of cognitive decline. Although several cohort studies have reported a high prevalence of cognitive impairment in ICU survivors8,33,6 none have had available premorbid neuropsychological data with which to compare postcritical illness performance. Therefore, to our knowledge, this is the first investigation to objectively quantify the extent of cognitive decline after an ICU hospitalization compared to premorbid neuropsychological data.
Our patient had an initial decline in intelligence of approximately 2 SD on measures of verbal, performance, and full-scale intelligence after hospital discharge–a decline from >99th to the 61st percentile. This decline is remarkable as it is significantly larger than the decline observed among patients after cardiopulmonary bypass surgery and other serious medical procedures or illnesses.34–37 Along with a pattern of global intellectual decline, the patient had mild impairments in attention and visual memory and significant impairment in executive functioning. Her executive functioning difficulties were particularly notable in light of both their severity and their correspondence with subjective complaints of deficits in planning and problem solving. If an individual with average intellectual abilities at baseline (IQ of 100, 34th percentile) experienced the precipitous decline observed in our patient, he or she would function at a level consistent with a diagnosis of mild mental retardation (IQ of 70, 2nd percentile). Concomitant with her decline in cognitive function are the neuroimaging findings that show initial significant signal abnormalities in white matter and specific structures such as the hippocampus, a critical structure for memory. These findings likely reflect brain injury due to critical illness with sepsis and ARDS and its treatment. Such findings have been unreported to this point and supplement existing data regarding brain imaging abnormalities in ARDS survivors.38,39
At the time of her second follow-up evaluation (3.5 years after ICU discharge), she continued to report the presence of neuropsychological difficulties severe enough to negatively impact her daily functioning. She demonstrated executive dysfunction, displayed cognitive abilities far below her pre-ICU baseline, and manifested a variable neuropsychological profile by both slight improvement and slight decline. The persistent, and likely permanent, cognitive impairments experienced by the patient are supported by significant brain atrophy and ventricular enlargement (Fig. 3, Table 3).
Although the etiology of her cognitive dysfunction is unknown, it is likely multi-factorial. Possible contributors include delirium40,41 and sepsis-related inflammation, as well as hypoxia4 and glucose dysregulation.42 Inflammation in the brain results in the activation of microglial cells that function as “scavengers” and eliminate dead or injured neurons.20–22 Animal models have demonstrated learning and memory impairment as well as executive dysfunction following sepsis,43,44 and Hopkins et al4,8 reported that a majority of survivors of ARDS, itself characterized by severe inflammation, have persistent cognitive impairment after discharge. As noted in the introduction, delirium, which occurred in varying manifestations in our patient for almost 2 weeks, may be associated with cerebral hypoperfusion in subcortical regions populated by structures exquisitely sensitive to slight blood flow changes.17,18 Evidence from clinical investigations suggests that executive dysfunction, as well as other forms of impairment, commonly develops secondary to reduced blood flow to vulnerable subcortical structures, even in the absence of frank ischemic injury.19
While the precise cause of her intellectual decline and ongoing executive function and memory deficits remains unclear, it seems very likely that these difficulties are the result of events associated with her critical illness and/or its treatment. This conclusion is bolstered by her history of robust premorbid functioning; the absence of learning disabilities, prior brain injury, cardiac disease, meningitis, psychiatric or neurologic disorders; and her relatively young age—all factors inconsistent with dementia or other insidious etiologies of cognitive impairment. Although her intellectual functioning was assessed 17 years prior to her ICU admission, it is unlikely that her scores would have changed significantly insofar as IQ scores (particularly composite scores) are typically stable across time and do not significantly decline between young adulthood and middle age.45–47
Conclusion
The cognitive impairments and intellectual decline experienced by our subject 3.5 years following ICU discharge— and concomitant significant brain atrophy and ventricular enlargement—appear to be related to the effects of her critical illness with sepsis and ARDS. This decline resulted in a clinically significant disruption to her overall functioning and led to difficulties with organization, multitasking and efficiency. That the striking decline from premorbid to postcritical illness IQ scores mirrors the interval development of marked atrophy on brain imaging (computed tomography and MRI) suggests a potential mechanistic role of neuronal loss in the development of cognitive impairment. Whether the primary contributors to her cognitive dysfunction and brain atrophy are delirium, the toxic effects of sepsis on the brain, the sedative medications used while on the ventilator, hypoxia, or some other etiology is unclear. Nonetheless, this case raises awareness of the brain injury and concomitant cognitive decline following critical illness and highlights a number of key clinical and research-related implications. Notably, this case demonstrates that cognitive decline can be profound even in middle-aged and robustly healthy ICU patients who, in the context of their critical illness, do not experience frank neurologic traumas or insults. Such a finding is important because individual physicians and medical teams may be relatively less attuned to the needs of these patients than to frail or elderly ICU survivors or those with obvious forms of cognitive impairment. Further, it suggests that cognitive impairment in survivors of ARDS and sepsis may include memory and executive dysfunction—an important insight as this kind of impairment involves a disruption of abilities such as planning and organizing and, as such, may be particularly detrimental to daily functioning. Finally, it points to the need for further research focusing on issues including more fully identifying cognitive impairment associated with ARDS and sepsis, identifying modifiable risk factors that contribute to such impairment, and beginning to pilot the development of ICU follow-up clinics which could facilitate routine neuropsychological screening and psychological intervention to survivors of critical illness at high risk for cognitive decline.
Key Points.
Critical illnesses such as acute respiratory distress syndrome and sepsis are associated with substantial declines in intellectual functioning as well as significant brain atrophy.
Cognitive decline following severe critical illness appears to be persistent and only partially improves over time, rarely returning to baseline levels.
Memory and executive functioning abilities (abilities required for the successful management of complex tasks) may be particularly vulnerable to decline and impairment following critical illness.
The precise mechanisms contributing to cognitive decline after critical illness are unclear but may include prolonged ventilation, hypoxia, delirium, and inflammation.
Intensive care unit follow-up clinics focused on the identification and management of critical illness-related cognitive decline and impairment may be an important component of patient care in the future.
Acknowledgments
Supported by VA-Clinical Research Center of Excellence Fellowship (CRCOE) (to J.C.J.), by the NIA sponsored R01 (AG0727201) BRAIN-ICU, VA-MERIT MIND-ICU, and the VA-GRECC (to E.W.E.), and by the NHLBI (1R01HL091760-01A1) ALTOS and (N01-HR-5670) ARDS Long Term Outcome (LTO) Project (to R.O.H.).
References
- 1.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins RO, Brett S. Chronic neurocognitive effects of critical illness. Curr Opin Crit Care. 2005;11:369–375. doi: 10.1097/01.ccx.0000166399.88635.a5. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RO, Weaver LK, Pope D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 5.Rothenhäusler HB, Ehrentraut S, Stoll C, et al. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RO, Weaver LK, Chan KJ, et al. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10:1005–1017. doi: 10.1017/s135561770410711x. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 9.Marquis KA, Curtis JR, Caldwell ES, et al. Neuropsychological sequelae in survivors of ARDS compared with critically ill control patients (abstract) Am J Respir Crit Care Med. 2000;161:A383. [Google Scholar]
- 10.Hopkins RO, Jackson JC. Assessing neurocognitive outcomes after critical illness: are delirium and long-term cognitive impairments related? Curr Opin Crit Care. 2006;12:388–394. doi: 10.1097/01.ccx.0000244115.24000.f5. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins RO, Ely EW, Jackson JC. The role of future longitudinal studies in ICU survivors: understanding determinants and pathophysiology of brain dysfunction. Curr Opin Crit Care. 2007;13:497–502. doi: 10.1097/MCC.0b013e3282efd19c. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neuro-degenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunther ML, Jackson JC, Ely EW. Loss of IQ in the ICU brain injury without the insult. Med Hypotheses. 2007;69:1179–1182. doi: 10.1016/j.mehy.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Milbrandt EB, Angus DC. Potential mechanisms and markers of critical illness-associated cognitive dysfunction. Curr Opin Crit Care. 2005;11:355–359. doi: 10.1097/01.ccx.0000170508.63067.04. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 17.Fong TG, Bogardus ST, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 18.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 20.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Semmler A, Okulla T, Sastre M, et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 23.Selnes OA, McKhann GM. Coronary-artery bypass surgery and the brain. N Engl J Med. 2001;344:451–452. doi: 10.1056/NEJM200102083440609. [DOI] [PubMed] [Google Scholar]
- 24.Stump DA, Rogers AT, Hammon JW. Neurobehavioral tests are monitoring tools used to improve cardiac surgery outcome. Ann Thorac Surg. 1996;61:1295–1296. doi: 10.1016/0003-4975(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 25.Stump DA, James RL, Murkin JM. Is that outcome different or not? The effect of experimental design and statistics on neurobehavioral outcome studies. Ann Thorac Surg. 2000;70:1782–1785. doi: 10.1016/s0003-4975(00)02202-5. [DOI] [PubMed] [Google Scholar]
- 26.Murkin JM, Newman SP, Stump DA, et al. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 27.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT. BDI-II Depression Inventory Manual. 2 ed. New York: Harcourt Brace; 1996. [Google Scholar]
- 29.Weiss D, Marmar CR. The Impact of Events Scale—Revised. In: Wilson J, Keane TM, editors. Assessing Psychological Trauma and PTSD. New York: Guilford; 1996. pp. 399–411. [Google Scholar]
- 30.Wechsler D. The Wechsler Memory Scale-III Manual. New York: Har-court Brace Jovanovich; 1987. [Google Scholar]
- 31.Lexak MD. Neuropsychological Assessment. 3 ed. ew York: Oxford University Press; 1995. [Google Scholar]
- 32.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 33.Devlin JW, Boleski G, Mlynarek M, et al. Motor Activity Assessment Scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27:1271–1275. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287:1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 35.van Dijk D, Moons KG, Keizer AM, et al. Octopus Study Group. Association between early and three month cognitive outcome after off-pump and on-pump coronary bypass surgery. Heart. 2004;90:431–434. doi: 10.1136/hrt.2002.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selnes OA, Goldsborough MA, Borowicz LM, et al. Neurobehavioural sequelae of cardiopulmonary bypass. Lancet. 1999;353:1601–1606. doi: 10.1016/S0140-6736(98)07576-X. [DOI] [PubMed] [Google Scholar]
- 37.Selnes OA, Grega M, Borowicz LM, Jr., et al. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:1377–1384. doi: 10.1016/s0003-4975(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins RO, Gale SD, Johnson SC, et al. Severe anoxia with and without concomitant brain atrophy and neuropsychological impairments. J Int Neuropsychol Soc. 1995;1:501–509. doi: 10.1017/s135561770000059x. [DOI] [PubMed] [Google Scholar]
- 39.Hopkins RO, Gale SD, Pope D, et al. Ventricular enlargement in patients with acute respiratory distress syndrome. J Int Neuropsychol Soc. 2000;6:229. [Google Scholar]
- 40.Jackson JC, Gordon SM, Girard TD, et al. Delirium as a risk factor for long term cognitive impairment in mechanically ventilated ICU survivors (abstract) Am J Respir Crit Care Med. 2007;175:A22. [Google Scholar]
- 41.Jackson JC, Gordon SM, Hart RP, et al. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins RO, Suchyta MR, Jephson A, et al. Hyperglycemia and neurocognitive outcome in ARDS survivors (abstract) Proc Am Thorac Soc. 2005;2:A36. [Google Scholar]
- 43.Barichello T, Martins MR, Reinke A, et al. Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med. 2005;33:221–223. doi: 10.1097/01.ccm.0000150741.12906.bd. [DOI] [PubMed] [Google Scholar]
- 44.Larsson A, Lipcsey M, Sjölin J, et al. Slight increase of serum S-100B during porcine endotoxemic shock may indicate blood-brain barrier damage. Anesth Analg. 2005;101:1465–1469. doi: 10.1213/01.ANE.0000180193.29655.6A. [DOI] [PubMed] [Google Scholar]
- 45.Livingston RB, Jennings E, Reynolds CR, et al. Multivariate analyses of the profile stability of intelligence tests: high for IQs, low to very low for subtest analyses. Arch Clin Neuropsychol. 2003;18:487–507. [PubMed] [Google Scholar]
- 46.Matarazzo JD, Herman DO. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol. 1984;6:351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- 47.Raguet ML, Campell DA, Berry DTR, et al. Stability of intelligence and intellectual predictors in older persons. Psychol Assess. 1996;8:154–160. [Google Scholar]