Summary
Haemophagocytic lymphohistiocytosis (HLH) is a life threatening complication of Epstein-Barr virus (EBV) infection. The anti-CD20 antibody rituximab depletes B cells, leading to improved outcomes for patients with EBV-associated B-lymphoproliferative disorders. To gather data on the use of rituximab in EBV-HLH, we performed a retrospective investigation involving 42 EBV-HLH patients who had received treatment with rituximab-containing regimens. On average, patients received 3 rituximab infusions (range 1 – 10) at a median dose of 375 mg/m2. In all patients, rituximab was administered with other HLH-directed medications, including steroids, etoposide and/or ciclosporin. Rituximab-containing regimens appeared well tolerated and improved clinical status in 43% of patients. Examination of laboratory data obtained prior to and within 2 – 4 weeks after the first rituximab dose revealed significant reductions in EBV load (median load pre-rituximab: 114,200 copies/ml, median post-rituximab: 225 copies/ml, p=0.0001) and serum ferritin levels (median ferritin pre-rituximab: 4,260 μg/l, median post-rituximab: 1,149 μg/l, p=0.001). Thus, when combined with conventional HLH-directed therapies, rituximab improves symptoms, reduces viral load and diminishes inflammation. These data support the incorporation of rituximab into future prospective clinical trials for patients with EBV-HLH.
Keywords: Epstein-Barr virus, haemophagocytic lymphohistiocytosis, macrophage activation syndrome, rituximab, x-linked lymphoproliferative disease
Introduction
Haemophagocytic lymphohistiocytosis (HLH) comprises a rare group of disorders typified by activation of CD8+ T cell and macrophages and secretion of high levels of pro-inflammatory cytokines (Janka 2012, Lykens, et al 2011, Risma and Jordan 2012). HLH occurs as a hereditary condition caused by germline mutations that impair lymphocyte cytotoxic function or as a nonhereditary disorder triggered by infection, malignancy or autoimmune disease (Coffey, et al 1998, Cote, et al 2009, Feldmann, et al 2003, Janka 2012, Nichols, et al 1998, Rigaud, et al 2006, Stepp, et al 1999, zur Stadt, et al 2009, zur Stadt, et al 2005). Currently, a two-tiered approach is used to treat HLH: chemo-immunotherapeutic agents are administered to dampen inflammation and targeted therapies are given to eliminate HLH trigger(s) (Janka 2012).
Epstein-Barr virus (EBV) is a common trigger of HLH, particularly in Asian individuals (Imashuku 2002, Kawaguchi, et al 1993, Yachie, et al 2003) and in patients with congenital or acquired immunodeficiencies (McClain, et al 1988, Pasic, et al 2003, Rezaei, et al 2011). EBV is poorly responsive to antiviral agents; however, it resides in B lymphocytes, which can be rapidly depleted using the B cell-targeting monoclonal antibody rituximab. Based on its efficacy in lowering disease burden in patients with B-lymphoproliferative disorders (DiNardo and Tsai 2010, Maloney 2012), some investigators are using rituximab to treat EBV-HLH. It is not known whether this is an effective strategy for this disorder.
To understand current practice and prepare for the possible incorporation of rituximab into future HLH protocols, we performed this retrospective investigation, which describes 42 patients with EBV-induced disease, who were treated with regimens containing rituximab, steroids, etoposide and/or ciclosporin. Rituximab-containing regimens improved clinical status in most patients, exhibited no toxicities beyond those normally encountered and significantly reduced EBV load and serum ferritin levels. These data suggest that rituximab can be safely added to standard therapies and provide the evidence needed to move forward with a prospective clinical trial.
Materials and Methods
Data collection
Survey documents were developed, approved by the Institutional Review Board at The Children's Hospital of Philadelphia and distributed to members of the Histiocyte Society.
Patients
This study included 42 patients with EBV-HLH who received treatment with rituximab-containing regimens between June 1, 2000 and October 31, 2011 (Table I). HLH was diagnosed based on established criteria (Henter, et al 2007). The median age at diagnosis was 6.75 years (range 1.2 – 44) and there was a predominance of males (n=30; 71%). Positive EBV status was documented by monospot (n=9), serological evidence of acute infection (n=28) and/or evidence of EBV DNA in the blood by real time polymerase chain reaction (n=41; median viral load 114,200 copies of EBV genome/ml [range 500 - 4×107]). Thirty-seven patients underwent mutational analysis for at least 1 HLH gene, and in 16 patients, mutations were identified. The gene most commonly mutated was SH2D1A (n=8), followed by PRF1 (n=2, each monoallelic), XIAP (n=2), UNC13D (n=2) and STXBP2 (n=2). Both patients with heterozygous PRF1 mutations developed disease recurrence, suggesting a genetic form of HLH.
Table I.
Laboratory Features at diagnosis with EBV-HLH
| HLH genetic testing | Number of patients with positive result/number of patients tested (%) |
|---|---|
| PRF1 | 2/28 (7) |
| SH2D1A | 8/24 (33) |
| XIAP | 2/21 (10) |
| UNC13D | 2/25 (8) |
| STX11 | 0/21 (0) |
| STXBP2 | 2/4 (50) |
| RAB27A | 0/3 (0) |
| ITK | 0/1 (0) |
| Assay to detect EBV | Number of patients with positive result/number of patients tested (%) |
|---|---|
| Monospot | 9/12 (75) |
| EBV serology | 28/30 (93) |
| EBV PCR | 41/41 (100) |
| Complete blood count (normal values) | Median (range) |
|---|---|
| WBC (4.5 -13 × 109/l) | 2.85 × 109/l (0.1-85.2 × 109/l) |
| ANC (≥ 1 × 109/l) | 1.25 × 109/l (0.014-11.3 × 109/l) |
| Hb (130-160 g/l) | 89 (44 -146) |
| Plt (150-450 × 109/l) | 69 × 109/l (9-31000 × 109/l) |
| Hepatic panel (normal values) | Median (range) |
|---|---|
| AST (15-45 u/l) | 410 (46-7,334) |
| ALT (10-40 u/l) | 205 (14-5,227) |
| Total bilirubin (10-24 μmol/l) | 62 (6.8-282) |
| Total protein (62-81 g/l) | 52 (40-71) |
| Albumin (37-56 g/l) | 23 (16-38) |
| Other HLH tests (normal values) | |
|---|---|
| soluble interleukin 2 receptor (<2,000 u/ml) | 12,412 (1,891-206,567) |
| Fibrinogen (1.7-4.7 g/l) | 1.5 (0.5-7.9) |
| Ferritin (10-70 μg/l) | 6,334 (210-121,379) |
| Triglycerides (0.3-1.4 mmol/l) | 3.3 (0.9-14) |
| NK function (Normal/ depressed) | Depressed in 12 pts |
| Haemophagocytosis (Present/absent) | Present in 30 pts |
EBV, Epstein-Barr virus; HLH, haemophagocytic lymphohistiocytosis; PCR, polymerase chain reaction; WBC, white blood cells; ANC, absolute neutrophil count; Hb, haemoglobin concentration; Plt, platelets; AST, aspartate transaminase; ALT, alanine transaminase; NK, natural killer cell.
Clinical and laboratory manifestations
All patients exhibited fever, hepatomegaly and/or splenomegaly and were cytopenic in one or more lineages (Table I). Natural killer (NK) cell cytotoxicity was examined in 20 patients, and in 12 (60%), activity was reduced or absent. Among these patients, 3 harboured HLH-associated mutations. Twenty-three patients (96% of those tested) exhibited elevated levels of soluble IL2 receptor (sIL2RA; median 12,412 u/ml; range 1,891 – 206,567 u/ml). Similarly, ferritin levels were high in all 41 patients for whom data were provided (median 6,334 μg/l; range 210 – 121,379 μg/l). Haemophagocytosis in the bone marrow was present in 30 patients (73%).
Management of HLH
All patients received treatment with rituximab-containing regimens (Table II). The median dose was 375 mg/m2 (range 175 – 412) and the average number of infusions was 3 (range 1 – 10). Twenty-eight patients (67%) received the first dose of rituximab within 1 month of HLH diagnosis (median 5.5 days; range 1 – 28), while the remaining patients received it later (median 73.5 days; rage 30 – 210). Nine patients received the first dose of rituximab as therapy for recurrent, not primary, HLH (median 90 days; range 53 – 210). No rituximab infusions followed haematopoietic stem cell transplantation (HSCT). Twenty-six patients (62%) received rituximab weekly, while 1 patient received it monthly. Eleven patients received a single dose, 1 patient received 2 doses given 10 days apart, and for 3 patients the schedule was not specified.
Table II.
Dosing and Schedule of Rituximab Administration
| Dosage information | Patients (n) | Median, range |
|---|---|---|
| Dose (mg/m2) | 42 | 375 (175-412) |
| Days between diagnosis & first rituximab | 42 | 13 (1-210) |
| • < 30 days | 28 | 5.5 (1-28) |
| • ≥ 30days | 14 | 73.5 (30-210) |
| Number of doses$ | 42 | 3 (1-10) |
| Schedule | Patients (n) | % of total cohort |
|---|---|---|
| • Weekly | 26 | 64 |
| • Monthly | 1 | 2 |
| • Other* | 15 | 34 |
| Other medications | ||
|---|---|---|
| Chemotherapy | 38 | 90 |
| Steroids | 41 | 98 |
| IVIG | 33 | 78 |
| Ciclosporin | 32 | 76 |
| SCT | 20 | 48 |
| Antiviral therapies | ||
|---|---|---|
| Acyclovir | 13 | 31 |
| Ganciclovir | 14 | 33 |
| Other# | 7 | 17 |
IVIG, intravenous immunoglobulin; SCT, stem cell transplantation
All patients received concomitant therapy with etoposide (n=38), dexamethasone (n=41) and/or ciclosporin (n=32). Twenty-six patients (62%) received anti-viral medications and 33 (79%) received intravenous immunoglobulin. Twenty patients (48%) underwent allogeneic (HSCT) and among these, 11 (55%) harboured HLH gene mutations. The remaining 9 patients had at least 1 reactivation, suggesting a genetic form of disease. Consistent with the presence of a verified or suspected diagnosis of familial HLH, the median age at presentation was 3.5 years for those going on to HSCT and 14.5 years for the non-transplanted patients.
Statistical analysis
Comparisons of laboratory parameters before and after the first rituximab dose were completed using the Wilcoxon matched-pairs signed rank test. Univariate associations between clinical and laboratory features at diagnosis and response to rituximab were analysed using the log-rank test. Kaplan-Meier curves were created using Stata 11.1 (StataCorp, College Station, TX) software with time to last follow-up or death as endpoints. Statistical significance was declared at P ≤ 0.05.
Results
Clinical effects of rituximab-containing regimens
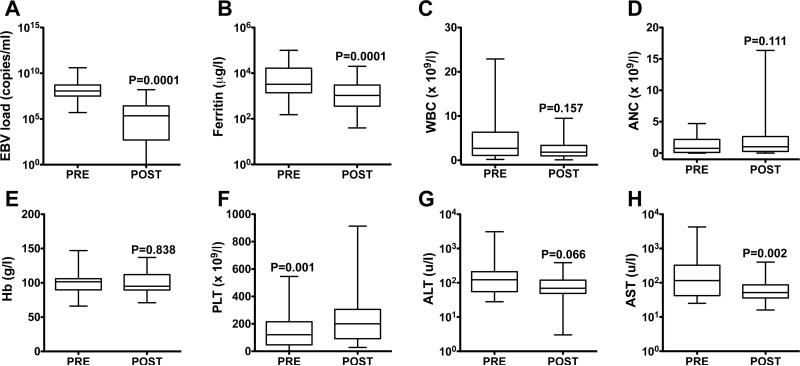
After initiation of a rituximab-containing regimen, 18 of the 42 patients (43%) improved clinically, with resolution of fever and reduction in hepatosplenomegaly and fluid retention. To assess quantitatively whether rituximab-containing regimens improved HLH manifestations, laboratory data were collected prior to and within 2 – 4 weeks after the first course of rituximab-based chemo-immunotherapy (Fig 1). Among 36 patients for whom serial data were available, treatment was associated with a 500-fold reduction in EBV load (median load pre-rituximab: 114,200 copies/ml; median load post-rituximab: 225 copies/ml; p=0.0001). In 22 patients (61%), the EBV load dropped to <1,000 copies/ml (n=14) or fell below the limits of detection (n=8). Similarly, ferritin, a surrogate for HLH activity (Allen, et al 2008, Lin, et al 2011), exhibited a 3.7-fold reduction (median ferritin pre-rituximab: 4,260 μg/l; median post-rituximab: 1,149.5 μg/l; p=0.0001). Other changes included a significant increase in platelet count and reduction in aspartate transaminase (AST), and a lowering of alanine transaminase (ALT) that neared but did not reach statistical significance.
Fig 1. Laboratory parameters before and after administration of the first dose of rituximab.
Box plots representing laboratory values prior to and within 2 – 4 weeks after delivery of the first dose of rituximab. The central line of the box plots represents the median value with the whiskers representing the minimum and maximum levels. Laboratory parameters included: Epstein-Barr virus (EBV) DNA levels (n=36; A), ferritin (n=35; B), white blood cell count (WBC, n=37; C), absolute neutrophil count (ANC, n=36; D); haemoglobin concentration (Hb, n=37; E), platelet count (PLT, n=37; F), alanine transaminase (ALT, n=36; G), aspartate transaminase (AST, n=36; H). Statistical associations between pre- and post-rituximab values were calculated using the Wilcoxon matched-pairs signed rank test.
Side effects of rituximab
Fifteen patients (36%) experienced immediate (within 1 day) or later (all other times) side effects. Of these, 8 patients developed 1 or more infusion-related toxicities, including fever (n=7), chills (n=2) or other symptoms (n=5; respiratory distress, facial flushing/swelling, myalgia, urticaria). Two patients developed tachycardia and hypotension, requiring a decrease in the rate of infusion or discontinuation of the rituximab. Later side effects included hypogammaglobulinaemia (n=5), neutropenia (n=4) and transaminitis (n=1). Of the 5 patients with hypogammaglobulinaemia, 4 underwent genetic analysis and 3 harboured mutations (1 each in XIAP, SH2D1A, STXBP2). No patients died within 24 h after receiving a dose of ritumximab or experienced reactivation of hepatitis B, hepatitis C, cytomegaolvirus, herpes simplex virus, parvovirus, varicella zoster virus or West Nile virus.
Overall survival
Twenty-six (62%) patients were alive at the time of analysis with a median duration of survival of 1,120 days since EBV-HLH diagnosis (range 230-3,750). Sixteen patients died at a median of 97.5 days (range 22-900) due to HLH/multi-system organ dysfunction (n=10), with or without infection (n=5), toxicities of stem cell (n=3) or multi-visceral transplantation (n=1), cerebral bleeding (n=1), lymphoma (n=1) or EBV-associated lymphoproliferative disease (n=1). Of the 26 surviving patients, 24 exhibited no evidence of active disease. Fourteen of these 24 (58%) had received an allogeneic HSCT. Within the 10 non-transplanted patients, 2 experienced ≥1 HLH reactivation and in both patients, repeat treatment with rituximab-containing regimens was sufficient to control disease.
Prognostic factors
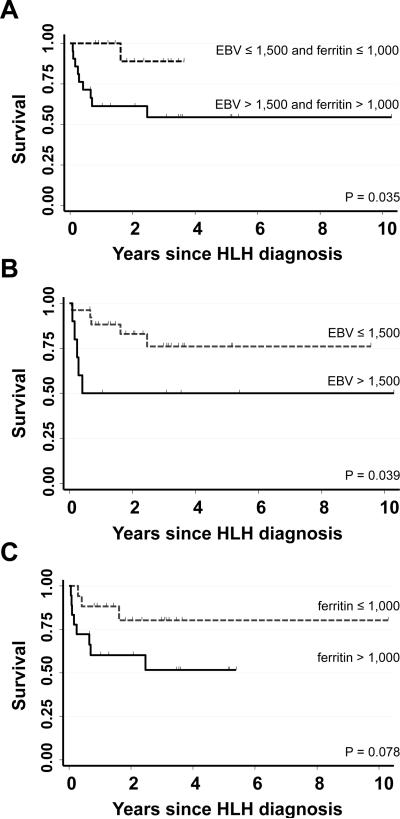
To ascertain whether specific clinical or laboratory factors at diagnosis predicted response to the first course of a rituximab-containing regimen, we examined the relationship between presenting parameters (age, gender, EBV load, ferritin, white blood cell count, absolute neutrophil count [ANC], haemoglobin, platelet count, sIL2RA level, NK function, presence of HLH mutations, bilirubin, AST, ALT, albumin, number of days until the first rituximab dose) and reduction in EBV load and/or ferritin (defined as a drop in viral load to ≤1,500 copies/ml and/or ferritin to ≤1,000 μg/l). Although none of the features examined were predictive of a combined response, higher diagnostic ferritin levels inversely correlated with a drop in ferritin to ≤1,000 μg/l post-rituximab (odds ratio 0.38, 95% confidence interval [CI] 0.19-0.75; p=0.005). Conversely, a higher ANC at presentation significantly correlated with a drop in viral load to ≤1,500 copies/ml post-rituximab (odds ratio 2.14, 95% CI 1.14-4.00; p=0.018). To determine whether the degree of response to the first course of a rituximab-containing regimen impacted upon long-term outcomes, Kaplan Meier analyses were completed and revealed that patients whose EBV load dropped to ≤1,500 copies/ml (alone or combined with a drop in ferritin) were significantly more likely to survive compared to those whose viral loads remained >1,500 copies/ml (Fig 2).
Fig 2. Probability of survival.
Kaplan-Meier estimates of the probability of survival for the 42 patients in this cohort based on a combined reduction in viral load to ≤1,500 copies/ml and ferritin to ≤1,000 μg/l (A), reduction in viral load alone (B) or reduction in ferritin alone (C). Statistical differences between groups were evaluated using the log-rank test.
Discussion
The management of EBV-HLH remains challenging, with ≥30% patients dying of the disease or its complications (Imashuku 2011, Qin, et al 2012, Shiraishi, et al 2012). It is proposed that outcomes in infection-associated HLH are improved if one targets the inciting pathogen. We hypothesized that the depletion of B cells, which serve as a reservoir for EBV, might ameliorate the signs and symptoms and improve the survival rate for patients with EBV-HLH. To test this hypothesis, we analysed data from 42 patients who had received treatment with rituximab-containing regimens. Chemo-immunotherapeutic approaches incorporating rituximab appeared well tolerated and, in most patients, significantly reduced EBV load and/or serum ferritin levels. In addition, patients whose viral load dropped to ≤1,500 copies/ml were significantly more likely to survive their disease than those whose viral loads remained elevated. These data lend credence to the potential beneficial effects of rituximab and they concur with prior reports linking a drop in viral load with improved survival in EBV-HLH (Teramura, et al 2002).
EBV infects B as well as non-B cell populations in HLH patients (Kawaguchi, et al 1993, Yang, et al 2012; Beutel, et al 2009) and there has been concern that a B cell depleting agent might not be effective in HLH. Nonetheless, physicians reported an immediate improvement in the signs and symptoms of HLH in 43% of patients. From a molecular perspective, treatment with the initial course of a rituximab-containing regimen led to a significant drop in viral load, and in 78% of patients for whom serial data were available, viral DNA fell within the normal range or below the limits of detection. As rituximab does not deplete T or NK cells, we presume that EBV was residing within a significant proportion of the B cells in responding patients. Alternatively, the administration of etoposide and/or dexamethasone could have reduced T and NK cell numbers and facilitated the clearance of virus residing in these cell populations.
Immediate toxicities of rituximab include infusion-related fever, chills, hypotension and bronchospasm (Kimby 2005). Surprisingly, only 7 patients (17%) in the current cohort were reported to develop a new fever while receiving rituximab and only two (5%) developed hypotension. After treatment with rituximab, B cell numbers are depleted for months, but immunoglobulin levels generally remain within the normal range (Kimby 2005). Consistent with these data, only 5 patients in this cohort developed hypogammaglobulinaemia. Notably, of these individuals, 3 harboured mutations in SH2D1A, XIAP or STXBP2, genes associated with the development of humoral immune defects (Booth, et al 2011, Meeths, et al 2010, Pachlopnik Schmid, et al 2011). As a result, it is not possible to determine whether the low immunoglobulin levels in these patients resulted from the rituximab itself or instead from an underlying genetic predisposition. Other rare yet significant side effects of rituximab include development of thrombocytopenia, neutropenia and anaemia (Kimby 2005, Wolach, et al 2010), and reactivation of specific viral infections (Aksoy, et al 2007). Within this cohort, 4 patients developed neutropenia; however, none experienced reactivation of hepatitis B, hepatitis C or other viral infections. Collectively, these data suggest that rituximab is safe, even when given to HLH patients who are often critically ill and receiving concomitant cytotoxic and/or immunosuppressive agents.
There are several limitations of this study that warrant consideration. First, the sample size is small, which limits power to detect associations between diagnostic parameters or variations in treatment and response. By completing this investigation on an international level, we attempted to capture as many EBV-HLH patients as possible. Second, this cohort is retrospective and contains patients with familial as well as non-familial HLH. While all patients received rituximab, it was given at varying schedules and dosages and always along with other medications. Thus, there are potential reporting biases and confounding factors, which make interpretation of these data challenging. Nonetheless, this study demonstrates that rituximab-containing regimens significantly reduce EBV load and signs of inflammation. The retrospective nature of this study does not allow us to determine the efficacy of rituximab as a treatment for EBV-HLH; however, it provides the evidence needed to move forward with a prospective clinical trial to address this important and clinically relevant, but currently unanswered question.
Acknowledgements
We thank Richard Aplenc, Alix Seif, Ed Behrens and John Maris for their critical review of the manuscript. This work was supported in part by the XLP Research Trust (KEN).
Appendix 1
Investigators from the EBV-HLH Rituximab Study Group include: Gregory Hale (All Children's Hospital, Pediatric Cancer and Blood Disorders Center, St. Petersburg, FL); Milen Minkov, Susanne Karlhuber (St. Anna's Children's Hospital, University Clinic of Paediatrics, Medical University of Vienna, Austria), Joanna L. Weinstein (Ann & Robert H. Lurie Children's Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, IL); Sheila Weitzman (University of Toronto, The Hospital for Sick Children, Toronto, Canada); Despina Moshous, Alain Fischer (Unité d'Immunologie, Hématologie et Rhumatologie Pédiatriques, APHP, Hôpital Necker-Enfants Malades, Paris, France); Michael Jeng (Stanford University School of Medicine, Stanford, CA); Michael Henry (Center for Cancer and Blood Disorders, Phoenix Children's Hospital, Phoenix, AZ); Riccardo Haupt, Joanna Svahn (Istituto G. Gaslini, Genova, Italy); Ester Zapotocka (University Hospital Motol and Charles University 2nd Facility of Medicine, Prague, Czech Republic); Julie Wolfson (City of Hope, Duarte, CA); Joanne Yacobovich (Schneider Children's Medical Center of Israel and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel); Jan A.M. Van Laar (Departments of Immunology and Internal Medicine, Erasmus MC University Hospital, Rotterdam, The Netherlands); Julie Talano (Medical College of Wisconsin, Milwaukee, WI); Itziar Astigarraga (Servicio de Pediatria, BioCruces Health Research Institute, Hospital Universitario Cruces, Barakaldo, Bizkaia, Spain); Karin Beutel (University Children's Hospital Muenster, Paediatric Haematology and Oncology, Muenster, Germany); Elisabet Berglöf, Jan-Inge Henter (Department of Women's and Children's Health, Karolinska Institutet, Karolinska University Hospital Solna, Stockholm, Sweden); Shinsaku Imanshuku (Takasaga-Seibu Hospital, Takasaga, Japan); Marco Hok-kung Ho (Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China); Chris Fraser (Queensland Children's Cancer Center, Royal Children's Hospital, Brisbane, Australia); Jennifer Greene Welch (Hasbro Children's Hospital, Alpert Medical School, Brown University, Providence, RI); Brenda Kitchen, Rama Jasty (Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Michigan Medical Center, Ann Arbor, MI); Barbara A. Degar (Dana-Farber Cancer Center, Harvard University School of Medicine, Boston, MA); Maria Winther Gunnes (Department of Paediatrics, Haukeland University Hospital, Bergen, Norway); Corrina McMahon (Our Lady's Children's Hospital, Dublin, Ireland); Timothy Garrington (Children's Hospital Colorado, Center for Cancer and Blood Disorders, Denver CO); Alexandra H. Filipovich, Michael B. Jordan, Jacob J. Bleesing, Rebecca A. Marsh (Cincinnati Children's Hospital Medical Center, Cincinnati, OH).
Footnotes
Author contributions
D.C. designed the research, analysed data and wrote the manuscript. S.W. and K.Z. collected and analysed data. R.D. assisted with data interpretation and preparation of figures. H.Z. completed statistical analyses. K.E.N. oversaw all analyses, and edited and revised the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, Barista I. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–1312. doi: 10.1080/10428190701411441. [DOI] [PubMed] [Google Scholar]
- Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50:1227–1235. doi: 10.1002/pbc.21423. [DOI] [PubMed] [Google Scholar]
- Beutel K, Gross-Wieltsch U, Wiesel T, Stadt UZ, Janka G, Wagner HJ. Infection of T lymphocytes in Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in children of non-Asian origin. Pediatr Blood Cancer. 2009;53:184–190. doi: 10.1002/pbc.22037. [DOI] [PubMed] [Google Scholar]
- Booth C, Gilmour KC, Veys P, Gennery AR, Slatter MA, Chapel H, Heath PT, Steward CG, Smith O, O'Meara A, Kerrigan H, Mahlaoui N, Cavazzana-Calvo M, Fischer A, Moshous D, Blanche S, Pachlopnik Schmid J, Latour S, de Saint-Basile G, Albert M, Notheis G, Rieber N, Strahm B, Ritterbusch H, Lankester A, Hartwig NG, Meyts I, Plebani A, Soresina A, Finocchi A, Pignata C, Cirillo E, Bonanomi S, Peters C, Kalwak K, Pasic S, Sedlacek P, Jazbec J, Kanegane H, Nichols KE, Hanson IC, Kapoor N, Haddad E, Cowan M, Choo S, Smart J, Arkwright PD, Gaspar HB. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 2011;117:53–62. doi: 10.1182/blood-2010-06-284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, Al-Harbi M, Alangari A, Le Deist F, Gennery AR, Prince N, Cariou A, Nitschke P, Blank U, El-Ghazali G, Menasche G, Latour S, Fischer A, de Saint Basile G. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo CD, Tsai DE. Treatment advances in posttransplant lymphoproliferative disease. Curr Opin Hematol. 2010;17:368–374. doi: 10.1097/MOH.0b013e328339018c. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002;44:259–272. doi: 10.1016/s1040-8428(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Imashuku S. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. J Pediatr Hematol Oncol. 2011;33:35–39. doi: 10.1097/MPH.0b013e3181f84a52. [DOI] [PubMed] [Google Scholar]
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–246. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Miyashita T, Herbst H, Niedobitek G, Asada M, Tsuchida M, Hanada R, Kinoshita A, Sakurai M, Kobayashi N. Epstein-Barr virus-infected T lymphocytes in Epstein-Barr virus-associated hemophagocytic syndrome. J Clin Invest. 1993;92:1444–1450. doi: 10.1172/JCI116721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimby E. Tolerability and safety of rituximab (MabThera). Cancer Treat Rev. 2005;31:456–473. doi: 10.1016/j.ctrv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154–155. doi: 10.1002/pbc.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118:618–626. doi: 10.1182/blood-2010-12-324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366:2008–2016. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- McClain K, Gehrz R, Grierson H, Purtilo D, Filipovich A. Virus-associated histiocytic proliferations in children. Frequent association with Epstein-Barr virus and congenital or acquired immunodeficiencies. Am J Pediatr Hematol Oncol. 1988;10:196–205. [PubMed] [Google Scholar]
- Meeths M, Entesarian M, Al-Herz W, Chiang SC, Wood SM, Al-Ateeqi W, Almazan F, Boelens JJ, Hasle H, Ifversen M, Lund B, van den Berg JM, Gustafsson B, Hjelmqvist H, Nordenskjold M, Bryceson YT, Henter JI. Spectrum of clinical presentations in familial hemophagocytic lymphohistiocytosis type 5 patients with mutations in STXBP2. Blood. 2010;116:2635–2643. doi: 10.1182/blood-2010-05-282541. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F, Kanegane H, Lopez-Granados E, Mejstrikova E, Pellier I, Galicier L, Galambrun C, Barlogis V, Bordigoni P, Fourmaintraux A, Hamidou M, Dabadie A, Le Deist F, Haerynck F, Ouachee-Chardin M, Rohrlich P, Stephan JL, Lenoir C, Rigaud S, Lambert N, Milili M, Schiff C, Chapel H, Picard C, de Saint Basile G, Blanche S, Fischer A, Latour S. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood. 2011;117:1522–1529. doi: 10.1182/blood-2010-07-298372. [DOI] [PubMed] [Google Scholar]
- Pasic S, Micic D, Kuzmanovic M. Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis in Wiskott-Aldrich syndrome. Acta Paediatr. 2003;92:859–861. doi: 10.1080/08035250310003631. [DOI] [PubMed] [Google Scholar]
- Qin Q, Xie Z, Shen Y, Yang S, Liu C, Huang Z, Xu J, Al J, Shen K. Assessment of immunochemotherapy and stem cell transplantation on EBV-associated hemophagocytic lymphohistiocytosis in children: a systematic review and meta analysis. Eur Rev Med Pharmacol Sci. 2012;16:672–678. [PubMed] [Google Scholar]
- Rezaei N, Hedayat M, Aghamohammadi A, Nichols KE. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J Allergy Clin Immunol. 2011;127:1329–1341. e1322. doi: 10.1016/j.jaci.2011.02.047. quiz 1342-1323. [DOI] [PubMed] [Google Scholar]
- Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- Risma K, Jordan MB. Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr. 2012;24:9–15. doi: 10.1097/MOP.0b013e32834ec9c1. [DOI] [PubMed] [Google Scholar]
- Shiraishi A, Ohga S, Doi T, Ishimura M, Takimoto T, Takada H, Miyamoto T, Abe Y, Hara T. Treatment choice of immunotherapy or further chemotherapy for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2012;59:265–270. doi: 10.1002/pbc.24039. [DOI] [PubMed] [Google Scholar]
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- Teramura T, Tabata Y, Yagi T, Morimoto A, Hibi S, Imashuku S. Quantitative analysis of cell-free Epstein-Barr virus genome copy number in patients with EBV-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2002;43:173–179. doi: 10.1080/10428190210176. [DOI] [PubMed] [Google Scholar]
- Wolach O, Bairey O, Lahav M. Late-onset neutropenia after rituximab treatment: case series and comprehensive review of the literature. Medicine (Baltimore) 2010;89:308–318. doi: 10.1097/MD.0b013e3181f2caef. [DOI] [PubMed] [Google Scholar]
- Yachie A, Kanegane H, Kasahara Y. Epstein-Barr virus-associated T-/natural killer cell lymphoproliferative diseases. Semin Hematol. 2003;40:124–132. doi: 10.1053/shem.2003.50012. [DOI] [PubMed] [Google Scholar]
- Yang X, Wada T, Imadome K, Nishida N, Mukai T, Fujiwara M, Kawashima H, Kato F, Fujiwara S, Yachie A, Zhao X, Miyawaki T, Kanegane H. Characterization of Epstein-Barr virus (EBV)-infected cells in EBV-associated hemophagocytic lymphohistiocytosis in two patients with X-linked lymphoproliferative syndrome type 1 and type 2. Herpesviridae. 2012;3:1. doi: 10.1186/2042-4280-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nurnberg P, Janka G, Hennies HC. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- Zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]