Abstract
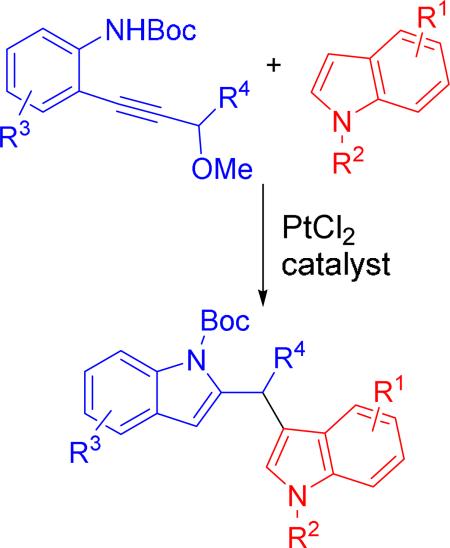
Various diindolylmethanes were prepared from propargylic ethers and substituted indoles via a platinum-catalyzed tandem indole annulation/arylation cascade. The resulting diindolylmethanes could be converted to natural product malassezin by formylation or indolo[3,2-b]carbazoles by cyclization.
Indole is one of the most abundant heterocycles in bioactive natural products and pharmaceutical agents. Not surprisingly, numerous efforts have been devoted to the preparation of indoles from a diverse range of starting materials.1,2 Most previous efforts, however, have focused on indole annulation alone.1 The efficiency of the synthesis can be increased significantly if the event of indole annulation is coupled with other transformations in a cascade manner. We recently developed a tandem indole annulation/(4+3) cycloaddition for the construction of both indole and a seven-membered ring simultaneously in the synthesis of cyclohepta[b]indoles.3 We herein report a synthesis of diindolylmethanes via a platinum-catalyzed indole annulation/arylation cascade as our continued efforts in this area.
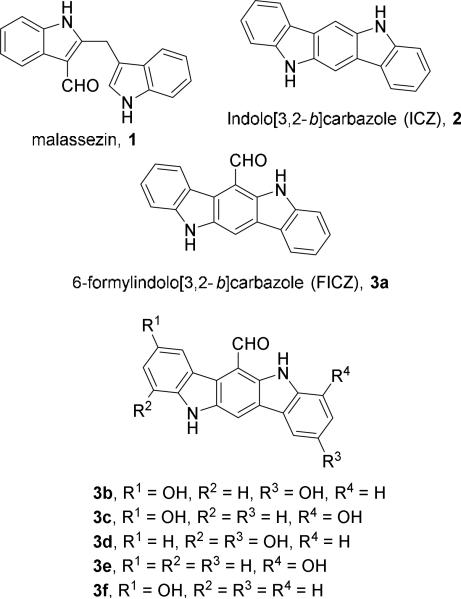
Diindolylmethanes are not only present in natural products such as malassezin4 but also important precursors for other naturally occurring heterocycles such as indolocarbazoles 2 and 3a-3f5 shown in Figure 1.6 Malassezin, ICZ, and FICZ are potent agonists of aryl hydrocarbon receptor (AhR), which is best known for mediating the toxicity of dioxin and related environmental toxins.7 Recent studies showed that AhR also played a critical role in immune cell differentiation,8 promoting intestinal immune function,9 and the development of prostate.10 It has been demonstrated that selective AhR modulators inhibit prostate tumor metastasis11 and have anti-asthmatic effects12 in animal models. Indolo[3,2-b]carbazoles are also important class of organic electroluminescent compounds.6,13
Figure 1.
Diindolylmethanes and Indolo[3,2-b]carbazoles
Rearrangement of the symmetrical 3,3’-diindolylmethanes to 2,3-diindolylmethanes could be realized using iodine as the catalyst.14 Low yields, however, were observed when substituted indoles were employed as the substrates. Synthesis of non-symmetric 2,3-diindolylmethanes requires the joining of two different indoles in multiple steps.15
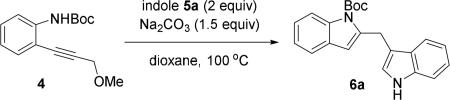
A metal carbene intermediate was generated from annulation of propargylic ether 4 and trapped previously by a diene in a (4+3) cycloaddition.3a We envisioned that this metal carbene intermediate could also be trapped by other nucleophiles. In the presence of another indole, diindolylmethanes could then be prepared conveniently. We first examined the conditions that were employed previously (entries 1 and 2, Table 1). We were pleased to find that both Pt- and Rh-complexes promoted the formation of 2,3’-diindolylmethane product 6a, though the former provided a higher yield. A slightly lower yield was obtained with lower catalyst loading (entry 3). The electron-poor phosphine ligand was proved to be critical in previous indole annulation/(4+3) cycloaddition cascade. In the case of indole annulation/arylation to form diindolylmethanes, using PtCl2 alone as the catalyst appeared to be sufficient (entry 4). The yield again became slightly lower if the amount of catalyst was lowered to 5 mol% (entry 5). Other metal complexes did not produce any desired product (entries 6-10).
Table 1.
Screening of Catalysts and Conditionsa
| ||
|---|---|---|
| entry | conditions | yield (%) |
| 1 | PtCl2 (10 mol %), P(C6F5)3 (20 mol %) | 84 |
| 2 | [Rh(CO)2Cl]2 (10 mol %), P[OCH(CF3)2]3 (20 mol %) | 56 |
| 3 | PtCl2 (5 mol %), P(C6F5)3(10 mol %) | 77 |
| 4 | PtCl2 (10 mol %) | 83b |
| 5 | PtCl2 (5 mol %) | 76 |
| 6 | AgBF4 (10 mol %) | 0 |
| 7 | AgOTf (10 mol %) | 0 |
| 8 | CuOTf (10 mol %) | 0 |
| 9 | AgOTf (10 mol %), P(C6F5)3(20 mol %) | 0 |
| 10 | CuOTf (10 mol %), P(C6F5)3(20 mol %) | 0 |
Unless noted otherwise, the yield of 6a was determined by 1H NMR of crude product.
Isolated yield.
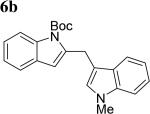
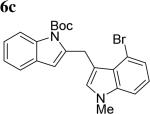
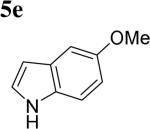
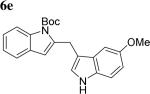
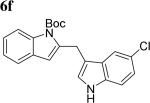
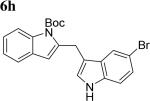
The scope of different indoles was examined for this tandem indole annulation/arylation cascade using propargylic ether 4 as the starting material (Table 2). N-Methyl indole 5b afforded a high yield of the 2,3’-diindolyl methane product 6b. The yield became 20% lower for 4-Br substituted N-methylindole 5c. Interestingly, the 4-cyano substituted indole 5d did not interfere with the efficiency of the tandem reaction. The indole annulation/arylation reaction could also tolerate electron-donating methoxy or halogen substituents on the 5- or 6-position of the indole (e.g. 5e, 5f, 5g, and 5h).
Table 2.
Scope of Indoles for Pt-Catalyzed Tandem Indole Annulation/Arylation of Propargylic Ether 4a
| indole substrates | products | yield(%)b |
|---|---|---|

|
|
88 |

|
|
68 |

|

|
81 |
|
|
77 |

|
|
84 |

|

|
85 |
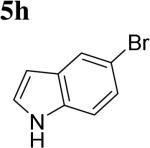
|
|
82 |

|

|
80 |

|

|
60 |
Conditions: 4 (1 equiv), indole 5 (2 equiv), PtCl2 (10 mol %), Na2CO3 (1.5 equiv), 100 °C, dioxane.
Isolated yield.
We then studied the effect of substituent on the 2- and 3-position of indole 5. With a 2-phenyl substituent on indole 5i, the yield of product 6i is comparable to 6a. When the intrinsic more reactive 3-position of the indole is blocked in substrate 5j, 2,2’-diindolylmethane was obtained in 60% yield.
The scope of aniline and propargylic ether was also investigated with different substituents (Table 3). We focused on the para-position of the aniline to examine how the change of pKa of the aniline influenced the efficiency of the reaction. To our delight, anilines with either an electron-donating methoxy group or an electron-withdrawing ester group on the para-position participated in the tandem reaction. Aniline 7a with an electron-donating para methoxy group provided a higher yield of the desired product than the one with a para electron-withdrawing group (7b). A moderate 55% yield was observed when aniline 7c with a free hydroxyl group was employed as the substrate. Secondary propargylic ether 7d could also participated in the tandem reaction and afforded diindolylethane 8d.
Table 3.
Scope of Aniline and Propargylic Ether for Pt-Catalyzed Tandem Indole Annulation/Arylationa
| indole substrates | products | yieldb |
|---|---|---|
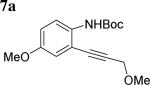
|

|
77% |
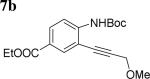
|

|
62% |

|

|
55% |
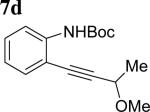
|
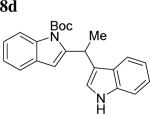
|
70% |
See Table 1 for conditions.
Isolated yield.
We next examined nucleophiles besides indoles. We were pleased to find that N-methylpyrrole could also serve as the nucleophile and product 9 was isolated in 68% yield (eq 1). We previously reported that various substituted furans underwent tandem indole annulation/(4+3) cycloaddition with substrate 4.3 When 1,3-dimethoxybenzene or thiophene was employed, a complex mixture was obtained.
 |
(1) |
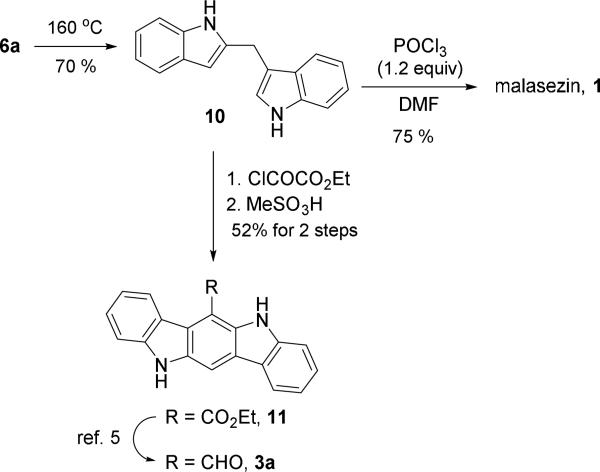
To demonstrate the utility of the Pt-catalyzed indole annulation/arylation method, we finished the synthesis of natural product malassezin and a formal synthesis of natural product FICZ as shown in Scheme 1. Removal of the Boc-protecting group in product 6a under thermal conditions yielded diindolylmethane 10, which could undergo formylation to afford natural product malassezin 1.4a Acylation of diindolylmethane 10 followed by acid-mediated cyclization produced indolo[3,2-b]carbazole 11, which has been converted to natural product FICZ 3a through a known sequence of reduction and oxidation.5 The spectroscopic data of compounds 1, 10 and 11 are all in accordance with literature.17 The structure of 2,3’-diindolylmethane 6a is then further confirmed.
Scheme 1.
Synthesis of Malassezin and FICZ
The proposed mechanism for the indole annulation/arylation is shown in Scheme 2. After the coordination of the metal catalyst to propargylic ether 4, 5-endo-cyclization of metal complex 12 will lead to the formation of indole intermediate 13. Elimination of a methanol can then produce metal carbene intermediate 14, which has been proposed previously by us and others.3a,16 This electrophilic metal carbene is then captured by an indole nucleophile to form adduct 15. Protonation and re-aromatization can then lead to final diindolylmethane product 5a. A (3+2) cycloaddition between metal carbene 14 and vinyl ethers has been reported.16a In our case, we did not observe any (3+2) cycloaddition product between metal carbene 14 and indole.
Scheme 2.
Proposed Mechamism
In summary, we have developed an efficient method for the synthesis of various highly substituted 2,3’-diindolylmethanes. The indole annulation event is accompanied by the coupling of two indole units. An electrophilic platinum carbene intermediate was proposed to be involved in this cascade reaction. We also demonstrated that the method can be applied to the synthesis of natural products malassezin and indolo[3,2-b]carbazoles.
Supplementary Material
Acknowledgment
We thank the NIH (R01GM088285) and the University of Wisconsin for financial support.
Footnotes
Supporting Information Available 1H NMR, 13C NMR, IR, HRMS for starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews on indole synthesis, see: Barluenga J, Rodriguez F, Fananas FJ. Chem. Asian J. 2009;4:1036. doi: 10.1002/asia.200900018.Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195. doi: 10.1016/j.tet.2011.06.040.Vicente R. Org. Biomol. Chem. 2011;9:6469. doi: 10.1039/c1ob05750b.Platon M, Amardeil R, Djakovitch L, Hierso J-C. Chem. Soc. Rev. 2012;41:3929. doi: 10.1039/c2cs15350e.
- 2.For recent examples of indole annulations, see: Cui X, Li J, Fu Y, Liu L, Guo Q-X. Tetrahedron Lett. 2008;49:3458.Oda Y, Hirano K, Satoh T, Miura M. Org. Lett. 2012;14:664. doi: 10.1021/ol203392r.Ackermann L, Lygin AV. Org. Lett. 2012;14:764. doi: 10.1021/ol203309y.Inamoto K, Asano N, Nakamura Y, Yonemoto M, Kondo Y. Org. Lett. 2012;14:2622. doi: 10.1021/ol300958c.Xia X-F, Wang N, Zhang L-L, Song X-R, Liu X-Y, Liang Y-M. J. Org. Chem. 2012;77:9163. doi: 10.1021/jo301741j.Song W, Ackermann L. Chem. Commun. 2013;49:6638. doi: 10.1039/c3cc43915a.
- 3.Shu D, Song W, Li X, Tang W. Angew. Chem. Int. Ed. 2013;52:3237. doi: 10.1002/anie.201209266. For a synthesis of cyclohepta[b]indoles by a different type of (4+3) cycloaddition, see: Han X, Li H, Hughes RP, Wu J. Angew. Chem. Int. Ed. 2012;51:10390. doi: 10.1002/anie.201205238.
- 4.a Wille G, Mayser P, Thoma W, Monsees T, Baumgart A, Schmitz HJ, Schrenk D, Polborn K, Steglich W. Bioorg. Med. Chem. 2001;9:955. doi: 10.1016/s0968-0896(00)00319-9. [DOI] [PubMed] [Google Scholar]; b Kramer HJ, Podobinska M, Bartsch A, Battmann A, Thoma W, Bernd A, Kummer W, Irlinger B, Steglich W, Mayser P. Chembiochem. 2005;6:860. doi: 10.1002/cbic.200400247. [DOI] [PubMed] [Google Scholar]
- 5.Wahlstrom N, Romero I, Bergman J. Eur. J. Org. Chem. 2004:2593. [Google Scholar]
- 6.Knolker HJ, Reddy KR. Chem. Rev. 2002;102:4303. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]; Schmidt AW, Reddy KR, Knoelker H-J. Chem. Rev. 2012;112:3193. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- 7.Denison MS, Soshilov AA, He G, Degroot DE, Zhao B. Toxicol. Sci. 2011;124:1. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Nature. 2008;453:65. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]; b Stevens EA, Mezrich JD, Bradfield CA. Immunology. 2009;127:299. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Cell. 2011;147:629. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]; b Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Science. 2011;334:1561. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 10.Mehta V, Vezina CM. Differentiation. 2011;82:211. doi: 10.1016/j.diff.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz WA, Lin T-M, Safe S, Moore RW, Peterson RE. Biochem. Pharmacol. 2009;77:1151. doi: 10.1016/j.bcp.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong K-T, Hwang S-J, Oh G-S, Park J-H. Int. Immunopharmacol. 2012;13:377. doi: 10.1016/j.intimp.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 13.a Gu R, Robeyns K, Van Meervelt L, Toppet S, Dehaen W. Org. Biomol. Chem. 2008;6:2484. doi: 10.1039/b807255h. [DOI] [PubMed] [Google Scholar]; b Boudreault PLT, Wakim S, Tang ML, Tao Y, Bao ZA, Leclerc M. J. Mater. Chem. 2009;19:2921. [Google Scholar]
- 14.a Gu R, Hameurlaine A, Dehaen W. Synlett. 2006:1535. [Google Scholar]; b Gu R, Hameurlaine A, Dehaen W. J. Org. Chem. 2007;72:7207. doi: 10.1021/jo0711337. [DOI] [PubMed] [Google Scholar]
- 15.a Tholander J, Bergman J. Tetrahedron Lett. 1998;39:1619. [Google Scholar]; b Tholander J, Bergman J. Tetrahedron. 1999;55:6243. [Google Scholar]; c Wahlstrom N, Stensland B, Bergman J. Synthesis. 2004:1187. [Google Scholar]
- 16.a Saito K, Sogou H, Suga T, Kusama H, Iwasawa N. J. Am. Chem. Soc. 2011;133:689. doi: 10.1021/ja108586d. [DOI] [PubMed] [Google Scholar]; b Allegretti PA, Ferreira EM. Org. Lett. 2011;13:5924. doi: 10.1021/ol202649j. [DOI] [PubMed] [Google Scholar]; c Allegretti PA, Ferreira EM. Chem. Sci. 2013;4:1053. [Google Scholar]
- 17.See Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.