Abstract
Purpose
Early-stage non-small cell lung cancer (NSCLC) incidence among older adults is expected to increase due to demographic trends and CT-based screening, yet optimal treatment in the elderly remains controversial. Using the SEER-Medicare cohort spanning 2001–2007, we compared survival outcomes associated with five strategies used in contemporary practice: lobectomy, sublobar resection, conventional radiation, stereotactic ablative radiotherapy (SABR) and observation.
Methods and Materials
Treatment strategy and covariates were determined in 10,923 patients age≥66 with stage IA-IB NSCLC. Cox regression, adjusted for patient and tumor factors, compared overall and disease-specific survival for the five strategies. In a second, exploratory analysis, propensity-score matching was used for comparison of SABR with other options.
Results
Median age was 75 years and 29% had moderate-to-severe comorbidities. Treatment distribution was lobectomy (59%), sublobar resection (11.7%), conventional radiation (14.8%), observation (12.6%), and SABR (1.1%). In Cox regression with median follow up of 3.2 years, SABR was associated with the lowest risk of death within six months of diagnosis (HR 0.48; 95%CI 0.38–0.63; referent is lobectomy). After six months, lobectomy was associated with the best overall and disease-specific survival. In the propensity-score matched analysis, survival after SABR was similar to lobectomy (HR 0.71; 95%CI 0.45–1.12). Conventional radiation and observation were associated with poor outcomes in all analyses.
Conclusions
In this population-based experience, lobectomy was associated with the best long-term outcomes in fit elderly patients with early-stage NSCLC. Exploratory analysis of SABR early-adopters suggests efficacy comparable to surgery in select populations. Evaluation of these therapies in randomized trials is urgently needed.
Introduction
Though advanced non-small cell lung cancer is associated with poor prognosis, early-stage presentations are potentially curable with five-year overall survival rates approaching 50% (1). In the United States, two public health developments will increase the burden of early lung cancer and strain limited health care dollars. First, the overall incidence of NSCLC among adults over 65 is expected to rise dramatically from a level of 163,000 in 2010 to 271,000 by 2030 due to demographic changes associated with population aging (2). Second, recent evidence showing a mortality benefit from computed tomography (CT) screening may lead to a rise of newly diagnosed early-stage (T1a-T2a N0) lung cancers as screening disseminates into routine care (3).
Patients with NSCLC are frequently older and experience a high burden of comorbid illness. Surgical resection for early-stage disease affords a high likelihood of cure, but is often precluded by comorbid illness that renders patients medically inoperable. New minimally invasive methods for thoracic surgery and a novel radiotherapy treatment, stereotactic ablative radiotherapy (SABR), promise to improve outcomes in elderly patients who previously would not have been candidates for curative surgical therapy. However, no phase III randomized data are available to guide integration of these newer therapies into treatment selection for the elderly.
Given the urgency of this health policy question and the lack of randomized data to guide therapy, we used the Surveillance, Epidemiology, and End Results (SEER)-Medicare cohort to identify patients older than 65 treated for early-stage NSCLC between 2001 and 2007, during which time all major contemporary treatment strategies were in use. We sought to determine the comparative effectiveness of lobectomy, sublobar resection, conventional radiation, SABR, and observation with respect to overall survival (OS) and lung cancer-specific survival (LCSS).
Methods
Data Source
The Surveillance, Epidemiology, and End Results (SEER)-Medicaredatabase captures clinical, pathological, and insurance claims data for incident cancers diagnosed in Medicare beneficiaries who reside within one of 16 geographic catchment areas that account for 26% of the US population. The case ascertainment rate for the SEER data is approximately 98% (4). In this study, demographic and tumor characteristics for incident malignancies diagnosed from January 1, 2001 to December 31, 2007 were linked to Medicare claims for treatment and outcomes from January 1, 2000 to December 31, 2009.
Study Sample
From 2001–2007, 168,475 patients aged ≥ 66 years without prior malignancy were diagnosed with lung cancer and reported in the SEER-Medicare cohort. To facilitate use of Medicare billing claims, patients with inadequate Medicare records were excluded from the study as were patients with any second cancer diagnosed within 120 days of the index lung cancer, as billing records could not discriminate between procedures performed for the index cancer versus the second cancer (Table e1). Other exclusion criteria included histologies other than NSCLC, tumors larger than 5 cm, distant metastases or nodal disease at presentation, absence of pathologic confirmation, and the use of non-standard therapies for early-stage NSCLC (chemotherapy, radiofrequency ablation, pneumonectomy, multimodality therapy). To ensure that SABR was not directed at intracranial targets, we excluded patients with diagnosis codes for brain metastasis. These criteria yielded a final sample of 10,923 patients (Table e1).
Outcome
OS was determined from Medicare records with follow-up through May 2010. LCSS was determined using cause of death data abstracted from death certificates and reported by SEER with follow up through December 2007. In the United States, the observed sensitivity and specificity of death certificates for reporting lung cancer as the cause of death have been recently reported as approximately 89 and 99 percent, respectively (5).
Treatment Strategies
Lung surgery was determined from SEER and Medicare claims and classified as lobar and sublobar resection (Table e2). The definitive surgery was defined as the most extensive surgical procedure reported by SEER or Medicare during the first four months following diagnosis. SABR use was extracted from Medicare claims using International Classification of Diseases, 9th Revision, Clinical Modification codes 92.3, 92.30–92.39 and Current Procedural Terminology/Healthcare Common Procedure Coding System (CPT) codes 77373, G0173, G0251, G0339, G0340, 61793, and 0082T (Table e3). We classified patients as having received SABR if the code indicated delivery of radiosurgery (as opposed to planning or management). Conventional radiation was defined as radiation treatment other than SABR (Table e2).
Other Covariates
Tumor characteristics extracted from the SEER data included size, histology, grade, and location within the lung. Claims were used to identify patients who underwent positron emission tomography as part of their diagnostic evaluation from 2 months prior to 4 months after diagnosis (eTable 2). Demographic variables included year of diagnosis, age at diagnosis, race, median income of census tract or zip code, and percent of adults in census tract or zip code with some college education. Race was dichotomized as white or non-white because approximately 90% of the study population was white. A modified Charlson comorbidity index using the Klabunde modification was calculated with Part A and Part B claims spanning a pre-diagnosis interval of 12 months to 1 month, with scores of 2 or more indicating moderate to severe comorbidity (6).
Statistical Analysis
Baseline characteristics across the five treatment strata were compared with Pearson’s χ2 test. Unadjusted survival rates by covariate strata were determined using the Kaplan-Meier method and differences across strata were assessed using the log-rank test. Cox regression determined the associations of treatment strategy with OS and LCSS adjusted for prespecified, clinically relevant patient, tumor, and treatment characteristics. The proportional hazards assumption was assessed by visual inspection of the log-log plots and suspected violations were confirmed by testing the significance of a time-interaction variable (7). Changing care patterns over time, for example increased use of lung cancer screening, could potentially bias comparisons of treatments used more commonly in recent years, such as SABR, to treatments used more commonly in earlier years, such as lobectomy. To account for this possibility, we conducted a sensitivity analysis in which the multivariate analysis was limited to patients diagnosed in 2007. Because baseline covariate differences of the smaller SABR cohort were unlikely to have been adequately addressed by Cox regression, we performed a second, exploratory analysis wherein propensity-score matching was used to compare SABR patients with matched controls. Propensity scores were calculated using a logistic model with SABR versus the non-SABR treatment as the dependent variable and the independent variables being race, gender, education level, median income, comorbidity score, histology, tumor grade, tumor size, and receipt of lymph node sampling. Patients were matched 1:1 using an 8- to 1- digit greedy matching algorithm to avoid bias introduced by many-to-one matching (8). The maximum caliper distance allowed was 0.1. Differences in covariate strata by treatment group in the matched cohort were assessed using the McNemar χ2 test and the Wilcoxon ranked sum test for paired data. Covariate balance was also assessed with the standardized because it has been shown not to be influenced by sample size (9). P-value less than 0.05 and standardized difference less than 20% were used to indicate similarity in distributions of covariates (9). Cox regression stratified by matched pairs and adjusted for unbalanced covariates was used to compare survival between case and control cohorts. A propensity-score analysis comparing lobectomy with sublobar resection was also performed but is not shown because the findings were not substantively different from the multivariable Cox model.
Assuming 124 patients treated with SABR and 6,531 treated with lobectomy, accrual spanning 6 years with 2 additional years of follow-up, a median survival of 4 years in patients treated with lobectomy, and a true hazard ratio (HR) of 1.44 for patients treated with SABR, this study was able to reject the null hypothesis that lobectomy and SABR are associated with an equal risk of death over the long-term with power of 80%. The Type I error probability associated with this test of the null hypothesis is 0.05. All statistical analyses were 2-sided with P ≤ 0.05 and conducted using SAS v. 9.3 (Cary, NC). Our institutional review board granted this study exempt status.
Results
Baseline Characteristics and Unadjusted Outcomes
Among the 10,923 patients, median age was 75 years, 54.1% were female, and 29% had moderate-to-severe comorbidity. Treatment strategy was as follows: 6,531 lobectomy (58.9%), 1,277 sublobar resection (11.7%), 1,613 conventional radiation (14.8%), 1,378 supportive care (12.6%) and 124 SABR (1.1%). Nodal sampling to establish pathologic node negative status was accomplished in 94% of the lobectomy patients, 42% of the sublobar resection patients, and fewer than 10% of the non-surgical cohorts. Baseline characteristics are summarized in Table 1.
Table 1.
Characteristics of Patients with Early Stage NSCLC Stratified by Treatment
| Overall Cohort N = 10,923 | SABR | Conventional Radiation | Sublobar Resection | Lobectomy | Observation | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | P>X2 |
| Sociodemographics | |||||||
| Age, y | |||||||
| 66–69 | 1939 (18) | 11 (9) | 163 (10) | 234 (18) | 1408 (22) | 123 (9) | <.001 |
| 70–74 | 3043 (28) | 20 (16) | 338 (21) | 362 (28) | 2055 (31) | 28 (19) | |
| 75–79 | 3115 (29) | 29 (23) | 428 (27) | 392 (31) | 1907 (29) | 359 (26) | |
| ≥80 | 2826 (26) | 64 (52) | 684 (42) | 289 (23) | 1161 (18) | 628 (46) | |
| Race | |||||||
| White | >9790b (>90) | >11 (>90) | 1416 (88) | 1184 (93) | 5927 (91) | 1151 (84) | <.001 |
| Black/Other | <1135 (<10) | <11 (<10) | 197 (12) | 93 (7) | 604 (10) | 227 (16) | |
| Sex | |||||||
| Male | 5016 (46) | 49 (40) | 753 (47) | 571 (45) | 3011 (46) | 632 (46) | 0.52 |
| Female | 5907 (54) | 75 (60) | 860 (53) | 706 (55) | 3520 (54) | 746 (54) | |
| Comorbidity | |||||||
| 0 | 3940 (36) | 28 (23) | 387 (24) | 339 (27) | 2814 (43) | 372 (27) | <.001 |
| 1 | 3466 (32) | 42 (34) | 503 (31) | 447 (35) | 2042 (31) | 432 (31) | |
| ≥2 | 3151 (29) | 54 (44) | 652 (40) | 457 (36) | 1495 (23) | 493 (36) | |
| missing | 366 (3) | ||||||
| Educational Attainment of zip code or county | |||||||
| Quartile 1 | 2731 (25) | 34 (27) | 315 (20) | 323 (25) | 1781 (27) | 278 (20) | <.001 |
| Quartile 2 | 2738 (25) | 29 (23) | 396 (25) | 339 (27) | 1651 (25) | 323 (23) | |
| Quartile 3 | 2720 (25) | 36 (29) | 422 (26) | 323 (25) | 1597 (24) | 342 (25) | |
| Quartile 4 | 2734 (25) | 25 (20) | 480 (30) | 292 (23) | 1502 (23) | 435 (32) | |
| Median income of zip code or county | |||||||
| Quartile 1 | 2741 (25) | 27 (22) | 513 (32) | 284 (22) | 1494 (23) | 423 (31) | <.001 |
| Quartile 2 | 2741 (25) | 38 (31) | 423 (26) | 322 (25) | 1603 (25) | 355 (26) | |
| Quartile 3 | 2724 (25) | 31 (25) | 379 (23) | 340 (27) | 1627 (25) | 347 (25) | |
| Quartile 4 | 2717 (25) | 28 (23) | 298 (18) | 331 (26) | 1807 (28) | 253 (18) | |
| Tumor Characteristics | |||||||
| Tumor size | |||||||
| ≤ 2.0 cm | 4393 (40) | 48 (39) | 437 (27) | 820 (64) | 2723 (42) | 365 (26) | <.001 |
| 2.1 – 3.0 cm | 3595 (33) | 48 (39) | 576 (36) | 316 (25) | 2188 (34) | 467 (34) | |
| 3.1 – 5.0 cm | 2935 (27) | 28 (23) | 600 (37) | 141 (11) | 1620 (25) | 546 (40) | |
| Histology | |||||||
| NSCLC, NOS | 1389 (13) | 34 (27) | 475 (29) | 84 (7) | 373 (6) | 423 (31) | <.001 |
| Adenocarcinoma | 5763 (53) | 53 (43) | 500 (31) | 749 (59) | 3931 (60) | 530 (38) | |
| Squamous | 3361 (31) | 36 (29) | 580 (36) | 389 (30) | 1982 (30) | 374 (27) | |
| Grade | |||||||
| Low-intermediate | 5054 (46) | 31 (25) | 329 (20) | 690 (54) | 3722 (57) | 282 (20) | <.001 |
| High | 3477 (32) | 23 (19) | 453 (28) | 431 (34) | 2215 (34) | 355 (26) | |
| Unknown | 2392 (22) | 70 (56) | 831 (52) | 156 (12) | 594 (9) | 741 (54) | |
| Laterality | |||||||
| Right | 6354 (58) | 57 (46) | 941 (58) | 716 (56) | 3866 (59) | 774 (56) | <.001 |
| Left | 4567 (42) | 67 (54) | 672 (42) | 561 (44) | 2665 (41) | 602 (44) | |
| Subsite in lung | |||||||
| Upper lobe | 6599 (60) | 76 (61) | 995 (62) | 782 (61) | 3995 (61) | 751 (54) | <.001 |
| Middle lobe | 530 (5) | <11 (<10) | >73 (<10) | 39 (3) | 336 (5) | 71 (5) | |
| Lower lobe | 3461 (32) | 36 (29) | 468 (29) | 425 (33) | 2067 (32) | 465 (34) | |
| Bronchus | 45 (<1) | ||||||
| Other | 288 (3) | ||||||
| PET scanning | |||||||
| Not Perfomed | 5785 (53) | 32 (26) | 741 (46) | 684 (54) | 3407 (52) | 921 (67) | <.001 |
| Performed | 5138 (47) | 92 (74) | 872 (54) | 593 (46) | 3124 (48) | 457 (33) | |
| Treatment Characteristics | |||||||
| Number of lymph nodes sampled | |||||||
| 0 | 4160 (38) | >110 (>90) | 1552 (96) | 736 (58) | 432 (7) | 1321 (96) | <.001 |
| 1 or more | 6741 (62) | <11 (<10) | 55 (4) | 538 (42) | 6095 (93) | 49 (4) | |
| Unknown | 22 (<1) | ||||||
Abbrev: SABR (stereotactic ablative radiation); NSCLC, NOS (Nonsmall-cell lung cancer, not otherwise specified).
Exact figures not specified in some cells due to SEER-Medicare terms of use.
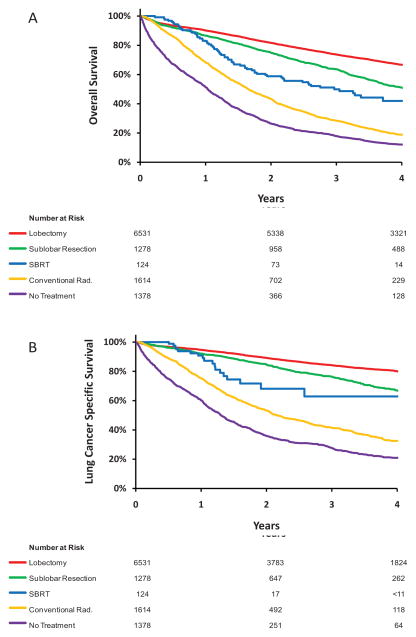
Unadjusted 30-day mortality was lowest for SBRT (0%) followed by conventional radiation (0.6%), sublobar resection (1.2%), lobectomy (1.3%), and observation (8.6%). At 90-days, unadjusted mortality was 0.8%, 4.1%, 4.1%, 5.6%, and 20.7%, respectively. At six months, unadjusted mortality was 4%, 6.5%, 7.8%, 13.8% and 32.8%, respectively (P<0.001). At two years, however, unadjusted mortality was lowest for lobectomy (18.3%), followed by sublobar resection (25.1%), SABR (41.1%), conventional radiation (56.7%) and observation (73.4%). Unadjusted survival curves are presented in Figure 1.
Figure 1.
Unadjusted Kaplan Meier curves for (A) overall survival and (B) lung cancer-specific survival stratified by treatment type.
Multivariable Analysis
Based on the statistical significance of the time-interaction terms, the proportional hazards assumption was violated for both the OS (p<0.001) and LCSS (p<0.001) models. Therefore, stratified models for the follow-up periods 0–6 months and after 6 months are presented: During the initial six months, SABR was associated with the lowest risk of death (adjusted hazard ratio [HR], 0.48; 95%CI, 0.38 to 0.63, P<0.001) when compared to the baseline modality, lobectomy (Table 2). After the initial six months, lobectomy was associated with the lowest risk of death. Sublobar resection was associated with a modestly increased risk of death (HR, 1.40; 95%CI, 1.28 to 1.54). SABR outcomes were similar to sublobar resection (P=0.51) while conventional radiation and observation were associated with poor outcomes (Table 2). Findings were similar for LCSS (Table 2). Similar findings were noted in a sensitivity analysis limited to patients diagnosed in 2007.
Table 2.
Proportional Hazards Model with Time-Dependent Variable for Treatment Categories.
| Overall Survival | Lung Cancer-Specific Survival | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | HR | 95%CI | P>X2 | HR | 95%CI | P>X2 |
| Treatment (t ≤ 6 Months) | ||||||
| Lobectomy (baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| Sublobar Resection | 0.95 | (0.86 to 1.05) | 0.31 | 1.07 | (0.91 to 1.25) | 0.42 |
| SABR | 0.48 | (0.38 to 0.63) | <.001 | 0.59 | (0.36 to 0.96) | 0.03 |
| Conventional XRT | 1.22 | (1.09 to 1.36) | <.001 | 1.65 | (1.39 to 1.95) | <.001 |
| Observation | 22.6 | (19.9 to 25.7) | <.001 | 28.8 | (23.8 to 34.6) | <.001 |
| Treatment (t > 6 Months) | ||||||
| Lobectomy (baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| Sublobar Resection | 1.40 | (1.28 to 1.54) | <.001 | 1.55 | (1.33 to 1.82) | <.001 |
| SABR | 1.56 | (1.21 to 2.00) | <.001 | 1.81 | (1.11 to 2.95) | 0.02 |
| Conventional XRT | 2.65 | (2.38 to 2.96) | <.001 | 3.50 | (2.96 to 4.13) | <.001 |
| Observation | 2.18 | (1.93 to 2.46) | <.001 | 3.01 | (2.51 to 3.60) | <.001 |
| Age | 1.02 | (1.02 to 1.03) | <.001 | 1.01 | (1.00 to 1.02) | 0.002 |
| Race | ||||||
| White | 1.00 | -- | -- | 1.00 | -- | -- |
| Black/Other | 0.98 | (0.89 to 1.08) | 0.70 | 0.95 | (0.82 to 1.09) | 0.51 |
| Gender | ||||||
| Male | 1.00 | -- | -- | 1.00 | -- | -- |
| Female | 1.27 | (1.21 to 1.34) | <.001 | 1.25 | (1.16 to 1.36) | <.001 |
| Charleson Comorbidity Score | ||||||
| 0 (Baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| 1 | 1.26 | (1.18 to 1.34) | <.001 | 1.16 | (1.05 to 1.27) | 0.002 |
| ≥2 | 1.59 | (1.50 to 1.70) | <.001 | 1.25 | (1.16 to 1.35) | <.001 |
| Missing | 1.23 | (1.07 to 1.41) | 0.003 | 1.03 | (0.84 to 1.25) | 0.80 |
| Size | ||||||
| 0.0–2.9 cm (Baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| 2.1 – 3.0 cm | 1.23 | (1.16 to 1.30) | <.001 | 1.40 | (1.27 to 1.54) | <.001 |
| 3.1 – 5.0 cm | 1.47 | (1.38 to 1.57) | <.001 | 1.82 | (1.65 to 2.00) | <.001 |
| Grade (High v. Other) | ||||||
| High | 1.00 | -- | -- | 1.00 | -- | -- |
| Other | 1.07 | (1.01 to 1.13) | 0.02 | 1.11 | (1.01 to 1.21) | 0.03 |
| Nodal Sampling | ||||||
| Performed | 1.00 | -- | -- | 1.00 | -- | -- |
| Not Performed | 0.80 | (0.73 to 0.88) | <.001 | 0.77 | (0.67 to 0.89) | <.001 |
| Histology | ||||||
| NSCLC, nos (Baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| Adenocarcinoma | 0.92 | (0.85 to 0.99) | 0.03 | 0.88 | (0.79 to 0.99) | 0.03 |
| Squamous carcinoma | 1.12 | (1.04 to 1.22) | 0.003 | 1.08 | (0.97 to 1.20) | 0.17 |
| Large cell | 1.02 | (0.89 to 1.17) | 0.82 | 1.04 | (0.86 to 1.26) | 0.72 |
| PET Imaging | ||||||
| Not Performed | 1.00 | -- | -- | |||
| Performed | 0.91 | (0.87 to 0.96) | <.001 | 0.84 | (0.78 to 0.91) | <.001 |
| Income | ||||||
| First Quartile (Baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| Second Quartile | 1.04 | (0.97 to 1.12) | 0.29 | 1.04 | (0.93 to 1.16) | 0.49 |
| Third Quartile | 0.99 | (0.91 to 1.07) | 0.77 | 0.94 | (0.83 to 1.06) | 0.32 |
| Fourth Quarile | 0.95 | (0.86 to 1.06) | 0.36 | 0.89 | (0.76 to 1.03) | 0.12 |
| Educational Level | ||||||
| First Quartile (Baseline) | 1.00 | -- | -- | 1.00 | -- | -- |
| Second Quartile | 1.01 | (0.93 to 1.09) | 0.84 | 1.07 | (0.95 to 1.21) | 0.28 |
| Third Quartile | 1.05 | (0.96 to 1.14) | 0.28 | 1.07 | (0.93 to 1.22) | 0.34 |
| Fourth Quartile | 1.15 | (1.04 to 1.27) | 0.005 | 1.15 | (0.99 to 1.34) | 0.07 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SABR, stereotactic ablative radiation; XRT, radiation; NSCLC, NOS, non-small cell lung cancer, not otherwise specified; PET, positron emission tomography
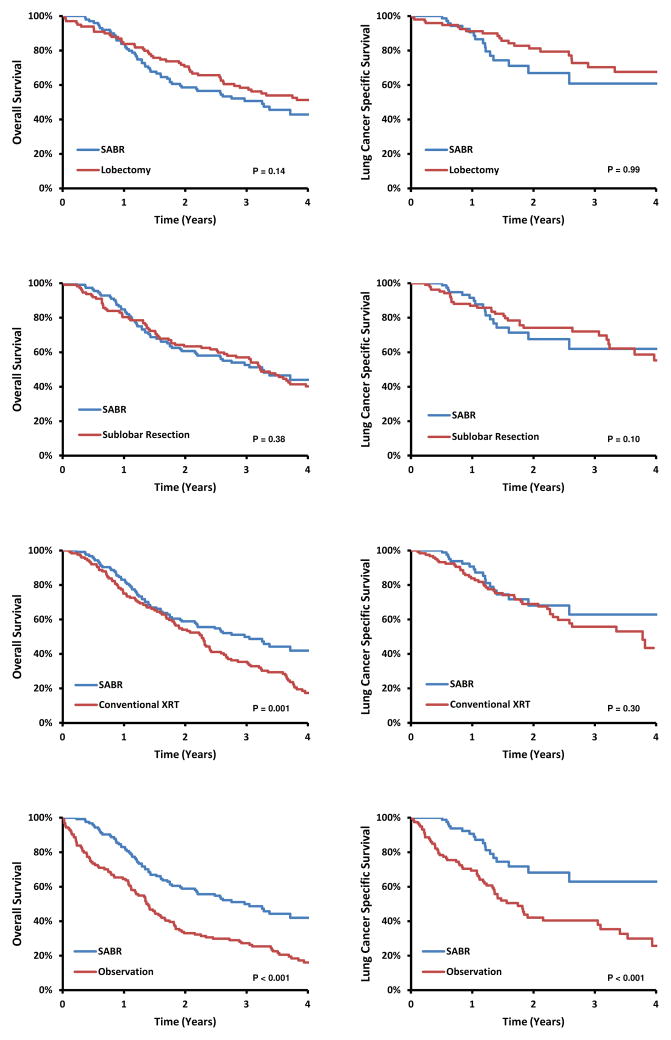
Matched Comparison of SABR with other Strategies
The majority of SABR case patients were successfully matched to lobectomy, sublobar resection, conventional radiation, and observation control patients, respectively (Table 3). The paired cohorts were well-balanced with the exception of modest differences in comorbidity score when comparing SABR with observation (Table 3). Using SABR as the referent, OS and LCSS were not significantly different between lobectomy and SABR (OS HR, 0.71; 95%CI 0.45–1.12, P=0.14; LCSS HR 1.00, 95%CI, 0.40–2.52, P=0.99) or between sublobar resection and SABR (OS HR 0.82, 95%CI, 0.53–1.27, P=0.38; LCSS HR 2.14, 95%CI, 0.87–5.26, P=0.10) (Table 4). SABR was associated with significantly better overall survival than either conventional radiation or observation (Table 4).
Table 3.
Baseline Characteristics of Propensity-Matched SBRT Patients and Non-SBRT Control Patients
| Non-SBRT Control Cohort | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobectomy | Sublobar Resection | Conventional XRT | Observation | |||||||||||||
|
|
||||||||||||||||
| Variable | SBRT N=99 | Control N=99 | Pa | SD (%) | SBRT N=112 | Control N=112 | P | SD (%) | SBRT N=124 | Control N=124 | P | SD (%) | SBRT N=124 | Control N=124 | P | SD (%) |
| Age | ||||||||||||||||
| Mean ± SD | 78.1±6.2 | 78.2±5.7 | 0.23 | 2.21 | 78.8±6.2 | 78.6±5.5 | 0.78 | −4.24 | 79.3±6.3 | 80.9±6.2 | 0.27 | 25.30 | 79.3±6.3 | 79.5±6.3 | 0.59 | 2.95 |
| Median (Range) | 78 (66,90) | 78 (66,94) | 79 (66,90) | 78 (66,91) | 80 (66,91) | 81 (66,95) | 80 (66,91) | 80 (66,94) | ||||||||
| White | >85 | >85 | >.99 | 0.00 | >100 | >100 | >.99 | 0.00 | >100 | >100 | 0.61 | −7.59 | >100 | >100 | >.99 | 0.00 |
| Male Gender | 59 | 65 | 0.45 | 10.30 | 70 | 66 | 0.67 | −5.96 | 75 | 73 | 0.90 | −2.68 | 75 | 77 | 0.90 | 2.72 |
| Comorbidity | ||||||||||||||||
| 0 | 26 | 24 | 0.87 | −3.81 | 26 | 22 | 0.64 | −7.18 | 28 | 29 | >.99 | 1.57 | 28 | 29 | >.99 | 1.57 |
| 1 | 32 | 34 | 0.88 | 3.49 | 40 | 35 | 0.58 | −7.77 | 42 | 48 | 0.50 | 8.19 | 42 | 39 | 0.78 | −4.23 |
| ≥2 | 41 | 41 | >.99 | 0.00 | 46 | 55 | 0.29 | 13.20 | 54 | 47 | 0.43 | −9.44 | 54 | 56 | 0.90 | 2.64 |
| Tumor Size | ||||||||||||||||
| Mean ± SD | 25.1±9.9 | 25.9±10.0 | 0.69 | 8.64 | 22.6±10.3 | 24.5±9.8 | 0.78 | 18.35 | 24.9±9.6 | 26.4±9.9 | 0.62 | 15.15 | 24.9±9.6 | 27.3±10.2 | 0.08 | 24.42 |
| Median (Range) | 25 (9,50) | 25 (10,50) | 21 (7,50) | 22 (9,50) | 23.5 (9,50) | 25 (4,50) | 23.5 (9,50) | 26 (5,50) | ||||||||
| High Grade | 70 | 67 | 0.74 | −5.34 | 83 | 87 | 0.61 | 6.88 | 93 | 106 | 0.05 | 22.45 | 93 | 96 | 0.78 | 4.67 |
| Nodal Sampling Done | <11 | <11 | >.99 | 3.89 | <11 | <11 | >.99 | 3.64 | <11 | <11 | >.99 | 0.00 | <11 | <11 | >.99 | −4.09 |
| PET Staging Done | 73 | 69 | 0.63 | −7.28 | 80 | 83 | 0.73 | 4.94 | 92 | 90 | 0.86 | −2.97 | 92 | 93 | >.99 | 1.52 |
| Histology | ||||||||||||||||
| NSCLC, NOS | 18 | 23 | 0.49 | 10.05 | 26 | 29 | 0.76 | 5.06 | 34 | 36 | 0.89 | 2.91 | 34 | 35 | >.99 | 1.47 |
| Adenocarcinoma | 48 | 46 | 0.88 | −3.30 | 52 | 47 | 0.58 | −7.37 | 53 | 51 | 0.89 | −2.67 | 53 | 50 | 0.79 | −4.02 |
| Squamous | >30 | >30 | 0.88 | −3.57 | >30 | >30 | >.99 | 1.60 | >30 | >30 | >.99 | 1.45 | >30 | >30 | >.99 | 1.45 |
| Large cell | <11 | <11 | >.99 | −14.28 | <11 | <11 | >.99 | 6.07 | <11 | <11 | >.99 | 12.78 | <11 | <11 | >.99 | 5.68 |
| Income | ||||||||||||||||
| First Quartile | 24 | 26 | 0.88 | 3.78 | 22 | 25 | 0.74 | 5.34 | 27 | 25 | 0.86 | −3.25 | 27 | 27 | >.99 | 0.00 |
| Second Quartile | 30 | 27 | 0.77 | −5.50 | 34 | 37 | 0.74 | 4.69 | 38 | 37 | >.99 | −1.44 | 38 | 43 | 0.59 | 6.99 |
| Third Quartile | 24 | 24 | >.99 | 0.00 | 31 | 28 | 0.77 | −5.00 | 31 | 28 | 0.77 | −4.67 | 31 | 22 | 0.20 | −14.83 |
| Fourth Quartile | 21 | 22 | >.99 | 1.99 | 25 | 22 | 0.75 | −5.42 | 28 | 34 | 0.45 | 9.04 | 28 | 32 | 0.64 | 6.12 |
| Educational Level | ||||||||||||||||
| First Quartile | 25 | 25 | >.99 | 0.00 | 31 | 29 | 0.89 | −3.31 | 34 | 37 | 0.78 | 4.35 | 34 | 32 | 0.88 | −2.98 |
| Second Quartile | 23 | 24 | >.99 | 1.93 | 25 | 25 | >.99 | 0.00 | 29 | 27 | 0.87 | −3.18 | 29 | 29 | >.99 | 0.00 |
| Third Quartile | 30 | 27 | 0.76 | −5.50 | 34 | 33 | >.99 | −1.61 | 36 | 33 | 0.77 | −4.43 | 36 | 36 | >.99 | 0.00 |
| Fourth Quartile | 21 | 23 | 0.87 | 3.95 | 22 | 25 | 0.76 | 5.34 | 25 | 27 | 0.85 | 3.21 | 25 | 27 | 0.86 | 3.21 |
Abbreviations: SD, standardized difference; XRT, radiation; SBRT, stereotactic body radiotherapy; NSCLC, NOS, non-small cell lung cancer, not otherwise specified.
P-values derived from McNemar’s exact test for categorical variables and the Wilcoxon ranked sign test for continuous variables.
Exact figures not specified in some cells due to Surveillance, Epidemiology, and End Results (SEER)-Medicare terms of use.
Table 4.
Proportional Hazards Models for Propensity Matched Pairs of SABR Cases and Non-SABR Controls.
| Overall Survival | Lung Cancer-Specific Survival | |||||
|---|---|---|---|---|---|---|
| Comparison | HR | 95%CI | P>X2 | HR | 95%CI | P>X2 |
| Lobectomy v. SABR1 | 0.71 | (0.45–1.12) | 0.14 | 1.00 | (0.40–2.52) | >.99 |
| Sublobar Resection v. SABR | 0.82 | (0.53–1.27) | 0.38 | 2.14 | (0.87–5.26) | 0.10 |
| Conventional XRT v. SABR | 1.97 | (1.31–2.96) | 0.001 | 1.56 | (0.67–3.59) | 0.30 |
| Adj for Age and Grade | 1.96 | (1.28–3.00) | 0.002 | 1.59 | (0.67–3.80) | 0.30 |
| Observation v. SABR | 2.10 | (1.37–3.08) | <.001 | 3.88 | (1.78–8.43) | <.001 |
| Adj for Tumor Size | 2.03 | (1.34–3.07) | <.001 | 3.90 | (1.76–8.61) | <.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; adj, adjustment; SABR, stereotactic ablative radiation; XRT, radiation.
SABR is the referent group for all comparisons.
Discussion
The median age of patients with NSCLC is 70 years (10), and the most prevalent risk factor is chronic smoking which is associated with many systemic medical conditions including chronic obstructive pulmonary disease and coronary artery disease. This combination of advanced age and comorbid illness poses therapeutic challenges and increases the morbidity and mortality risks of treatment. In the absence of randomized data, clinical decision-making for the rising number of elderly patients can be swayed by a temptation to de-escalate treatment to avoid treatment-related injury. However, lung cancer relapse due to inferior therapy also carries high costs for the patient and the health care system.
Currently, the 2012 National Comprehensive Cancer Network guidelines recommend surgery for patients able to undergo an operation and conventionally fractionated radiotherapy or SABR for patients who are medically inoperable (11). Regarding the choice of surgery, the superiority of lobectomy over sublobar resection is based upon the North American Lung Cancer Study Group 821 trial. This trial assigned early-stage patients, a third of whom were nonelderly (<60 years), to either lobectomy or limited resection (12). A statistically significant improvement in local control was seen in the lobectomy arm, but the trial was not adequately powered to detect a difference in overall survival. Previous population-based cohort analysis likewise revealed no statistically significant differences in overall survival between these surgical options (13). This absence of survival benefit and a perception of greater safety has recently prompted interest in reintroducing sublobar resections as a standard of care for elderly patients.
In contrast to these prior analyses, our multivariable model suggests that lobectomy is associated with improved long-term OS and LCSS for patients older than 65 when compared to sublobar resection. Better patient selection, improved perioperative care in community centers, and dissemination of improved operative technology including video-assisted thoracoscopic surgery during the last decade may have resulted in fewer complications and better survival outcomes following lobectomy in the elderly. Supporting this premise, we observed no differences in post-operative outcomes between sublobar and lobar resection during the initial six month period. These results suggest that for most elderly patients, the benefit of lobectomy may extend to disease-specific and overall survival in addition to the better local control outcomes observed in randomized trials.
We identified 124 patients who underwent SABR in the early-adoption phase before 2007. In multivariable analysis, these patients were observed to have promising short-term outcomes, perhaps as a consequence of avoiding perioperative mortality. Over the long-term, mortality in this group may have been driven by baseline differences as these patients were mostly octogenarians with multiple co-morbidities (consistent with the practice of reserving SABR for medically inoperable patients). Therefore, in a second analysis, we more robustly adjusted for the baseline imbalances in the SABR cohort via propensity-score matching. This analysis revealed no statistically significant difference in OS or LCSS in the comparison of matched lobectomy and SABR patients. Likewise SABR was associated with outcomes similar to sublobar resection, in accordance with retrospective single-institution studies (11, 14).
An important observation is that most lobectomy patients in the matched analysis did not undergo pathological nodal evaluation to confirm stage I disease, in contrast to the broader lobectomy population. Because patients without nodal evaluation may harbor occult disease, stage migration may have accounted for the finding that SABR outcomes were inferior to lobectomy in the unmatched analysis but similar in the matched comparison. Another possibility is that lobectomy patients who did not undergo nodal dissection constituted a subset predisposed to poor outcomes. However, because SABR patients in this era were ostensibly selected because of expected poor outcomes, we do not believe this possibility undermines the premise that the two cohorts were balanced.
While these findings should be tempered by the small number of SABR patients, they provide a measure of support for SABR as an alternative to definitive surgical therapy among very elderly patients (>75 years) with comorbid illness, a group that accounts for up to one third of NSCLC patients (10). Moreover, SABR technique is now more sophisticated than in the study interval, and a reasonable hypothesis is that SABR outcomes are comparable to surgery for additional patient subsets. Unfortunately, randomized trials of surgery and SABR have been beset by poor enrollment, and one, the Dutch ROSEL study, has been terminated (15). We hope that the promising outcomes among the early-adopters of SABR observed in this study encourage stronger recruitment in such trials, particularly as over half of American radiation oncologists now deliver this treatment (16).
Finally, the findings regarding conventional radiation and observation bear mention as they pertain to the important public health issue of triaging patients ineligible for surgery. A previous SEER analysis of patients treated between 1988 and 2001 reported no impact on long-term overall survival when conventional radiation was compared to observation alone (17). In the contemporary period conventional radiation was associated with better outcomes in the first six months of therapy but we infer that this was driven by early death in the observation group rather than treatment efficacy. Interestingly, SABR was associated with superior outcomes when compared to these options in all analyses, which supports the trend toward SABR among medically inoperable patients who desire definitive therapy.
This analysis adds to a growing literature regarding utilization of SABR in the contemporary era. In addition to the single-institution studies mentioned previously (11, 14), two population-based analyses have been performed in the Netherlands. The first by Palma et al. was limited to 2 specialized centers with a study interval through 2007 (18). The second study by Haasbeek et al. extended the analysis to 4605 elderly in the entire Netherlands Cancer Registry with a study interval through 2009 (19). In both investigations, the authors found that the rates of patients not receiving any treatment fell while survival improved in patients undergoing radiotherapy. The authors concluded that SABR introduction accounted for these trends, but an important caveat is that individual treatment data was unavailable in these registries to separately analyze those receiving SABR versus those receiving conventional radiation. Our findings with respect to SABR in the United States are concordant with the Netherlands experience and provide evidence that SABR is responsible for the improved outcomes seen with modern radiotherapy.
Our study has several limitations. Confounders pertinent to the care of lung cancer patients including pulmonary function and performance status were not available for adjustment in our models. We conjecture that patients with the best pulmonary function underwent a surgical strategy. A second limitation is the relatively small sample size for the SABR cohort. This reflects the fact that SABR for primary lung tumors was introduced and slowly adopted between 2001 and 2007 with the inflection point of utilization occurring after 2007 (16). Updated SEER-Medicare data with more recent diagnosis years is expected soon and will be important to more definitively elucidate the comparative effectiveness of SABR versus other treatment strategies. Finally, despite the statistical adjustments performed in this study, it remains difficult to fully account for potential confounding by indication in population-based analyses (20). For this reason, prospective trials are still needed to verify the findings reported here.
In summary, our analysis of patients with early-stage NSCLC lung cancer in the contemporary period supports lobectomy as the optimal treatment strategy for fit older adults. Our findings also raise intriguing questions regarding the comparative effectiveness of SABR in certain patient subsets. Surgical intervention comes at the price of perioperative mortality and SABR may offer an alluring compromise, namely a lower risk of early periprocedural mortality with promising long-term survival outcomes.
Supplementary Material
Figure 2.
Overall survival and lung cancer-specific survival for propensity matched SABR cases and non-SABR cohorts.
Summary.
Comparative effectiveness of five treatment strategies (lobectomy, sublobar resection, conventional radiation, SBRT, and observation) with regard to overall and disease-specific survival was determined using the SEER-Medicare database. In Cox regression, SBRT was associated with superior outcomes in the periprocedural period while lobectomy was associated with the best outcomes over the long-term. In propensity-matched analysis, SBRT outcomes were not statistically different than lobectomy, suggesting that SBRT may offer lower morbidity without compromising efficacy in certain populations.
Acknowledgments
Dr Smith is supported by grants from the Cancer Prevention & Research Institute of Texas [Grant RP101207] and the Department of Health and Human Services National Cancer Institute [Grants CA16672, T32CA77050].
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflicts of Interest: A portion of this study was funded by a research grant from Varian Medical Systems (SR2011-00034954RG 01). This entity had no role in the study design, data analysis, or data interpretation. Dr. Welsh reports a compensated consultory role to Reflexion Medical. Other conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 5.Doria-Rose VP, Marcus PM. Death certificates provide an adequate source of cause of death information when evaluating lung cancer mortality: an example from the Mayo Lung Project. Lung Cancer. 2009;63:295–300. doi: 10.1016/j.lungcan.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 7.Klein JP, Moeschberger ML. Survival analysis techniques for censored and truncated data. New York: Springer; 2003. [Google Scholar]
- 8.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172:1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institutes. [Accessed on April 1, 2012];Cancer of the Lung and Bronchus - SEER Stat Fact Sheet. 2011 http://seer.cancer.gov/statfacts/html/lungb.html.
- 11.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2011;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg R, Rubinstein L. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of Thoracic Surgery. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 13.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (ROSEL) http://clinicaltrials.gov/ct2/show/NCT00687986.
- 16.Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005;128:1461–1467. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 18.Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 19.Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: A population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012 doi: 10.1093/annonc/mds081. [DOI] [PubMed] [Google Scholar]
- 20.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63:64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.