Abstract
Prader–Willi syndrome (PWS) is a genetic imprinting disorder characterized mainly by hyperphagia and early childhood obesity. Previous functional neuroimaging studies used visual stimuli to examine abnormal activities in the eating-related neural circuitry of patients with PWS. It was found that patients with PWS exhibited both excessive hunger and hyperphagia consistently, even in situations without any food stimulation. In the present study, we employed resting-state functional MRI techniques to investigate abnormal brain networks related to eating disorders in children with PWS. First, we applied amplitude of low-frequency fluctuation analysis to define the regions of interest that showed significant alterations in resting-state brain activity levels in patients compared with their sibling control group. We then applied a functional connectivity (FC) analysis to these regions of interest in order to characterize interactions among the brain regions. Our results demonstrated that patients with PWS showed decreased FC strength in the medial prefrontal cortex (MPFC)/inferior parietal lobe (IPL), MPFC/precuneus, IPL/precuneus and IPL/hippocampus in the default mode network; decreased FC strength in the pre-/postcentral gyri and dorsolateral prefrontal cortex (DLPFC)/orbitofrontal cortex (OFC) in the motor sensory network and prefrontal cortex network, respectively; and increased FC strength in the anterior cingulate cortex/insula, ventrolateral prefrontal cortex (VLPFC)/OFC and DLPFC/VLPFC in the core network and prefrontal cortex network, respectively. These findings indicate that there are FC alterations among the brain regions implicated in eating as well as rewarding, even during the resting state, which may provide further evidence supporting the use of PWS as a model to study obesity and to provide information on potential neural targets for the medical treatment of overeating.
Keywords: Prader, Willi syndrome, eating disorder, obesity, amplitude of low-frequency fluctuation, resting-state networks, functional MRI
INTRODUCTION
Approximately 90 million Americans are overweight or obese, and more than 400 000 deaths are related to obesity or its associated diseases per year in the USA (1). Although much attention has been given to obesity in the Western world (2), developing countries are not immune to the ‘globesity’ epidemic (3,4). As obesity is associated with an increased risk of morbidity and mortality, there is a sense of urgency to understand the processes contributing to this epidemic.
Studies utilizing both human and animal models have revealed different aspects of this complicated disease (5–12). A genetic imprinting disorder, Prader–Willi syndrome (PWS), results in profound hyperphagia and early-childhood-onset obesity caused by excessive and pathological overeating and reinforcement of food (13–20). It is important to note that PWS is one of very few human genetic models for the study of obesity. PWS has been associated with substance dependence and has been chosen as a well-defined genetic model as it may help to explain certain neurophysiological mechanisms that affect appetite and food addiction (20,21). Thus, this model can be investigated more comprehensively and applied to obesity research in a better way than using animal models of obesity, especially as a neuroimaging model (12).
Patients with PWS can be diagnosed genetically. About 70% of cases are caused by a paternal genetic deletion on chromosome 15 (15q11-13) and 25% are from a maternal uniparental disomy of chromosome 15 (22). The remaining 1–5% of cases of PWS result from certain imprinting defects, which have a 50% potential risk of reoccurring in future offspring (23–25). In patients diagnosed with PWS, specific brain genes, such as MAGEL2, MKRN3, NDN, SNURF-SNRPN and sno-RNA, are misrouted or lost, resulting in abnormal cortical development (26).
Individuals with PWS are characterized as having dolichocephaly, almond-shaped eyes, small mouth, small appendages, decreased muscle tone (27), infantile hypotonia, hypogonadia, short stature and early onset of obesity as a result of central dysfunction (around 18–36 months of age) (28). They also show major disturbances in appetite, sleep, breathing and metabolism regulation. The abnormal eating behavior of patients with PWS is manifested by delayed satiety, premature return of hunger after eating a meal, the seeking and hoarding of food and food-related objects and the ingestion of inanimate items (17). This irregular pattern of food consumption leads to symptoms displayed as excessive daytime drowsiness, poor ventilation, hypercapnia and dental cavities (29). In addition to the common propensity of overeating, compulsive and ritualistic behaviors, patients with PWS also show pronounced emotional volatility and a striking inability to control their emotions, which results in frequent temper outbursts (30).
Functional neuroimaging studies of obesity and/or eating disorders have been performed in patients with PWS using visual cues to investigate the abnormalities of eating-related neural circuitry (13–17,19,31–33). In response to high- versus low-calorie food stimulation, these studies mainly found that patients with PWS exhibited a delayed signal reduction after glucose administration which was located in the hypothalamus (HPAL), insula, ventromedial prefrontal cortex (VMPFC) and nucleus accumbens (NAc) (19); hyperactivity in the limbic and paralimbic regions that drive eating behavior [e.g. the amygdala (AMY)] and in regions that suppress food intake [e.g. the medial prefrontal cortex (MPFC)] (15,31); and increased activation in HPAL and the orbitofrontal cortex (OFC) (13,14,32), VMPFC (17) and bilateral middle frontal, right inferior frontal, left superior frontal and bilateral anterior cingulate cortex (ACC) (16,32).
Most of the aforementioned studies investigated the abnormalities of eating-related neural circuitry using food cues. Recently, Kullmann et al. (34) used resting-state functional MRI (RS-fMRI) to investigate the functional connectivity (FC) integrity of resting-state networks (RSNs) related to food intake in lean and obese subjects; García-García et al. (35) found that the FC strength of the putamen nucleus in the salience network was increased in the obese group during the resting state. The relationship between the abnormalities in patients with PWS and intrinsic or resting brain function involved in the regulation of food-related and reward processing has yet to be evaluated. In this study, we applied RS-fMRI to assess the baseline brain activity levels and to examine the FC of brain networks (36–38) in patients with PWS compared with their sibling control group. We focused on four RSNs that involve brain areas reported previously in food and reward processing. The default mode network (DMN) includes the MPFC, precuneus, hippocampus (HIPP), posterior cingulate cortex (PCC) and inferior parietal cortex (34–39). The second network, termed the core network, includes the ACC and bilateral insula, which are part of the primary gustatory cortex (40). The third network is the prefrontal lobe network, which includes the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and OFC. The prefrontal cortex is involved in inhibitory processes (13,17,19,31). The fourth network, termed the motor sensory network, includes the precentral and postcentral gyri (36,41,42).
Based on previous findings, we hypothesized that there are abnormalities which may contribute to overeating in patients with PWS during the resting state. These abnormalities alter the FC of the four RSNs (DMN, core, prefrontal and motor sensory), especially in brain regions known to be important in food and reward regulation.
METHODS
Subjects
Twenty-seven patients with PWS and 21 of their siblings participated in the study of both functional changes in the brain and neuroanatomical variability across their lifespan (43,44). Among them, 21 children with PWS (10 girls, 11 boys; mean age, 7.3 ±9.3years) and 18 healthy siblings of these patients with PWS (control group; 10 girls, 8 boys, p = 0.632; mean age, 11.1 ±8.4years, p = 0.184) were selected. Other participants were removed from the current analysis because of either excessive head motion or failure to perform functional scanning. The subjects with PWS were significantly more obese than controls, as assessed by the body mass index (BMI; mean BMI, 33.1 versus 24.8; p = 0.02). None of the patients with PWS were being treated with growth hormone or estrogen/androgen replacement at the time of MRI scanning. Molecular testing was performed on all subjects with PWS. Fourteen had a deletion of the chromosomal 15q11-13 region, whereas the remainder had maternal uniparental disomy of chromosome 15 (17). The overall research protocol was approved by the Institutional Review Board at the University of Florida, and informed consent was obtained from each participant or from his/her legal guardian.
Image acquisition
The experiments were carried out on a 3.0-T head-dedicated Siemens Allegra MRI scanner (Siemens, Munich, Germany). A set of T1-weighted, high-resolution structural images was acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with a matrix size of 512 × 512, TR = 1500 ms, TE = 4.38 ms, field of view of 240 mm × 240 mm, flip angle of 8° and 160 contiguous slices (1.1–1.4 mm thick). Then, a gradient echo T2*-weighted echo planar imaging (EPI) sequence was used to acquire resting-state functional images with the following parameters: TR = 3 s; TE = 25 ms; flip angle, 90°; matrix size, 64 × 64; field of view, 240 mm × 240 mm; in-plane resolution, 3.75 mm × 3.75 mm; 36 axial slices (3.8 mm thick with no gap). The scan for RS-fMRI lasted for 300 s (5 min), containing 100 brain volumes.
Image processing and measurement
All of the imaging data were preprocessed and analyzed using Statistical Parametric Mapping 5 (SPM5, http://www.fil.ion.uclac.uk/spm). The functional images first underwent slice-timing correction for within-scan acquisition time differences between slices, and were then realigned to the first volume to correct for interscan head motion. Next, we spatially normalized the realigned images to the standard EPI template and resampled them to a voxel size of 3 mm × 3 mm × 3 mm. Finally, the functional images were spatially smoothed with a Gaussian kernel of 6 mm × 6 mm × 6 mm full width at half-maximum (FWHM) to decrease spatial noise. After the linear trends had been removed, a band-pass filter between 0.01 and 0.08 Hz was applied to the data to remove the effects of very low-frequency drift and high-frequency noise using REST software (http://resting-fmri.sourceforge.net). Finally, the nuisance covariates, including head motion parameters, global mean signals, white matter signals and cerebrospinal fluid signals, were regressed and removed from the blood oxygenation level-dependent signals.
Amplitude of low-frequency fluctuation (ALFF) analysis and definition of regions of interest (ROIs)
The ALFF analysis was carried out using REST software (http://resting-fmri.sourceforge.net) to define the ROIs. The calculation procedure was the same as that reported in a previous study (45). Specifically, a filtered time series was transformed to the frequency domain with a fast Fourier transform, and thus the power spectrum was obtained. Because the power of a given frequency is proportional to the square of the amplitude of this frequency component, the square root was calculated at each frequency of the power spectrum and the averaged square root was obtained across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF measurement. For standardization, the ALFF of each voxel was further divided by the global mean of the ALFF values. Then, voxel-wise two-sample t-tests were employed to compare the differences in ALFF between the PWS and control groups using SPM5 software. The brain regions showing significant ALFF alterations were selected as a mask to define the ROIs (p < 0.05, false discovery rate corrected). The ALFF values of each ROI were then averaged across subjects in the PWS and control groups, respectively. The significant ALFF difference of each ROI between the two groups was detected by a two-sample t-test.
FC analysis
After obtaining the ROIs that showed significant ALFF differences between the two groups, an FC analysis was carried out. We registered the ROIs backward to the original space of each subject, and then the time series of each ROI from the resting-state scan was extracted. For a given pair of ROIs, the zero-lag temporal correlation between them was calculated on the voxel time series for all pair-wise combinations between X and Y, and then averaged (46). After averaging the strength across the subjects of the two groups, we obtained the FC value between ROIs within the four RSNs, respectively. The significant FC alteration of each pair-wise ROI between the two groups was detected by a two-sample t-test (p < 0.0001).
RESULTS
Altered resting-state ALFF in patients with PWS
Compared with the controls, the patients with PWS exhibited a significant difference in ALFF in the MPFC (Brodmann area, BA 9/10), ACC (BA 32/24), parahippocampus (BA 28/34), HIPP, AMY, insula, thalamus, caudate, HPAL, DLPFC, VLPFC, OFC, midbrain, pre- and postcentral gyri, the temporal gyri, inferior and superior parietal gyri, precuneus, cuneus and fusiform gyrus (p < 0.05, false discovery rate corrected). No significant difference was found in the PCC. Among these brain areas, the MPFC, ACC, insula, HIPP, DLPFC, VLPFC, OFC, IPL, precuneus, and pre- and postcentral gyri (Fig. 1 and Table 1) were chosen as the ROIs to be used in further FC analyses.
Figure 1.
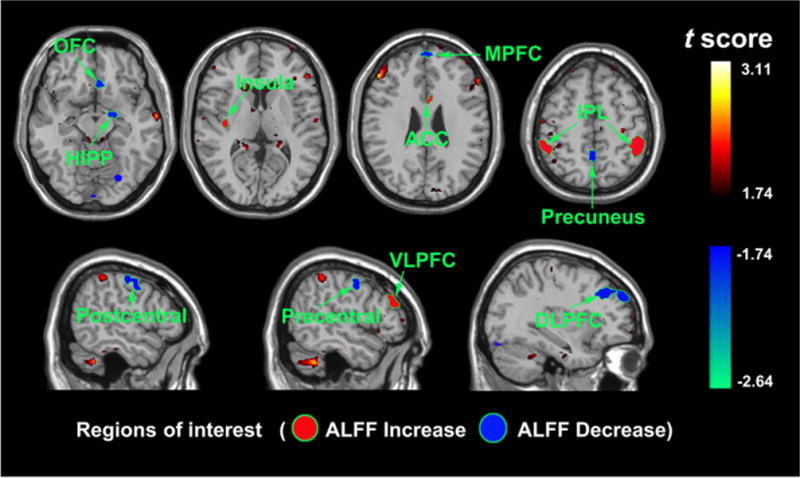
Functional mapping of the brain areas showing significant amplitude of low-frequency fluctuation (ALFF) alterations between the Prader–Willi syndrome (PWS) and control groups during the resting state. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; HIPP, hippocampus; IPL, inferior parietal lobe; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex.
Table 1.
Brain regions of interest (ROIs) showing significant amplitude of low-frequency fluctuation (ALFF) alterations between patients with Prader–Willi syndrome (PWS) and controls in the resting state. The results were obtained using two-sample t-tests (p < 0.05, false discovery rate corrected)
| ROI | Hemisphere | Brodmann area | Montreal Neurological Institute atlas
|
t value (max) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Medial prefrontal cortex | L | 9 | −3 | −24 | 63 | −3.07 |
| R | 9/10 | 6 | 54 | 42 | −2.91 | |
| Inferior parietal lobe | L | 40 | −51 | −42 | 48 | 2.31 |
| R | 40 | 51 | −39 | 54 | 2.63 | |
| Hippocampus | L | −30 | −36 | −9 | −2.08 | |
| R | 27 | −42 | 3 | −1.77 | ||
| Precuneus | L | 7 | −3 | −54 | 51 | −1.96 |
| R | 7 | 15 | −45 | 54 | −1.92 | |
| Anterior cingulate cortex | L | 32/24 | −9 | 39 | 15 | 2.86 |
| R | 32 | 6 | 9 | 24 | 3.11 | |
| Insula | L | −36 | −15 | 6 | 1.93 | |
| Dorsolateral prefrontal cortex | L | 9/46 | −38 | 49 | 31 | −2.23 |
| R | 9/46 | 37 | 50 | 33 | −2.89 | |
| Ventrolateral prefrontal cortex | L | 45 | −52 | 32 | 25 | 2.85 |
| R | 45 | 46 | 42 | 32 | 2.60 | |
| Orbitofrontal cortex | L | 10 | −34 | 62 | 5 | −1.76 |
| Precentral | L | 4/6 | −21 | −24 | 61 | −2.39 |
| R | 4/6 | 38 | −11 | 65 | −1.80 | |
| Postcentral | L | −47 | −31 | 51 | −1.92 | |
| R | 46 | −28 | 56 | −2.28 | ||
Further quantitative analyses of the mean ALFF values were calculated to compare the baseline brain activity level changes in these ROIs using a two-sample t-test (Fig. 2). Our results showed a trend of higher ALFF values in the IPL, ACC, insula and VLPFC, and lower ALFF values in the MPFC, HIPP, precuneus, pre- and postcentral gyri, DLPFC and OFC, in the PWS group relative to the controls. All of the regions showed statistically significant ALFF alterations between the two groups, except in the insula and DLPFC (p < 0.001).
Figure 2.
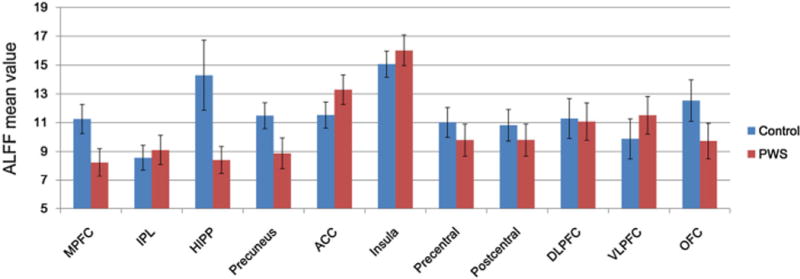
Comparison of the mean amplitude of low-frequency fluctuation (ALFF) values in each region of interest (ROI) between the Prader–Willi syndrome (PWS) and control groups. The mean ALFF values were obtained by cross-subject averaging for PWS (n = 21, red bar) and control (n = 18, blue bar) groups. Error bars denote the standard deviation. Two-sample t-tests showed that there existed significant ALFF alterations between the two groups in all of the regions (p < 0.001), except in the insula and DLPFC. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; HIPP, hippocampus; IPL, inferior parietal lobe; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex.
Altered FC in patients with PWS
Correlation values of the pair-wise ROIs from the two groups were obtained using an FC analysis. Compared with the control group, the PWS group showed decreased FC strength in the MPFC/IPL, MPFC/precuneus, IPL/precuneus and IPL/HIPP in the DMN; decreased FC strength in the pre-/postcentral gyri and DLPFC/OFC in the motor sensory network and prefrontal cortex network, respectively; and increased FC strength in the ACC/insula, VLPFC/OFC and DLPFC/VLPFC in the core network and prefrontal cortex network, respectively (p < 0.001) (Fig. 3). No significant FC alterations were observed in the MPFC/HIPP and HIPP/precuneus.
Figure 3.
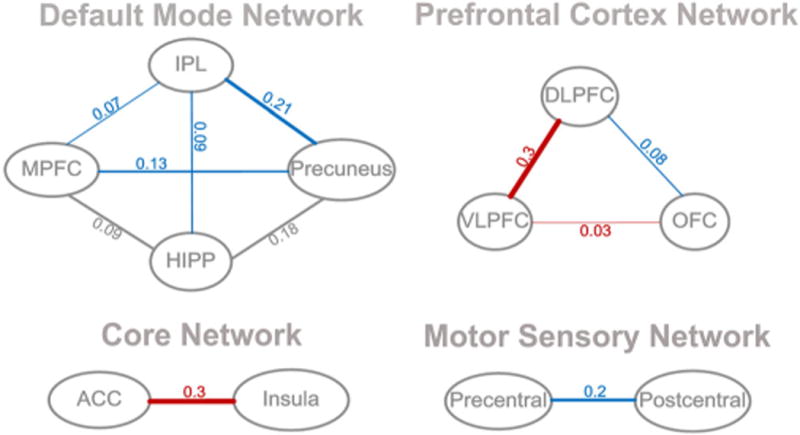
Functional connectivity between each pair-wise region of interest (ROI), showing the functional connectivity (FC) strength alterations from one brain region to the other. Compared with the control group, patients with Prader–Willi syndrome (PWS) showed decreased FC strength in the medial prefrontal cortex (MPFC)/inferior parietal lobe (IPL), MPFC/precuneus, IPL/precuneus and IPL/hippocampus (HIPP) in the default mode network (DMN) (blue); decreased FC strength in the pre-/postcentral gyri and dorsolateral prefrontal cortex (DLPFC)/orbitofrontal cortex (OFC) in the motor sensory network and prefrontal cortex network, respectively (blue); and increased FC strength in the anterior cingulate cortex (ACC)/insula, ventrolateral prefrontal cortex (VLPFC)/OFC and DLPFC/VLPFC in the core network and prefrontal cortex network, respectively (red, p < 0.001). No significant FC alterations were observed in the MPFC/HIPP and HIPP/precuneus.
DISCUSSION
The aim of this study was to explore the effects of PWS on the integrity of FC within the DMN, core, prefrontal lobe and motor sensory networks. In the present study, we used RS-fMRI to investigate the FC alterations of RSNs in children with PWS. We first used ALFF analysis to define the ROIs that showed significant alterations of resting-state brain activity levels in the patients. Then, based on the selected ROIs, we applied the FC method to characterize interactions among these brain regions. The evidence indicated that there were substantial differences during the resting state in the brain networks related to reward and food regulation compared with sibling controls.
In the DMN, patients with PWS showed decreased FC strength in the MPFC/IPL, MPFC/precuneus, IPL/precuneus and IPL/HIPP. The MPFC is closely connected to the OFC, which is involved in stimulus reward and evaluation in sync with direct multisensory input (47). Early reports on the neural mechanisms of hyperphagia noted strong MPFC activation in individuals with PWS (14,17,31,48). Hinton et al. (48) reported a lack of association between activation in the MPFC and ratings of the reward value of various foods in a group of individuals with PWS with deletion, suggesting that, although these individuals did not have higher ratings on food desirability compared with the control group, they displayed hyperactivity in regions associated with behavioral disinhibition and lack of self-control. The MPFC is also involved in the inhibition of emotion and regulation of appetitive drives. Results indicate that food motivation in obese individuals is associated with increased self-reported disinhibition and hunger, and increased activation in the ACC and MPFC, which is strongly implicated in motivational processing (49). There is a greater need for inhibitory control, and the activation in the MPFC is greater (50,51). This may be the reason why the FC strength between MPFC and other regions within the DMN decreased. Traditionally, HIPP is regarded as an important substrate for learning and memory. The consequences of the involvement of memory conditioning/habit circuits are that repeated consumption of large quantities of high-density food results in the formation of new linked memories, which condition the individuals to expect a pleasurable response, not only when exposed to the food, but also from exposure to stimuli conditioned to the food. These stimuli trigger automatic responses that frequently induce relapses in food bingeing (52). However, HIPP also participates in sensing the metabolic and hormonal status of the body (53), and in the regulation of food intake (54,55). In humans, HIPP stimulation generates autonomic and endocrine effects, such as gastric secretion (56) and increased food consumption (55). HIPP activation also occurs in a number of studies involving food-related stimuli, hunger and food craving (57–61).
In the core network, the insula and ACC showed increased FC strength in patients with PWS. The insular cortex is considered to be the primary gustatory cortex (62,63) and is also involved in motivational processes (64,65), representing an important relay of the neural circuitry connecting HPAL, OFC and the limbic system (66). It is possible that the delayed satiety response is the result of disturbance in the perception of inner physiological states. This would relate to the suggestion that people with PWS may have a problem with interoception (insula), or the perception of internal sensation (67). The insula is also known to receive gustatory, olfactory and visceral afferents involved in taste memory (68), and has been implicated in the experience of emotion (69). Activation in the insula has also been reported in studies using other methodologies (e.g. hunger) to manipulate the desire for food (70–72). Insular activation in response to food cues has also been found to be correlated with the desire to eat, as well as to hunger and prospective food intake. These findings indicate that the insula is a brain region that is important in the processing of food-related cues, both internal and external, and appears to be important in processing the motivational value of food and feeding. The ACC is involved in the executive control of internal and external stimuli regulating context-dependent behaviors (73–75). The ACC has both cognitive (e.g. working memory or attention) and affective functions. The affective subdivision is involved in the assessment of the salience of emotional information and in the regulation of emotional responses, and is connected to a number of other areas that show liking- or craving-related activation, including AMY, insula and HIPP (76). The ACC can contribute to an increased risk for overeating through an imbalance between cognitive and emotional processing.
In the prefrontal lobe network, the VLPFC/OFC and DLPFC/VLPFC exhibited increased FC strength, whereas the DLPFC/OFC showed decreased FC strength. The DLPFC is linked to numerous aspects of top-down control, especially ‘executive functions’, goal selection, planning, manipulation of information and response inhibition (77–79). Top-down processing in the lateral PFC allows regions such as the DLPFC to influence processing in other regions to obtain a common goal. This role in cognitive control might extend to decision-making related to food intake, as suggested by findings of increased DLPFC activity during satiation in a group of successful dieters (41). The DLPFC is also involved in working memory, including holding the reward value in mind whilst judging potential outcomes of a given set of behavioral responses (80). The VLPFC is involved in more cognitive aspects of emotional material. The findings of a positive correlation between increased heart rate deceleration and increasing activity in the VLPFC indicated that the more intense the orienting response, especially to food cues, the greater the activation of the VLPFC. The OFC is believed to play a role in stimulus-reinforcement learning (81), with extensive connections to AMY that have been implicated in affect-driven motivational behavior (31). In addition to the integration of information from multiple sensory inputs, the OFC serves a role in reward learning. The FC strength alteration of the OFC indicated dysfunction in the rewarding-learning circuit in the PWS group.
In addition, in the motor sensory network, the pre- and postcentral gyri showed decreased FC strength. These two regions drive the control of motor activity and are involved in the execution of gestural or graphic sequences in motor program elaboration, and therefore are involved in visually guided movements (82). Abnormal FC strength between the regions results in perceptive agnosia, particularly in incorrect perceptions of the consistency, size, weight and shape of objects. Patients with PWS showed a significant delay in the ‘block design scale’ of the Wechsler test, which targets the ability to combine visual and motor processes (16).
The present study has several limitations. First, PWS is a rare disease and the small sample size limits the generalization of our findings. As a result of the low prevalence of the disease, it is difficult to find large numbers of participants, particularly when recruiting for an fMRI study which requires strict inclusion criteria. In addition, although we balanced the gender, the age range among the patients with PWS and the control samples is still relatively large.
CONCLUSIONS
In the present study, we sought to investigate the abnormal brain networks in children with PWS using RS-fMRI. Our results demonstrated that patients with PWS showed decreased FC strength in the MPFC/IPL, MPFC/precuneus, IPL/precuneus and IPL/HIPP in the DMN; decreased FC strength in the pre-/postcentral gyri and DLPFC/OFC in the motor sensory network and prefrontal cortex network, respectively; and increased FC strength in the ACC/insula, VLPFC/OFC and DLPFC/VLPFC in the core network and prefrontal cortex network, respectively. These findings indicated that there were FC alterations among those brain regions implicated in eating as well as rewarding in patients with PWS during the resting state, which may provide further evidence supporting the use of PWS as a model to study food addiction and to provide information on potential neural targets for use in medical treatment for overeating.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant Nos. 60901064, 81271549, 60903127 and 61131003, the Fundamental Research Funds for the Central Universities and the 2010 Independent Investigator Award from the Brain and Behavior Research Foundation (NARSAD).
Abbreviations used
- ACC
anterior cingulate cortex
- ALFF
amplitude of low-frequency fluctuation
- AMY
amygdala
- BA
Brodmann area
- BMI
body mass index
- DLPFC
dorsolateral prefrontal cortex
- DMN
default mode network
- EPI
echo planar imaging
- FC
functional connectivity
- HIPP
hippocampus
- HPAL
hypothalamus
- IPL
inferior parietal lobe
- MPFC
medial prefrontal cortex
- MPRAGE
magnetization prepared rapid acquisition gradient echo
- NAc
nucleus accumbens
- OFC
orbitofrontal cortex
- PCC
posterior cingulate cortex
- PWS
Prader–Willi syndrome
- ROI
region of interest
- RS-fMRI
resting-state functional MRI
- RSN
resting state network
- VLPFC
ventrolateral prefrontal cortex
- VMPFC
ventromedial prefrontal cortex
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Gold MS. From bedside to bench and back again: a 30-year saga. Physiol Behav. 2011;104(1):157–161. doi: 10.1016/j.physbeh.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P. Diabetes and obesity worldwide – epidemics in full flight; Presented at the 60th Scientific Sessions of the American Diabetes Association; San Antonio, Texas. 10 June 2000. [Google Scholar]
- 4.von Deneen KM, Qin W, Tian J, Liu Y. Obesity in China: what are the causes? Curr Pharm Des. 2011;17(12):1132–1139. doi: 10.2174/138161211795656765. [DOI] [PubMed] [Google Scholar]
- 5.Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010;55(3):734–737. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30(9):1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- 7.Gold MS, Graham NA, Cocores JA. Food addiction? J Addict Med. 2009;3(1):42–45. doi: 10.1097/ADM.0b013e318199cd20. [DOI] [PubMed] [Google Scholar]
- 8.Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, Jacobs WS, Kadish W, Manso G. Refined food addiction: a classic substance use disorder. Med Hypotheses. 2009;72(5):518–526. doi: 10.1016/j.mehy.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, von Deneen KM, Kobeissy FH, Gold MS. Food addiction and obesity: evidence from bench to bedside. J Psychoactive Drugs. 2010;42(2):133–145. doi: 10.1080/02791072.2010.10400686. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3(1):8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, von Deneen KM, Tian J, Gold MS, Liu Y. Food addiction and neuroimaging. Curr Pharm Des. 2011;17(12):1149–1157. doi: 10.2174/138161211795656855. [DOI] [PubMed] [Google Scholar]
- 13.Dimitropoulos A, Blackford J, Walden T, Thompson T. Compulsive behavior in Prader–Willi syndrome: examining severity in early childhood. Res Dev Disabil. 2006;27(2):190–202. doi: 10.1016/j.ridd.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Dimitropoulos A, Schultz RT. Food-related neural circuitry in Prader–Willi syndrome: response to high- versus low-calorie foods. J Autism Dev Disord. 2008;38(9):1642–1653. doi: 10.1007/s10803-008-0546-x. [DOI] [PubMed] [Google Scholar]
- 15.Holsen LM, Zarcone JR, Chambers R, Butler MG, Bittel DC, Brooks WM, Thompson TI, Savage CR. Genetic subtype differences in neural circuitry of food motivation in Prader–Willi syndrome. Int J Obes (Lond) 2009;33(2):273–283. doi: 10.1038/ijo.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantoulan C, Payous P, Diene G, Glattard M, Roge B, Molinas C, Sevely A, Zilbovicius M, Celsis P, Tauber M. PET scan perfusion imaging in the Prader–Willi syndrome: new insights into the psychiatric and social disturbances. J Cereb Blood Flow Metab. 2011;31(1):275–282. doi: 10.1038/jcbfm.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JL, James GA, Goldstone AP, Couch JA, He AG, Driscoll DJ, Liu Y. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader–Willi syndrome. J Neurol Neurosurg Psychiatry. 2007;78(6):615–619. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogura K, Shinohara M, Ohno K, Mori E. Frontal behavioral syndromes in Prader–Willi syndrome. Brain Dev. 2008;30(7):469–476. doi: 10.1016/j.braindev.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y. Satiety dysfunction in Prader–Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry. 2005;76(2):260–262. doi: 10.1136/jnnp.2004.039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Deneen KM, Gold NS, Liu Y. Food addiction and cues in Prader–Willi syndrome. J Addict Med. 2009;3(1):19–25. doi: 10.1097/ADM.0b013e31819a6e5f. [DOI] [PubMed] [Google Scholar]
- 21.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68(8):808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunay-Aygun M, Schwartz S, Heeger S, O’Riordan MA, Cassidy SB. The changing purpose of Prader–Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics. 2001;108(5):E92. doi: 10.1542/peds.108.5.e92. [DOI] [PubMed] [Google Scholar]
- 23.Benarroch F, Hirsch HJ, Genstil L, Landau YE, Gross-Tsur V. Prader–Willi syndrome: medical prevention and behavioral challenges. Child Adolesc Psychiatr Clin N Am. 2007;16(3):695–708. doi: 10.1016/j.chc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Glenn CC, Driscoll DJ, Yang TP, Nicholls RD. Genomic imprinting: potential function and mechanisms revealed by the Prader–Willi and Angelman syndromes. Mol Hum Reprod. 1997;3(4):321–332. doi: 10.1093/molehr/3.4.321. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader–Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- 26.Pagliardini S, Ren J, Wevrick R, Greer JJ. Developmental abnormalities of neuronal structure and function in prenatal mice lacking the Prader–Willi syndrome gene necdin. Am J Pathol. 2005;167(1):175–191. doi: 10.1016/S0002-9440(10)62964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassidy SB. Prader–Willi syndrome. J Med Genet. 1997;34(11):917–923. doi: 10.1136/jmg.34.11.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstone AP. Prader–Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15(1):12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Nixon GM, Brouillette RT. Sleep and breathing in Prader–Willi syndrome. Pediatr Pulmonol. 2002;34(3):209–217. doi: 10.1002/ppul.10152. [DOI] [PubMed] [Google Scholar]
- 30.Koenig K, Klin A, Schultz R. Deficits in social attribution ability in Prader–Willi syndrome. J Autism Dev Disord. 2004;34(5):573–582. doi: 10.1007/s10803-004-2551-z. [DOI] [PubMed] [Google Scholar]
- 31.Holsen LM, Zarcocne JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying hyperphagia in Prader–Willi syndrome. Obesity. 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SE, Jin DK, Cho SS, Kin JH, Hong SD, Paik KH, Oh YJ, Kin AH, Kwon EK, Choe YH. Regional cerebral glucose metabolic abnormality in Prader–Willi syndrome: a 18 F-FDG PET study under sedation. J Nucl Med. 2006;47(7):1088–1092. [PubMed] [Google Scholar]
- 33.Ogura K, Fujii T, Abe N, Hosokai Y, Shinohara M, Takahashi S, Mori E. Small gray matter volume in orbitofrontal cortex in Prader–Willi syndrome: a voxel-based MRI study. Hum Brain Mapp. 2010;32(7):1059–1066. doi: 10.1002/hbm.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, Fritsche A, Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-García I, Jurado MA, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I, Pueyo R, Sender-Palacios MJ, Vernet-Vernet M, Narberhaus A, Ariza M, Junqué C. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckmann CF, Deluca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantini D, Perruco MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104(32):13, 170–13, 175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer T, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104(47):18, 760–18, 765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 42.Damoiseaux JS, Bkackford J, Walden T, Thompson T. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13, 848–13, 853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JL, Couch JA, Schmalfuss I, He G, Liu Y, Driscoll DJ. Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader–Willi syndrome. Am J Med Genet A. 2007;143:476–483. doi: 10.1002/ajmg.a.31508. [DOI] [PubMed] [Google Scholar]
- 44.Miller JL, Couch JA, Leonard CM, Schwenk K, Towler SD, Shuster J, Goldstone AP, He G, Driscoll DJ, Liu Y. Sylvian fissure morphology in Prader–Willi syndrome and early-onset morbid obesity. Genet Med. 2007;9:536–543. doi: 10.1097/gim.0b013e31812f720d. [DOI] [PubMed] [Google Scholar]
- 45.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Wen X, Mo J, Ding M. Exploring resting-state functional connectivity with total interdependence. Neuroimage. 2012;60(2):1587–1595. doi: 10.1016/j.neuroimage.2012.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 48.Hinton EC, Holland AJ, Gellatly MS, Soni S, Owen AM. An investigation into food preferences and the neural basis of food-related incentive motivation in Prader–Willi syndrome. J Intellect Disabil Res. 2006;50(Pt 9):633–642. doi: 10.1111/j.1365-2788.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 49.Martin LE, Holson LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 50.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 51.Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- 52.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lathe R. Hormones and the hippocampus. J Endocrinol. 2001;169(2):205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- 54.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127(1–2):13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 56.Halgren E. Mental phenomena induced by stimulation in the limbic system. Hum Neurobiol. 1982;1(4):251–260. [PubMed] [Google Scholar]
- 57.Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T, Considine RV, Kareken DA. Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity. 2010;18(8):1566–1571. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- 58.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4(7):e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gautier JF, Chen K, Uecker A, Bandy D, Frost J, Salbe AD, Pratley RE, Lawson M, Ravussin E, Reiman EM, Tataranni PA. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: a positron emission tomography study. Am J Clin Nutr. 1999;70(5):806–810. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- 60.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 61.Pelchat ML, Johnson A, Chan R, Valder J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Kringelbach ML, de Araujo LE, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21(2):781–788. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 63.Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66(1):96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- 64.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32(3):1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 66.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 68.Levy LM, Henkin RI, Lin CS, Finley A, Schellinger D. Taste memory induces brain activation as revealed by functional MRI. J Comput Assist Tomogr. 1999;23(4):499–505. doi: 10.1097/00004728-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 70.Gordon CM, Dougherty DD, Rauch SL, Emans SJ, Grace E, Lamm R, Alpert NM, Majzoub JA, Fischman AJ. Neuroanatomy of human appetitive function: a positron emission tomography investigation. Int J Eat Disord. 2000;27(2):163–171. doi: 10.1002/(sici)1098-108x(200003)27:2<163::aid-eat4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 71.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 72.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21(4):1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 73.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 74.Fan J, McCandiss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Lane RD, Reiman EM, Axelrod B, Yun LS, Holman A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10(4):525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 76.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 77.Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Narayanan NS, Laubach M. Top–down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52(5):921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88(1):37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 80.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 81.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 82.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dichstein RJ, Dichstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]


